Abstract
In active tuberculosis, T-cell response to Mycobacterium tuberculosis is known to be reduced. In the course of Mycobacterium tuberculosis infection in mice, we observed that T-cell proliferation in response to M. tuberculosis purified protein derivative (PPD) reached the maximum level on day 7, then declined to the minimal level on day 14, and persisted at a low level through day 28 postinfection. The frequency of PPD-specific CD4 T cells in the spleen on day 28 decreased to one-sixth on day 7. To further investigate the mechanism of this T-cell hyporesponsiveness, we next analyzed the suppressive activity of spleen macrophages on T-cell function. The nonspecific proliferative response of naive T cells and the PPD-specific proliferative response of T cells were suppressed by day 28 macrophages, but not by day 7 macrophages or naive macrophages. This reduction of proliferative response was restored by addition of nitric oxide synthesis inhibitor, NG-monoethyl-l-arginine monoacetate, but not by monoclonal antibody against interleukin 10 or transforming growth factor β. These data indicate that the macrophages from mice chronically infected with M. tuberculosis suppress T-cell response through production of nitric oxide, suggesting that nitric oxide-induced elimination mediated by activated macrophages may reduce the T-cell response and the number of mycobacterium-specific CD4 T cells in vivo.
Mycobacterium tuberculosis is an acid-fast intracellular pathogen that resides mainly in the macrophages of hosts and that causes a chronic infection. It is known that M. tuberculosis induces T-cell-mediated immunity. The primed mycobacterium-reactive T cells, which consist mainly of CD4 T cells, activate infected macrophages in granulomatous lesions by Th1-type cytokines (21, 34). The activated macrophages subsequently produce bactericidal effector molecules, such as nitric oxide (6, 9, 16). Thus, the interaction between T cells and macrophages is critical for prevention of bacterial growth (4, 8, 34). It is also possible that macrophages in infected sites influence the T-cell response in vivo. Our group and others have previously reported that induction of T-cell-mediated immune response was highly influenced by the activated macrophages (26, 30, 31, 36, 45, 48).
Some patients with tuberculosis showed depressed immune response (41). Depressed proliferative response and interleukin 2 (IL-2) production of PBMC against purified protein derivative (PPD) of M. tuberculosis (25, 43) was found in 40 to 60% of patients with active pulmonary tuberculosis. In experimental murine tuberculosis, it was reported that gamma interferon (IFN-γ) production by CD4 T cells declined after the third or fourth week of infection (35). Nonspecific response to concanavalin A or antigen (Ag)-specific proliferative response by splenocytes also declined after mycobacterial infection (36). Other chronic disease pathogens, such as Leishmania, Trypanosoma, and Toxoplasma, are also reported to induce T-cell hyporesponsiveness (23, 28, 39, 46). Nitric oxide derived from macrophages was reported to be a major cause of T-cell suppression in murine Salmonella (3, 10, 11) and Trypanosoma (38) infections. In M. tuberculosis infection in mice, the mechanisms of the reduction of T-cell responses are not clearly understood. Furthermore, it remains unknown whether the immune suppression contributes to exacerbation of the disease.
The purpose of this study is to determine the mechanisms of T-cell hyporesponsiveness during M. tuberculosis infection. We report here that, in murine experimental tuberculosis, T-cell-mediated immune response was suppressed in the chronic stage of infection. The hyporesponsiveness was associated with a reduced number of mycobacterium-specific CD4 T cells. We also show that the activated macrophages from mice at day 28 postinfection suppressed T-cell response to anti-T-cell receptor (TCR) monoclonal antibodies (MAb) and PPD by NO.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice bred under specific-pathogen-free conditions were purchased from Japan SLC (Shizuoka, Japan). Seven- to 9-week-old mice were used for experiments.
Bacteria and infection.
Mice were infected intravenously with 106 CFU of M. tuberculosis (H37Rv) harvested at the mid-log phase of growth in Middlebrook 7H9 medium (Difco Laboratories, Detroit, Mich.) supplemented with 0.05 mg of oleic acid (Wako Pure Chemical Industries Ltd., Osaka, Japan)/ml, 0.05% Tween 80 (Difco), 2 mg of dextrose/ml, 0.85 mg of NaCl/ml, and 5 mg of bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.)/ml and kept frozen.
Preparation of lymphocytes and macrophages.
Spleens were aseptically removed from mice and teased between two sterile glass slides. After erythrocytes were lysed by treatment with 0.83% ammonium chloride in 0.17 mM Tris-HCl (pH 7.6), the spleen cells were washed twice with Hanks balanced salt solution and suspended in RPMI 1640 medium (Gibco, Grand Island, N.Y.) supplemented with 10% fetal calf serum (Hyclone, Logan, Utah), 50 μM 2-mercaptoethanol, 20 μM HEPES, and 0.02% sodium bicarbonate. Nylon wool-passed cells and plastic-adherent cells were used as T cells and macrophages, respectively. Lymph node T cells were prepared from the mesenteric lymph nodes of uninfected mice. In some experiments, CD4 T cells were enriched from T cells by depletion of CD8 T cells with a supernatant of anti-CD8 MAb-producing hybridoma (83.12.5) plus complement.
Proliferation assay.
T cells (2 × 105/well) prepared from spleens were cultured in 96-well tissue culture plates (Coster, Cambridge, Mass.) in triplicate, with or without 10 μg of PPD of M. tuberculosis (Aoyama strain) (Japan BCG Inc., Tokyo, Japan), for 72 h in the presence of 4 × 105 irradiated splenocytes/well as antigen-presenting cells (APC). In some experiments, recombinant human IL-2 (rIL-2; generously provided by Takeda Chemical Industries Ltd., Osaka, Japan) was added. Whole spleen cells (4 × 105/well) instead of purified T cells were used in proliferation assays in some experiments. Cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting.
Cytokine measurements.
T cells (106/well) from spleens were cultured in 24-well tissue culture plates in triplicate, with or without 10 μg of PPD/ml for 72 h in the presence of 2 × 106 irradiated splenocytes/well, and the culture supernatants were collected and used for cytokine measurements by standard sandwich enzyme-linked immunosorbent assay (ELISA), as described in a previous report with minor modification (24). Briefly, the supernatants were added to the wells of enzyme immunoassay plates (Greiner, Frickenhausen, Germany), precoated overnight with 2 μg of rat anti-mouse IFN-γ MAb (R4-6A2) (PharMingen, San Diego, Calif.) or rat anti-mouse IL-4 MAb (11B11) (PharMingen). After incubation for 60 min at room temperature, the plates were washed with phosphate-buffered saline containing 0.05% Tween 20 and incubated at room temperature for 60 min with 2 μg of biotin-conjugated anti-mouse IFN-γ (XMG1.2) (PharMingen) or IL-4 MAb (BVD6-24G2) (PharMingen)/ml. The plates were then washed, and streptavidin-β-galactosidase (Gibco) was added at a dilution of 1/1,000 to each well and incubated for 45 min. After the plates were washed, substrate solution containing 0.2 mM 4-methylumbelliferyl-β-d-galactopyranoside (Wako) was added to the wells, which were left for 45 to 60 min while being protected from direct light. Finally, after the addition of 0.1 M glycin-NaOH (pH 10.2), absorbance was measured with a fluorescence microplate reader (MTP-32; Corona Co., Ltd., Ibaragi, Japan). The values for IFN-γ and IL-4 were calculated from a standard curve of recombinant mouse IFN-γ and IL-4 (PharMingen).
Limiting-dilution assay.
The frequency of PPD-reactive cells was determined by limiting-dilution assay as previously described (5, 29). Briefly, CD4 T cells prepared from spleens were plated in 96-well tissue culture plates in 12 replicates for each concentration and cultured for 72 h with or without 10 μg of PPD/ml in the presence of APC. The cultures were pulsed with 1 μCi of [3H]thymidine, and [3H]thymidine uptake was measured by liquid scintillation counting. Wells of culture with PPD were defined as positive when [3H]thymidine uptake was higher than the mean ± 3 standard deviations (SD) of Ag-negative wells containing the same cell concentrations.
Inhibition of T-cell-proliferative response by macrophages.
Splenocytes were analyzed for their Mac-1 expression by fluorescence-activated cell sorter and plated in 96-well-plates, each containing 3 × 105 Mac1-positive cells as macrophages. The plates were then incubated for 60 min to allow the macrophages to adhere to the plates and washed twice with warm phosphate-buffered saline to remove nonadherent cells. Naive lymph node T cells (5 × 104/well) were added to the macrophage monolayer and cultured for 72 h in the presence of 20 μg of anti-TCR β MAb (H57-597)/ml and 20 μg of streptomycin (Meiji)/ml. In some experiments, spleen T cells from infected mice on day 7 were used instead of naive lymph node T cells and stimulated with 10 μg of PPD/ml instead of anti-TCR β MAb. The cultures were pulsed with 1 μCi of [3H]thymidine, and [3H]thymidine uptake was measured by liquid scintillation counting. To neutralize the cytokines, anti-IL-10 (SXC-1) (PharMingen), and anti-pan transforming growth factor β (TGF-β) (R & D Systems, Minneapolis, Minn.) were added at 20 μg/ml. Ten millimolar NG-monoethyl-l-arginine monoacetate (L-NMMA) (Wako) and NG-monoethyl-d-arginine monoacetate (D-NMMA) (Wako) were used as an inhibitor of NO synthase and its control, respectively.
NO assay.
Macrophages prepared by the method mentioned above were cultured for 72 h with medium only. The production of NO was estimated by measuring the amount of nitrite in the culture supernatant according to the method of Green et al. (17) with Greiss reagent (Wako). Absorbance was measured with a microplate reader. The titer was determined by the standard curve generated by the absorbance of serial dilutions of NaNO2.
Statistical analysis.
Student’s t test (two tailed) was used for statistical analysis; a P value of less than 0.05 was considered significant.
RESULTS
Kinetics of PPD-reactive T-cell response after infection with M. tuberculosis.
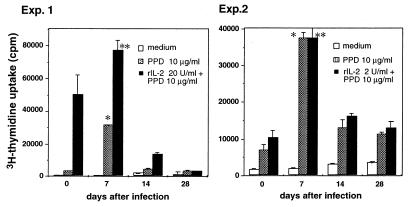
In the first set of experiments, we examined the M. tuberculosis-specific proliferative response of spleen T cells in mice during the course of M. tuberculosis infection (Fig. 1). T cells obtained on day 7 of infection showed a high level of proliferation in response to PPD in the presence of naive syngeneic irradiated splenocytes. On day 14 of infection, however, the T-cell proliferative response to PPD was significantly decreased, and the low level of T-cell proliferative response persisted through day 28 of infection. This reduced response cannot be explained by typical T-cell anergy, since the addition of 20 (experiment 1) and 2 (experiment 2) U of rIL-2 to the cultures failed to restore the proliferative response on day 14 and day 28. Since a high number of mycobacteria was detected in spleens on both day 7 [(4.3 ± 0.8) × 106 CFU/spleen], and day 28 [(2.8 ± 0.6) × 106 CFU/spleen], the lack of responsiveness on day 28 cannot be explained by differences in bacterial load or amount of Ag. The suppressed proliferative response of spleen T cells to PPD in mice infected with M. tuberculosis is observed for at least 90 days after infection (data not shown).
FIG. 1.
Kinetic analysis of proliferative response to PPD by spleen T cells from mice infected with M. tuberculosis. The mice were infected intravenously with 106 CFU of M. tuberculosis H37Rv. On the indicated days, nylon wool-passed spleen cells (2 × 105) from four to six spleens were cultured in triplicate with or without 10 μg of PPD/ml for 72 h in the presence of irradiated syngeneic naive splenocytes as APC and 20 (experiment 1) or 2 (experiment 2) μg of rIL-2/ml was added to some of the cultures. The cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting. The results are presented as the mean values of triplicate wells ± SD. ∗, P < 0.05 versus T cells cultured with PPD on days 0, 14, and 28. ∗∗, P < 0.05 versus T cells cultured with PPD plus IL-2 on days 0, 14, and 28. Exp., experiment.
Kinetics of cytokine production by T cells.
To determine if T cells on day 28 produce suppressive cytokines in an Ag-specific manner, culture supernatants were assayed for cytokine production by the standard sandwich ELISA (Table 1). IFN-γ production in response to PPD was detected from day 7 and reached a peak on day 14. On day 28 of infection, IFN-γ production by spleen T cells was decreased compared to that on day 14, but significant IFN-γ production was still detected. IL-4 (Table 1) and IL-10 (data not shown), which were thought to be suppressive for Th1 response, were not detected in the samples. These data did not support Th2-type T-cell influence on T-cell proliferation and IFN-γ production on day 28. It is notable that there were high levels of IFN-γ production on day 14 and day 28, when PPD-specific proliferative response was low.
TABLE 1.
Kinetics of cytokine production by T cells in mice infected with M. tuberculosis
| Stimulus | IFN-γ production (U/ml)a
|
IL-4 production (U/ml)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Naive | Day 7 | Day 14 | Day 28 | Naive | Day 7 | Day 14 | Day 28 | |
| Medium | <2.0 | <2.0 | <2.0 | <2.0 | <1.0 | <1.0 | <1.0 | <1.0 |
| PPD | <2.0 | 142.6 ± 12.0b | 343.2 ± 14.9 | 101.7 ± 3.2c | <1.0 | <1.0 | <1.0 | <1.0 |
Mice were infected intravenously with 106 CFU of H37Rv. At the indicated days, nylon wool-passed cells from the spleen (2 × 105) were cultured in triplicate with or without 10 μg of PPD/ml for 72 h in the presence of irradiated splenocytes as APC. Culture supernatants were assayed for IFN-γ and IL-4 by standard sandwich ELISA.
Results are presented as the mean values of triplicate wells ± SD.
P < 0.05 versus day 14.
Frequency of PPD-reactive CD4 T cells in spleens after infection with M. tuberculosis.
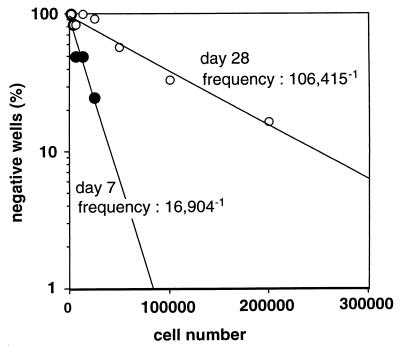
We next checked if the frequency of PPD-responsive T cells had decreased in vivo on day 28 of infection. As shown in Fig. 2, we found, by limiting-dilution assay, that the frequency of PPD-reactive CD4 T cells in spleens on day 28 (1/106,415) was lower than that on day 7 (1/16,904). This result suggests that the number of mycobacterium-reactive CD4 T cells in the spleen declines in the course of M. tuberculosis infection from day 7 to day 28.
FIG. 2.
Frequencies of PPD-reactive CD4 T cells in the spleen after infection with M. tuberculosis. Mice were infected intravenously with 106 CFU of M. tuberculosis H37Rv. On day 7 or day 28, a graded number of CD4 T cells (1,560 to 200,000/well) from four pooled spleens were cultured with or without 10 μg of PPD/ml for 72 h in the presence of irradiated syngeneic naive splenocytes as APC. The cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting. Positive wells (PPD-reactive wells) were counted as those with cpm of more than the mean plus 3 SD of wells without PPD in each dilution. The results are representative of one of two similar experiments.
Inhibition of T-cell proliferation induced by macrophages from mice infected with M. tuberculosis.
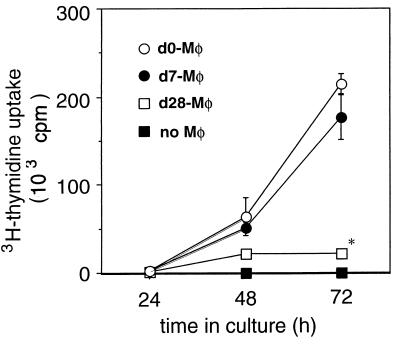
Since it was reported that activated macrophages induce T-cell apoptosis (31), we hypothesized that the activated macrophages on day 28 of infection can kill or suppress the mycobacterium-specific T cells. To address this hypothesis, we analyzed the inhibition of T-cell stimulation by the macrophages from mice infected with M. tuberculosis by using an in vitro system. In the experiments, naive lymph node T cells were stimulated by anti-TCR β MAb in the presence of spleen macrophages derived from naive mice or M. tuberculosis-infected mice on day 7 and day 28. As shown in Fig. 3, although there was high proliferative response of naive T cells to anti-TCR MAb in wells to which naive or day 7 macrophages were added, the T-cell proliferative response significantly decreased in the presence of day 28 macrophages. Furthermore, we found that PPD-specific proliferative response of spleen T cells from mice on day 7 postinfection significantly decreased when they were cultured with day 28 macrophages (Fig. 4B).
FIG. 3.
Inhibition of T-cell proliferation induced by macrophages from mice infected with M. tuberculosis on day 28. Nylon wool-passed lymph node cells (5 × 104) from naive mice were stimulated with 20 μg of anti-TCR β MAb/ml in the absence (solid squares) or presence of splenic adherent cells from naive mice (open circles) on day 7 (solid circles), and day 28 (open squares) after infection. The cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting. The results are presented as the mean values of triplicate wells ± SD. The results are representative of one of three similar experiments. ∗, P < 0.05 versus T cells with day 0 and day 7 macrophages.
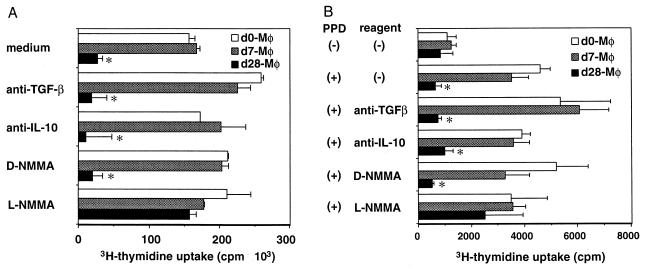
FIG. 4.
Inhibition of T-cell proliferation by day 28 macrophages was restored by L-NMMA. Nylon wool-passed lymph node cells from naive mice (A) and nylon wool-passed spleen cells from mice on day 7 post-M. tuberculosis infection (B) were stimulated with anti-TCR MAb (A) or PPD (B) for 72 h with splenic adherent cells (macrophages) from mice on day 0, day 7, and day 28 of infection. Anti-IL-10 MAb, anti-pan-TGF-β MAb, L-NMMA, or D-NMMA was added to the cultures. The cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting. The results are presented as the mean values of triplicate wells ± SD. The results are representative of one of three similar experiments. ∗, P < 0.05 versus T cells cultured with day 0 and day 7 macrophages (Mφ). (+), present; (−), absent.
Inhibition of T-cell proliferation induced by day 28 macrophages was restored by L-NMMA.
From the data mentioned above, it is possible that day 28 macrophages actively suppress T-cell response. NO is a possible suppressor because NO is produced mainly by activated macrophages in infections with mycobacteria (33, 47) or other intracellular parasites (37). NO is not only a bactericidal effector molecule (6, 7, 9, 16) but also a suppressive factor for Th1 (39, 40, 46). Other candidates are macrophage-derived suppressive cytokines, such as TGF-β and IL-10. We studied the effects of blocking these substances on suppression of T-cell response mediated by day 28 macrophages.
The cpm values of anti-TCR β MAb-stimulated T-cell proliferation when T cells were cultured with day 28 macrophages were restored by the addition of L-NMMA, an NO synthase inhibitor (Fig. 4A). Those of PPD-specific T cell proliferation when T cells were cultured with day 28 macrophages were partially restored by the addition of L-NMMA (Fig. 4B). In contrast, an isomer of L-NMMA, D-NMMA, which lacks NO synthesis-blocking activity, showed no effect on the T-cell responses. Furthermore, macrophage-derived nitrite on day 28 of infection (4.40 ± 1.19 μM), measured in culture supernatant, was approximately fourfold higher than that from naive macrophages (1.26 ± 0.44 μM) or that from macrophages on day 7 (1.45 ± 0.29 μM). In contrast, cytokine-neutralizing experiments showed that neither anti-IL-10 nor TGF-β MAb restored the T-cell proliferative response against anti-TCR β MAb (Fig. 4A) or PPD (Fig. 4B). These data strongly suggest that day 28 macrophages suppress the T-cell proliferation through NO production. The absolute cpm values of proliferative responses shown in Fig. 4B were lower than those in Fig. 4A, since PPD stimulation was weaker than mitogenic stimulation of anti-TCR MAb. In addition, the Ag (PPD)-presenting capacity of macrophages was considered to be lower than that of irradiated splenocytes, as shown in Fig. 1.
The effect of L-NMMA on the proliferative response of total spleen cells from mice infected with M. tuberculosis.
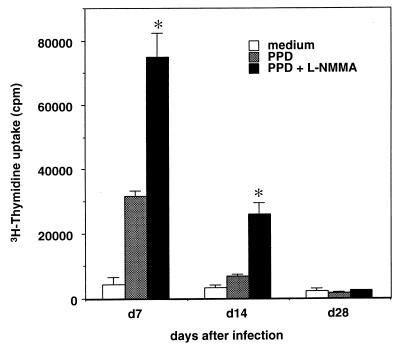
To demonstrate the significance of NO in an in vivo situation, we analyzed the proliferative response of total spleen cells in response to PPD (Fig. 5). On day 28 of infection, when NO production from macrophages was increased, L-NMMA did not restore the proliferation in response to PPD. This result may be explained by NO-mediated elimination of PPD-reactive T cells on day 28 of infection, as shown in Fig. 2. Interestingly, L-NMMA restored the proliferative response of day 7. This result is probably due to the low levels of NO produced by macrophages, which can suppress T-cell proliferation but are not sufficient to eliminate T cells.
FIG. 5.
Proliferative response of total spleen cells from mice infected with M. tuberculosis in the presence or absence of L-NMMA. On the indicated days, total spleen cells (4 × 105/well) were cultured in triplicate with medium alone, PPD, or PPD plus L-NMMA. The cultures were pulsed with 1 μCi of [3H]thymidine for the last 6 h of culture, and [3H]thymidine uptake was measured by liquid scintillation counting. The results are presented as the mean values of triplicate wells ± SD. ∗, P < 0.05 versus T cells cultured with PPD in the absence of L-NMMA.
Failure to transfer the suppressive effect on proliferative response by supernatants obtained from cultures with activated macrophages.
To investigate whether the suppressive effect of macrophages on T cells is transferable by culture supernatant, we performed the following experiment. The macrophages from mice infected with M. tuberculosis on day 90 were cultured for 72 h, and the supernatant was collected. The supernatant was transferred to the wells with the whole spleen cells from mice on day 7 of infection and cultured for 72 h with PPD or anti-TCR MAb. Transferred supernatant had no suppressive effect against the proliferative response to the stimuli (data not shown). This result is consistent with NO-mediated suppression, because the half-life of NO is too short for it to transfer by culture supernatant.
DISCUSSION
Our study demonstrated that activated macrophages obtained on day 28 from mice infected with M. tuberculosis have strong suppressive activity on T-cell response against mycobacterial Ag and mitogenic anti-TCR MAb. Furthermore, the frequency of mycobacterial Ag-reactive T cells in the spleen on day 28 of M. tuberculosis infection was decreased compared to that at earlier stages of infection. These observations suggest that activated macrophages in mice chronically infected with M. tuberculosis suppress T-cell response, which may subsequently induce apoptosis of mycobacterial Ag-reactive T-cell populations. There are several possible mechanisms to explain the macrophage-mediated suppression of T-cell response, as discussed below.
Our results show that NO is involved in the suppression of T-cell response by the macrophages from mice chronically infected with M. tuberculosis. Macrophages from M. tuberculosis-infected mice on day 28 produced three to four times more NO than macrophages obtained on day 7 of infection and those from naive mice. Blocking of NO synthesis by L-NMMA restored the proliferative response of T cells cultured with the day 28 macrophages. These observations demonstrated that the large amount of NO produced by the suppressor macrophages on day 28 of M. tuberculosis infection suppresses T-cell response in vitro. It has been shown in other systems that NO contributes not only to killing pathogens but also to suppressing T-cell responses, especially Th1-type responses (39, 40, 46), which further supports our data showing NO-mediated T-cell suppression. In the present study, the restoration of proliferative response of total spleen cells by L-NMMA was not observed on day 28 (Fig. 5), when a larger amount of NO was produced by macrophages. This was explained by the reduced number of PPD-reactive T cells on day 28 of infection in vivo. On the other hand, it is surprising that L-NMMA restored PPD-specific proliferation on day 7 (Fig. 5), although NO production by macrophages was at low levels on day 7 of infection. We can explain this discrepancy by the fact that NO-induced T-cell suppression is observed even on day 7 with a small amount of NO, although the number of PPD-specific cells is not reduced.
NO-induced apoptosis may explain both the in vivo decline in the frequency of mycobacterial Ag-reactive CD4 T cells in the spleen observed on day 28 of M. tuberculosis infection and the in vitro macrophage-mediated suppression of T-cell response. There are several reports showing NO as the mediator of apoptosis. NO has been shown to induce apoptosis of human lymphoblastoid cells (32), mouse thymocytes (13), mouse peritoneal macrophages (1), rat lung epithelial cells (19), rat hepatoma cells (27), and rat islet cells (12), possibly through excessive activation of poly(ADP-ribose) polymerase and a consequent depletion of its substrate NAD+ (18). Apoptosis was also induced on peripheral T cells by macrophage colony-stimulating factor-stimulated macrophages in a Fas- and Bcl-2-independent manner when the T cells recognized Ag expressed by the macrophages (31). Therefore, it is highly possible that apoptosis is also induced in T cells by the high level of NO produced by the activated macrophages from mice chronically infected with M. tuberculosis both in vivo and in vitro. However, it is still debatable whether in vitro suppression of naive T-cell response by macrophages from chronically infected mice, as shown in Fig. 3 to 5, is accompanied by apoptosis. We stained T cells cultured with macrophages from naive or infected mice on day 7 or day 28 with an in situ nicking and end-labeling technique and used flow cytometry to detect apoptotic cells. However, the T-cell preparation contained a large amount of dead cells and cell debris even in the control culture with naive macrophages, which made the analysis difficult (data not shown). More sensitive methods to detect apoptotic cells or an improved culture system that induces less non-specific cell death would be required to detect differences of apoptosis in vitro.
Another possible mechanism of macrophage-mediated suppression of T-cell response is the lack of costimulation of T cells. It is known that T-cell activation in the absence of costimulation by the B-7–CD28 system induces T-cell anergy (22). Saha et al. reported that B-7 expression on BALB/c macrophages was downregulated after in vitro M. tuberculosis infection (36), which supports T-cell hyporesponsiveness induced by the lack of costimulation after M. tuberculosis infection. In our M. tuberculosis in vivo experiments, there is a possibility that the lack of costimulation may induce T-cell anergy on day 28 of infection. Further analysis of B-7 expression on macrophages and CD28 expression on T cells is needed. However, it is suggested that anergy induced by the lack of costimulation may not be the cause of T-cell suppression, because addition of IL-2 failed to restore the proliferation of T cells from mice on day 28 postinfection (Fig. 1). Previously, it was also reported that the addition of IL-2 did not restore the suppression of anti-sheep erythrocyte plaque-forming cell response of spleen cells after infection with Salmonella typhimurium in mice (2).
Macrophages are also known to produce cytokines that suppress T-cell responses. It was reported, in patients with active tuberculosis, that blood monocytes release molecules suppressive for T-cell response, such as soluble IL-2 receptor (44) and TGF-β (42). The monocyte-derived TGF-β was shown to suppress proliferation and IFN-γ production by peripheral blood mononuclear cells in response to PPD (20). It was also reported that IL-10, another potent cytokine suppressive for Th1 response (14), was produced by macrophages infected with mycobacteria (15). However, neutralization of TGF-β and IL-10 did not restore the T-cell hyporesponsiveness induced by the macrophages from mice chronically infected with M. tuberculosis. Prostaglandins are another candidate for molecules which suppress T-cell response. At present, there is no direct data on the role of prostaglandins in T-cell suppression in our system, because we did not use indometacin to block the synthesis of prostaglandins in experiments such as those shown in Fig. 1, 4, and 5. However, we do not consider that prostaglandins are important in the suppression of T-cell response, because culture supernatant obtained from the culture with the activated macrophages from mice on day 90 of infection did not suppress the proliferative response of spleen cells from mice on day 7 of infection. These observations do not support T-cell suppression caused by cytokines produced by macrophages.
Although our results clearly demonstrate low T-cell proliferative response against PPD in the spleens of chronically M. tuberculosis-infected mice and T-cell suppression by macrophages, it is debatable whether the immunosuppression contributes to persistence of the infection or exacerbation of the disease. The number of bacteria in the spleens of M. tuberculosis-infected mice showed no increase on day 28 of infection, although the immune response of the spleen was significantly suppressed. Evidence for the bactericidal effect of NO in mycobacterial infection in mice has been reported by Chan et al. (6). Although NO is necessary for host defense, the significance of NO-mediated T-cell suppression in host defense is unknown. It has been reported that protective cells were induced and maintained even if NO-mediated suppression was demonstrated in vaccination with some Salmonella strains (11). In the present study, it is noteworthy that the IFN-γ production of spleen T cells was clearly demonstrated in response to PPD when the proliferative response to PPD was low. Therefore, protective immunity against mycobacteria could be maintained through IFN-γ production, even when the proliferative response of T cells to mycobacterial Ag is decreased. These IFN-γ-producing T cells are considered to be protective and independent of proliferation, and to have the capacity to avoid apoptosis. To confirm this hypothesis, we need further analysis of T-cell function in the chronic phase of M. tuberculosis infection by such tests as ELISPOT assay of IFN-γ production.
ACKNOWLEDGMENT
This work was supported in part by Japan-U.S. Cooperative Medical Science.
REFERENCES
- 1.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 2.Al-Ramadi B K, Chen Y, Meissler J J, Eisenstein T K. Immunosuppression induced by attenuated Salmonella reversal by IL-4. J Immunol. 1991;147:1954–1961. [PubMed] [Google Scholar]
- 3.Al-Ramadi B K, Meissler J J, Jr, Huang D, Eisenstein T K. Immunosuppression induced by nitric oxide and its inhibition by interleukin-4. Eur J Immunol. 1992;22:2249–2254. doi: 10.1002/eji.1830220911. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P F, Modlin R L. Human cellular immune responses to Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 1996;215:197–219. doi: 10.1007/978-3-642-80166-2_9. [DOI] [PubMed] [Google Scholar]
- 5.Brett S J, Kingston A E, Colston M J. Limiting dilution analysis of the human T cell response to mycobacterial antigens from BCG vaccinated individuals and leprosy patients. Clin Exp Immunol. 1987;68:510–520. [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J, Tanaka K, Carroll D, Flynn J L, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannenberg A M J. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–233. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 9.Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991;132:150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- 10.Eisenstein T K, Huang D, Meissler J J, Al-Ramadi B. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein T K, Meissler J J, Jr, Miller S I, Stocker B A D. Immunosuppression and nitric oxide production induced by parenteral live Salmonella vaccines do not correlate with protective capacity: a phoP::Tn10 mutant does not suppress but does protect. Vaccine. 1998;16:24–32. doi: 10.1016/s0264-410x(97)00160-6. [DOI] [PubMed] [Google Scholar]
- 12.Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes. 1993;42:496–500. doi: 10.2337/diab.42.3.496. [DOI] [PubMed] [Google Scholar]
- 13.Fehsel K, Kröncke K-D, Meyer K L, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 14.Fiorentino D F, Bond M A, Mosmann T R. Two types of mouse helper T cells. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flesch I E A, Hess J H, Oswald I P, Kaufmann S H E. Growth inhibition of Mycobacterium bovis by IFN-γ simulated macrophages: regulation by endogenous tumor necrosis factor-α and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 16.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green L C, Wagner D A, Glogowski J, Skipper P L, Whisnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15-N]-nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 18.Heller B, Wang Z-Q, Wagner E F, Radons J, Bürkle A, Fehsel K, Burkart V, Kolb H. Inactivation of the poly(ADP-ribose) polymerase gene affects oxygen radical and nitric oxide toxicity in islet cells. J Biol Chem. 1995;270:11176–11180. doi: 10.1074/jbc.270.19.11176. [DOI] [PubMed] [Google Scholar]
- 19.Hirano S. Nitric oxide-mediated cytotoxic effects of alveolar macrophages on transformed lung epithelial cells are independent of the β2 integrin-mediated intercellular adhesion. Immunology. 1998;93:102–108. doi: 10.1046/j.1365-2567.1998.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 22.Kaye P M. Costimulation and the regulation of antimicrobial immunity. Immunol Today. 1995;16:423–427. doi: 10.1016/0167-5699(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 23.Khan I A, Matsuura T, Kasper L H. Activation-mediated CD4+ T cell unresponsiveness during acute Toxoplasma gondii infection in mice. Int Immunol. 1996;8:887–896. doi: 10.1093/intimm/8.6.887. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura K, Matsuda K, Ide M, Tokunaga T, Honda M. A fluorescence sandwich ELISA for detecting soluble and cell-associated human interleukin-2. J Immunol Methods. 1989;121:281–288. doi: 10.1016/0022-1759(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 25.Kleinhenz M E, Ellner J J. Antigen responsiveness during tuberculosis: regulatory interaction of T-cell subpopulations and adherent cells. J Lab Clin Med. 1987;110:31–40. [PubMed] [Google Scholar]
- 26.Koga T, Mitsuyama M, Watanabe Y, Yamada A, Yoshikai Y, Nomoto K. Effect of increase in macrophage Ia expression on subsequent immune response in vivo. J Clin Lab Immunol. 1986;20:29–35. [PubMed] [Google Scholar]
- 27.Kurose I, Higuchi H, Yonei Y, Ebinuma H, Watanabe N, Hokari R, Fukumura D, Miura S, Takaishi M, Saito H, Nakatsumi R C, Ishii H. Rat Kupffer cell-derived nitric oxide suppresses proliferation and induces apoptosis of syngeneic hepatoma cells. Gastroenterology. 1996;111:1058–1070. doi: 10.1016/s0016-5085(96)70075-6. [DOI] [PubMed] [Google Scholar]
- 28.Lopes M F, da Veiga V F, Santos A R, Fonseca M E F, DosReis G A. Activation-induced CD4+ T cell death by apoptosis in experimental Chagas’ disease. J Immunol. 1995;154:744–752. [PubMed] [Google Scholar]
- 29.Manca F, Rossi G, Valle M T, Lantero S, Pira G L, Fenoglio D, De Bruin J, Costantini M, Damiani G, Balbi B, Celada F. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect Immun. 1991;59:503–513. doi: 10.1128/iai.59.2.503-513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyata M, Mitsuyama M, Ogata N, Nomoto K, Takeya K. Two steps in the generation of acquired cellular resistance against Listeria monocytogenes: accumulation and activation of macrophages. Immunology. 1982;47:247–253. [PMC free article] [PubMed] [Google Scholar]
- 31.Munn D H, Pressey J, Beall A C, Hudes R, Alderson M R. Selective activation-induced apoptosis of peripheral T cells imposed by macrophages: a potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol. 1996;156:523–532. [PubMed] [Google Scholar]
- 32.Nguyen T, Brunson D, Crespi C L, Penman B W, Wishnok J S, Tannenbaum S R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien L, Roberts B, Andrew P W. In vitro interaction of Mycobacterium tuberculosis and macrophages: activation of anti-mycobacterial activity of macrophages and mechanisms of anti-mycobacterial activity. Curr Top Microbiol Immunol. 1996;215:97–130. doi: 10.1007/978-3-642-80166-2_5. [DOI] [PubMed] [Google Scholar]
- 34.Orme I M, Anderson P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 35.Orme I M, Roberts A D, Griffin J P, Abrams J S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–525. [PubMed] [Google Scholar]
- 36.Saha B, Das G, Vohra H, Ganguly N K, Mishra G C. Macrophage-T cell interaction in experimental mycobacterial infection: selective regulation of co-stimulatory molecules on Mycobacterium-infected macrophages and its implication in the suppression of cell-mediated immune response. Eur J Immunol. 1994;24:2618–2624. doi: 10.1002/eji.1830241108. [DOI] [PubMed] [Google Scholar]
- 37.Stenger S, Donhauser N, Thüring H, Röllinghoff M, Bogdan C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J Exp Med. 1996;183:1501–1514. doi: 10.1084/jem.183.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternberg J, McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg J M, Mabbott N A. Nitric oxide-mediated suppression of T cell response during Tripanosoma brucei infection: soluble tripanosome products and interferon-γ are synergistic inducers of nitric oxide synthase. Eur J Immunol. 1996;26:539–543. doi: 10.1002/eji.1830260306. [DOI] [PubMed] [Google Scholar]
- 40.Taylor-Robinson A W, Liew F Y, Severn A, Xu D, McSorley S J, Garside P, Padron J, Phillips R S. Regulation of the immune response by nitric oxide differentially produced by T helper type 1 and T helper type 2 cells. Eur J Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 41.Toosi Z, Ellner J J. Mechanism of anergy in tuberculosis. Curr Top Microbiol Immunol. 1996;215:221–238. doi: 10.1007/978-3-642-80166-2_10. [DOI] [PubMed] [Google Scholar]
- 42.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 43.Toossi Z, Kleinhenz M E, Ellner J J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toossi Z, Sedor J R, Lapurga J P, Ondash R J, Ellner J J. Expression of functional interleukin 2 receptors by peripheral blood monocytes from patients with active pulmonary tuberculosis. J Clin Investig. 1990;85:1770–1784. doi: 10.1172/JCI114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuru S, Nomoto K, Taniguchi M, Kitani H, Watanabe M, Zinnaka Y. Depression of macrophage functions and T-cell-mediated immunity to Listeria infection in tumor-bearing mice and its prevention by PSK. Cancer Immunol Immunother. 1985;18:160–163. doi: 10.1007/BF00205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei X-Q, Charles I G, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Kawamura I, Zhu H, Mitsuyama M. Involvement of natural killer cells in nitric oxide production by spleen cells after stimulation with Mycobacterium bovis BCG. J Immunol. 1995;155:5728–5735. [PubMed] [Google Scholar]
- 48.Yoshikai Y, Miake S, Matsumoto K, Nomoto K, Takeya K. Effect of stimulation and blockade of mononuclear phagocyte system on the delayed footpad reaction to SRBC in mice. Immunology. 1979;38:577–582. [PMC free article] [PubMed] [Google Scholar]