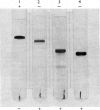
Abstract
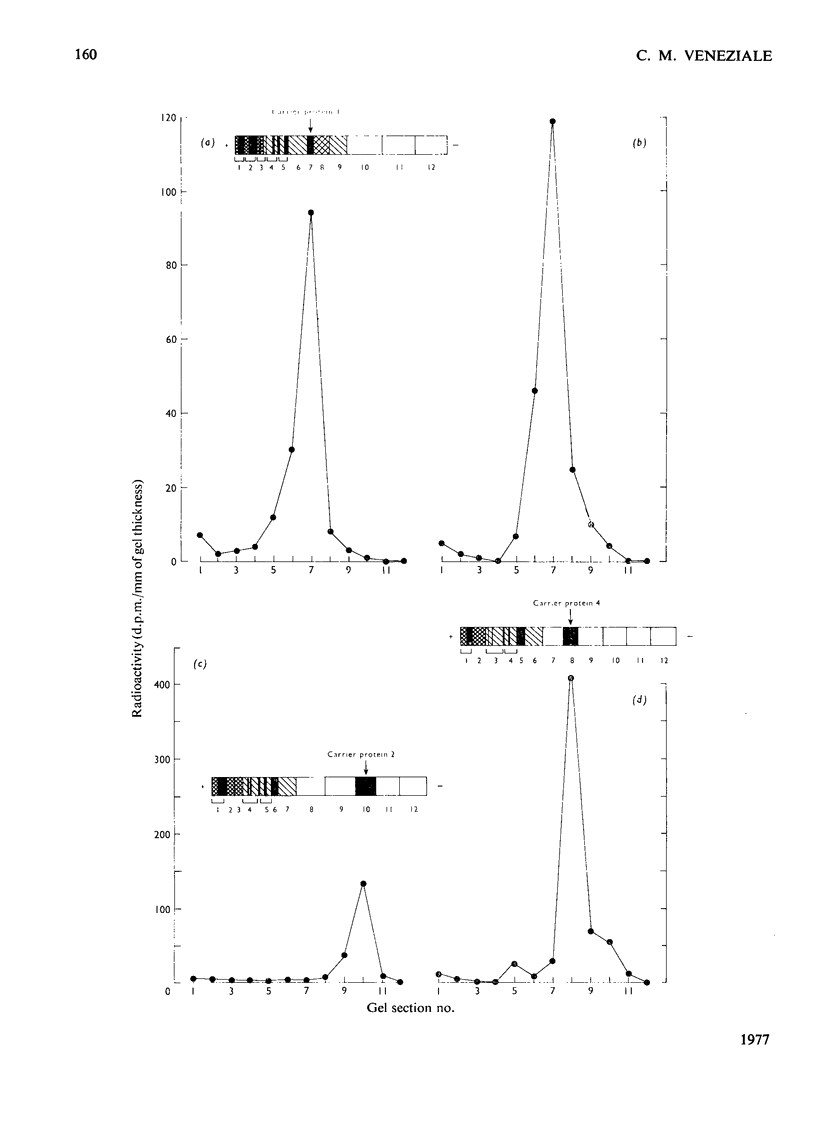
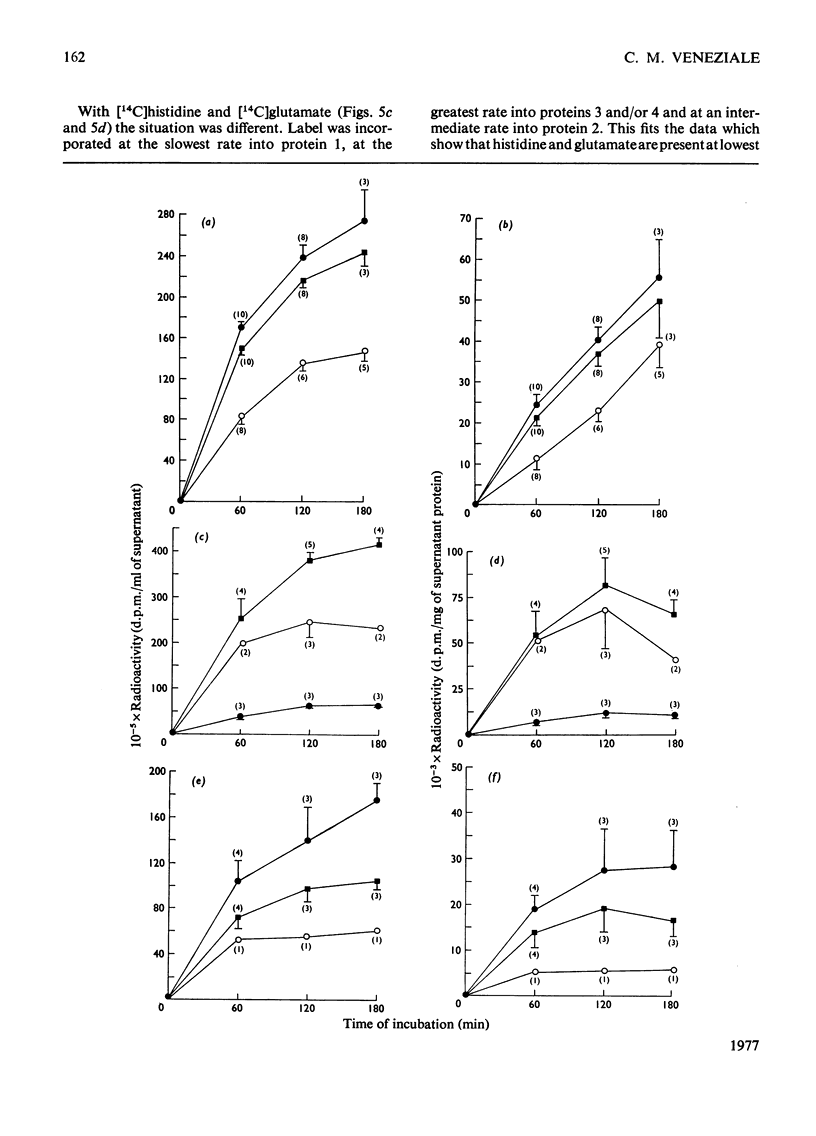
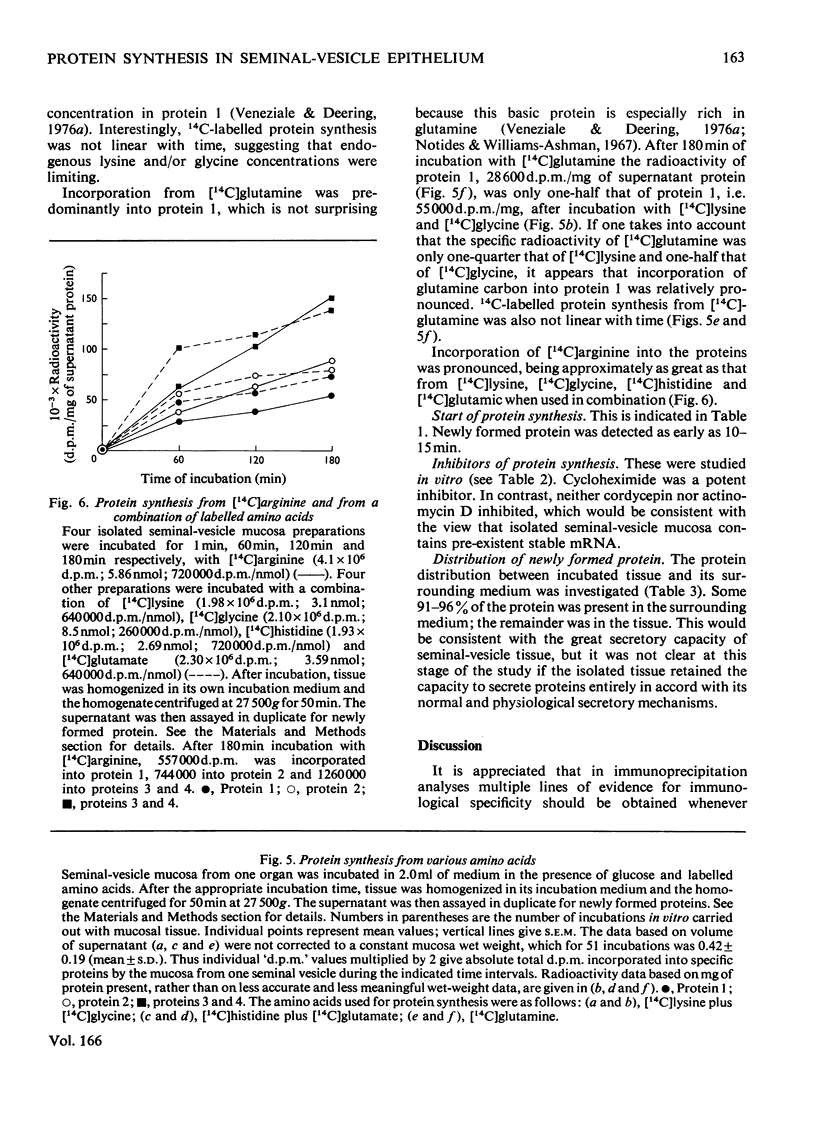
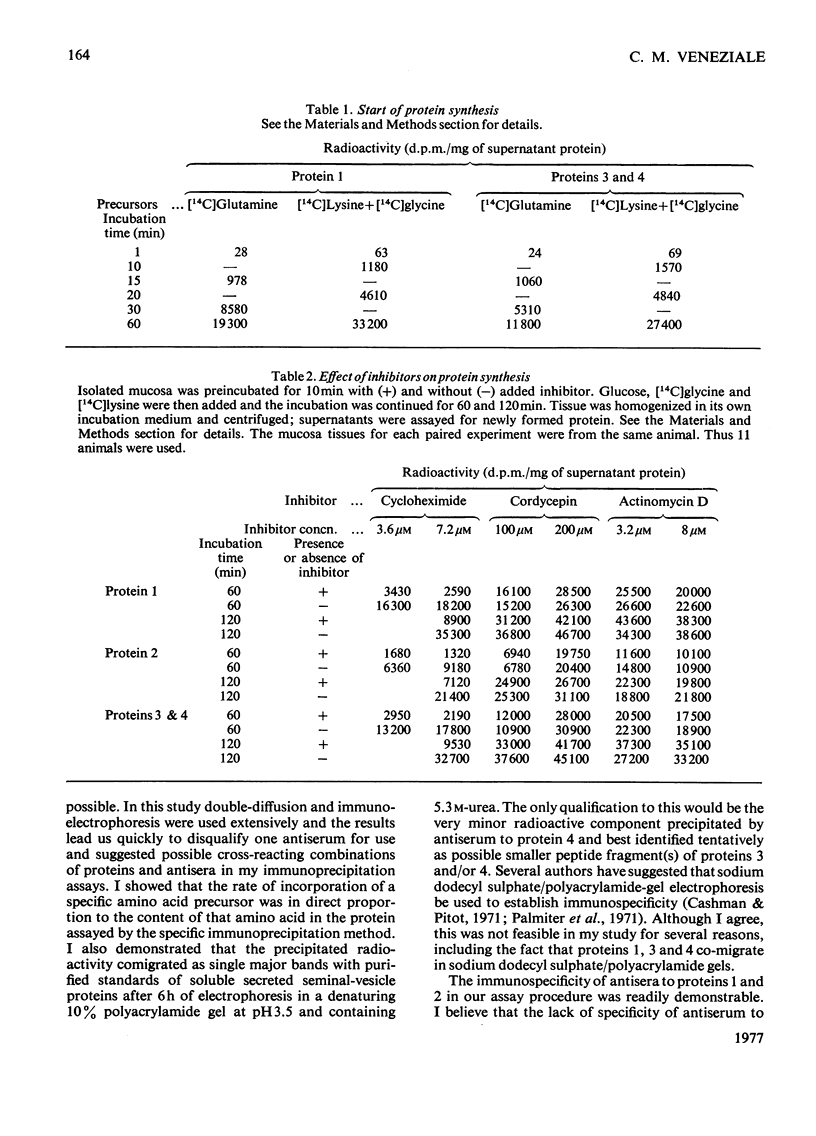
Four intrinsic soluble proteins are synthesized and secreted by sexually mature guinea-pig seminal-vesicle mucosa, which comprises a monolayer of a homogeneous columnar epithelial cell. All four proteins can be extracted readily in 154mm-NaCl from the organ's luminal constituents in which they are present in high concentration. They are referred to as proteins 1, 2, 3 and 4 in order of their elution during DEAE-cellulose column chromatography. Specific primary antibodies were harvested from goats that had been inoculated with the purified vesicular proteins; secondary antibodies were obtained from a donkey inoculated with goat γ-globulins. Double-antibody-immunoprecipitation techniques were developed to precipitate the vesicular proteins. Thus proteins newly synthesized from 14C-labelled amino acids could be precipitated and the incorporated radioactivity assessed. Isolated seminal-vesicle mucosa, incubated in only a buffered salt solution containing glucose, readily synthesized the soluble secreted proteins from added [14C]lysine plus [14C]glycine, [14C]histidine plus [14C]glutamate, [14C]glutamine alone and [14C]arginine alone. The rates of incorporation (d.p.m./mg of total soluble protein) of labelled lysine and glycine and of labelled arginine were linear with time over 180min. With the other labelled precursors, rates diminished between 60 and 180min. Labelled protein could be detected after only 10–15min of incubation. Only 4–9% of the newly synthesized protein remained associated with the mucosa; the remainder was found in the cell-free incubation medium. The isolated seminal-vesicle mucosal preparation will provide a unique opportunity to study the synthesis and secretion of abundant cell-specific proteins by this androgen-dependent tissue.
Full text
PDF

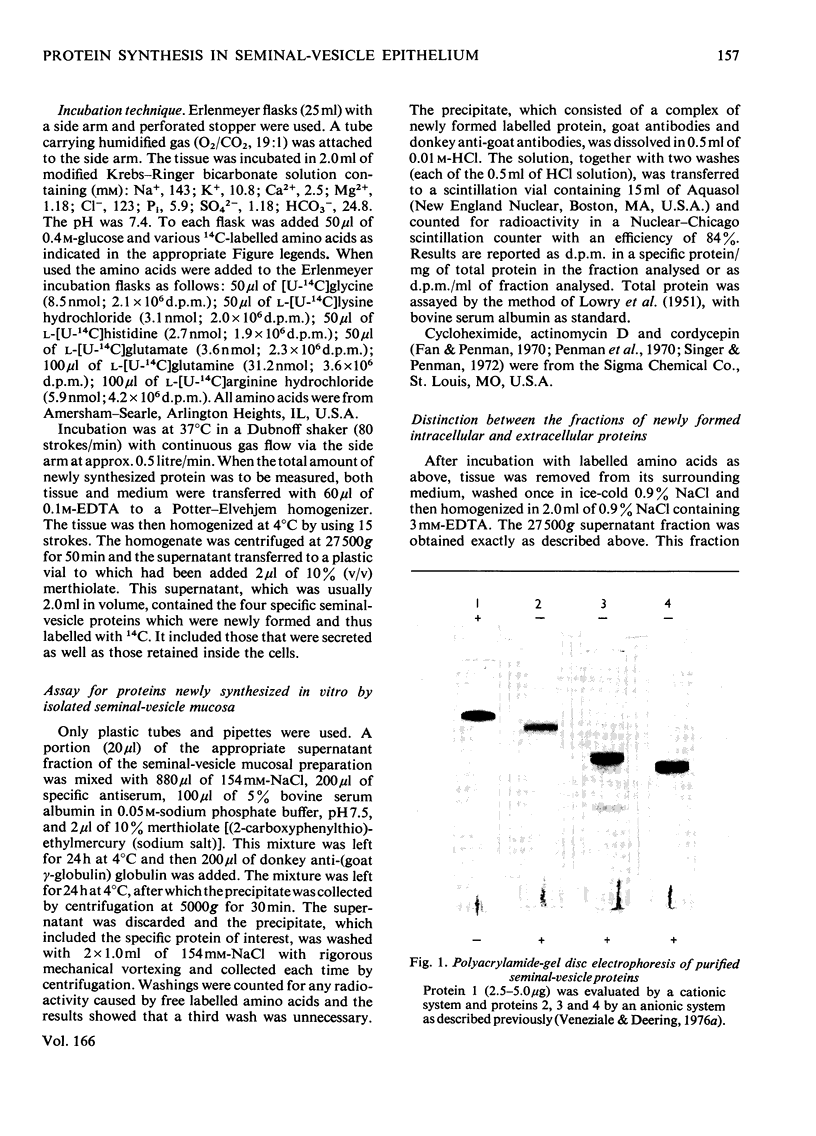
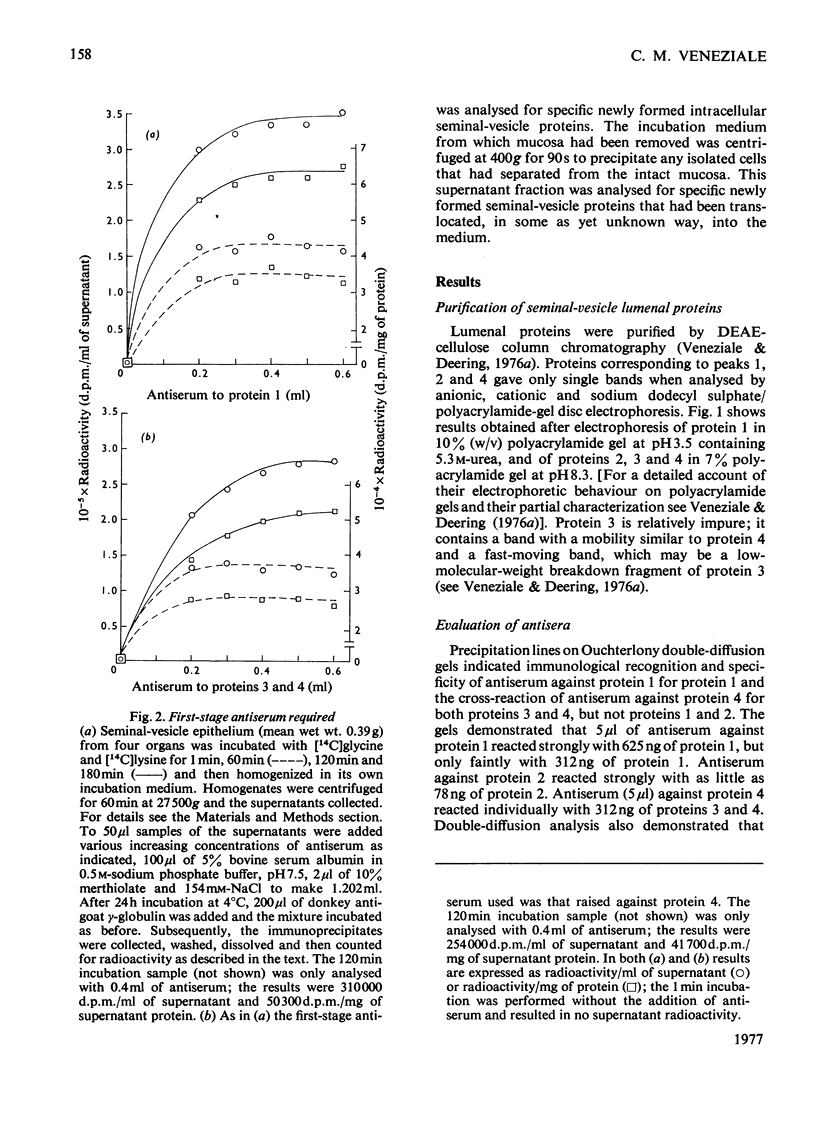
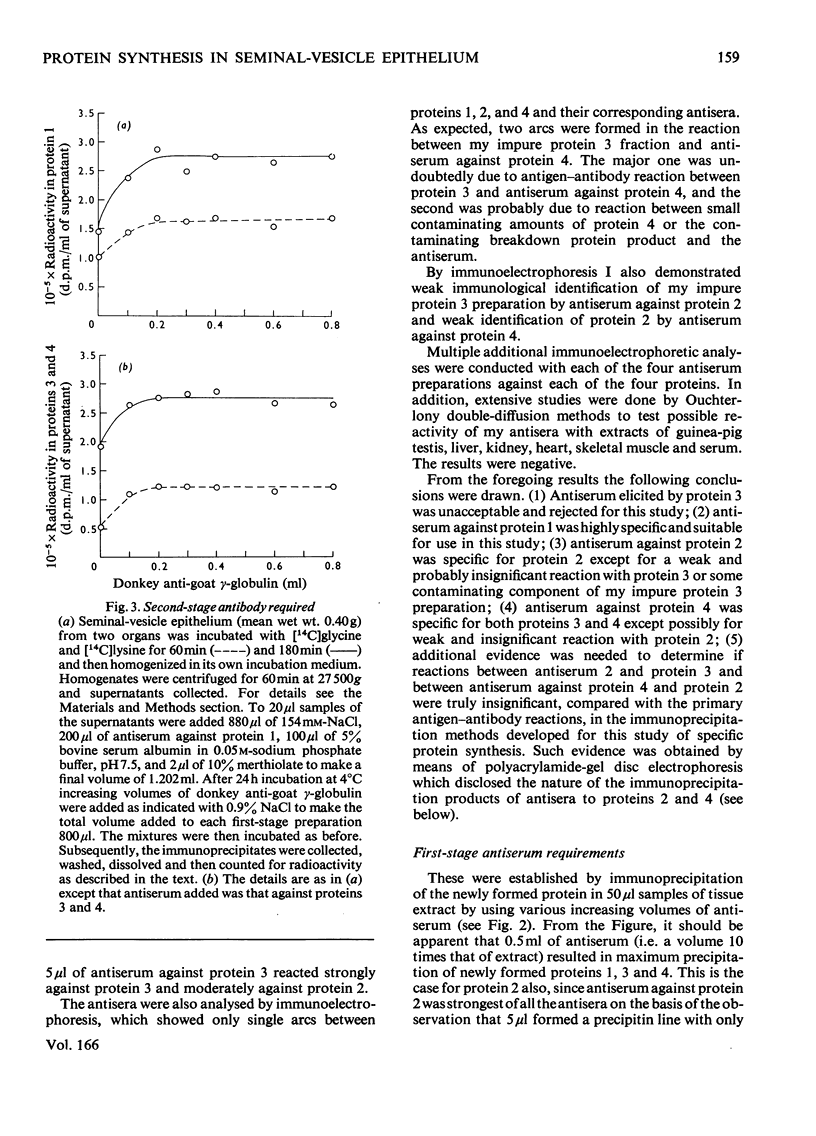



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashman D., Pitot H. C. Characterization of radioactive immunochemically precipitated protein antigens using SDS-polyacrylamide gels. Anal Biochem. 1971 Dec;44(2):559–569. doi: 10.1016/0003-2697(71)90245-4. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Flickinger C. J. Synthesis, intracellular transport, and release of secretory protein in the seminal vesicle of the rat, as studied by electron microscope radioautography. Anat Rec. 1974 Nov;180(3):407–425. doi: 10.1002/ar.1091800302. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. Biochem J. 1976 Aug 15;158(2):271–282. doi: 10.1042/bj1580271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf R., Michel I., Bell C. Biochemical and morphological responses of normal and neoplastic mammary tissue to hormonal treatment. Recent Prog Horm Res. 1967;23:229–295. doi: 10.1016/b978-1-4831-9826-2.50009-x. [DOI] [PubMed] [Google Scholar]
- KORNER A. GROWTH HORMONE CONTROL OF BIOSYNTHESIS OF PROTEIN AND RIBONUCLEIC ACID. Recent Prog Horm Res. 1965;21:205–240. [PubMed] [Google Scholar]
- Kohler P. O., Grimley P. M., O'Malley B. W. Estrogen-induced cytodifferentiation of the ovalbumin-secreting glands of the chick oviduct. J Cell Biol. 1969 Jan;40(1):8–27. doi: 10.1083/jcb.40.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz D. T., Sippel A. E., Feigelson P. Effect of thyroid hormones on the level of the hepatic mRNA for alpha2u globulin. Biochemistry. 1976 Mar 9;15(5):1031–1036. doi: 10.1021/bi00650a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANYAI S. PROTEIN SYNTHESIS IN THE SEMINAL VESICLE OF THE RAT. 1. RAPIDLY LABELLED PROTEIN IN THE RNA FRACTION. Acta Physiol Acad Sci Hung. 1963;24:11–28. [PubMed] [Google Scholar]
- Notides A. C., Williams-Ashman H. G. The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1991–1995. doi: 10.1073/pnas.58.5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Palmiter R. D., Wrenn J. T. Interaction of estrogen and progesterone in chick oviduct development. 3. Tubular gland cell cytodifferentiation. J Cell Biol. 1971 Sep;50(3):598–615. doi: 10.1083/jcb.50.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast F. G., Veneziale C. M. Control of fructose and citrate synthesis in guinea pig seminal vesicle epithelium. J Biol Chem. 1975 Feb 25;250(4):1282–1289. [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Singhal R. L., Ling G. M. Metabolic control mechanisms in mammalian systems. IV. Androgenic induction of hexokinase and glucose-6-phosphate dehydrogenase in rat seminal vesicles. Can J Physiol Pharmacol. 1969 Mar;47(3):233–239. doi: 10.1139/y69-043. [DOI] [PubMed] [Google Scholar]
- Singhal R. L., Valadares J. R. Metabolic control mechanisms in mammalian systems. Hormonal regulation of phosphofructokinase in the rat prostate and seminal vesicles. Biochem J. 1968 Dec;110(4):703–711. doi: 10.1042/bj1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A. E., Feigelson P., Roy A. K. Hormonal regulation of the hepatic messenger RNA levels for alpha2u globulin. Biochemistry. 1975 Feb 25;14(4):825–829. doi: 10.1021/bi00675a028. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Hormonal regulation of growth and protein synthesis. Nature. 1968 Jul 27;219(5152):331–337. doi: 10.1038/219331a0. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M., Brown A. L., Jr, Prendergast F. G. Histology and fine structure of guinea pig seminal vesicle. Mayo Clin Proc. 1974 May;49(5):309–313. [PubMed] [Google Scholar]
- Veneziale C. M., Deering L. C. Soluble proteins of guinea pig seminal vesicle lumen: purification and partial characterization. Andrologia. 1976;8(2):73–82. doi: 10.1111/j.1439-0272.1976.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M., Deering N. G. Metabolism of ATP during anoxia in guinea pig seminal vesicle mucosa. Andrologia. 1976;8(4):271–276. doi: 10.1111/j.1439-0272.1976.tb01652.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS-ASHMAN H. G., LIAO S., HANCOCK R. L., JURKOWITZ L., SILVERMAN D. A. TESTICULAR HORMONES AND THE SYNTHESIS OF RIBONUCLEIC ACIDS AND PROTEINS IN THE PROSTATE GLAND. Recent Prog Horm Res. 1964;20:247–301. [PubMed] [Google Scholar]
- WILSON J. D. Localization of the biochemical site of action of testosterone on protein synthesis in the seminal vesicle of the rat. J Clin Invest. 1962 Jan;41:153–161. doi: 10.1172/JCI104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYDECK F. A., MUIRHEAD E. E., SCHNEIDER H. A RAPID TECHNIQUE FOR DETECTING MULTIPLE ANTIGEN-ANTIBODY PRECIPITIN REACTIONS. Nature. 1965 Jan 9;205:189–190. doi: 10.1038/205189a0. [DOI] [PubMed] [Google Scholar]