Summary
Background
Dengue is a significant global public health concern that poses a threat in Africa. Particularly, African countries are at risk of viral introductions through air travel connectivity with areas of South America and Asia in which explosive dengue outbreaks frequently occur. Limited reporting and diagnostic capacity hinder a comprehensive assessment of continent-wide transmission dynamics and deployment of surveillance strategies in Africa. In this study, we aimed to identify African airports at high risk of receiving passengers with dengue from Asia, Latin America, and other African countries with high dengue incidence.
Methods
For this modelling study, air travel flow data were obtained from the International Air Transport Association database for 2019. Data comprised monthly passenger volumes from 14 high-incidence countries outside of Africa and 18 countries within the African continent that reported dengue outbreaks in the past 10 years to 54 African countries, encompassing all 197 commercial airports in both the source and destination regions. The risk of dengue introduction into Africa from countries of high incidence in Asia, Latin America, and within Africa was estimated based on origin–destination air travel flows and epidemic activity at origin. We produced a novel proxy for local dengue epidemic activity using a composite index of theoretical climate-driven transmission suitability and population density, which we used, in addition to travel information in a risk flow model, to estimate importation risk.
Findings
Countries in eastern Africa had a high estimated risk of dengue importation from Asia and other east African countries, whereas for west African countries, the risk of importation was higher from within the region than from countries outside of Africa. Some countries with high risk of importation had low local transmission suitability, which is likely to hamper the risk that dengue importations would lead to local transmission and establishment of a dengue outbreak. Mauritius, Uganda, Côte d'Ivoire, Senegal, and Kenya were identified as countries susceptible to dengue introductions during periods of persistent transmission suitability.
Interpretation
Our study improves data-driven allocation of surveillance resources, in regions of Africa that are at high risk of dengue introduction and establishment, including from regional circulation. Improvements in resource allocation will be crucial in detecting and managing imported cases and could improve local responses to dengue outbreaks.
Funding
Rockefeller Foundation, National Institute of Health, EDCTP3 and Horizon Europe Research and Innovation, World Bank Group, Medical Research Foundation, Wellcome Trust, Google, Oxford Martin School Pandemic Genomics programme, and John Fell Fund.
Introduction
Dengue is an arthropod-borne virus with four main serotypes and is a member of the genus Flavivirus, of the Flaviviridae family.1, 2 The virus is estimated to cause between 5·2 million and 390 million infections around the world every year, mostly transmitted by Aedes aegypti and Aedes albopictus mosquitoes.3, 4 Approximately half of the global human population is estimated to currently live in areas that are environmentally suitable for dengue transmission.3, 5, 6 Dengue is endemic in more than 100 countries, with reported outbreaks predominantly in central America, South America, and southeast Asia, and in the past decade, epidemic cycles have been established in parts of Africa and North America.6, 7, 8
Despite the widespread distribution of mosquito vectors in Africa9 and favourable transmission conditions characterised by high temperatures and increased urbanisation,7, 10, 11 severe gaps exist in our understanding of the transmission intensities of the virus on the continent. Limited diagnostic capacity and reporting on dengue incidence make it difficult to assess the true burden of dengue in Africa.3, 5 Similarity of symptoms with other febrile illnesses such as malaria also contribute to underdiagnosis.12, 13 Consensus based on data from previous studies shows that dengue transmission is endemic in 34 countries in the African region.14, 15 It is therefore speculated that dengue is more widespread in Africa than previously thought. In 2023 alone, large dengue outbreaks were reported in 16 African countries.16
Risk assessment for dengue in Africa must consider several factors. Considering the frequent explosive dengue outbreaks in South America and Asia, African countries are at risk of constant viral introductions through air travel connectivity. Once introduced, dengue can theoretically spread rapidly in large parts of Africa due to the presence of suitable vectors, appropriate environmental conditions, and low population immunity. Many initial dengue infections are often asymptomatic, which can lead to underreporting and unnoticed spread.1 Although primary dengue infections are rarely fatal, secondary infections with different dengue virus serotypes can be highly problematic, potentially causing severe dengue fever.17 Even in countries where dengue is thought to be endemic, the importation of genetic variants of the dengue virus from countries with high dengue incidence in South America or Asia can exacerbate the burden of disease, favour serotype diversity, and initiate new explosive outbreaks.
Research in context.
Evidence before this study
Despite the significant global burden of dengue virus, Africa remains relatively understudied due to limited reporting and diagnostic capabilities. We searched PubMed from database inception to May 6, 2024, for articles published in English using the search terms “Dengue OR dengue”, “Africa”, and “importation OR imported”. Few studies have investigated the introduction of dengue into African countries. Limited evidence includes phylogeographic studies describing a potential introduction of dengue from Brazil into Angola in 2013 and evidence of multiple historical introductions of dengue from Asia to Africa in the past decade. Before our study, no studies had used a modelling framework to investigate the continental risks of importing dengue via viraemic travellers into African countries from other regions of high dengue incidence.
Added value of this study
This study provides a novel approach to assessing the risk of dengue importation into Africa, integrating climate-dependent transmission suitability and air travel data. By identifying high-risk regions and highlighting the complex interplay between travel patterns, population density, and climatic factors, our findings enhance the understanding of ongoing dengue dynamics in Africa. This information enables targeted allocation of surveillance resources, improving preparedness and response to potential dengue outbreaks in susceptible regions.
Implications of all the available evidence
The integration of transmission suitability as a proxy for local epidemic activity and air travel data into a risk flow metric provides valuable insights into the risk of dengue importation into African airports from high-incidence countries. These findings have implications for tailored surveillance and prevention strategies in high-risk regions, facilitating early detection and management of potential imported dengue outbreaks in Africa.
The potential for dengue importation into a specific location via viraemic travellers is a function of transmission occurring in the origin country and the volume of travellers from the origin country to the destination country of interest. It is well documented that increased international travel and trade have facilitated the global spread of dengue virus and mixing of viral serotypes and genotypes.6, 10, 18, 19 The influence of human mobility on pathogen spread has previously been characterised for influenza viruses,20, 21 Zika virus,22 and SARS-CoV-2.23, 24 Human mobility is also important for shaping individuals’ interactions with disease-carrying vectors and consequently affects viral transmission.25, 26 Understanding the combination of factors driving dengue introduction and onward transmission is crucial for effective surveillance, prevention, and control in Africa in the absence of antiviral treatment, adequate diagnosis, and widespread vaccine coverage. This study aimed to identify African airports at high risk of receiving passengers with dengue virus from Asia, Latin America, and other African countries with high dengue incidence, which could subsequently identify potential high-risk areas and optimal months during which enhanced disease surveillance could have a larger effect on targeted, localised public health strategies.
Methods
Data sources
For this modelling study, we obtained air travel flow data from the International Air Transport Association. The data comprise the number of origin–destination passenger tickets and account for any connections at intermediate airports for the year 2019. The year 2019 was chosen to reflect a typical year of travel preceding disruptions due to the COVID-19 pandemic. The data comprise monthly passenger volumes from 14 high-incidence countries outside of Africa and 18 countries within the African continent that reported dengue outbreaks in the past 10 years to 54 African countries, encompassing all 197 commercial airports in both the source and destination regions.
Transmission suitability estimation
We obtained spatiotemporal estimates of climate-based transmission suitability of dengue (referred to as the P index hereafter) from Nakase and colleagues.7 The P index used in this study quantifies the transmission capacity of a single adult female mosquito throughout its lifetime in a completely susceptible host population, incorporating factors such as infectious periods and oviposition (ie, the number of eggs laid by a female mosquito). The P index metric utilises local temperature and humidity time series (spatial pixel resolution of 0·25° × 0·25° [around 28 km2]) as primary inputs, which enables its application to any location with available climate data. Detailed explanations about how climatic factors such as temperature and rainfall affect mosquito biology and dengue transmission have been published previously.27, 28, 29
We post-processed raw climate-based transmission suitability P estimates to define periods of persistence suitability. Since P measures the number of hosts a single infected female might transmit dengue virus to during its infectious lifespan, a P index value greater than 1 represents the potential for epidemic expansion (ie, single infected mosquitoes would transmit to more than one host). Following this rationale, periods of persistence suitability were defined as the months in which the P index was greater than 1.
Population density
Population density at a given location influences dengue transmission on the basis of the availability of human hosts for dengue transmission via mosquitoes. To consider population density, we extracted the population count of each district within countries from the gridded population of the world, using administrative (level 1) boundary data from the Global Administrative Areas database. To calculate the population density of a province, we aggregated the population count values of grid cells intersecting the province boundary and divided by the respective areas.
Transmission suitability as a proxy for case counts
To address the challenge of missing or insufficient dengue case data, we explored the possibility of using climate-based transmission suitability (P index) as a proxy. To assess the viability of replacing dengue case data with transmission suitability, we conducted a preliminary correlation test between transmission suitability and monthly dengue cases for countries in which monthly dengue case data were available (appendix p 1). Moreover, using transmission suitability instead of case data would allow for a more granular understanding of dengue activity in a country, surpassing the resolution typically available at the national level.
For this study, we considered countries outside of Africa reported to have had large outbreaks of dengue in 2019 according to the European Centre for Disease Prevention and Control. Outbreaks were predominantly in Latin America and Asia. Monthly dengue case data from these countries were extracted from public health organisations such as the Pan American Health Organisation, from governmental health reports or bulletins, and from statistical bureau websites (governmental reporting websites, and WHO reports); a full list of sources is provided in the appendix (p 12). The inclusion criteria for countries with high dengue incidence used in this study were based on the availability of monthly case data in 2019, which allowed for reliable testing of the transmission suitability proxy and use of travel data for the same year. This resulted in a final selection of 14 countries with high dengue incidence in Latin America and Asia: Brazil, Bolivia, Peru, Sri Lanka, Viet Nam, Malaysia, Colombia, Thailand, Nicaragua, India, Bangladesh, Cambodia, Singapore, and Belize. We also extracted province-level case data where openly available. In the correlation coefficient computation, we also incorporated data from two African countries, Burkina Faso and Mauritius, for which monthly data for the year 2019 were available.
We used the Spearman's rank correlation coefficient to examine the association between transmission suitability and monthly dengue case counts for 16 countries. Following the methodology of Nasake and collagues,7 we computed correlation coefficients between case counts and transmission suitability with various month lags (no lag, 1-month lag, 2-month lag). We found a significant positive correlation between transmission suitability and dengue cases for most countries (appendix p 1), with the highest association at a lag of 1 month (12 of 16 countries), corroborating the findings of previous analyses.7 The strength of the association ranged from –0·204 to 0·954. For three countries, we identified a weak correlation between transmission suitability and dengue cases, which could be caused by several factors, such as: (1) paucity of case data obscuring seasonal patterns, which results in poor alignment with the index, (2) the national-level index failing to accurately reflect the overall case distribution if cases are concentrated in specific regions. The correlation between case data and transmission suitability was also calculated at higher resolution (state level) for Brazil, and the strongest association was also found at a 1-month lag (appendix p 2). Thus, we used transmission suitability with a 1-month lag for the analyses presented in this study. Subsequently, we included 18 African countries that had dengue outbreaks in the past decade as reported by WHO Africa.16 The 18 countries were: Angola, Benin, Burkina Faso, Chad, Comoros, Ethiopia, Ghana, Guinea, Kenya, Mali, Mauritania, Mauritius, Niger, Nigeria, Senegal, Tanzania, Togo, and São Tomé and Príncipe.
Although the transmission suitability index serves as a valuable indicator of dengue risk in a given region, consideration of the index alone only indicates whether a climate is suitable for transmission. Additional factors, such as the presence of the host and vector, are crucial for a more accurate assessment. Specifically, Aedes mosquito density data, and host density and various other environmental factors (eg, precipitation and vector habitat distribution) that influence the spatial distribution of vectors, remain relevant for a complete understanding of the biological system. The main reason why such factors are often excluded from modelling approaches is the paucity of sufficient data with adequate spatiotemporal resolution. In this study, we incorporated human population density alongside the transmission suitability index. Using Spearman's rank correlation coefficient, we also examined the correlation between dengue cases and the composite index at a provincial level (appendix p 3). We thus defined a composite index (t):
which captures the combined influence of the transmission suitability and the population density at the district level, by multiplying both elements (appendix p 4).
Risk flow metric
The importation risk to each airport (destination) in each African country was estimated as the probability of importing a case from each state (origin) within each country of high dengue incidence, accounting for the origin–destination travel flows and their different, estimated transmission suitability from the originating state, region, or province. For cross-country comparisons, we aggregated the resulting risk of introduction from each airport to the country level, and for visualisation purposes, we calculated the average risk across each airport over the 12-month period (January to December, 2019).
The methodology was adapted from Gilbert and colleagues:30 risk flow (ria) from origin state i to destination airport α is calculated by:
where ti is the combined transmission suitability and population density of state i, ni is the travel flux from the origin state i, and Aiα is the probability of a traveller flying from i to α, conditioned on travelling internationally from i (ΣαAiα=1; ie, the sum of the probabilities [Aiα] for all destination airports [α] from origin state [i] must equal 1). The denominator accounts for the transmission suitability in various states and the potential risk associated with travellers from different origin states or regions. It is constructed as the sum product of all transmission suitability and travel flows originating from each individual state (denoted by j). This normalisation process ensures that the impact of each state's transmission suitability and travel flow is proportionally represented in relation to the collective risk from all origin states.
The total risk of case importation to destination α is then given by:
This risk is normalised such that ΣαAiα=1.
To summarise and compare the overall introduction risks of dengue into African countries from high-incidence countries in Asia and South America, we aggregated the risk originating from Asia, South America, and Africa separately by summing it across all airports to the district level and then calculating the mean value over all months. We then computed the proportions of risk originating from Asia, South America, and Africa for each district. To further explore the dynamics between introduction risk and the local transmission suitability, we applied cosine similarity, a measure of synchrony between two time series. Cosine similarity helped us evaluate how closely the temporal patterns of introduction risk align with local transmission suitability. The cosine similarity ranges between 0 and 1, with 0 being less synchronous and 1 being highly synchronous.
Additionally, in aggregating the risk for destination countries, we conducted a secondary computation of risk emphasising the locations characterised by persistence suitability. This secondary computation was done by excluding any estimate of risk that occurred outside of times of persistence suitability (ie, when the P index ≤1) in respective destination locations. We filtered only for estimated risks that were most likely to lead to onward transmission and local outbreaks (defined as the introduction suitability hereafter). The risk based on the non-filtered values was defined as the raw introduction risk. It is important to note that the resulting risk values should not be interpreted in absolute terms but rather as indicators of the direction and timing of risk introduction, highlighting where and when the risk is introduced.
Role of the funding source
The funders had no role in data collection, analysis, interpretation of data, writing of the manuscript, or the decision to submit it for publication.
Results
Several countries in Africa are estimated to have high transmission suitability for dengue and simultaneously high population density, indicating elevated potential for local transmission after viral introductions (appendix p 4). Additionally, some African countries receive high volumes of international passengers from Asian and South American countries (appendix p 5). In 2019, 1 924 614 travellers entered the African continent from 14 countries with high dengue incidence. Of these 1 924 614 travellers, 750 599 (39%) originated from southeast Asia, 654 369 (34%) from south Asia, and 519 646 (27%) from South America (appendix p 5). The highest numbers of travellers entering the African continent from these countries were observed in November, December, and January, 2019.
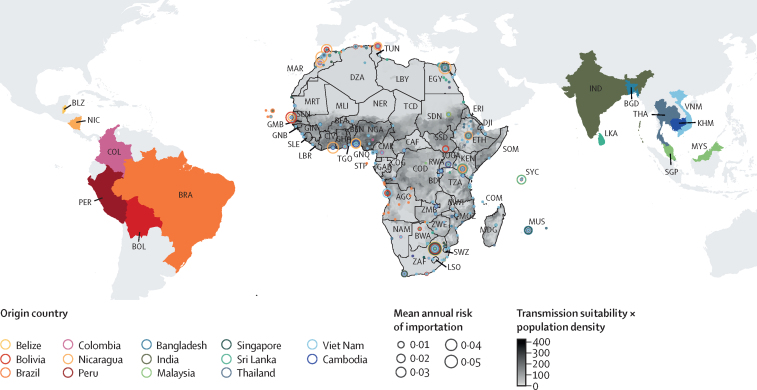
The total risk flow of dengue from Asian and South American countries with high dengue incidence showed that South Africa and Egypt were the countries at higher risk of dengue importation, generally considered as popular tourist destinations that receive large volumes of travellers, followed by Kenya, Angola, Morocco, Seychelles, and Mauritius (figure 1). Egypt had a high risk of dengue importation, with 67% of this risk originating from Asia. Of that, 19% was attributed to passengers travelling from Malaysia. The risk of dengue importation was high from India into Kenya (11% of risk), Mauritius (20% of risk), Tanzania (23% of risk), and Uganda (16% of risk). The risk of dengue importation from Singapore was also high, especially into Mauritius (20% of risk), South Africa (11% of risk), and Egypt (19% of risk). Importation risks from Viet Nam were relatively low across the entire continent, with the exception of South Africa (7% of risk) and Angola (49% of risk). Additionally, Morocco and Nigeria were identified as regions with a considerable risk of dengue importation from Nicaragua (37% of risk in Morocco and 12% of risk in Nigeria).
Figure 1.
Mean risk of dengue introduction into African countries in 2019 from 14 countries in Asia and Latin America
The size of the circles represents the mean risk of dengue importation in 2019 for each airport in Africa. The colour of the circles represents the country from which the risk is coming from and the fill colour of those countries are consistently matched. The fill colour of the African continent represents the index of transmission suitability multiplied by population density to highlight the hotspots of high transmission at the destination. AGO=Angola. BDI=Burundi. BEN=Benin. BFA=Burkina Faso. BGD=Bangladesh. BLZ=Belize. BOL=Bolivia. BRA=Brazil. BWA=Botswana. CAF=Central African Republic. CIV=Côte d’Ivoire. CMR=Cameroon. COD=Democratic Republic of the Congo. COG=Republic of the Congo. COL=Colombia. COM=Comoros. DJI=Djibouti. DZA=Algeria. EGY=Egypt. ERI=Eritrea. ETH=Ethiopia. GAB=Gabon. GHA=Ghana. GIN=Guinea. GMB=The Gambia. GNB=Guinea-Bissau. GNQ=Equatorial Guinea. IND=India. KEN=Kenya. KHM=Cambodia. LBR=Liberia. LBY=Libya. LKA=Sri Lanka. LSO=Lesotho. MAR=Morocco. MDG=Madagascar. MLI=Mali. MOZ=Mozambique. MRT=Mauritania. MUS=Mauritius. MWI=Malawi. MYS=Malaysia. NAM=Namibia. NER=Niger. NGA=Nigeria. NIC=Nicaragua. PER=Peru. RWA=Rwanda. SDN=Sudan. SEN=Senegal. SGP=Singapore. SLE=Sierra Leone. SOM=Somalia. SSD=South Sudan. STP=São Tomé and Príncipe. SWZ=Eswatini. SYC=Seychelles. TCD=Chad. TGO=Togo. THA=Thailand. TUN=Tunisia. TZA=Tanzania. UGA=Uganda. VNM=Viet Nam. ZAF=South Africa. ZMB=Zambia. ZWE=Zimbabwe.
Generally, the risk of dengue importation from Asia was high in countries in the southern and eastern African region, whereas the risk of introduction from South America was higher in countries in central and western Africa, and a portion of northern Africa. In South Africa, the risk of importation was high from both Asia and South America. The discrepancies between the raw risks of introduction and the local transmission suitability index across Africa suggest that a high risk of introduction is not necessarily introduced in areas with high transmission suitability (figure 1). For example, the high risks of introduction into South Africa might not necessarily translate to transmission locally due to low dengue transmission suitability.
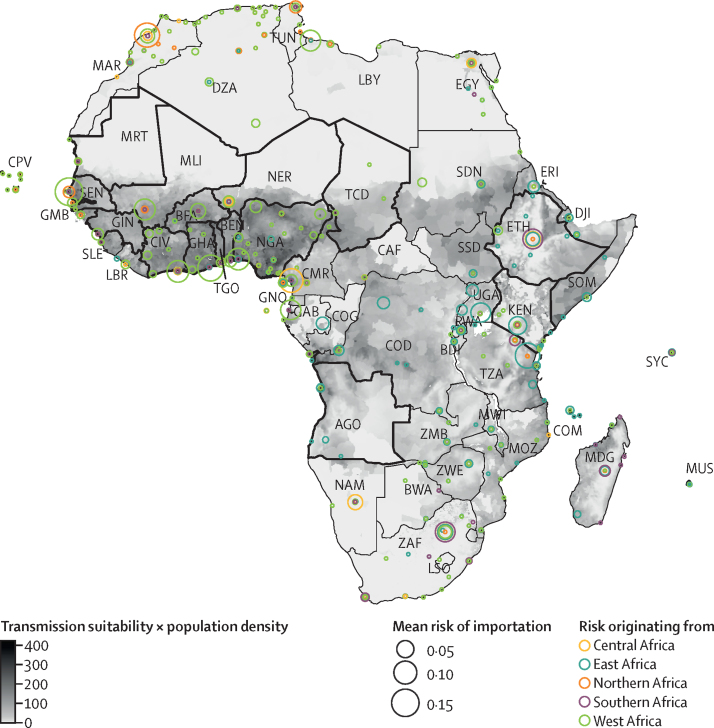
The analysis of dengue introduction risk into African countries from other African countries with dengue circulation highlighted regional patterns. We identified that importation risk was higher in west and eastern African countries, particularly as a result of regional transmission, when compared with other regions (figure 2). High risk of importation was estimated in countries such as Kenya, Tanzania, Cameroon, and Gabon. A notable pattern is that the importation risk within the east African region primarily originated from within the region itself, a pattern that was also observed in west Africa. In contrast, countries in northern Africa, such as Libya and Tunisia, and those in southern Africa, such as South Africa, had a low risk of dengue importation risks from the African continent itself.
Figure 2.
Mean risk of dengue introduction into African countries in 2019 from 18 African countries with dengue circulation
The risk of dengue introduction into African countries is represented by circles on the African continent coloured by the corresponding region of the originating African country. The size of the circles represents the size of the risk averaged over the 12 months for each airport in Africa. The borders for African countries considered as originating locations in the study are outlined in black. The fill colour of the African continent represents the index of transmission suitability multiplied by population density to highlight the hotspots of high transmission at the destination. AGO=Angola. BDI=Burundi. BEN=Benin. BFA=Burkina Faso. BWA=Botswana. CAF=Central African Republic. CIV=Côte d’Ivoire. CMR=Cameroon. COD=Democratic Republic of the Congo. COG=Republic of the Congo. COM=Comoros. CPV=Cabo Verde. DJI=Djibouti. DZA=Algeria. EGY=Egypt. ERI=Eritrea. ETH=Ethiopia. GAB=Gabon. GHA=Ghana. GIN=Guinea. GMB=The Gambia. GNQ=Equatorial Guinea. KEN=Kenya. LBR=Liberia. LBY=Libya. LSO=Lesotho. MAR=Morocco. MDG=Madagascar. MLI=Mali. MOZ=Mozambique. MRT=Mauritania. MUS=Mauritius. MWI=Malawi. NAM=Namibia. NER=Niger. NGA=Nigeria. RWA=Rwanda. SDN=Sudan. SEN=Senegal. SLE=Sierra Leone. SOM=Somalia. SSD=South Sudan. SYC=Seychelles. TCD=Chad. TGO=Togo. TUN=Tunisia. TZA=Tanzania. UGA=Uganda. ZAF=South Africa. ZMB=Zambia. ZWE=Zimbabwe.
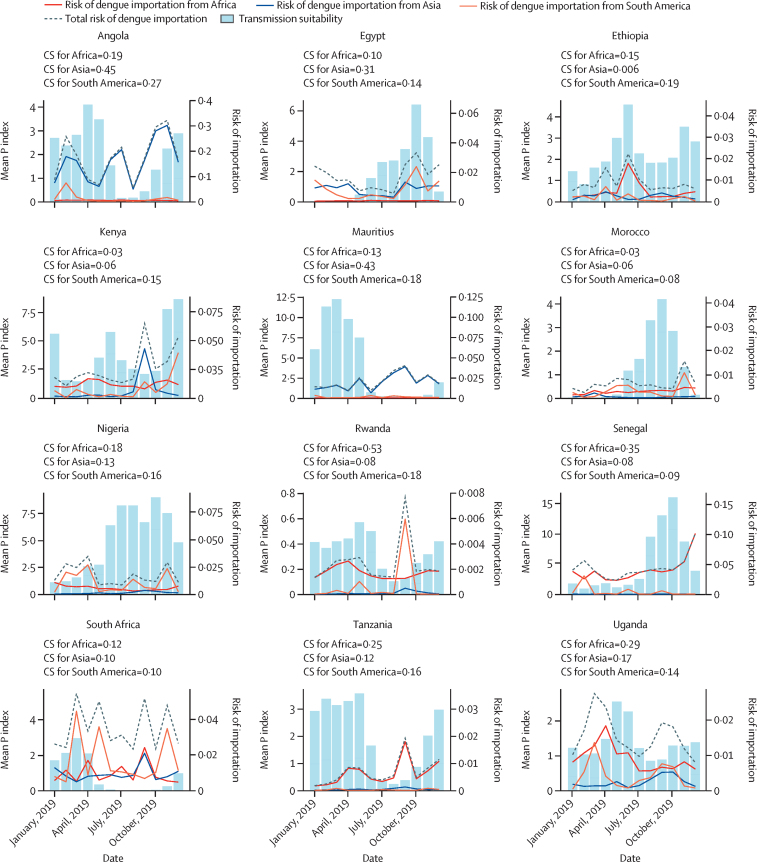
In addition to quantifying the risk of dengue introductions from countries of high incidence in Asia and South America, we evaluated the timing of these introductions and whether they would arrive at periods of high local transmission suitability in Africa. For this, the monthly introduction risk was mapped to local transmission suitability at the airport level in Africa. Egypt was estimated to be at high risk of dengue introduction during the second half of the year from both Asia and South America (figure 3). This temporal pattern aligns with a concurrent period of heightened transmission suitability within the country, which could indicate a higher likelihood of viral introductions leading to onward transmission. To quantify the synchrony between the estimated viral importation risk and the local transmission suitability, we computed the cosine similarity between the different time series: for Egypt, the cosine similarity of estimated risks was 0·311 from Asia and 0·147 from Latin America (appendix p 10). A large dengue outbreak was observed in Egypt between September and November, 2023,30 which coincided with peaks in estimated risk and transmission suitability. Angola and Ethiopia both were estimated to have a continuous risk of disease introduction from Asia throughout the year. However, in Angola, the transmission suitability was predicted to decline between July and September. The risk of introduction from Asia was also estimated to be high in Mauritius. The cosine similarity between the risk of introduction and transmission suitability for Mauritius was 0·437 for Asia and 0·183 for South America. The risk of dengue introduction into South Africa was high, but transmission suitability remained low throughout the year, indicating these potential introductions as likely impasses for transmission in the country. In Nigeria, the highest risk of introduction from South America was estimated to occur during periods of lower local transmission suitability. Angola and Mauritius were identified as being at high risk of suitable introductions, considering the combination of high and synchronous risk of introduction from Asia (appendix p 1).
Figure 3.
Time-varying risk of dengue introduction into African countries in 2019
The risk of importation was aggregated at the national level by summing the individual risks from all airports within each African destination country. The blue bars represent the time-varying transmission suitability index. For CS, a value of 0 indicates low synchrony and a value of 1 indicates high synchrony. In this figure, the countries shown had the most synchronicity between transmission suitability and risk of introduction. Additional countries are shown in the appendix (p 6). CS=cosine similarity.
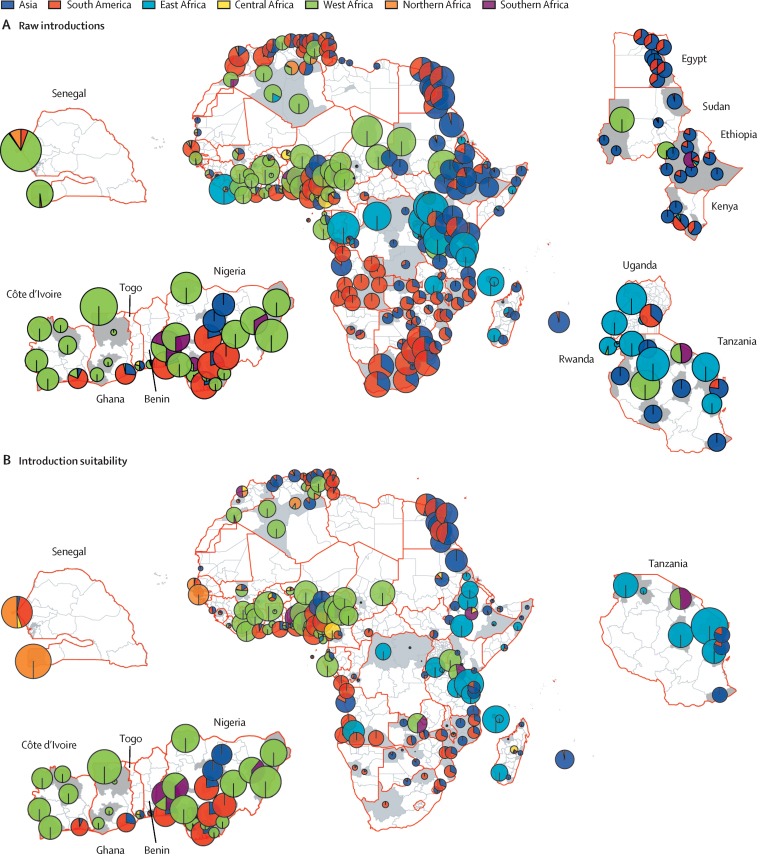
We integrated the risk of dengue importation from 14 countries outside of Africa with that from 18 African countries to gain a clearer understanding of the proportion of importation risk originating from within the African continent compared with external sources. We filtered for risk estimates that are most likely to lead to onward transmission: introduction suitability risk (ie, risks that occur during periods of persistence suitability [P index >1]). Figure 4A shows, based on the raw risk of dengue introduction, risks were introduced across Angola and South Africa, with the largest proportion originating from South America. In South Africa, where the transmission suitability (P index) remained consistently below 1 throughout the year, the risk of dengue evolving into an outbreak from an introduction was negligible (figure 4B).
Figure 4.
Overall proportional risk of dengue importation into African countries before and after adjusting for period of persistence suitability (transmission suitability >1)
(A) The proportion of risk from Asian, South American, and African regions throughout the year for each district. The size of the pie charts is proportional to the mean risk of importation and the colours correspond to the origin continent or region. (B) The proportion of risk from Asian, South American, and African regions across the year, for months with high persistence suitability.
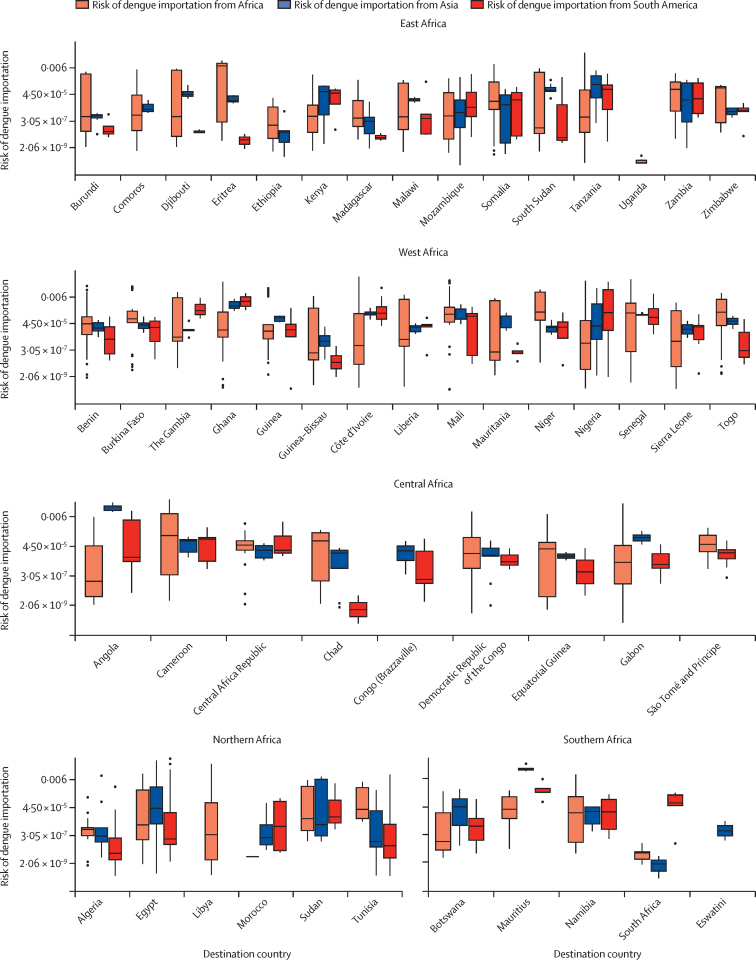
In our analysis, a shift in risk proportionality emerged when focusing on introduction suitability compared with raw risk. For example, in the case of Dakar in Senegal, initially, 87·7% of the total risk was attributed to the west African region, followed by 3·9% from South America and 6·0% from northern Africa. However, when focusing on periods of persistence suitability for Senegal, the risk distribution shifted and the proportion of risk attributed to northern African regions increased to 50·4%, followed by South America with 41·5%. This outcome underlines the importance of understanding not only the geographical origin of importation risks but also the specific temporal and spatial context in which the potential introductions can occur. Focusing on introduction suitability risk, the risk of dengue importation was high in countries in the west and east African regions. The highest risks for these countries were associated with regions within Africa itself (figure 5).
Figure 5.
Risk of dengue importation into African countries in 2019
Boxplots of dengue importation risk in 2019 in African districts with high persistence suitability (P index >1). Boxes indicate the 25th and 75th percentiles, the horizontal lines in the boxes indicate the median values, whiskers indicate the range, and the datapoints outside of the whiskers are outliers.
Discussion
In this study, we investigated the temporal and spatial intersections between high risk of introduction and heightened local transmission suitability of dengue in African countries. The exploration of dengue introduction into Africa is imperative due to the potential implications for onward transmission. We constructed an importation suitability map to depict the spatial and temporal hotspots of dengue introduction and establishment into Africa from high-incidence countries in Asia, Latin America, and regionally from other African countries.
We identified that the variation in risk across Africa is influenced by the spread and intensity of dengue activity in different provinces or states within high-incidence countries, and their connections to destination airports in Africa (appendix p 9). Furthermore, we found that seasonal variation is crucial. For example, in India, major outbreaks tend to occur after the monsoon season and thus have a higher transmission suitability during these seasons (monsoon season spans from June to September)31, 32, 33 and thus we also estimated a higher risk of dengue importation from India to African countries after the monsoon season (appendix p 9).
This research presents an alternative risk assessment by considering modelled dengue transmission suitability7 rather than reported incidence, compared with other studies using case incidence, which might be subject to substantial underreporting.34, 35, 36, 37 The correlation analyses showed a robust association between transmission suitability and dengue cases for countries where high resolution spatiotemporal case records were available, with a significant correlation observed at a 1-month lag (appendix p 1). Our findings are consistent with previous research, demonstrating that 96% of municipalities in Brazil and 95% of provinces in Thailand had incidence dynamics with 0–3-month delays relative to estimated transmission suitability.7 Our findings further support the utility of using transmission suitability as a proxy for local dengue circulation.
Increased international travel and trade have facilitated the global spread of dengue from endemic to non-endemic regions, by enabling the movement of infected individuals and mosquito vectors between regions.6, 7, 8, 10 The risk flow results presented here highlight the substantial risks of dengue introduction into certain parts of the African continent from Asia, South America, or regionally from other African countries through human mobility. We show that the risk of importation to African countries is highly heterogeneous. The results produced by our model suggest that major airports in South Africa, Egypt, Morocco, Kenya, Seychelles, and Mauritius are potential hotspots for the importation of travellers with dengue from high-incidence countries. However, the analysis also highlighted important geographical nuances: for example, the broader eastern African region, and parts of southern Africa have elevated risks of dengue importation from Asia, while central Africa, and parts of northern and southern Africa are more susceptible to dengue importation from South America (albeit these importations are not likely to cause a widespread dengue outbreak). This pattern is consistent with a previous phylogeographic study that found that all four dengue serotypes were introduced on multiple occasions to Africa, primarily from south Asia or southeast Asia since the 1940s.38 This highlights the significance of our study in obtaining high-resolution and temporal and spatial trends for the susceptibility of Africa to dengue introductions.
In contrast, our analysis of the risk of importing dengue from within the continent highlights areas of the continent where a substantial portion of the risks tends to originate from within the region, underscoring the importance of intra-continental transmission dynamics. This is estimated to be the major driver of cross-country introduction in west Africa, and to a lesser extent in east Africa. This is supported by phylogeographic reconstructions that showed frequent introductions and reintroductions of the dengue virus within the western African region, and similar patterns in the eastern region of the continent.38, 39 While Asia represents the second largest contributor to dengue risk, particularly affecting the east, west and central African regions, the internal risk within Africa remains a primary concern. To put this in context, Africa has relatively similar or closely aligned spatial characteristics in transmission suitability across its regions and, therefore, it is expected that intra-continental risk remains predominant. This spatial similarity reinforces the interconnectedness of African regions in the context of disease transmission dynamics. However, there is also a substantial contribution from Asia particularly in east Africa, and towards the island nation of Mauritius, indicating that these countries are susceptible to introductions both from within the continent and from Asia.
It remains crucial not to disregard regions with high population density that currently have low transmission suitability, such as Ethiopian highlands or high altitude regions of Tanzania. With the potential impacts of future global climate change, these areas might become increasingly susceptible to local dengue outbreaks. By integrating appropriate travel models, this approach can be adapted to predict dengue risk under various climatic conditions, providing valuable insights for future public health planning.
To translate the raw risk of introduction into introduction suitability—ie, considering potential introductions that are highly likely to cause onward transmission in the destination countries—it was important to understand the specific temporal and spatial patterns of the risk flow estimates. The temporal analysis of estimated importation risks and local transmission suitability indicated the optimum time periods to increase disease surveillance, when the risk of introduction and potential for transmission are highest. Therefore, the temporal synchrony between estimated introduction risks and transmission suitability has important implications for which viral introductions are predicted to have the ability to contribute to local outbreak risks.
Epidemiological and genomic surveillance are crucial public health measures to mitigate disease burden, and also to understand the spread and evolution of pathogens. At present, there is no approved antiviral treatment for dengue and supportive care is the only option.17, 18, 19 However, two dengue vaccines have shown great promise (Takeda and Butantan vaccines).20 Genomic surveillance can provide crucial information about the viral transmission landscape before upcoming dengue vaccine roll-outs. To determine the optimal locations for conducting genomic surveillance in Africa, it is also important to have a quantitative understanding of the actual risk of a dengue virus epidemic at country level, particularly considering the limited resources available to implement surveillance programmes. The findings of this study highlight that emphasis should be placed on targeted monitoring during periods when the likelihood of risks escalating into an outbreak is higher, and within areas prone to introductions during time of persistence suitability. Considering the risks of dengue introduction, genomic surveillance for dengue enables public health officials to monitor the circulating serotypes while remaining vigilant for the emergence of novel strains or genotypes. In the particular case of dengue, introductions could lead to outbreaks with worsened disease outcomes in a population that has already been exposed to a different serotype of the virus, and depending on the temporal sequence of serotype circulation. This is a growing concern in Africa and elsewhere considering the number of countries in which dengue is well established and serotype co-circulation is becoming universal. Importantly, our modelling approach can also be used for other continents, namely Europe and North America where dengue epidemic activity is increasing.
This study had several limitations and thus findings should be interpreted with caution. First, this study presents relative introduction and establishment risks calculated from ecological, statistical, and mathematical models but does not present direct estimates of the number of expected introductions. Second, due to paucity of genomic and traveller testing data the models presented here have yet to be validated. In countries that perform disease surveillance of incoming travellers, the history of travel of positive dengue cases could serve as validation data to this type of modelling study. Rapidly expanding the volume of available dengue genomes will soon make it possible to reconstruct high resolution transmission dynamics to validate introduction models. Additionally, the measure of transmission suitability used for the source locations does not account for specific control measures in place, potentially affecting the accuracy of the estimated dengue incidence. Also, the measure does not account for the presence and abundance of mosquito vectors. Despite this limitation, the chosen transmission suitability P index seems to produce realistic estimates for dengue incidence in source locations, considering variations in testing and reporting practices globally. Third, while our findings are founded on the current travel networks, it is imperative to acknowledge the dynamic nature of travel patterns. Considering the expansive reach of air travel and the transformative impact observed during the COVID-19 pandemic, we must remain vigilant to the possibility of shifts in dengue introduction risks. This is particularly relevant as more areas are becoming suitable for transmission of dengue. Furthermore, the connectivity between African countries is only captured here through air travel data, while the true connection between neighbouring countries would also be dependent on road travel networks and information about the porosity of borders, data which are much more difficult to obtain. Finally, models similar to that used in this study rely on air travel data and do not account for the role of trade in moving infected mosquitoes between countries (eg, in tyres); this should be considered in future work.
In conclusion, this study provides valuable insights into the complex dynamics of dengue importation risks into Africa, while making a distinction as to the source of those potential introductions. The incorporation of estimated transmission suitability for dengue and population density in risk assessment enhances the accuracy of predictions. The temporal and spatial analyses highlight specific regions and times that warrant intensified surveillance and public health interventions considering the likelihood of potential introductions that would lead to local outbreaks. These findings contribute to a more nuanced understanding of global dengue dynamics, and importantly focus on informing further surveillance on the Africa continent and globally.
Contributors
Data sharing
Proprietary air travel data are commercially available on request from the International Air Transport Association databases (https://www.iata.org/). Transmission suitability (P index) estimates are available online (https://doi.org/10.6084/m9.figshare.21502614). Risk estimates computed from this study are available online (https://github.com/CERI-KRISP/Dengue_Importation_Risk_Modelling.git).
Declaration of interests
TdO reports travel expenses from WHO, Novo Nordisk, and the Coalition for Epidemic Preparedness Innovations. HT reports travel expenses from Ecology and Evolution of Infectious Diseases and VBioM conference organisers. All other authors declare no competing interests.
Acknowledgments
The authors acknowledge that this work is an initiative of the CLIMADE consortium. The Centre for Epidemic Response and Innovation and the Kwazulu-Natal Research Innovation and Sequencing Platform are supported in part by grants from the Rockefeller Foundation (HTH 017), the National Institute of Health USA (U01 AI151698) for the United World Antiviral Research Network, and the INFORM Africa project through the Institute of Human Virology Nigeria (U54 TW012041), Global Health EDCTP3 Joint Undertaking and its members and the Bill & Melinda Gates Foundation (101103171), European Union (EU) Horizon Europe Research and Innovation Programme (101046041), the Health Emergency Preparedness and Response Umbrella Program, managed by the World Bank Group (TF0B8412), the Medical Research Foundation (MRF-RG-ICCH-2022-100069), and the Wellcome Trust (228186/Z/23/Z). MUGK acknowledges funding from The Rockefeller Foundation (PC-2022-POP-005), Google, the Oxford Martin School Programmes in Pandemic Genomics & Digital Pandemic Preparedness, EU Horizon Europe programme project MOOD (874850), E4Warning (101086640), the Wellcome Trust (225288/Z/22/Z, 226052/Z/22/Z, and 228186/Z/23/Z), UK Research and Innovation (APP8583), and the Medical Research Foundation (MRF-RG-ICCH-2022-100069); and a Branco Weiss Fellowship. JL-HT is supported by a Yeotown Scholarship from New College at the University of Oxford. VC acknowledges funding from Horizon Europe (grants ESCAPE 101095619 and VERDI 101045989), and an EU Horizon 2020 grant (MOOD [H2020-874850, paper 874850]). The content and findings reported herein are the sole deductions, views, and responsibility of the researchers and do not necessarily reflect the official position and sentiments of the funding agencies.
Acknowledgments
HT and JP conceptualised and designed the study. JP analysed data, did all primary data visualisations, and wrote the original draft. VC accessed the travel data. YR collected and curated epidemiological data. MD, TdO, VC, and MUGK interpreted data. JL and JL-HT contributed to data analysis and visualisation. CB and TdO acquired funding for this project. HT, MD, and TdO supervised the study. All authors had full access to all the data in this study and HT, JP, JL, VC, MD, and MUGK verified the data. All authors reviewed and edited the final draft. All authors had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paz-Bailey G, Adams LE, Deen J, Anderson KB, Katzelnick LC. Dengue. Lancet. 2024;403:667–682. doi: 10.1016/S0140-6736(23)02576-X. [DOI] [PubMed] [Google Scholar]
- 3.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6 doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakase T, Giovanetti M, Obolski U, Lourenço J. Global transmission suitability maps for dengue virus transmitted by Aedes aegypti from 1981 to 2019. Sci Data. 2023;10:275. doi: 10.1038/s41597-023-02170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Quam MBM, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med. 2021;28 doi: 10.1093/jtm/taab146. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae albopictus. Elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health. 2011;39(suppl):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin CW, Comrie AC, Ernst K. Climate and dengue transmission: evidence and implications. Environ Health Perspect. 2013;121:1264–1272. doi: 10.1289/ehp.1306556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan W, Zakai HA, Khan K, Kausar S, Aqeel S. Discriminating clinical and biological features in malaria and dengue patients. J Arthropod Borne Dis. 2018;12:108–118. [PMC free article] [PubMed] [Google Scholar]
- 13.Nyenke CU, Nnokam BA, Esiere RK, Nwalozie R. Dengue fever: etiology, diagnosis, prevention and treatment. Asian J Res Infect Dis. 2023;14:26–33. [Google Scholar]
- 14.Bosire C, Mutuku F, Ndenga B, Nzaro M, Mwendwa K, LaBeaud AD. A narrative review of dengue fever infection and epidemic activity in Kenya (2010 to 2020) PAMJ One Health. 2023 doi: 10.11604/pamj-oh.2023.12.10.40416. published online Oct 16. [DOI] [Google Scholar]
- 15.Gainor EM, Harris E, LaBeaud AD. Uncovering the burden of dengue in Africa: considerations on magnitude, misdiagnosis, and ancestry. Viruses. 2022;14:233. doi: 10.3390/v14020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO African Region Outbreak and other emergencies. https://www.afro.who.int/health-topics/disease-outbreaks/outbreaks-and-other-emergencies-updates
- 17.Halstead SB. WHO Regional Office for South-East Asia; 2000. Global perspectives on dengue research.https://iris.who.int/handle/10665/148787 [Google Scholar]
- 18.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17:e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 19.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charu V, Zeger S, Gog J, et al. Human mobility and the spatial transmission of influenza in the United States. PLoS Comput Biol. 2017;13 doi: 10.1371/journal.pcbi.1005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merler S, Ajelli M. The role of population heterogeneity and human mobility in the spread of pandemic influenza. Proc Biol Sci. 2010;277:557–565. doi: 10.1098/rspb.2009.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogoch II, Brady OJ, Kraemer MUG, et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect Dis. 2016;16:1237–1245. doi: 10.1016/S1473-3099(16)30270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraemer MUG, Hill V, Ruis C, et al. Spatiotemporal invasion dynamics of SARS-CoV-2 lineage B.1.1.7 emergence. Science. 2021;373:889–895. doi: 10.1126/science.abj0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tegally H, Wilkinson E, Tsui JLH, et al. Dispersal patterns and influence of air travel during the global expansion of SARS-CoV-2 variants of concern. Cell. 2023;186:3277–3290. doi: 10.1016/j.cell.2023.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soriano-Paños D, Arias-Castro JH, Reyna-Lara A, Martínez HJ, Meloni S, Gómez-Gardeñes J. Vector-borne epidemics driven by human mobility. Phys Rev Res. 2020;2 [Google Scholar]
- 26.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, et al. The role of human movement in the transmission of vector-borne pathogens. PLoS Negl Trop Dis. 2009;3:e481. doi: 10.1371/journal.pntd.0000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu-Helmersson J, Stenlund H, Wilder-Smith A, Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebi KL, Nealon J. Dengue in a changing climate. Environ Res. 2016;151:115–123. doi: 10.1016/j.envres.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Mordecai EA, Cohen JM, Evans MV, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert M, Pullano G, Pinotti F, et al. Preparedness and vulnerability of African countries against importations of COVID-19: a modelling study. Lancet. 2020;395:871–877. doi: 10.1016/S0140-6736(20)30411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank C, Lachmann R, Wilking H, Stark K. Increase in dengue fever in travellers returning from Egypt, Germany 2023. Euro Surveill. 2024;29 doi: 10.2807/1560-7917.ES.2024.29.5.2400042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barik P, Goswami P. Quantifying the impact of rainfall categories on dengue occurrence: a multi-site analysis in India. Preprints. 2023 doi: 10.20944/preprints202312.0239.v1. published online Dec 5. (preprint). [DOI] [Google Scholar]
- 33.Mutheneni SR, Morse AP, Caminade C, Upadhyayula SM. Dengue burden in India: recent trends and importance of climatic parameters. Emerg Microbes Infect. 2017;6:e70. doi: 10.1038/emi.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shil P. Rainfall and dengue occurrences in India during 2010–2016. Biomed Res J. 2019;6:56. [Google Scholar]
- 35.Gardner LM, Fajardo D, Waller ST, Wang O, Sarkar S. A predictive spatial model to quantify the risk of air-travel-associated dengue importation into the United States and Europe. J Trop Med. 2012;2012 doi: 10.1155/2012/103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang YM. Contributions to the mosquito fauna of southeast Asia. The subgenus Stegomyia of Aedes in southeast Asia I—the scutellaris group of species. Contrib Amer Ent Inst. 1972;9:1–109. [Google Scholar]
- 37.Semenza JC, Sudre B, Miniota J, et al. International dispersal of dengue through air travel: importation risk for Europe. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasitol. 2006;62:293–343. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfsnes K, Eldholm V, Gaunt MW, de Lamballerie X, Gould EA, Pettersson JHO. Tracing and tracking the emergence, epidemiology and dispersal of dengue virus to Africa during the 20th century. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Proprietary air travel data are commercially available on request from the International Air Transport Association databases (https://www.iata.org/). Transmission suitability (P index) estimates are available online (https://doi.org/10.6084/m9.figshare.21502614). Risk estimates computed from this study are available online (https://github.com/CERI-KRISP/Dengue_Importation_Risk_Modelling.git).