Abstract
A new accurate, precise thin layer chromatographic (TLC densitometric) approach was developed for the simultaneous analysis of ergotamine tartrate (ERG), phenobarbital sodium (PHEN), caffeine anhydrous (CAF), dipyrone sodium (DIP) and meprobamate (MEP) in their pure form and in their combined formulation; (MIGRANIL tablets). TLC separation depended on using a stationary phase of TLC plates F254 (20 × 10 cm) and using a developing system of ethyl acetate: methanol: n- hexane (8:2:3, by volume) with UV scanning at 254 nm for ERG, PHEN, CAF and DIP. For MEP, detection was done at 560 nm after spraying with 0.3% ethanolic solution of para -dimethylaminobenzaldehyde (PDAB) and heating at 110 ºC for 30 min. Analysis was performed in the concentration intervals of 0.06-2, 0.15–5 µg/band for ERG and PHEN, respectively; while for CAF, DIP and MEP it was done in the ranges of 0.3-9, 0.5–10, 1–100 µg/band, respectively. Moreover, the method was used for the quantitation of the five studied drugs in their unique formulation and the recovery% was calculated and found to be 102.04, 100.73, 100.65, 99.91, 1nd 101.77% for ERG, PHEN, CAF, DIP, and MEP, in order and all these outcomes proved excellent selectivity of the method. Other validation parameters were tested following ICH guidelines. Accuracy ranged from 98.02 to 100.53% while the obtained %RSD of interday day precision ranged from 1.215 to 1.940%. The environmental impact and performance of the method was tested with several tools; Analytical eco-scale, green analytical procedure index (GAPI), Analytical GREEnness Metric Approach (AGREE), Blue applicability grade index (BAGI), and RGB additive color model. Results ensured minimum hazardous effect on health and environment. This is the first analytical approach developed for simultaneous analysis of the quinary mixture which can be easily applied in any analytical laboratory.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79518-5.
Keywords: TLC densitometry, Ergotamine, Phenobarbital, Caffeine, Dipyrone, Meprobamate
Subject terms: Chemistry, Analytical chemistry
Introduction
Strong headache is a common side effect of the neurological condition; migraine. The headache occurs in spurts and occasionally is accompanied by light sensitivity, vomiting and nausea1. Numerous comorbidities, such as anxiety, irritable bowel syndrome, overactive bladder, sleep difficulties, and obsessive-compulsive disorders are also linked to migraine2. Five compounds; ergotamine tartrate (ERG), phenobarbital sodium (PHEN), caffeine anhydrous (CAF), dipyrone sodium (DIP) and meprobamate (MEP); are formulated in single tablets dosage form which can control and decrease the migraine attack.
Ergotamine tartrate (ERG) is stated in the British Pharmacopeia as an ergot alkaloid3. It is utilized in the prevention or treatment of vascular headaches, including cluster headaches and migraines. While PHEN is a barbituric acid derivative and nonselective central nervous system depressant which helps in treating the stress associated with migraine and is prescribed to treat anxiety, seizures, and sleeplessness disorders4. CAF is a methylxanthine alkaloid4 that can boost the analgesic efficacy of the main ingredient in analgesic formulations by up to 40% because of its adjuvant properties5. DIP, also called analgin, is regarded as a prodrug and is frequently used as an analgesic and antipyretic6. MEP binds to GABA A receptors so produces sedation and alters perception of pain and has a skeletal muscle relaxant and tranquilizer effect7.
There were several previous publications for the determination of each analyte of this co-formulated mixture either as a single drug or with other components. There were publications that analyzed some of them in one mixture. ERG, CAF and DIP were determined together by HPTLC8, spectrophotometry9, and spectrofluorimetric method combined with chemometry10. Only one method was published for the analysis of DIP, CAF and PHEN together with paracetamol and codeine by HPLC11. Different combinations containing ERG and CAF in presence of other drugs were analyzed by various methods including TLC12,13, HPLC14–17, and capillary electrophoresis18.
Moreover, some approaches were published for quantification of DIP and CAF in the presence of other drugs as HPLC19–21 and electrophoresis22,23. Additionally, DIP together with PHEN and Khellin were analyzed by HPLC method24, whereas, PHEN and CAF along with phenacetin and aspirin were determined by spectrophotometry25. While HPLC methods were published for their analysis either with acetylsalicylic acid and paracetamol26 or with other drugs in presence of excipients27. In contrast, PHEN and MEP were determined by UPLC- MS28. Finally, CAF and MEP with morphine, 6-monoacetylmorphine and cyamemazine were determined by GC- MS29.
From the mentioned literature review, no publications were found for the concurrent determination of the quinary mixture under study. The challenge in this combination is the presence of MEP which lacks a chromophoric group making it difficult to be directly determined using traditional methods of analysis.
Most endeavors to diminish the environmental ramifications of chemical processes concentrate on using more ecologically friendly solvents or on getting rid of solvents using fewer reagents and auxiliaries. To develop a totally green analytical method is almost impossible due to the scarcity of available green solvents. Therefore, it is necessary to evaluate the environmental impact and applicability of any developed method using different metrics like Analytical Eco-scale30, green analytical procedure index (GAPI)31, Analytical GREEnness Metric Approach (AGREE)32, Blue applicability grade index (BAGI)33, and RGB additive color model34.
Hence, this work aims to establish for the first time a new, sensitive, time saving and an economical method for the concurrent analysis of the five studied drugs; ERG, PHEN, CAF, DIP and MEP. The developed method has the advantages of saving energy and consuming less amount of solvents, hence it has a low environmental impact. Additionally, the work is optimized and validated according to instructions of the International Conference on Harmonization (ICH) Guidelines35.
Experimental
Instruments
A TLC linomat V sample applicator was used along with A 100-µl syringe (CAMAG; Muttenz, Switzerland) for the application of samples on TLC plates (20 cm x 10 cm) coated with 0.25 mm thick silica gel 60 F254 (MERCK, Germany).
Chromatographic development was performed in a tank using an eluent consisting of ethyl acetate: methanol: n- hexane (8:2:3, by volume).
Plates were scanned using TLC Scanner 3 densitometer (CAMAG), with wavelength settings at 254 nm for ERG, PHEN, CAF, and DIP, and 560 nm for MEP. The winCATS program (V 3.15, CAMAG) was used to run the scanner, which had a slit size of 3.00 × 0.45 mm and a scanning speed of 20 mm/s.
A UV lamp with a short wavelength of 254 nm (VL-6.LC; Marne-la-Vallee Cedex 1, France) was used during method optimization.
Chemicals and samples
The used chemicals and solvents were of analytical grade.
Ethyl acetate, ethanol, methanol, n-hexane, hydrochloric acid (HCl) were obtained from El NASR Pharmaceutical Chemicals Co, Cairo, Egypt.
p-dimethylaminobenzaldehyde (PDAB) was supplied from Sigma-Aldrich, Chemie, Germany.
Pure raw material samples of ERG (100.20%), PHEN (100.53%), CAF (100.06%), DIP (100.07%), MEP (98.02%) (purity was evaluated according to the developed method), were kindly obtained from The NILE Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. Their purity was additionally evaluated according to methods of assay reported in USP36 and was found to be 99.55, 98.90, 100.41, and 99.32% for ERG, PHEN, CAF, and MEP, in order.
MIGRANIL tablets (batch no. t095003) were produced by The NILE Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. Each tablet contains 1 mg ergotamine tartarate, 10 mg phenobarbital sodium, 50 mg caffeine, 150 mg meprobamate, 200 mg dipyrone.
Prepared solutions
Standards solutions
Stock solutions: 0.01 g each of ERG, PHEN, CAF and DIP, 0.1 g of MEP were precisely weighed and transferred into five separate 10 mL measuring flasks. Methanol was added, mixed well and then completed to the volume to reach 1000 µg/mL each of ERG, PHEN, CAF and DIP and 10,000 µg/mL of MEP.
Working solutions: 1 mL each of ERG, PHEN, CAF, DIP, MEP were accurately taken from their corresponding stock solutions into five separate 10 mL measuring flasks, then the volume was completed with methanol to prepare diluted solutions of 100 µg/mL each of ERG, PHEN CAF, and DIP and of 1000 µg/ mL for MEP.
Sample solution
After ten MIGRANIL tablets were weighed, finely ground, and thoroughly mixed, a precisely weighed quantity of the powder equivalent to 0.5 mg ERG, 5 mg PHEN, 25 mg CAF, 75 mg MEP, and 100 mg DIP was transferred into a 50 mL calibrated flask, 30 mL methanol was added, thoroughly mixed, and the flask was filled to the mark with methanol. The resulting solution was sonicated for 15 min. and then filtered. The obtained stock sample concentration was 0.01 mg/mL ERG, 0.1 mg/mL PHEN, 0.5 mg/mL CAF, 1.5 mg/mL MEP and 2 mg/mL DIP. Diluted sample was prepared from the stock sample solution by transferring 2 mL to a 10 mL volumetric flask using methanol.
PDAB solution
0.3% ethanolic solution of PDAB was prepared by dissolving 0.3 g of PDAB in 90 mL ethanol and 10 mL concentrated HCl. This reagent was used as a spray for visualization of MEP on TLC plates37,38.
Procedure
Construction of calibration graphs
Sample solutions in the concentration ranges of 6–200 µg/mL ERG, 15–500 µg/mL PHEN, 30–900 µg/mL CAF, 100–10,000 µg/mL MEP and 50–1000 µg/mL DIP were prepared in methanol in five separate series of 10 mL measuring flasks using their formerly prepared stock and working solutions. 10 µL of each sample was applied in triplicate as bands of 3 mm width to TLC plates (20 × 10 cm). Development was done using an eluent composed of ethyl acetate–methanol–n-hexane (8:2:3, by volume) at 25 °C. Plates were removed from the jars and were air dried after which scanning was performed at 254 nm. After that plates were sprayed with 0.3% ethanolic solution of PDAB and heated for 30 min at 110 º C then scanning was repeated at 560 nm. Peak areas were recorded and were plotted versus the corresponding concentrations for each component to establish the calibration curve and calculate the regression parameters.
Application to dosage form
10 µL of each of the stock and diluted sample solutions were applied each in sextuplets, to TLC plates and the process under construction of calibration curve was followed. Peak areas were recorded and concentrations were computed from the corresponding regression equations. Moreover, standard addition was performed on three different levels; 80, 100 and 120% to test the accuracy of the method.
Results and discussion
After extensive reviewing in the literature, no publications were found for the concurrent analysis of the mixture under investigation. The difficulty in the analysis of this mixture being the presence of MEP which lack UV absorbance so derivatization of this component must be done. TLC is broadly used for the separation and estimation of multicomponent mixtures39,40. In this manuscript a TLC chromatographic method was proposed and validated for concurrent analysis of the five components under study.
Method development and optimization
Meprobamate has no UV absorbance and therefore after developing the system, the spots of ERG, CAF, PHEN and DIP were visualized under UV lamp. Reviewing the published methods, MEP reacts with 1% ethanolic solution of PDAB to give a yellow color that can be seen with the naked eye37,38, hence the plate was then sprayed with the mentioned reagent to detect the spots of MEP.
Several experimental trials were done to study different factors that affect the proposed method and to reach optimum separation.
Different eluents were tested with varying solvent composition and fractions, starting with chloroform - methanol, followed by chloroform-acetone then ethyl acetate-acetone; all the previous systems gave bad resolution for the five components under study. Ethyl acetate- methanol was then tried as a developing system in a ratio of (8:2, v/v) and it gave good resolution, however PHEN was at the solvent front due to its partially high lipophilicity. In a trial to reduce the Rf of PHEN, a lower polarity solvent mixture of ethyl acetate-hexane was used in ratios of (6:4, v/v) and (7:3, v/v), good resolution was observed. Since, DIP has the least Log P value, it is the most polar one among the studied components and therefore it is more attached to the polar TLC stationary phase and appeared near the baseline. Finally, combination was made for the last two systems consisting of ethyl acetate- methanol- n-hexane in different ratios, the best resolution with reasonable Rf for all the five components was obtained when using the ratio of (8:2:3, by volume).
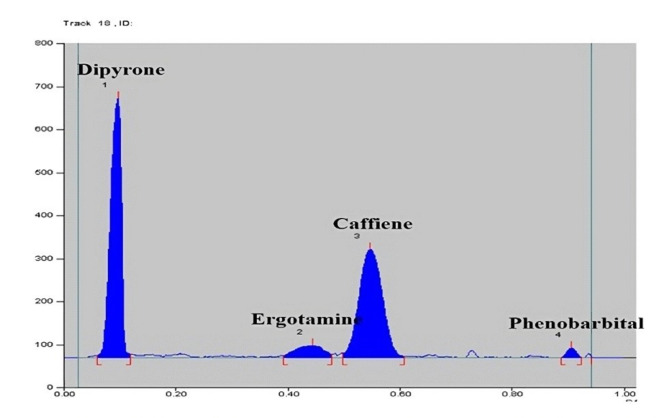
The highest sensitivity was determined by experimenting with various wavelengths for the four analytes ERG, PHEN, CAF and DIP including 210, 254 and 275 nm. ERG and PHEN were less sensitive at 275 nm and DIP was less sensitive at 210 nm. So the optimum sensitivity was attained at 254 nm with least noise as presented in Fig. 1.
Fig. 1.
2D densiometric chromatogram of pure drugs scanned at 254 nm of 0.03 µg/band ergotamine, 3 µg/band of phenobarbital, 1.5 µg/band caffeine, 6 µg/band dipyrone.
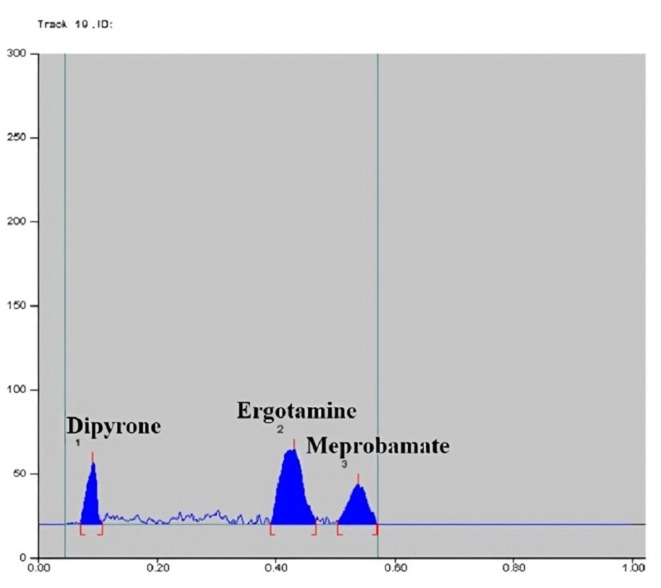
Reaction conditions of PDAB with MEP were then optimized; different PDAB concentrations (1%, 0.5% and 0.3%), various temperatures (50, 70 and 110 °C) and several time intervals (15, 20, 30 and 35 min) were tried, the optimum result was obtained by spraying the plates with 0.3% ethanolic solution of PDAB and then drying at 110 °C for 30 min. Scanning the sprayed plates was tried at several wavelengths including 400, 410, 420 and 560 nm for MEP. The best sensitivity and minimum noise were obtained at 560 nm, Fig. 2. Components were well separated at Rf of 0.06, 0.36, 0.50, 0.55 and 0.86 for DIP, ERG, CAF, MEP and PHEN, in order.
Fig. 2.
2D densiometric chromatogram of pure drugs scanned at 560 nm of 8 µg/band Dipyrone, 0.04 µg/band ergotamine, 6 µg/band Meprobamate.
Method validation
The developed TLC densitometric approach was validated in agreement with ICH recommendations35. Different samples in different concentration ranges were prepared and quantified by the recommended approach. To test linearity; calibration curves representing peak areas against concentrations were plotted. First linear regression equations were applied, where linearity was proved in the intervals of 0.06–2 µg/band regarding ERG and 0.15–5 µg/band regarding PHEN. As for CAF, DIP and MEP, polynomial regression covered wider calibration range with good correlation coefficient in the ranges of 0.3–9 µg/band for CAF, 0.5–10 µg/band for DIP and 1–100 µg/band for MEP. Good correlations were obtained as conferred in Table 1.
Table 1.
Regression and analytical parameters of thin layer chromatographic method for the determination of the studied components.
| Parameter | ERG | PHEN | CAF* | DIP* | MEP* |
|---|---|---|---|---|---|
| Calibration range (µg/band) | 0.06-1 | 0.15-5 | 0.3-9 | 0.5–10 | 1-100 |
| Slope | 8268.20 | 664.22 | -339.8a | 125.91a | -0.2944a |
| 6108.6b | 2805b | 98.667b | |||
| Intercept | 665.78 | 158.78 | 1012.30 | 1267.30 | 234.01 |
| Correlation coefficient (r) | 0.9999 | 1 | 0.9999 | 0.9997 | 0.9997 |
| Accuracy (mean ± RSD %) | 100.20 ± 0.876 | 100.53 ± 0.709 | 100.06 ± 0.920 | 100.07 ± 1.414 | 98.02 ± 2.008 |
| Precision (RSD%)** | |||||
| *Repeatability | 1.525 | 1.782 | 0.912 | 1.1039 | 1.276 |
| *Intermediate precision | 1.940 | 1.886 | 1.215 | 1.783 | 1.324 |
| Robustness (%RSD)*** | |||||
| Ethyl acetate (8 ± 0.1 mL) | 1.447 | 1.136 | 0.970 | 1.525 | 1.840 |
| Methanol (2 ± 0.05 mL) | 1.048 | 1.567 | 0.920 | 0.570 | 1.620 |
| N-hexane (3 ± 0.05 mL) | 0.870 | 1.180 | 1.435 | 1.210 | 2.008 |
| Wavelength (245 ± 1 nm) | 1.135 | 0.709 | 1.285 | 1.414 | - |
*The linearity was achieved using the polynomial regression equation: A = aX2 + bX + C,where a: coefficient 1. b: coefficient 2.
**Precision (RSD of inter- and intraday of three concentrations: ERG (0.03,0.1,1 μg/band),PHEN (0.35,2.5,5μg/band), CAF (0.3,1,7μg/band), MET (0.5,3,10, 7μg/band),MEP(1,30,70μg/band).
***Average of three determination, the %RSD was calculated for the Rf values
Process accuracy was carried out by analyzing seven different concentrations for each of the tested drugs, the already calculated regressions were applied for recording the concentrations from which mean percentage recoveries were obtained, as shown in Table 1 all values were close to the true value confirming accuracy of the proposed method. Besides, accuracy was assessed by performing standard addition technique at three scales; 80%, 100%, 120% on dosage form, results proved accuracy of the method and absence of intrusion from additives, Table 2.
Table 2.
Application of the proposed TLC densitometric method for the determination of Migranil® tablets.
| Taken µg/band | Found ± SD* | Pure added µg/band | Found %** | ||
|---|---|---|---|---|---|
|
MARGINAL Tablets Batch No. t095003 Containing1 mg ergotamine tartarate, 10 mg phenobarbital sodium, 50 mg caffeine, 150 mg meprobamate, 200 mg dipyrone |
ERG | 0.10 | 102.04 ± 1.55 | 0.08 | 101.50 |
| 0.10 | 103.80 | ||||
| 0.12 | 100.83 | ||||
| Mean ± SD | 102.04 ± 1.56 | ||||
| PHEN | 1.00 | 100.73 ± 1.80 | 0.80 | 100.16 | |
| 1.00 | 99.30 | ||||
| 1.20 | 102.75 | ||||
| Mean ± SD | 100.74 ± 1.80 | ||||
| CAF | 1.00 | 100.65 ± 0.92 | 0.80 | 101.12 | |
| 1.00 | 99.60 | ||||
| 1.20 | 101.25 | ||||
| Mean ± SD | 100.66 ± 0.917 | ||||
| DIP | 4.00 | 99.91 ± 1.781 | 3.20 | 99.46 | |
| 4.00 | 101.87 | ||||
| 4.80 | 98.39 | ||||
| Mean ± SD | 99.91 ± 1.78 | ||||
| MEP | 15.00 | 101.77 ± 1.299 | 12.00 | 103.17 | |
| 15.00 | 100.60 | ||||
| 18.00 | 101.55 | ||||
| Mean ± SD | 101.77 ± 1.30 |
*Average of 6 determinations.
**Average of 3 determinations.
Precision was evaluated by applying the prescribed chromatographic conditions for analysis of three various concentrations for each component on the one day (for testing repeatability) and on three consecutive days (for testing intermediate precision). All the obtained % RSD for all tested drugs by the proposed TLC method were < 2 which proves the precision of the suggested procedure, Table 1.
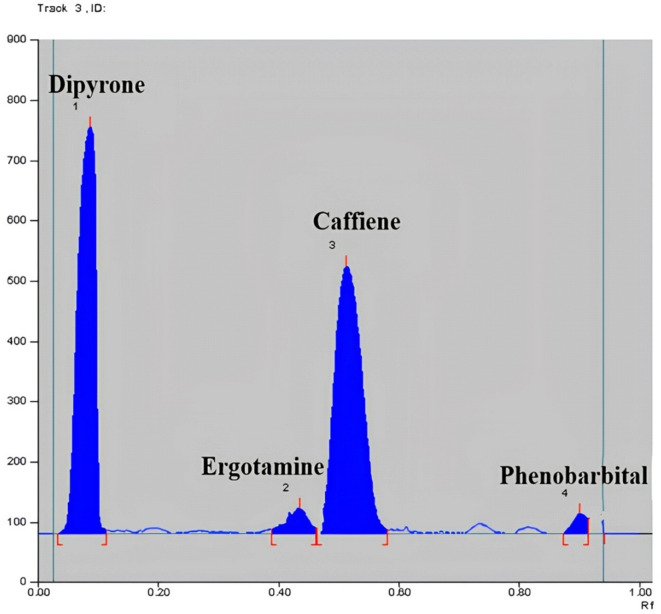
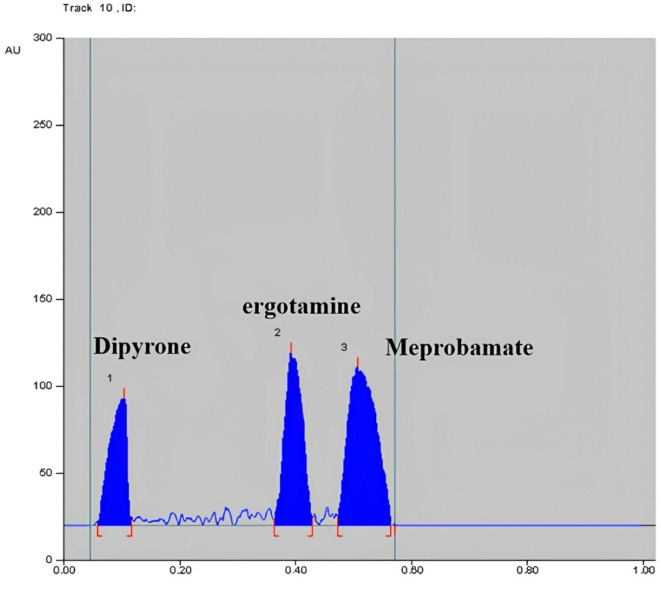
Method specificity was proved from the densitograms in Figs. 1 and 2, which show complete separation between the studied components. Moreover, specificity was also proved by method application for the intended drugs analysis in their combined tablets, Figs. 3 and 4 where there was no interference from tablets excipients. Moreover, results were within the acceptable values from 90 to 110% which proves absence of intrusion from additives, Table 2. Furtherly, the obtained resolution factors were more than 1.5, ensuring complete separation among the studied components.
Fig. 3.
2D densiometric chromatogram of dosage form scanned at 254 nm of 0.1 µg/band ergotamine, 1 µg/band of phenobarbital, 5 µg/band caffeine, 20 µg/band dipyrone.
Fig. 4.
2D densiometric chromatogram of dosage form scanned at 560 nm of 100 µg/band Dipyrone, 0.5 µg/band ergotamine, 75 µg/band Meprobamate.
Robustness presented as %RSD was tested by minor alterations in eluent composition as well as scanning wavelength and then calculating the Rf values after each change. The smaller the %RSD the more robust is the method. Amount of ethyl acetate (8 ± 0.1mL), methanol (3 ± 0.05 mL), and n-hexane (2 ± 0.05 mL) were the intended changes in the mobile phase composition while for scanning wavelength, it was changed by ± 1 nm. The obtained %RSD ranged from 0.57 to 2.008%. These small values ensured that Rf values of the studied components were not affected by these small intended changes previously mentioned, confirming robustness of the suggested approach, Table 1.
System suitability
Different aspects were checked to confirm the consistency of the system. Results in Table 3 confirm peak symmetry as well as good separation among the studied drugs.
Table 3.
Parameters of system suitability testing of the developed thin layer densitometric method.
| Parameters | DIP | ERG | CAF | PHEN | MEP | Reference46 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Retardation factor (Rf) | 0.06 | 0.36 | 0.50 | 0.86 | 0.55 | – | ||||
| Resolution (Rs) | 5.00 | 2.20 | 5.50 | – | R > 1.5 | |||||
| Retention factor (k) | 15.67 | 1.78 | 1.00 | 0.16 | 0.82 | – | ||||
| Selectivity (α) | 8.80 | 1.78 | 6.25 | – | α > 1 | |||||
| Symmetry factor | 1.03 | 1.00 | 1.02 | 1.00 | 1.05 | < 1.5 | ||||
The presented method was compared statistically with published methods17,24,38 using Student t test and F value at p-value of 95%. There was no noteworthy difference between them as shown in Table 4.
Table 4.
Statistical comparison of the results obtained by the developed TLC densitometric method and the reported methods for determination of ergotamine, phenobarbital, caffeine, dipyrone and meprobamate.
| Proposed TLC method | Reported method | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERG | PHEN | CAF | DIP | MEP | ERG17b | PHEN24c | CAF17b | DIP24c | MEP38d | |
| Accuracy | 10.20 | 100.53 | 100.06 | 100.07 | 98.90 | 100.55 | 99.29 | 100.07 | 100.66 | 100.00 |
| SD | 0.876 | 0.709 | 0.920 | 1.414 | 0.566 | 0.962 | 1.514 | 1.053 | 0.577 | 0.995 |
| Variance | 0.767 | 0.503 | 0.846 | 1.999 | 0.320s | 0.925 | 2.291 | 1.109 | 0.333 | 0.990 |
| n | 7 | 7 | 7 | 7 | 7 | 8 | 4 | 7 | 3 | 5 |
| Student s t-test | 0.766 (2.160)a | 1.544 (2.262)a | 0.019 (2.179)a | 0.936 (2.306)a | 2.227 (2.228)a | |||||
| F-test | 1.098 (4.210)a | 4.555 (4.760)a | 1.311 (4.280)a | 6.003 (19.330)a | 3.094 (4.530) a | |||||
a. The values between parenthesis are corresponding to the theoretical values of t and F (p = 0.05).
b. Reported RP-HPLC method for ergotamine and caffeine using Zobrax C18 (150 mm x 2.1 mm) column and gradient elusion was used with a mobile phase with consisting of water (adjusted to pH 3 using orthophosphoric acid): acetonitrile (80:20, v/v) for the first 3 min, (50:50, v/v) for the next 4.5 min, then (80:20, v/v) for the last 2.5 min. Flow rate was 0.7 mL/ min. and UV detection was at 230 nm.
c. Reported RP-HPLC for dipyrone using C18 column and a mobile phase consisting of methanol-water (38, 32, v/v) set at a flow rate of 0.7 mL/min. UV detection was at 254 nm. Whilst for phenobarbital C18 column was used with mobile phase of water-ammonia-methanol (94.5:0.5:5, by volume) and detection was done at 240 nm.
d. Colorimetric determination of meprobamate at 565 nm after its reaction with p-dimethylaminibenzaldehyde.
Greenness appraisal of the developed method
The fundamental majority of researchers have their primary goal to make analytical methods less harmful to the environment. Green chemistry and its tools are important to evaluate environmental impact of newly developed analytical method and to eradicate hazardous chemicals because their risks have serious effects on climate changes and global warming that the world is suffering from nowadays41–43.
It is not easy to use only one metric for the judgment of the overall process as each tool test certain parameters in the method and also some of them are qualitative while others are quantitative. So it is preferred to use more than one tool for evaluation to give a full picture of the impact of any new developed analytical approach.
Analytical eco-scale
It is a semi-quantitative method for assessing greenness, it is one of the simplest and most commonly used tool, it depends on computing penalty points for the quantity of reagents or solvents used, energy used, dangers involved, and overall waste produced throughout the analytical processes. Analytical Eco-Scale scores are totaled and ranked. It is represented by a score; the higher the score (close to 100), the better the method30,42,44,45. The developed TLC method has a score of 71 which indicates that the method is an acceptable green method, Table 5.
Table 5.
Results of assessment of greenness profile of the developed TLC method.
| Tool | Parameters | Penalty point | ||
|---|---|---|---|---|
| Analytical Eco-scale | Reagent |
PP of SOLVENT= subtotal pp * number of pictograms * signal word CONSUMED VOLUME = solvent volume in system/no. of spots |
Methanol | 6 |
| n-hexane | 6 | |||
| Ethyl acetate | 4 | |||
| PDAB | 1 | |||
| HCl | 4 | |||
| Ethanol | 4 | |||
| Instruments | Energy | 0 (≤ 60.1 kWh per sample) | 0 | |
| Occupational hazard | Analytical process hermitization | 0 | ||
| Wastes | < 1 mL (g) | 1 | ||
| No treatment | 3 | |||
| Total penalty points | 29 | |||
| Analytical Eco-scale total score | 71 | |||
| Agree |
|
|||
| GAPI |
|
|||
| BAGI |

|
|||
| RGB | White, 78.40% | |||
GAPI
This metric is a semi-quantitative one which evaluate the greenness of the whole analytical process from sample gathering to final determination. It is expressed as a pictogram with five pentagrams having color either of green, yellow or red regarding the environmental effect of the method31,43–45. The suggested tool was found to have six green fields, six yellow fields and three red ones. The red fields are due to that the method is offline, using the non-green solvent (n-hexane) and there was no waste treatment, Table 5.
AGREE
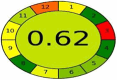
All twelve criteria of green analytical chemistry (GAC) are addressed by AGREE. Both qualitative and measureable findings are grounded on color and number provided by AGREE. Additionally, the outcome of AGREE is easily understood. The twelve criteria are distinctly converted into a 0–1 scale and the final score of AGREE is derived by computation of all the twelve criteria32,44,45. As the value approaches one, the procedure becomes more environmentally benign31,44,45. Table 5 shows that the newly created approach received a score of 0.62.
BAGI
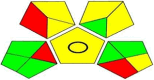
It is one of the more recent assessment tools that is thought to be an addition to the traditional green tools. This metric measures 10 principles to generate a pictogram and a score that describes the practicality and functionality of developed analytical approach. Concerning the attained total score, it is advisable to be ˃ than 6033,45. The developed approach achieved a score of 80 ensuring its practicality, Table 5.
RGB additive color model
Any analytical method or procedure can be evaluated globally using the RGB additive color model. Three primary colors are used to symbolize three key aspects of the assessed method: productivity/practical effectiveness (blue), conformance with “green” chemistry principles (green), and analytical performance (red). The additive synthesis of the primary colors yields the method’s final color. Additionally, the approach offers a quantifiable metric known as “method brilliance (MB)”35,45. Referring to results in Tables 5 and Table S1, it was observed that the method had white color and MB of 78.40%, indicating that the suggested approach can be considered as a good one and can be chosen for several applications.
The overall results of the used metrics confirm the low impact of the developed approach on health and environment.
Conclusion
A TLC chromatographic method was proposed for the simultaneous quantification of ERG, PHEN, CAF and DIP and MEP in their pure form and their pharmaceutical tablets. The approach has the advantages of using low amounts of solvents, consuming low energy and saving money and time. The drugs were determined with high sensitivity and selectivity. When testing the performance of the method using ICH guidelines, it showed high validity, moreover, the method showed low environmental impact when tested using different green assessment metrics. Moreover, method applicability and whiteness were tested using BAGI and RGB additive color models where the method proved to be the candidate of choice for all applications. This method can be easily applied in any laboratory.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M.A. performed the experimental part and wrote the draft manuscript. N.F.F., N.S.A. investigated the work, supervised, edited and reviewed the manuscript. M.M.Z.S. shared in investigation of the work and supervised the work.
Data availability
The datasets used during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.webmd.com/migraines-headaches/migraines-headaches-migraines
- 2.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2740949
- 3.The British Pharmacopeia Vols. I and II (Her Majesty’s Stationary Office, 2007).
- 4.Martindale The Complete drug Reference 33rd Edn (Pharmaceutical, 2002).
- 5.Sawynok, J. Caffeine and pain. Pain 152, 726–729 at (2011). 10.1016/j.pain.2010.10.011 [DOI] [PubMed]
- 6.Lal, A., Pandey, K., Chandra, P. & Pande, S. B. Dipyrone for treatment of post-operative pain. Anaesthesia. 28, 43–47 (1973). [DOI] [PubMed] [Google Scholar]
- 7.Karthikeyan, K., Arularasu, G. T., Murali, V. & Chandrasekara Pillai, K. Identification, isolation, characterization and response factor determination of process-related impurity in meprobamate drug substance. J. Pharm. Biomed. Anal.54, 208–212 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Aranda, M. & Morlock, G. Simultaneous determination of caffeine, ergotamine, and metamizol in solid pharmaceutical formulation by HPTLC-UV-FLD with mass confirmation by online HPTLC-ESI-MS. J. Chromatogr. Sci.45, 251–255 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Ayman, A., Zeid, A. M., Wahba, M. E. K. & EL-Shabrawy, Y. Simultaneous determination of ergotamine, caffeine and dipyrone in their ternary mixture by applying double divisor and first derivative ratio spectra methods. Ann. Pharm. Fr.80, 718–729 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Di Nezio, M. S. et al. A home-made hybrid system for the simultaneous determination of ergotamine, dipyrone and caffeine in pharmaceutical preparations. J. Braz. Chem. Soc.18, 1439–1442 (2007). [Google Scholar]
- 11.Golubitskii, G. B., Budko, E. & Ivanov, V. M. Quantitative analysis of pentalgin ICN tablets by gradient and isocratic high-performance liquid chromatography. J. Anal. Chem.60, 961–966 (2005). [Google Scholar]
- 12.Farid, N. F., Naguib, I. A., Abdelhamid, N. S., Anwar, B. H. & Magdy, M. A. Validated ecofriendly chromatographic method for quantitative determination of anti-migraine quaternary mixture. J. Sep. Sci.43, 2330–2337 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Ragab, M. T., Ramadan, N. K., El-Ragehy, N. A. & El-Zeany, B. A. Thin layer chromatography–spectrodensitometric determination of a three-component mixture of propyphenazone, caffeine, ergotamine tartrate, and two of their impurities with application to tablets, spiked human plasma, and green profile assessment. J. Planar. Chromatogr. Mod. TLC36, 295–305 (2023).
- 14.Issa, Y. M., Hassoun, M. E. M. & Zayed, A. G. Simultaneous determination of Paracetamol, caffeine, domperidone, ergotamine tartrate, propyphenazone, and drotaverine HCL by high performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol.35, 2148–2161 (2012). [Google Scholar]
- 15.Bhosale, D. & Nikalje, A. P. G. Development and validation of RP-Chromatography for simultaneous determination of Paracetamol, caffeine, ergotamine and prochlorperazine in tablets. Asian J. Pharm. Sci.7, 344–351 (2012). [Google Scholar]
- 16.Ibrahim, F. & Wahba, M. E. K. Liquid chromatographic determination of ergotamine tartrate in its combined tablets using fluorimetric and UV detection: Application to content uniformity testing. Sep. Sci. Technol. (Philadelphia)49, 2228–2240 (2014). [Google Scholar]
- 17.Farid, N. F., Abdelhamid, N. S., Naguib, I. A., Anwar, B. H. & Magdy, M. A. Quantitative determination of anti-migraine quaternary mixture in presence of p-Aminophenol and 4-Chloroacetanilide. J. Chromatogr. Sci.60, 538–544 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Sultan, M. A. et al. Capillary electrophoretic determination of antimigraine formulations containing caffeine, ergotamine, Paracetamol and domperidone or metoclopramide. J. Chromatogr. Sci.51, 502–510 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Belal, T. S., Khamis, E. F., el Yazbi, F. A. & Hamdy, M. M. A. High performance liquid chromatographic determination of the ternary mixture of caffeine, dipyrone and drotaverine hydrochloride in tablets dosage form. J. Appl. Pharm. Sci.3, 033–039 (2014). [Google Scholar]
- 20.Belal, F. F., El-Din, S., Tolba, M. K., Elmansi, H. & M. M. & Determination of two ternary mixtures for migraine treatment using HPLC method with ultra violet detection. Sep. Sci. Technol. (Philadelphia). 50, 592–603 (2015). [Google Scholar]
- 21.Vieira, J. C. et al. HPLC–DAD method for simultaneous determination of dipyrone (Metamizole) and caffeine in tablets and identification of major degradation product by direct infusion ESI–MS. Chromatographia80, 489–495 (2017). [Google Scholar]
- 22.Marra, M. C. et al. Ultra-fast determination of caffeine, dipyrone, and acetylsalicylic acid by capillary electrophoresis with capacitively coupled contactless conductivity detection and identification of degradation products. J. Chromatogr. A. 1327, 149–154 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Belal, F., El-Din, M. S., Tolba, M., El-Awady, M. & Elmansi, H. Analysis of four antimigraine drugs in two ternary mixtures by sweeping-micellar electrokinetic chromatography with retention factor gradient effect and dynamic pH junction. Microchem. J.127, 11–21 (2016). [Google Scholar]
- 24.Abounassif, M. A., Gad-Kariem, E. A. & Wahbi, A. M. High performance liquid chromatographic determinations of Khellin, phenobarbitone and dipyrone combination in tablets. Farmaco. 45, 465–472 (1990). [PubMed] [Google Scholar]
- 25.Wen, L. & Fang, H. H. Simultaneous determination of four components in the compound child phenobarbital tablet using ultraviolet spectrophotometry. Bull. Hunan Med. Univ.27, 83–84 (2002). [PubMed]
- 26.Franeta, J. T. et al. HPLC assay of acetylsalicylic acid, Paracetamol, caffeine and phenobarbital in tablets. Farmaco. 57, 709–713 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Aziz, A. A. & Ahmed, K. Simultaneous determination of paracetamol with different active pharmaceutical ingredient (Api) and excipient in. Sci. Int. (Lahore)27, 6173–6176 (2015).
- 28.Hass, U., Dünnbier, U., Massmann, G. & Pekdeger, A. Simultaneous determination of psychoactive substances and their metabolites in aqueous matrices by ultrahigh-performance liquid chromatography-tandem mass spectrometry. Anal. Methods3, 902–910 (2011). [Google Scholar]
- 29.Bévalot, F., Bottinelli, C., Cartiser, N., Fanton, L. & Guitton, J. Quantification of five compounds with heterogeneous physicochemical properties (morphine, 6-monoacetylmorphine, cyamemazine, meprobamate and caffeine) in 11 fluids and tissues, using automated solid-phase extraction and gas chromatography-tandem mass spectrometry. J. Anal. Toxicol.38, 256–264 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Gałuszka, A., Migaszewski, Z. M., Konieczka, P. & Namieśnik, J. Analytical Eco-scale for assessing the greenness of analytical procedures. TrAC - Trends Anal. Chem. vol. 37, 61–72 (2012). [Google Scholar]
- 31.Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta181, 204–209 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE - Analytical GREEnness Metric Approach and Software. Anal. Chem.92, 10076–10082 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manousi, N., Wojnowski, W., Płotka-Wasylka, J. & Samanidou, V. Blue applicability grade index (BAGI) and software: A new tool for the evaluation of method practicality. Green Chem.25, 7598–7604 (2023). [Google Scholar]
- 34.Paweł, M. N.& Paweł K. What color is your method? Adaptation of the RGB additive color model to analytical method evaluation. Anal. Chem.91(6), 10342–10352 (2019). [DOI] [PubMed]
- 35.ICH guideline Q14 on analytical procedure development. In International Conference on Harmonisation 31 (2022).
- 36.The United States Pharmacopeia, National Formulary 35, United States Pharmacopeia 30th Ed. (Convention Inc., 2012).
- 37.Moffat, A. C., Osselton, M. D., Widdop, B. & Watts, J. Clarke’s Analysis of Drugs and Poisons 4th edn. (Pharmaceutical Press, Berlin, 2011). [Google Scholar]
- 38.Sabir, N. A. & Al-Janabi, K. W. S. Colorimetric determination of meprobamate after a simple derivatization. Ibn AL-Haitham J. Pure Appl. Sci.32, 62–69 (2019). [Google Scholar]
- 39.AboulMagd, A. M., Abdelwahab, N. S., Abdelrahmam, M. M., Abdel-Rahman, H. H. & Farid, N. F. Lipophilicity study of diffirent cephalosporins: Computational prediction of minimum inhibitory concentration using salting-out chromatography. J. Pharm. Biomed. Anal. 206, 114358 (2021). [DOI] [PubMed]
- 40.Farid, N. F. & Abdelwahab, N. S. Development and validation of different chromatographic methods for analysis of cabergoline in the presence of its degradation products: Studying degradation profile. Chromatographia82 (2019).
- 41.Marzouk, H. M., Gouda, A. S., Rezk, M. R. & Abdel-Megied, A. M. Innovative eco-friendly stability-indicating HPLC-PDA method for simultaneous determination of the emerging antiviral drugs against COVID-19 infection molnupiravir and favipiravir; degradation kinetic studies along with LC-MS based structure elucidation. Microchem. J.205, 111197 (2024). [Google Scholar]
- 42.Farid, N. F. & Abdelwahab, N. S. A new HPLC methodology for the analysis of metronidazole and dexibuprofen: Application to pharmacokinetic study and comparative greenness assessment. Microchem. J.183, 108048 (2022). [Google Scholar]
- 43.Farid, N. F., Elgendy, M. O. & Abdelwahab, N. S. Sustainable TLC-densitometric method for pharmacokinetic study of the concurrently used ibuprofen and metronidazole: Green metric assessment. Microchem. J.179, 107582 (2022). [Google Scholar]
- 44.Shi, M. et al. Overview of sixteen green analytical chemistry metrics for evaluation of the greenness of analytical methods. TrAC - Trends Anal. Chem.166, 117211 (2023). [Google Scholar]
- 45.Sharkawi, M. M. Z., Safwat, M. T. & Abdelwahab, N. S. Ecofriendly UPLC-MS/MS method for simultaneous assay of veterinary drugs residues; erythromycin, sulfadiazine and trimethoprim in edible chicken tissues, evaluation of method greenness and whiteness. Microchem. J.204, 111060 (2024). [Google Scholar]
- 46.Srivastava, M. M. High-Performance Thin-Layer Chromatography (HPTLC) (Springer, Heidelberg, 2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study available from the corresponding author on reasonable request.