Abstract
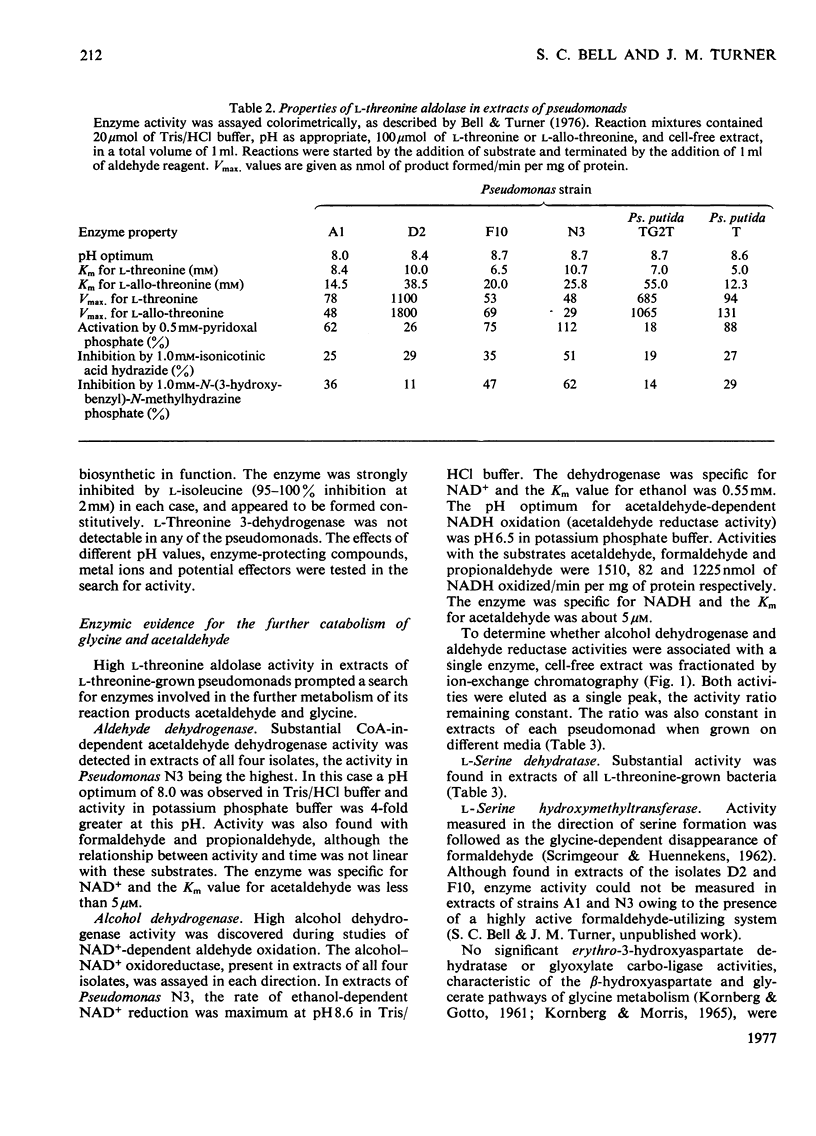
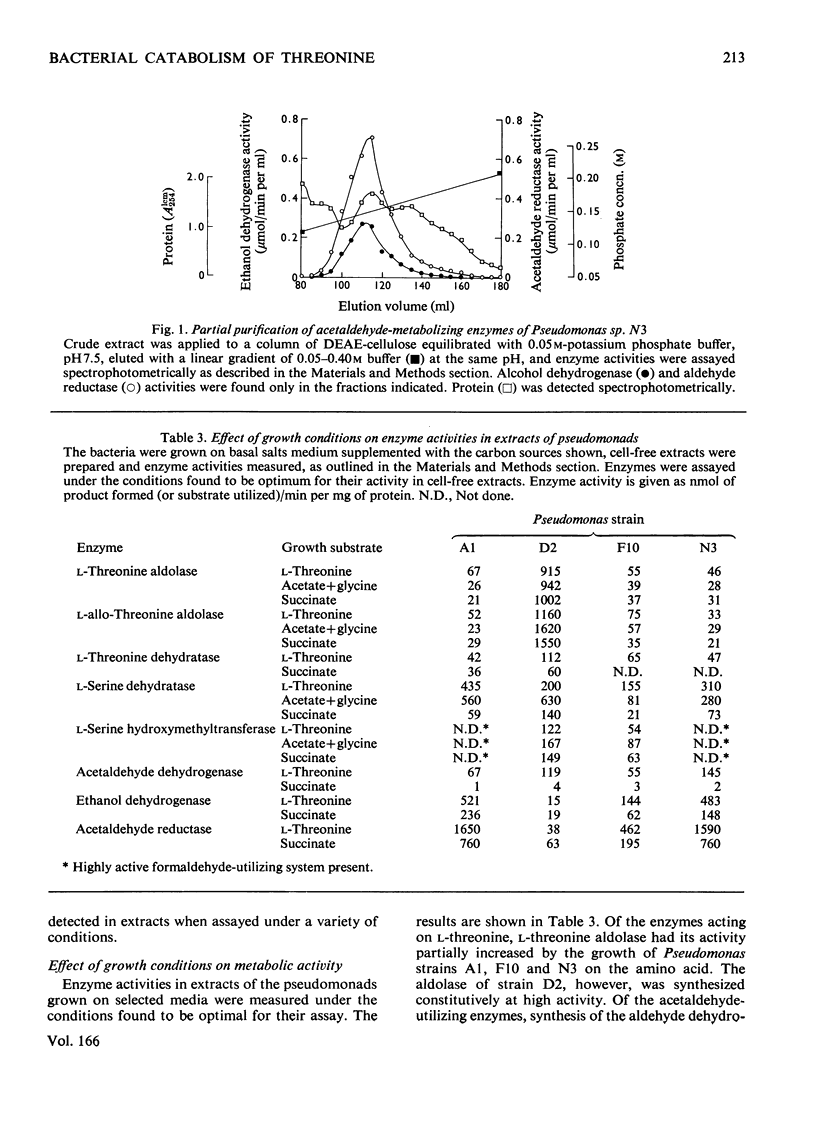
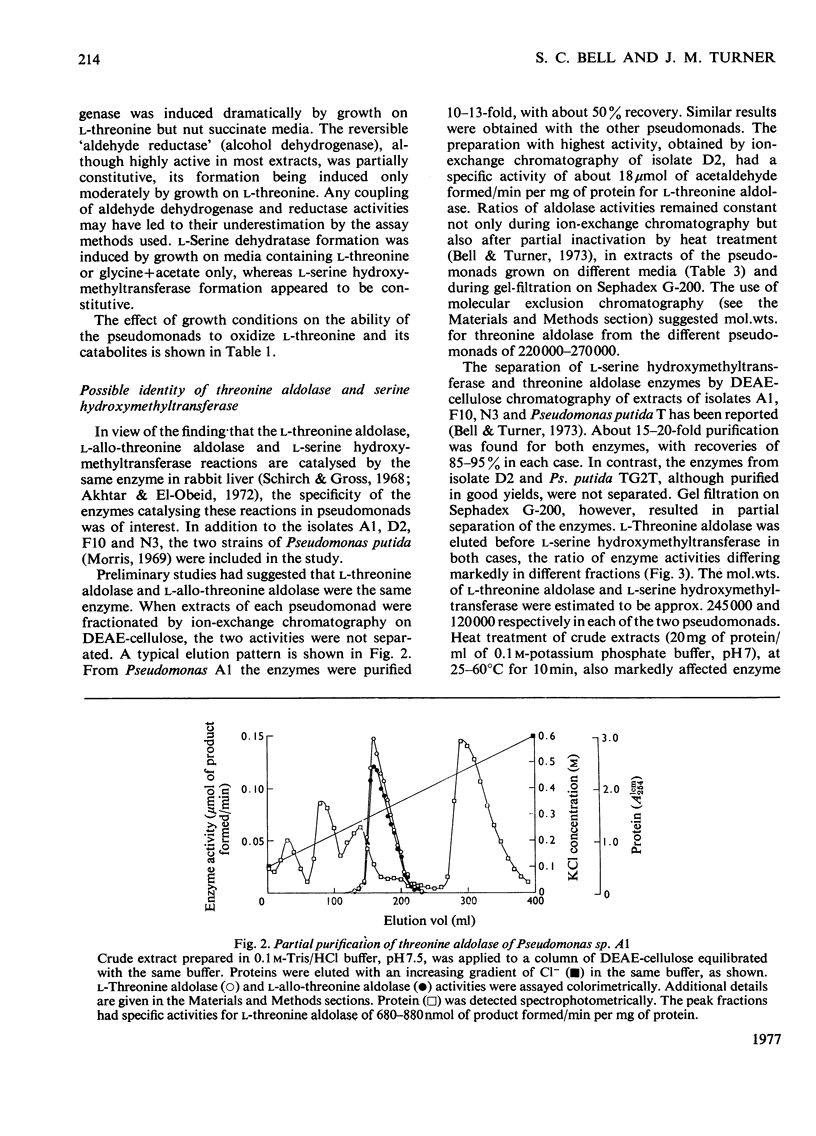
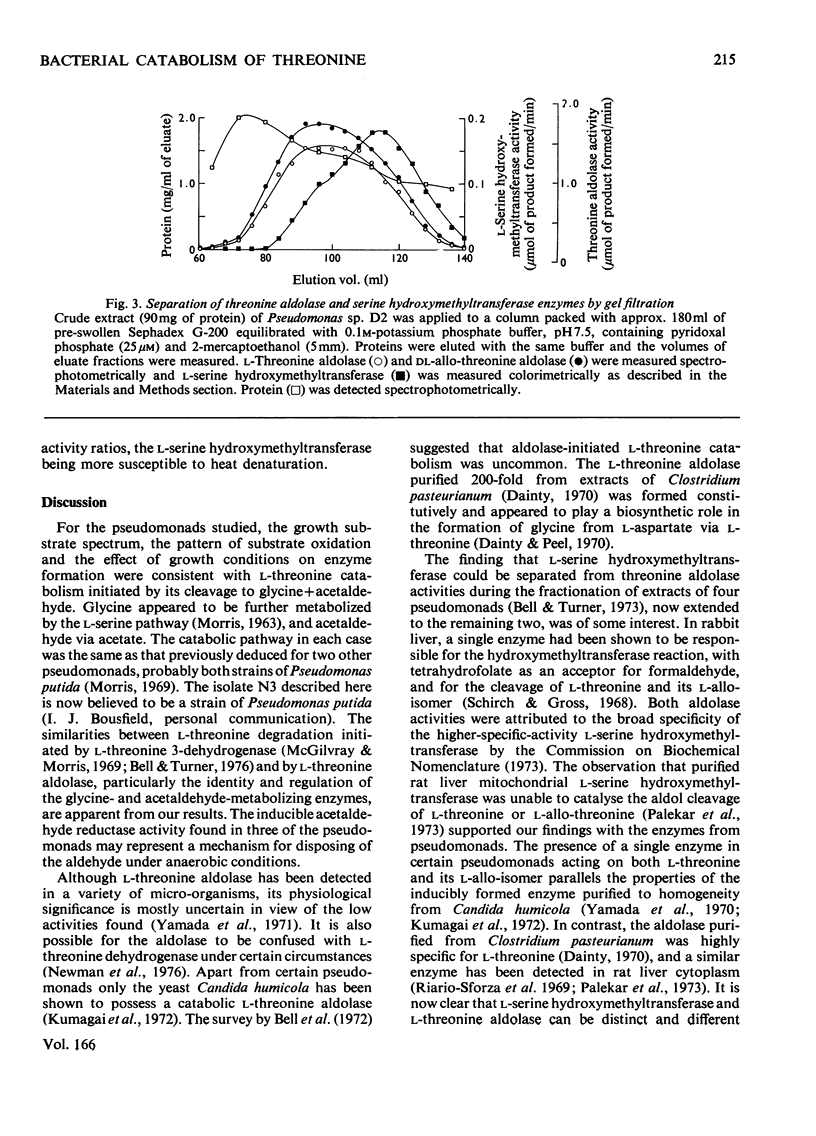
1. The route of l-threonine degradation was studied in four strains of the genus Pseudomonas able to grow on the amino acid and selected because of their high l-threonine aldolase activity. Growth and manometric results were consistent with the cleavage of l-threonine to acetaldehyde+glycine and their metabolism via acetate and serine respectively. 2. l-Threonine aldolases in these bacteria exhibited pH optima in the range 8.0–8.7 and Km values for the substrate of 5–10mm. Extracts exhibited comparable allo-l-threonine aldolase activities, Km values for this substrate being 14.5–38.5mm depending on the bacterium. Both activities were essentially constitutive. Similar activity ratios in extracts, independent of growth conditions, suggested a single enzyme. The isolate Pseudomonas D2 (N.C.I.B. 11097) represents the best source of the enzyme known. 3. Extracts of all the l-threonine-grown pseudomonads also possessed a CoA-independent aldehyde dehydrogenase, the synthesis of which was induced, and a reversible alcohol dehydrogenase. The high acetaldehyde reductase activity of most extracts possibly resulted in the underestimation of acetaldehyde dehydrogenase. 4. l-Serine dehydratase formation was induced by growth on l-threonine or acetate+glycine. Constitutively synthesized l-serine hydroxymethyltransferase was detected in extracts of Pseudomonas strains D2 and F10. The enzyme could not be detected in strains A1 and N3, probably because of a highly active `formaldehyde-utilizing' system. 5. Ion-exchange and molecular exclusion chromatography supported other evidence that l-threonine aldolase and allo-l-threonine aldolase activities were catalysed by the same enzyme but that l-serine hydroxymethyltransferase was distinct and different. These results contrast with the specificities of some analogous enzymes of mammalian origin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., el-Obeid H. A. Inactivation of serine transhydroxymethylase and threonine aldolase activities. Biochim Biophys Acta. 1972 Mar 8;258(3):791–799. doi: 10.1016/0005-2744(72)90180-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase. Biochem J. 1976 May 15;156(2):449–458. doi: 10.1042/bj1560449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. C., Turner J. M. Bacterial catabolism of threonine. Threonine degradation initiated by l-threonine hydrolyase (deaminating) in a species of Corynebacterium. Biochem J. 1977 Jun 15;164(3):579–587. doi: 10.1042/bj1640579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty R. H., Peel J. L. Biosynthesis of amino acids in Clostridium pasteurianum. Biochem J. 1970 Apr;117(3):573–584. doi: 10.1042/bj1170573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty R. H. Purification and properties of threonine aldolase from Clostridium pasteurianum. Biochem J. 1970 Apr;117(3):585–592. doi: 10.1042/bj1170585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. Utilization of single L-amino acids as sole source of carbon and nitrogen by bacteria. Can J Microbiol. 1972 Nov;18(11):1647–1650. doi: 10.1139/m72-255. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Pickard M. A., Turner J. M. Aminoacetone formation and utilization by pseudomonads grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):105–114. doi: 10.1099/00221287-54-1-105. [DOI] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols. 1-Aminopropan-2-ol and ethanolamine metabolism via propionaldehyde and acetaldehyde in a species of Pseudomonas. Biochem J. 1973 May;134(1):167–182. doi: 10.1042/bj1340167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Akhtar M. The mechanism of action of serine transhydroxymethylase. Biochem J. 1970 Jan;116(2):277–286. doi: 10.1042/bj1160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., GOTTO A. M. The metabolism of C2 compounds in micro-organisms. 6. Synthesis of cell constituents from glycollate by Pseudomonas sp. Biochem J. 1961 Jan;78:69–82. doi: 10.1042/bj0780069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L., MORRIS J. G. THE UTILIZATION OF GLYCOLLATE BY MICROCOCCUS DENITRIFICANS: THE BETA-HYDROXYASPARTATE PATHWAY. Biochem J. 1965 Jun;95:577–586. doi: 10.1042/bj0950577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H., Nagate T., Yoshida H., Yamada H. Threonine aldolase from Candida humicola. II. Purification, crystallization and properties. Biochim Biophys Acta. 1972 Mar 8;258(3):779–790. doi: 10.1016/0005-2744(72)90179-9. [DOI] [PubMed] [Google Scholar]
- Kumagai T., Omuda T., Nakaoka A., Yoshimura T. [A study on the quantity of amniotic fluid in the air passages and in the stomach]. Kango. 1972 Mar;24(3):164–166. [PubMed] [Google Scholar]
- MORRIS J. G. THE ASSIMILATION OF 2-C COMPOUNDS OTHER THAN ACETATE. J Gen Microbiol. 1963 Aug;32:167–170. doi: 10.1099/00221287-32-2-167. [DOI] [PubMed] [Google Scholar]
- McGilvray D., Morris J. G. Utilization of L-threonine by a species of Arthrobacter. A novel catabolic role for "aminoacetone synthase". Biochem J. 1969 May;112(5):657–671. doi: 10.1042/bj1120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. G. Utilization of L-threnonine by a pseudomonad: a catabolic role for L-threonine aldolase. Biochem J. 1969 Nov;115(3):603–605. doi: 10.1042/bj1150603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. B., Kapoor V., Potter R. Role of L-threonine dehydrogenase in the catabolism of threonine and synthesis of glycine by Escherichia coli. J Bacteriol. 1976 Jun;126(3):1245–1249. doi: 10.1128/jb.126.3.1245-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning C., Gest H. Regulation of L-isoleucine biosynthesis in the photosynthetic bacterium rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1823–1827. doi: 10.1073/pnas.56.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Palekar A. G., Tate S. S., Meister A. Rat liver aminomalonate decarboxylase. Identity with cytoplasmic serine hydroxymethylase and allothreonine aldolase. J Biol Chem. 1973 Feb 25;248(4):1158–1167. [PubMed] [Google Scholar]
- Pickard M. A., Higgins I. J., Turner J. M. Purification and properties of l-1-aminopropan-2-ol. NAD oxidoreductase from a pseudomonad grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):115–126. doi: 10.1099/00221287-54-1-115. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Stuart A., Hoskinson R. M., Khorana H. G. Studies on polynucleotides. 78. Yeast phenylalanine transfer ribonucleic acid: terminal sequences. J Biol Chem. 1968 Feb 10;243(3):565–574. [PubMed] [Google Scholar]
- Riario-Sforza G., Pagani R., Marinello E. Threonine aldolase and allothreonine aldolase in rat liver. Eur J Biochem. 1969 Mar;8(1):88–92. doi: 10.1111/j.1432-1033.1969.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Yamada H., Kumagai H., Nagate T., Yoshida H. Crystalline threonine aldolase from Candida humicola. Biochem Biophys Res Commun. 1970 Apr 8;39(1):53–58. doi: 10.1016/0006-291x(70)90756-4. [DOI] [PubMed] [Google Scholar]


