Abstract
Porphyromonas gingivalis fimbriae can mediate adherence to many of the available substrates in the oral cavity. Expression of P. gingivalis fimbriae is regulated at the transcriptional level by environmental signals, such as temperature and hemin concentration. The arrangement of the upstream promoter and regulatory sequences required for transcription and control of the fimbrial structural gene (fimA) was investigated. Primer extension analysis demonstrated that the transcriptional start site of the fimA gene is located 41 bp upstream from the translational start codon. A region (upf) spanning 648 bp upstream of the start codon to 44 bp downstream of the translational start site was cloned upstream of a promoterless lacZ reporter gene. A series of deletion and base substitution mutations were then generated in the upf region. The constructs were introduced into the chromosome of P. gingivalis, and promoter activity measured by assaying levels of β-galactosidase. The results showed that fimA contains sequences resembling ς70 promoter consensus sequences, consisting of a −10 region (TATGAC) located at −18 to −23 and a −35 region (TTGTTG) located at −41 to −46 from the transcriptional start point. The AT-rich upstream sequences spanning bases −48 to −85 and bases −90 to −240 were required for full expression of the fimA gene, indicating the existence of positive regulation regions. Moreover, the −48 to −64 region may constitute an UP element, contributing to promoter activity in P. gingivalis. Thus, our data suggest that the P. gingivalis fimA gene has a transcription complex consisting of −10 and −35 sequences, an UP element, and additional AT-rich upstream regulatory sequences.
Porphyromonas gingivalis is a primary causative agent in severe manifestations of periodontal disease, one of the most common bacterial infections in developed countries. Colonization of the periodontal area by P. gingivalis is facilitated by adherence to a variety of oral surfaces such as epithelial cells, extracellular matrix components, proline-rich proteins and statherin in enamel salivary pellicle, and antecedent plaque bacteria such as Streptococcus gordonii (9, 10, 12, 16). Fimbriae, which are among the major adhesins of P. gingivalis, are comprised of a major structural subunit protein with a molecular mass of approximately 43 kDa (fimbrillin, FimA). Much evidence suggesting an important role for P. gingivalis fimbrillin in pathogenicity has accumulated. In addition to directly mediating adhesion, fimbrillin-mediated attachment of P. gingivalis to gingival epithelial cells induces cytoskeletal rearrangements and modulates intracellular calcium-dependent signalling pathways, events that result in internalization of the bacteria within the epithelial cells (11, 15, 37). Fimbrillin has important immunomodulating properties and can stimulate the production of proinflammatory cytokines (such as interleukin-1, interleukin-6, and tumor necrosis factor alpha) in human monocytes and polymorphonuclear leukocytes (23, 24). Intracellular tyrosine phosphorylation-dependent signal transduction appears to be one of the targets of fimbrillin-induced cytokine production (21, 24). As a major surface protein, fimbrillin is strongly antigenic, and antifimbrillin immunoglobulin G titers are much higher in patients with adult periodontitis than in healthy individuals (25). The extent to which such antibodies contribute to protection or to antibody-mediated tissue destruction remains to be determined. Fimbriae are, therefore, considered pivotal in the multistep pathogenesis of periodontal disease. Indeed, insertional inactivation of the fimA gene, with concomitant loss of fimbrial production, results in a phenotype significantly less able to cause periodontal bone loss in the gnotobiotic rat model (18). Furthermore, immunization with purified fimbriae confers protection against periodontal destruction in gnotobiotic rats (7).
Many genes that are important for bacterial virulence are under tight transcriptional control and are regulated according to prevailing environmental conditions (6). Fimbrial genes from a variety of gram-negative bacteria are an illustrative model of how bacteria sense and respond to environmental cues. The fimbriae of P. gingivalis, however, lack any significant homology to fimbrial proteins from other bacteria and appear to constitute a unique class of gram-negative fimbriae (5). Despite the fact that the fimA gene was cloned more than a decade ago, little is known about gene expression and promoter architecture. Indeed, RNA polymerase binding sites and other regulatory sequences have not been functionally defined for any genes of this important oral anaerobe.
We have previously reported that expression of the fimA gene is regulated at the transcriptional level in P. gingivalis, as determined by analysis of a fimA:lacZ promoter-reporter fusion (38). Changes in environmental conditions, such as temperature and hemin concentration, were found to alter the level of fimA expression. Correspondingly, these small environmental fluctuations also modulated bacterial binding and invasive abilities. To further understand fimbrillin expression at the molecular level, we have generated a series of mutations in the fimA promoter region to determine specific DNA sequences recognized by the transcriptional machinery. In the study presented here, we demonstrate the characteristics and organization of the P. gingivalis fimA promoter. Our findings indicate that the P. gingivalis fimA gene contains both a ς70-like promoter sequence that carries out basal-level transcription and cis-acting regulatory elements required for maximal transcription of the fimA gene.
MATERIALS AND METHODS
Bacteria and plasmids.
Bacterial strains and plasmids used in this study are listed in Table 1. P. gingivalis 33277 and its derivatives were grown in Trypticase soy broth (TSB; BBL, Cockeysville, Md.) or on 1.5% TSB agar plates, supplemented with yeast extract (Difco, Detroit, Mich.) (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml), at 37°C in an anaerobic (85% N2, 10% H2, 5% CO2) chamber. All P. gingivalis strains harboring fimA:lacZ constructs were grown in TSB containing erythromycin (20 μg/ml). Escherichia coli DH5α was used as the host strain for recombinant plasmids and grown in L broth with appropriate antibiotics: ampicillin (100 μg/ml), kanamycin (50 μg/ml), trimethoprim (200 μg/ml), and tetracycline (10 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| P. gingivalis | ||
| 33277 | Type strain from ATCC | This laboratory |
| UPF | Derivative of 33277, upf:lacZ gene, Emr | This study |
| MPF10 | Derivative of 33277, mpf10:lacZ gene, Emr | This study |
| MPS10 | Derivative of 33277, mps10:lacZ gene, Emr | This study |
| MPF35 | Derivative of 33277, mpf35:lacZ gene, Emr | This study |
| MPS35 | Derivative of 33277, mps35:lacZ gene, Emr | This study |
| MP58 | Derivative of 33277, mp58:lacZ gene, Emr | This study |
| MP150 | Derivative of 33277, mp150:lacZ gene, Emr | This study |
| MP60 | Derivative of 33277, mp60:lacZ gene, Emr | This study |
| MP59 | Derivative of 33277, mp59:lacZ gene, Emr | This study |
| E. coli DH5α | endA1 hsdR17 supE44 thi-1 recA gyrA96 relA1 Δ(lacZYA-argF) U169λ-φ80 dlacZ ΔM15; recipient for recombinant plasmids | BRL |
| Cloning vectors | ||
| pTZBg21.1 | Containing a 2.5-kb SacI DNA fragment with the fimA gene, Amr | 32 |
| pUC19 | E. coli cloning vector, Amr | BRL |
| pDN19lac | Contains 3.6-kb BamHI-SalI fragment containing promoterless lacZ gene with ribosome binding site | 35 |
| pJRD215 | Wide-host-range cosmid vector, Kmr Smr Mob+, unable to replicate in P. gingivalis | 28 |
| pBF4 | Contains 3.8-kb EcoRI fragment in Tn4351 carrying two antibiotic resistance genes: Tcr expressed in E. coli and Emr expressed in P. gingivalis | 33 |
| R751 | IncP plasmid used to mobilize vectors from E. coli to Bacteroides recipient, Tpr Tra+ | 28 |
| pCR2.1-TOPO | Linearized plasmid with single 3′ dT residues, Kmr | Invitrogen |
| Recombinant plasmids | ||
| pUPF1 | P. gingivalis fimA promoter region (upf) containing sequence from −606 to +86 in plasmid pCR2.1-TOPO, Amr Kmr | This study |
| pUPF5 | upf:lacZ gene in pJRD215 with a 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMPF101 | upf:lacZ gene in pUC19 with 3-base change from TAT to CCG at positions −23 to −21 (mpf10:lacZ), Amr | This study |
| pMPF103 | mpf10:lacZ gene in pJRD215 with a 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMPS101 | upf:lacZ gene in pUC19 with 3-base change from TAA to GCC at positions −11 to −9 (mps10:lacZ), Amr | This study |
| pMPS103 | mps10:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMPF351 | upf:lacZ gene in pUC19 with 3-base change from TTG to CCA at positions −46 to −44 (mpf35:lacZ), Amr | This study |
| pMPF353 | mpf35:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMPS351 | upf:lacZ gene in pUC19 with 3-base change from TGG to CAC at positions −43 to −41 (mps35:lacZ), Amr | This study |
| pMPS353 | mps35:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMP1501 | upf:lacZ gene in pCR2.1-TOPO with 150-bp deletion from −240 to −90 (mp150:lacZ), Amr | This study |
| pMP1504 | mp150:lacZ, gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMP601 | mp150:lacZ gene with 15-bp deletion from −85 to −71 (mp60:lacZ) in pUC19, Amr | This study |
| pMP603 | mp60:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMP591 | pMP601 with 17-bp deletion from −64 to −48 (mp59:lacZ), Amr | This study |
| pMP593 | mp59:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
| pMP581 | upf:lacZ gene in pUC19 with 16-bp deletion from −23 to −8 (mp58:lacZ), Amr | This study |
| pMP583 | mp58:lacZ gene in pJRD215 with 3.8-kb EcoRI fragment from Tn4351, Tcr Kmr Smr | This study |
Kmr, Smr, Tcr, Emr, Tpr, and Amr, resistance to kanamycin, streptomycin, tetracycline, erythromycin, trimethoprim, and ampicillin; Mob+, can be mobilized; Tra+, capable of self-transfer.
DNA and RNA manipulations.
P. gingivalis chromosomal DNA was extracted by the procedure described by Sambrook et al. (31). All plasmid DNA was isolated by using a Promega miniprep kit and analyzed by 0.8% agarose gel electrophoresis. Restriction enzymes for DNA digestion were purchased from Gibco BRL (Grand Island, N.Y.). DNA fragments were purified from agarose gels by using a Geneclean kit (Bio 101, Inc., Vista, Calif.). P. gingivalis total RNA was isolated by using a TRIzol kit (Gibco BRL), and DNA contamination was eliminated following digestion with DNase I (Gibco BRL). RNA was visualized on 1.0% ethidium bromide-stained formaldehyde-agarose gels and quantitated spectrophotometrically.
DNA sequence analysis.
DNA sequencing was conducted by the dideoxy-chain termination procedure using Sequenase version 2 (U.S. Biochemical Corp., Cleveland, Ohio). For determination of the upstream sequence of the fimA gene, the template was plasmid pTZBg21.1 containing a 2.5-kb SacI fimA DNA fragment. For confirmation of the mutations in the fimA promoter region, the upf:lacZ fragment was subcloned into pUC19, and the recombinant plasmid was then used as the template. The synthetic oligonucleotide primers used in sequencing are described in Table 2.
TABLE 2.
Synthetic oligonucleotide primers
| Primer | Sequence (5′→3′)a | Position | Purpose |
|---|---|---|---|
| PE1 | GCTGGTCCTCAATACCACGCTGATGGTGGC | +30 to +60 | Primer extension |
| PE3 | AGCATTATCTAGAACCTCCTTAGGATCCCG | lacZ gene | Primer extension |
| FS1 | ATTGTGTTGTGCTCCGGGCTGGCCTTGCTG | −163 to −193 | DNA sequencing |
| FS2 | GATAGCTCTTGCGCTACGGGCTAAA | −422 to −446 | DNA sequencing |
| FP1 | GGAATTCCGAGCTATCGATGGCGGGTCTCT | −605 to −628 | Used with FP3 in PCR to obtain UPF fragment |
| EcoRI | |||
| FP3 | CGGGATCCCGCCAACTCCAAAAGCACGATTCGA | +87 to +61 | Used with FP1 in PCR to obtain UPF fragment |
| BamHI | |||
| MPF10 | CTTGCTGCTCTTGCCCGGACAGCTTGTAAC | −36 to −6 | Unique-site elimination mutagenesis |
| MPS10 | GCTATGACAGCTTGGCCCAAAGACGGCGAGGC | −24 to +8 | Same as above |
| MPF35 | CAAAGTTTTTCCCATTGGGACTTGCTGCTC | −55 to −25 | Same as above |
| MPS35 | CAAAGTTTTTCTTGCACGGACTTGCTGCTC | −55 to −25 | |
| MP58 | GTTTTTCTTGTTGGGACTTGCTGCTCTTGCAAAGA | −51 to +20 | Deletion from −22 to −7 |
| CAACGAGGCAGAACCCGTTACAG | |||
| MP59 | GTTGTTGGGCTTGCATAATTCACCGAGATGCTTG | −69 to −16 | Deletion from −63 to −45 |
| TTGGGACTTGCTGCTCTTGCTATGA | |||
| MP60 | GGATGTTGTTGGGCTTGCATAATTCACCGAGATC | −89 to −69 | Deletion from −84 to −69 |
| AAAAAAACAAAGTTTTTCTTGTTGGG | |||
| lacZ2 | GAAAGGGGGATGTGCTGCAAGGCGATTAAG | Corresponding to lacZ | Testing primer |
| MPF3 | TGTTGGGACTTGCTGCTCTTGCCCG | Testing primer for MPF10 | |
| MPS3 | ACTTGCTGCTCTTGCTATGACAGCTTGGCC | Testing primer for MPS10 | |
| TMPF35 | CAAAGTTTTTCCCAT | Testing primer for MPF35 | |
| TMPS35 | AGTTTTTCTTGCACG | Testing primer for MPS35 | |
| MP16 | CTTGCTGCTCTTGCAA | Testing primer for MP58 | |
| MP15 | ATTCACCGAGATGCT | Testing primer for MP59 | |
| MP150 | GCTTATGGATGTTGTTGGGCTTGCATATTCA | Testing primer for MP150 | |
| MP14 | TGGGCTTGCATAATTCACCG | Testing primer for MP60 |
Restriction sites are underlined; substituted nucleotides are italicized.
Primer extension analysis.
The transcriptional start site was investigated by primer extension. The avian myeloblastosis virus reverse transcriptase primer extension system (Promega, Madison, Wis.) was used, with modifications. Primers PE1 and PE3 (Table 2) were 5′ end labeled with [γ-32P]ATP (3,000 μCi/mmol; NEN, Boston, Mass.) with T4 polynucleotide kinase and annealed with approximately 50 μg of total RNA at 58°C for 20 min. The resulting heteroduplex was extended with avian myeloblastosis reverse transcriptase at 42 or 50°C for 30 min. The length of the extension was measured by polyacrylamide gel (8%) electrophoresis calibrated with a sequencing reaction using the same primer.
PCR and Southern blot analyses.
PCR mixtures contained 10 pmol of template DNA, 30 pmol of each primer, 1.5 mM MgCl2, 10 mM deoxynucleoside triphosphate, and 5 U of Taq DNA polymerase (Bethesda Research Laboratories [BRL]). The amplification was performed in a thermal cycler (Techne) at 94°C for 45 s, 42°C for 1 min, and 72°C for 1 min for a total 30 cycles, followed by 10 min of elongation at 72°C. Southern blotting was performed by using the PhotoGene detection system (BRL), with minor modifications. After UV cross-linking, the membrane was hybridized with the biotin-labeled 1.4-kb fimA fragment at 65°C overnight.
Construction of pUPF5 and derivatives carrying different mutations.
Standard recombinant DNA techniques were used in all plasmid construction (31). The fimA upstream region (upf) between nucleotides −648 and +44 from the translational initiation codon was amplified by PCR using P. gingivalis chromosomal DNA as the template, FP1 as the forward primer, and FP3 as the reverse primer (Table 2). Primers were tagged with EcoRI and BamHI restriction sites, respectively. The PCR product was cloned into pCR2.1-TOPO as instructed by the manufacturer (Invitrogen), creating pUPF1. To generate the upf:lacZ gene fusion, the upf fragment was cloned into plasmid pDN19lac (35), which contains a promoterless lacZ gene. A 4.3-kb EcoRI and BamHI upf:lacZ fragment of the resulting plasmid pUPF2 was cloned into the broad-host-range vector pJRD215 to generate pUPF4. A 3.8-kb EcoRI fragment of Tn4351 with Tcr and Emr genes was then cloned into pUPF4 to create pUPF5. For pUPF5 derivatives (with the exception of pMP1504), the upf:lacZ fragment from pUPF2 was cloned into pUC19 to generate pUPF3 and a series of site-specific mutations was generated (see below) prior to cloning into pJRD215.
Site-specific mutagenesis.
An 150-bp deletion mutation (MP150 [Fig. 2B]) was generated by exploiting unique restriction sites in pUPF1. After digestion with NdeI and SspI, the linearized plasmid with an NdeI overhang was blunt ended by the large fragment of DNA polymerase I and religated with T4 DNA ligase. Site-specific small deletion and base substitution mutations were generated by using a unique-site elimination mutagenesis kit (Pharmacia Biotech, Piscataway, N.J.). The general procedure was to use a pair of primers for each mutation; one was to introduce the desired mutation, and the other was a selection primer which could change a unique ScaI site to MluI in pUPF3. When both primers annealed to the same strand of the denatured pUPF3, a new strand was synthesized and selected by digestion of reaction mixture with ScaI. The authenticity of the mutated sequence was verified by DNA sequencing and PCR analysis. To identify mutations by using PCR, specific pairs of primers for each mutation were designed. The forward primer corresponded to the fimA promoter sequence except for the last two or three nucleotides at the 3′ end matching the mutated bases; the reverse primer was complementary to the lacZ gene. The results from both DNA sequencing and PCR analyses were always consistent.
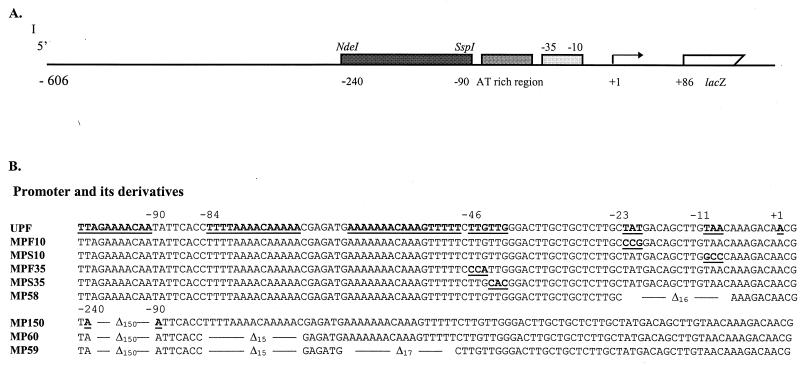
FIG. 2.
Core promoter region of the fimA gene. (A) Schematic map of the fimA promoter region fused with the lacZ reporter gene. (B) Sequences of the core promoter region of fimA with and without mutations. UPF represents wild-type promoter sequence; the sequences subsequently mutated are underlined and in boldface. The sequences of eight mutated promoters are also shown. Base substitutions are represented by underlined and bolded letters and deletion mutations are shown by the symbol ▵, with the number representing deleted base pairs.
Introduction of the upf:lacZ fusion and its derivatives into P. gingivalis.
The upf:lacZ fusion was introduced into P. gingivalis by conjugal transfer of the suicide plasmid pUPF5 from E. coli, resulting in integration of the fusion construct into the chromosome by a Campbell insertion. The conjugation experiments were performed with E. coli DH5α containing plasmids pUPF5 (or derivatives) and R751 as the donor and with P. gingivalis as the recipient. Briefly, E. coli DH5α containing pUPF5 and R751 was cultured aerobically in L broth for 2 to 4 h to an A600 of 0.2, and P. gingivalis was grown anaerobically in TSB medium for 8 h to an A600 of 0.3 (early logarithmic growth). The conjugation mixture had a donor-to-recipient ratio of 0.2 and was spotted onto a 0.45-μm-pore-size HAWP filter (Millipore, Bedford, Mass.). The mating was performed aerobically on TSB sheep blood plates for 16 h and then anaerobically in TSB for 8 h. Transconjugants were selected on TSB blood plates containing gentamicin (100 μg/ml) and erythromycin (20 μg/ml). Since P. gingivalis is naturally resistant to this concentration of gentamicin and E. coli is naturally sensitive to gentamicin, colonies growing on the antibiotic plates were P. gingivalis with pUPF5 integrated into the chromosomal DNA.
To confirm that the P. gingivalis transconjugants possessed a chromosomal integration of pUPF5 immediately upstream of the fimA gene, a Southern blot analysis was performed. P. gingivalis chromosomal DNA was digested with BamHI and analyzed by Southern hybridization with a 1.4-kb fimA fragment (generated by PCR and labeled with biotin) as the probe. The hybridized probe was detected by the Photogene nucleic acid detection system (BRL).
β-Galactosidase assays.
Expression of the lacZ gene under control of the fimA promoter was measured by a spectrophotometric β-galactosidase assay with o-nitrophenyl galactosidase as the substrate, according to the standard protocol of Miller (19) as described previously (38). The recombinant strains of P. gingivalis were cultured anaerobically in TSB under a variety of defined conditions. Bacteria were recovered from late log phase (except where noted) and tested at an optical density at 600 nm of 0.4 to 0.6. Since P. gingivalis does not normally ferment lactose or other sugars, background levels of enzyme activity were low. To ensure that any differences in β-galactosidase activity were not the result of a spontaneous chromosomal mutation, assays were performed on at least two independent isolates of each strain.
Mobility shift DNA-binding assay.
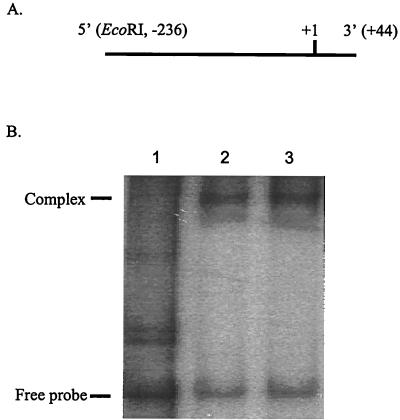
A 280-bp DNA fragment containing the wild-type fimA promoter (−44 to +236) was used as the probe and prepared by PCR. E. coli RNA polymerase (holoenzyme) saturated with ς70 was purchased from Epicentre Technologies (Madison, Wis.). The experiments were conducted with a Bandshift kit (Pharmacia Biotech). Briefly, the DNA fragment was digested with EcoRI and labeled with [α-32P]dATP (3,000 μCi/mmol; NEN), using Klenow enzyme. For the protein-DNA reaction, 1 μg of 32P-labeled DNA, 0.5 μg of RNA polymerase, and 3 μg of unrelated DNA (calf thymus DNA) were mixed and incubated at room temperature for 20 min; the mixture was then loaded onto a 5% nondenaturing polyacrylamide gel and electrophoresed in 0.5× Tris-borate-EDTA buffer at 10 V/cm. Finally, the gel was dried and exposed to X-ray film at −70°C.
RESULTS
Determination of the transcriptional start site.
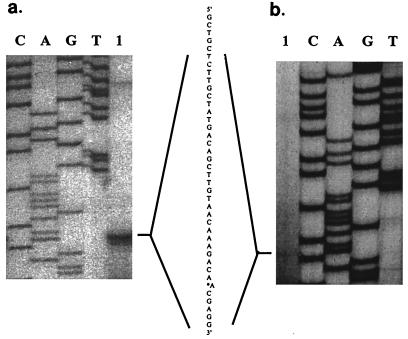
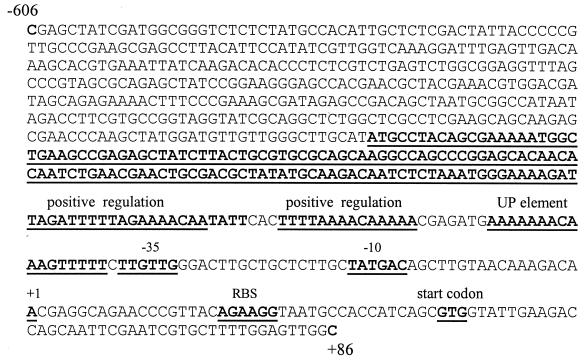
The transcription initiation site of the fimA gene was investigated by primer extension analysis. RNA isolated from P. gingivalis 33277 was analyzed with primer PE1, and RNA from the upf:lacZ-containing strain UPF was analyzed with primer PE3 (corresponding to sequence of the lacZ gene) (Table 2); two identical extension products were detected (Fig. 1). Therefore, the transcriptional start site was mapped to an A residue 41 bp upstream of the translational initiation codon. Examination of the upstream sequence revealed potential sequences recognized by ς70-dependent RNA polymerase (−10 sequences TATGAC and TAACAA; −35 sequence TTGTTG). The functionality of these sequences was examined by site-specific mutagenesis (see below).
FIG. 1.
Transcriptional start site mapping of P. gingivalis fimA, using primers PE1 (a) and PE3 (b) Lanes: 1, primer extension with P. gingivalis 33277 (a) and P. gingivalis UPF (b) RNAs as templates; G, T, C, and A, nucleotide-specific sequencing reactions.
Development of promoter fusion reporters.
We have previously demonstrated (38) that sequences required for the promotion of transcription of the fimA gene reside within 236 bp upstream of the translational start site. However, to facilitate integration of mutated fimA:lacZ constructs (especially the deletion mutations) into P. gingivalis chromosomal DNA and to investigate the presence of additional upstream regulatory sequences, a longer upstream region was used in this study. As shown in Fig. 2A and 3, a 692-bp EcoRI and BamHI fragment was amplified by PCR, fused to a promoterless lacZ gene, and integrated into P. gingivalis chromosomal DNA. The fimA promoter region used in this study thus spanned 648 bp upstream to 44 bp downstream of the translational start codon. This DNA fragment was designated upf and contained sufficient homologous sequence to permit integration into the chromosome of P. gingivalis.
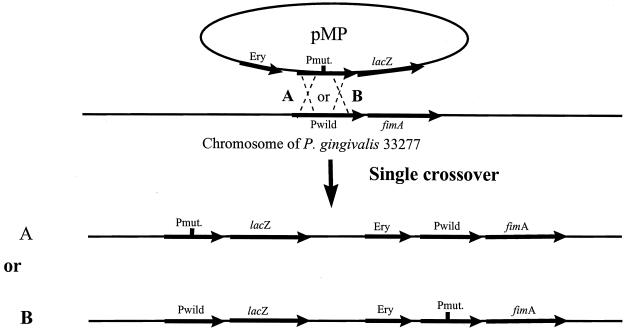
FIG. 3.
Homologous recombination between pUPF5 or its derivatives (pMP) and the P. gingivalis chromosome. The thicker lines represent the DNA fragment containing the fimA gene, fimA promoter region, lacZ gene, and erythromycin resistance gene (Ery). (A) The homologous recombination occurs upstream of the mutation. (B) The recombination occurs downstream of the mutation. Pmut. and Pwild, mutant and wild-type promoters, respectively.
Construction of upf:lacZ and its derivatives.
The strategy for identification of cis-acting regulatory elements of the fimA gene was to define the affect of DNA sequence alteration on fimA promoter activity in P. gingivalis. For this purpose, the fimA upstream region (upf) and its eight derivatives (Fig. 2B) with mutations generated in upf were individually fused with a promoterless lacZ gene and returned to P. gingivalis. The upstream region of the transcriptional start site determined by primer extension possesses potential −10 sequences centered at −8/−9 (TAACAA), −11/−12 (TTGTAA) or −20/−21 (TATGAC) and −35 sequences centered at −33/−34 (TTGCTG) or −43/−44 (TTGTTG). Thus, primers MPF10 and MPS10 were used in site-specific mutagenesis to generate two modified fimA promoters: one with a conversion of TAT to CCG at positions −23 to −21, and one with a conversion of TAA to GCC at −11 to −9. For delimiting the −35 sequence, primers MPF35 and MPS35 were designed to convert TTG at −46 to −44 to CCA and TTG at −43 to −41 to CAC, respectively. In each case, the impact of the mutations on lacZ expression would depend on the requirement of sequence for full promoter activity. A deletion mutation was also generated with the removal of bases −23 to −8 (MP58). This deletion mutation would decrease fimA promoter activity only partially, if additional upstream promoters were present.
We also constructed a series of upstream deletion mutations in order to detect any regulatory sequence(s) that contributes to fimA expression. The first large deletion mutation (MP150) entailed removal of 150 bp from −240 to −90. An AT-rich sequence located between −85 to −48 was also selected as a candidate regulatory region. Further deletions in this AT-rich sequence resulted in a double (MP60)- and triple (MP59)-deletion mutations in the upf region.
Selection of P. gingivalis strains with fused genes.
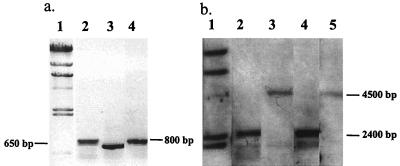
The upf:lacZ gene and its derivatives were introduced into P. gingivalis by conjugation between E. coli DH5α and P. gingivalis 33277. Plasmid pJRD215 carrying the upf:lacZ gene (pUPF5) or its derivatives cannot replicate in P. gingivalis due to the lack of a functional origin of replication. Southern blot analysis confirmed the integration of pUPF5 and its derivatives. Single crossover of pUPF5 could result in two genomic configurations (depicted in Fig. 3), depending on whether the crossover occurs proximal or distal to the mutation. If recombination occurs upstream of the mutation, the mutated fimA promoter would drive the lacZ gene (Fig. 3A). In contrast, the lacZ gene would be under control of the intact wild-type fimA promoter, leaving the mutated fimA promoter with the fimA structure gene, if recombination occurred downstream of the mutation (Fig. 3B). For the purpose of this study, P. gingivalis strains with the promoterless lacZ gene under control of the mutated fimA promoter (Fig. 3A) were required. Selection for the desired isolates was accomplished by PCR with a forward primer corresponding to the mutated promoter and a reverse primer (lacZ2) corresponding to the lacZ gene. A PCR product of the correct size could be obtained only when the mutated fimA promoter was directly upstream of the lacZ gene. The PCR results were confirmed by Southern blot analysis for the large deletion mutation, as shown in Fig. 4 for P. gingivalis MP150. The chromosomal DNAs from three isolates of P. gingivalis MP150 were used as templates, and FP1 (forward primer) and laCZ2 (reverse primer) were used for PCR analysis. Agarose gel electrophoresis shows two sizes of PCR products (Fig. 4a). The size (about 800 bp) of the larger product (lanes 2 and 4) indicated that the lacZ gene had a wild-type fimA promoter region, whereas the smaller fragment (lane 3) of 650 bp resulted from a 150-bp deletion. Thus, the DNA template used in lane 3 was from the mutant strain, and this was used in subsequent experiments for β-galactosidase activity. The results from Southern blotting also showed two different-size bands when the same P. gingivalis MP150 isolates were examined (Fig. 4b). Blotting was performed by digesting chromosomal DNA with SspI and HindIII and probing with 1.4 bp of the fimA gene. Since SspI was a unique restriction site in the fimA promoter that was lost during the deletion procedure (digestion and religation), the larger bands (lanes 3 and 5) indicated that the mutated fimA promoter was associated with the fimA gene. The small band (lane 4) indicated that the lacZ gene was associated with the mutated fimA promoter (mp150). A similar PCR analysis was performed for each small deletion or base pair substitution mutation.
FIG. 4.
PCR and Southern blot analyses of P. gingivalis MP150. (A) Chromosomal DNAs from three isolates of P. gingivalis MP150 analyzed by PCR with forward primer FP1 and reverse primer lacZ2. Lanes: 1, DNA standard; 2 to 4, isolates of P. gingivalis MP150. (B) DNA samples analyzed by Southern blotting. DNA was digested with SspI and HindIII and probed with a 1.4-bp fimA fragment. Lanes: 1, DNA standard; 2, UPF; 3 to 5, isolates of MP150.
Characterization of the fimA promoter.
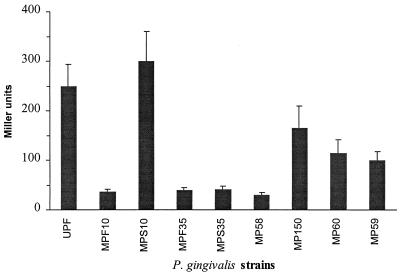
The effects of a series of mutations on fimA promoter activity in P. gingivalis are shown in Fig. 5. A 16-bp deletion from positions −23 to −8, which encompasses the putative −10 sequences (MP58), almost completely abolished fimA promoter activity. The contribution of the individual −10 consensus sequences was determined by using strains MPF10 and MPS10. The replacement of TAT with CGG at positions −23 to −21 (MPF10) decreased the level of fimA promoter activity dramatically. Moreover, the enzymatic activity of LacZ remained constant when P. gingivalis MPF10 was tested over a 50-h period, showing that the promoter deficiency is stable and not controlled by growth phase. In contrast, P. gingivalis MPS10, in which the mutation is in the −11 to −9 region (TAA replaced with GCC), did not show a reduction of fimA promoter activity; instead, we observed a slight increase in expression, most marked at 34°C. Thus, the −18 to −23 (TATGAC) region appears to be a ς70 functional site.
FIG. 5.
Effects of fimA mutations in the promoter region on transcriptional activity. See Fig. 2 for depiction of mutations. β-Galactosidase level is presented in Miller units as described in the text. Data represent the means and standard errors obtained from at least three independent experiments.
Further evidence that the fimA gene is controlled by a ς70-dependent promoter was provided by the results obtained with mutations in the putative −35 region and mobility shift DNA binding assay. Strains MPF35 (replacement of TTG at −44 to −46 with CAA) and MPS35 (replacement of TTG at −41 to −43 with CAC) showed a significant loss of promoter activity (Fig. 5). Moreover, as shown in Fig. 6, RNA holoenzyme containing ς70 was able to bind the fimA promoter (lane 2). This reaction showed specificity, since unrelated DNA (calf thymus DNA) was unable to compete with the fimA promoter region for enzyme binding (lane 3). These results strongly suggested that the fimA gene has a ς70-recognized promoter with a −10 sequence of TATGAC centered at −20/−21, and a −35 sequence of TTGTTG centered at −43/−44, from the transcriptional start site. The spacing between these two hexamers is 17 bp.
FIG. 6.
RNA polymerase (ς70)-fimA interaction. (A) DNA used in the mobility shift DNA binding assay. The region 5′ of the DNA fragment was tagged with an EcoRI site, and +1 corresponds to the transcriptional start site of the fimA gene. (B) Mobility shift DNA binding assay. Lanes: 1, fimA promoter fragment only; 2, fimA promoter fragment and E. coli RNA holoenzyme; 3, fimA promoter, RNA holoenzyme, and calf thymus DNA.
fimA upstream regulatory sequences.
P. gingivalis MP150, MP60, and MP59, which contained deletions upstream of the fimA promoter region (Fig. 2B), displayed only 66 to 40% of the total promoter activity displayed by the full-length fimA promoter (upf) (Table 3). AT-rich tracts in the region between −48 and −240 thus appear to be important for full expression of the fimA gene. These data support the concept that regulatory nucleotide sequences are involved in the control of fimA expression. Furthermore, the AT-rich −48 to −64 area, which begins 2 bp distal to the −35 region, may represent an UP element that interacts with the α subunit of RNA polymerase (Fig. 7).
TABLE 3.
Effects of mutations upstream of the fimA −35 region on promoter activity
| P. gingivalis strain | Relative % β-galactosidase levela
|
Ratiob
|
||||
|---|---|---|---|---|---|---|
| 34°C | 37°C | 39°C | 34°C | 37°C | 39°C | |
| UPF | 230 | 100 | 53 | 2.3 | 1 | 0.5 |
| MP150 | 200 | 66 | 37 | 3 | 1 | 0.56 |
| MP60 | 180 | 46 | 37 | 3.9 | 1 | 0.8 |
| MP59 | 150 | 40 | 32 | 3.75 | 1 | 0.8 |
The 100% activity is 250 ± 60 Miller units of β-galactosidase, which was obtained as P. gingivalis UPF grew at 37°C to log-phase growth.
Ratio of fimA promoter activity following growth at 34 or 39°C compared to that at 37°C.
FIG. 7.
Nucleotide sequence of the fimA promoter region. The underlined and boldface sequences represent −10, −35, UP element, and transcriptional start site regions. The −71 to −240 region contains positive regulatory sequences. RBS, ribosome binding site.
Activity of the fimA promoter increases as temperature decreases, being >4-fold greater at 34°C than at 39°C (Table 3). Upstream deletion mutants MP150, MP60, and MP59 responded similarly to culture temperature; however, a trend toward proportionally greater activity at 34°C was observed. Whereas the ratio of activity at 34°C to that at 37°C was 2.3 for strain UPF, ratios for mutants MP59 and MP60 were 3.75 and 3.9, respectively. This finding indicates that the AT-rich region spanning residues −71 to −85 may be involved in temperature control.
Interestingly, all of the fimA derivatives retained functional activity when they were expressed in E. coli. Moreover, E. coli RNA polymerase causes a mobility shift of the mutated constructs. This observation indicates that E. coli sigma factors can recognize alternative sequences in the fimA upstream region.
DISCUSSION
The ability of many pathogenic bacteria to sense important environmental cues and respond by regulating gene expression at the transcriptional level is well established. Previous reports show that expression of P. gingivalis fimbrillin, an important virulence factor, is regulated at the transcriptional level by certain nutritional and environmental signals (1, 38). It was proposed (38) that P. gingivalis optimizes expression of fimbrillin in the early stages of colonization to facilitate adherence and invasion and subsequently represses fimbrillin production to diminish the severity of the host immune response. In general, there are two major participants in the control of gene expression: trans-acting elements, including RNA polymerase and other protein regulators; and cis-acting elements, namely, specific DNA sequences involved in trans factor recognition and activity. The contribution of these elements to the control of virulence gene expression in P. gingivalis is not known. The putative promoter regions of many of the virulence genes of P. gingivalis that have been cloned and sequenced are deduced on the basis of DNA sequence (5, 13, 22, 29). Genes including those for fimbrillin (fimA), superoxide dismutase (sod), hemagglutinin A (hagA), and various proteases (prtR, prtRI, prtH, and dppIV) possess sequences for conventional ς70 recognition. The location of the promoter for the tpr gene was inferred on the basis of deletion mutational analysis (17); however, the RNA polymerase binding site was not resolved. Therefore, to date, functional definition of P. gingivalis promoters has not been established.
Promoter recognition for bacterial fimbrial genes can involve ς70 recognition (for example E. coli Pap and type 1 fimbriae [20]) or recognition by the alternative sigma factor ς54 (for example, the type 4 fimbrial family [34]). The upstream region of the P. gingivalis fimA gene has three potential −10 and two potential −35 ς70 recognition sequences partially matching the E. coli consensus sequences. By utilizing a specific mutagenesis scheme in combination with a transcriptional gene fusion assay in a P. gingivalis host, the functional promoter sequences were determined to be the hexamers centered around bases −20 and −21 (−10 region) and −43 and −44 (−35 region), as shown in Fig. 7. Although this −10 sequence is further upstream from the transcriptional initiation site than is commonly observed, there are additional features of this promoter arrangement that are consistent with ς70-dependent transcriptional promotion. The transcriptional start site is an adenine residue that was centered in AAC, a common start point for ς70 promoters. Furthermore, the spacing between the −10 and −35 regions, 17 bp, is optimal for E. coli ς70 promoters (8). Although the expression of the E. coli, Pap, and type 1 fimbriae is also under the control of the ς70 factor, differences in gene organization, regulation, and amino acid sequence tend to exclude P. gingivalis fimbriae from this grouping.
Deletion and base change mutations that reduced promoter activity in P. gingivalis had no effect on activity in E. coli. The transcriptional machinery in E. coli can, therefore, apparently recognize alternative sequences in the AT-rich fimA upstream region. These results may partially explain the observations of Onoe et al. (26), who reported that an E. coli recombinant strain containing the fimA gene and upstream sequences produced a prefimbrillin with an extremely long leader peptide (46 amino acids). This led to the proposal that the fimA promoter region is further upstream than the region we have defined. Evidence presented in this report, however, suggests that the extended prefimbrillin leader sequence observed in E. coli may be a consequence of promiscuous recognition of P. gingivalis sequences by E. coli RNA polymerases. Similarly, Boyd and Lory (2) demonstrated that Pseudomonas aeruginosa sequences are not faithfully recognized in E. coli. These findings emphasize the importance of performing promoter definition studies with the organism under investigation, rather than extrapolating from data obtained for E. coli. Such analyses have been problematic in studies of P. gingivalis due to the lack of the requisite genetic tools; thus, the genetic systems developed for this study may find utility in the investigation of other P. gingivalis promoters and regulatory mechanisms.
Since fimA expression is regulated in response to environmental conditions, it is likely that gene expression involves a regulatory DNA sequence(s). This concept is supported by the results of the deletion mutation analysis. Unlike promoter elements, regulatory sequences do not act without a promoter, nor does their loss completely abolish promoter activity (14). P. gingivalis MP150, bearing a large deletion from −240 to −90, showed a decrease in fimA promoter activity of approximately 35% at 37°C, suggesting that this 150-bp region contains a positive regulatory sequence. Such AT-rich regions are frequently involved in positive regulation of gene expression (27, 30). Additional AT-rich sequences in the −85 to −48 region also appeared to be involved in positive regulation at 37°C. Moreover, the −48 to −64 area may correspond to an UP element. This element is believed to be part of the promoter that interacts with C-termini of the α subunit of RNA polymerase (3). UP elements increase the strength of the overall RNA polymerase binding and thus enhance transcription. This may be important for fimA expression, as the −10 and −35 regions match the consensus sequences in only four and three of six bases, respectively.
Temperature fluctuation has been found to be a significant regulatory factor for fimA promoter activity, with expression increasing as temperature declines from 39 to 34°C (38). Although the −71 to −85 area may be involved in temperature-dependent control, the results did not allow a precise delineation of the elements of thermoregulation. It is possible, therefore, that more than one regulatory pathway is involved in fimA expression. For example, bacterial DNA supercoiling increases with increasing growth temperature (36). Changes in supercoiling can, in turn, affect the stability of binding between RNA polymerase and its promoter and thus modulate gene transcription. DNA topology-dependent control may be important in the thermoregulation of the P. gingivalis fimA gene.
In conclusion, transcription of the fimA gene in P. gingivalis is promoted by ς70-recognized sequences, including −10, −35, and UP elements (Fig. 7). AT-rich upstream regulatory sequences are required for full expression of fimA in P. gingivalis. More than one control pathway appears to be involved in environmental regulation of fimA expression.
ACKNOWLEDGMENTS
We thank Steve Lory and Yoonsuk Park for much helpful advice and for provision of plasmids.
The support of the NIDR (grants DE11111 and DE00401) is gratefully acknowledged.
REFERENCES
- 1.Amano A, Sharma A, Kuramitsu H K, Genco R J. Effects of temperature stress on expression of fimbriae and superoxide dismutase by Porphyromonas gingivalis. Infect Immun. 1994;62:4682–4685. doi: 10.1128/iai.62.10.4682-4685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd J M, Lory S. Dual function of PilS during transcriptional activation of the Pseudomonas aeruginosa pilin subunit gene. J Bacteriol. 1996;178:831–839. doi: 10.1128/jb.178.3.831-839.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busby S, Ebright R H. Promoter structure, promoter recognition, and transcription activity in prokaryotes. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 4.Cutler C W, Kalmar J R, Genco C A. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson D, Kubiniec M A, Yoshimura F, Genco R J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988;170:1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiRita J, Mekalanos J J. Genetic regulation of bacterial virulence. Annu Rev Genet. 1989;23:455–482. doi: 10.1146/annurev.ge.23.120189.002323. [DOI] [PubMed] [Google Scholar]
- 7.Evans R T, Klausen B, Sojar H T, Bedi G S, Sfintescu C, Ramamurthy N S, Golub L M, Genco R J. Immunization with Porphyromonas gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992;60:2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hideki N, Sharma A, Sojar H T, Amano A, Levine M I, Genco R J. Role of the carboxyl-terminal region of Porphyromonas gingivalis fimbrillin in binding to salivary proteins. Infect Immun. 1997;65:422–427. doi: 10.1128/iai.65.2.422-427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirose K, Isogai E, Mizugai H, Ueda I. Adhesion of Porphyromonas gingivalis fimbriae to human gingival cell line Ca9-22. Oral Microbiol Immunol. 1996;11:402–406. doi: 10.1111/j.1399-302x.1996.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 11.Izutsu K T, Belton C M, Chan A, Fatherazi S, Kanter J P, Park Y, Lamont R J. Involvement of calcium in interactions between gingival epithelial cells and Porphyromonas gingivalis. FEMS Microbiol Lett. 1996;144:145–150. doi: 10.1111/j.1574-6968.1996.tb08521.x. [DOI] [PubMed] [Google Scholar]
- 12.Kontani M, Kimura S, Nakagawa I, Hamada S. Adherence of Porphyromonas gingivalis to matrix proteins via a fimbrial cryptic receptor exposed by its own arginine-specific protease. Mol Microbiol. 1997;24:1179–1187. doi: 10.1046/j.1365-2958.1997.4321788.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuramitsu H K, Yoneda M, Madden T. Proteases and collagenases of Porphyromonas gingivalis. Adv Dent Res. 1995;9:37–40. doi: 10.1177/08959374950090010701. [DOI] [PubMed] [Google Scholar]
- 14.Kustu S, North A K, Weiss D S. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem Sci. 1991;16:397–402. doi: 10.1016/0968-0004(91)90163-p. [DOI] [PubMed] [Google Scholar]
- 15.Lamont R J, Chan A, Belton C M, Izutsu K T, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamont R J, Bevan C A, Gil S, Persson R E, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276. doi: 10.1111/j.1399-302x.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu B, McBride B C. Expression of the trp protease gene of Porphyromonas gingivalis is regulated by peptide nutrients. Infect Immun. 1998;66:5147–5156. doi: 10.1128/iai.66.11.5147-5156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek R, Fisher J G, Caleca A, Stinson M, Oss C J, Lee J Y, Cho M I, Genco R J, Evans R T, Dyer D W. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J Bacteriol. 1994;176:1052–1059. doi: 10.1128/jb.176.4.1052-1059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller J. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 20.Mol O, Oudega B. Molecular and structural aspects of fimbriae biosynthesis and assembly in Escherichia coli. FEMS Microbiol Res. 1996;19:25–52. doi: 10.1111/j.1574-6976.1996.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 21.Murakami Y, Hanazawa S, Watanabe A, Naganuma K, Iwasaka H, Kawakami K, Kitano S. Porphyromonas gingivalis fimbriae induce a 68-kilodalton phosphorylated protein in macrophages. Infect Immun. 1994;62:5242–5246. doi: 10.1128/iai.62.12.5242-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama K. Rapid viability loss on exposure to air in a superoxide dismutase-deficient mutant of Porphyromonas gingivalis. J Bacteriol. 1994;176:1939–1943. doi: 10.1128/jb.176.7.1939-1943.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa T, Uchida H. A peptide, ALTTE, within the fimbrial subunit protein from Porphyromonas gingivalis, induces production of interleukin 6, gene expression and protein phosphorylation in human peripheral blood mononuclear cells. FEMS Immunol Med Microbiol. 1993;11:197–206. doi: 10.1111/j.1574-695X.1995.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa T, Uchida H, Hamada S. Porphyromonas gingivalis fimbriae and their synthetic peptides induce proinflammatory cytokines in human peripheral blood monocyte cultures. FEMS Microbiol Lett. 1994;116:237–242. doi: 10.1111/j.1574-6968.1994.tb06707.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Kono Y, McGhee M L, McGhee J R, Roberts J E, Hamada S, Kiyono H. Porphyromonas gingivalis-specific serum IgG and IgA antibodies originated from immunoglobulin-secreting cells in inflamed gingiva. Clin Exp Immunol. 1991;83:237–244. doi: 10.1111/j.1365-2249.1991.tb05621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onoe T, Hoover C I, Nakayama K, Ideka T, Nakamura H, Yoshimura F. Identification of Porphyromonas gingivalis prefimbrillin possessing a long leader peptide: possible involvement of trypsin-like protease in fimbrillin maturation. Microb Pathog. 1995;19:351–364. doi: 10.1016/s0882-4010(96)80006-4. [DOI] [PubMed] [Google Scholar]
- 27.Owen-Huphes T A, Pavitt G D, Santos D S, Sidebotham J M, Hulton C S, Hinton J C, Higgins C F. The chromatin-associated protein H-NS-interacts with curved DNA topology and gene expression. Cell. 1992;71:255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- 28.Park Y, McBride B C. Characterization of the tpr gene product and isolation of a specific protease-deficient mutant of Porphyromonas gingivalis W83. Infect Immun. 1993;61:4139–4146. doi: 10.1128/iai.61.10.4139-4146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Progulske-Fox A, Tumwasorn T, Holt S C. The expression and function of Bacteroides gingivalis hemagglutinin gene in Escherichia coli. Oral Microbiol Immunol. 1989;4:121–131. doi: 10.1111/j.1399-302x.1989.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 30.Puente J L, Bieber D, Ramer S W, Murray W, Schoolnik K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol Microbiol. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Sharma A, Lee J Y, Bedi G S, Genco R J. PCR amplification and cloning of the Porphyromonas gingivalis fimbrillin gene. J Dent Res. 1992;71:293. [Google Scholar]
- 33.Shoemaker N B, Getty C, Gardner J F, Salyers A A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 35.Totten P A, Lory S. Characterization of the type a flagellin gene from Pseudomonas aeruginosa PAK. J Bacteriol. 1990;172:7188–7199. doi: 10.1128/jb.172.12.7188-7199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tse-Dinh Y C, Qi H, Menzel R. DNA supercoiling and bacterial adaptation: thermotolerance and thermoresistance. Trends Microbiol. 1997;5:323–326. doi: 10.1016/s0966-842x(97)01080-9. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg A, Belton C M, Park Y, Lamont R J. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie H, Cai S, Lamont R J. Environmental regulation of fimbrial gene expression in Porphyromonas gingivalis. Infect Immun. 1997;65:2265–2271. doi: 10.1128/iai.65.6.2265-2271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]