Abstract
Climate change is expected to alter the input of nitrogen (N) sources in the Eastern Canadian Arctic Archipelago and Baffin Bay due to increased discharge from glacial meltwater and permafrost thaw. Since dissolved inorganic N is generally depleted in surface waters, dissolved organic N (DON) could represent a significant N source fueling phytoplankton activity in Arctic ecosystems. Yet, few DON data for this region exist. We measured concentrations and stable isotope ratios of DON (δ15N) and nitrate (NO3 −; δ15N and δ18O) to investigate the sources and cycling of dissolved nitrogen in regional rivers and marine samples collected in the Eastern Canadian Arctic Archipelago and Baffin Bay during the summer of 2019. The isotopic signatures of NO3 − in rivers could be reproduced in a steady state isotopic model by invoking mixing between atmospheric NO3 − and nitrified ammonium as well as NO3 − assimilation by phytoplankton. DON concentrations were low in most rivers (≤4.9 μmol N L−1), whereas the concentrations (0.54–12 μmol N L−1) and δ15N of DON (−0.71–9.6‰) at the sea surface were variable among stations, suggesting dynamic cycling and/or distinctive sources. In two regions with high chlorophyll‐a, DON concentrations were inversely correlated with chlorophyll‐a and the δ15N of DON, suggesting net DON consumption in localized phytoplankton blooms. We derived an isotope effect of 6.9‰ for DON consumption. Our data helps establish a baseline to assess future changes in the nutrient regime for this climate‐sensitive region.
Keywords: dissolved organic and inorganic nitrogen, stable isotopes, eastern Canadian Arctic Archipelago, Baffin Bay, glacial rivers, biogeochemistry
Key Points
Nitrate in regional rivers was derived proximately from nitrification
Dissolved organic nitrogen concentrations in regional rivers were low
Dissolved organic nitrogen consumption was observed in the ECAA and Baffin Bay with the highest chlorophyll‐a
1. Introduction
Climate change is rapidly altering Arctic ecosystems. As air and seawater temperatures are rising, sea ice volume is decreasing and seasonal river discharge is increasing (Bintanja & Selten, 2014; Feng et al., 2021; Wassmann et al., 2011; Wu et al., 2005). The critical roles played by the Arctic Ocean in controlling the thermohaline circulation and supporting fisheries (Link & Tol, 2009) have stimulated scientific research in the region within the past few decades. While primary productivity is expected to increase for most Arctic shelves, similar changes are not ubiquitous across the entire Arctic (Arrigo & van Dijken, 2015; Le Fouest et al., 2013; Lewis et al., 2020). Decreased nutrient delivery through physical circulation as well as increased stratification due to higher freshwater input may decrease primary productivity overall in the Canada Basin and the Eastern Canadian Arctic Archipelago (ECAA), including Baffin Bay, the Nares Strait, Lancaster Sound, and Jones Sound (Lehmann et al., 2019; McLaughlin & Carmack, 2010). Nutrients in the surface waters of the ECAA are typically low unless there are localized sources, such as rivers and inputs from glacial buoyancy‐driven upwelling (Bhatia et al., 2021; Cape et al., 2019; Tank et al., 2012; Thibodeau et al., 2017). The availability of nutrients, as well as access to sunlight, are the key factors controlling primary productivity in the western Arctic basins (Tremblay et al., 2015). These factors can shift rapidly from season to season; for example, the spring freshet delivers more nutrient‐rich freshwater nearshore, while increased sunlight is observed during the summer months (Holmes et al., 2012; Juranek, 2022). Nitrogen is ultimately limiting in the western Arctic (Tremblay et al., 2006; Yamamoto‐Kawai et al., 2006) and thus sources and sinks of nitrogen need to be constrained to better understand the current N budget as a baseline to assess future changes.
The ECAA is fed predominantly by Pacific generated water from the Canada Basin moving eastward and exiting at Lancaster Sound. Arctic water also enters through the Nares Strait, carrying Pacific water and underlying Atlantic‐origin water. In Baffin Bay, Arctic water mixes with underlying Atlantic water directly from the North Atlantic via the West Greenland current. Freshwater is also introduced into the ECAA from river and glacial melt as the coastline of the region is peppered with glaciers and has high permafrost coverage. Based on stoichiometric nutrient tracers and nitrate isotope ratios (δ15N), nutrients are dominantly from Pacific‐derived water, albeit mixed with Atlantic‐derived waters supplying about 25% of the nitrate (NO3 −) (Lehmann et al., 2022, and references therein).
Dissolved organic nitrogen (DON) typically accounts for the largest pool of dissolved nitrogen in oligotrophic freshwater and marine surface waters (Sipler & Bronk, 2014). Although dominated by refractory species, the labile fraction of DON can be an essential source of N to primary producers, especially in N limited regions (Bronk et al., 2007; Knapp et al., 2018; Moschonas et al., 2017; Thibodeau et al., 2017). DON is composed of a highly refractory pool, a semi‐labile pool, and a smaller labile pool. The timescale for utilization of DON can vary depending on lability, with refractory or semi‐labile sources persisting from months to years. Labile DON can be utilized on time scales ranging from a few days to minutes in the case of amino acids (Bronk et al., 1998; Fuhrman, 1987; Sipler & Bronk, 2014). DON can be produced in situ in the oceans through mechanisms such as the viral lysing of bacteria or the loss of prey biomass during feeding by microzooplankton (Sipler & Bronk, 2014). Rivers are one of the major DON sources to the ocean due to the presence of terrestrial organic matter. As a result, DON tends to be higher in coastal areas than in the open ocean, although the utilization of DON is not limited to coastal regions (Sipler & Bronk, 2014). DON can be produced by surface plankton in productive areas and then transported out into N limited regions, fueling primary productivity (Bif et al., 2022; Knapp et al., 2018; Letscher, Hansell, Carlson, et al., 2013).
The Arctic Ocean is heavily impacted by rivers, receiving 10% of global river discharge despite accounting for only 4% of the global ocean surface (Holmes et al., 2012; Wu et al., 2005). River runoff, permafrost thaw and coastal erosion are significant sources of nutrients in Arctic coastal regions (Le Fouest et al., 2013; Tank et al., 2012; Terhaar et al., 2021; Thibodeau et al., 2017; Treat et al., 2016). Riverine input of DON is typically high in areas such as the Laptev Sea and the western Arctic, which are fed by large rivers. DON in the Lena River, which discharges into the Laptev Sea, can average between 10 and 13 μmol L−1 depending on the season (Sanders et al., 2022). Dissolved inorganic N delivered by rivers is immediately consumed as surface coastal Arctic waters are generally devoid of NO3 − (Emmerton et al., 2008; Tremblay et al., 2014, 2015). The fate of riverine DON in coastal marine waters is still unclear, whether it is utilized or simply diluted through mixing with DON‐depleted waters. For instance, in the Arctic surface ocean, DON concentrations are on average 4.7 μmol L−1, which is less than the concentrations in major Arctic rivers, which range from 7.4 to 18.4 μmol L−1 (Sipler & Bronk, 2014). Tank et al. (2012) suggested that riverine NO3 − and DON in the coastal Laptev Sea region contribute to overall Arctic Ocean primary productivity, albeit for a relatively small fraction (<10% each). They suggest that riverine DON in the Arctic is slowly remineralized, allowing for nutrient transport from the coastal areas into ocean basins. Thibodeau et al. (2017) found that DON concentrations discharged from the Lena River in the Eurasian Arctic were up to 6 times higher than riverine NO3 − concentrations and that DON was rapidly consumed nearshore, with over 50% of it disappearing before reaching the shelf. However, Dittmar et al. (2001) concluded that DON input from Siberian rivers did not substantially support primary productivity as it was largely recalcitrant. With riverine discharge increasing since around the 1960s (Peterson et al., 2002; Wu et al., 2005), the extent to which rivers influence the delivery of bioavailable DON in Arctic coastal waters needs to be further investigated.
While previous studies have largely focused on the inputs of nutrient from large rivers in past decades (e.g., Tank et al., 2012; Thibodeau et al., 2017), deliveries of NO3 − and dissolved organic nitrogen (DON) by small rivers and glacially fed rivers (e.g., proglacial rivers), and their impacts on coastal oceanic primary productivity in the ECAA and Baffin Bay have not been thoroughly explored. Inputs of NO3 − to surface waters have been shown to occur by upwelling induced by rising submarine glacial melt discharge in proximity to the Greenland Ice Sheet and Jones Sound (Bhatia et al., 2021; Cape et al., 2019) and glacial melt (Beaton et al., 2017). However, the input of exogenous DON to the surface ocean from glacial melt has not been considered thus far. Upwelling regions generally see elevated DON concentrations following the increase in biological activity due to NO3 − input from below (Sipler & Bronk, 2014). Since many rivers are of glacial origin in the ECAA and Baffin Bay, both glacial and terrestrial riverine end members must be constrained to identify the sources of DON in this rapidly changing region.
The naturally occurring stable N and O isotope ratios of the dissolved N species can be exploited to identify N sources and transformations in environmental samples. The nitrogen 15N/14N isotope ratios are reported in delta notation (δ) and units of per mil (‰), where the 15N/14N and 18O/16O references are atmospheric N2 (i.e., air) for N and Vienna Standard Mean Ocean Water (VSMOW) for O.
 |
The δ15N−NO3 − and DON are influenced originally by the δ15N of its source(s) and secondarily by the fractionation imposed during phytoplankton uptake and DON decomposition (see Knapp et al., 2005). The δ15N−NO3 − and DON can thus be diagnostic of dominant sources of new N in a system. The DON produced by N2‐fixing organisms as well as newly nitrified NO3 − from this source are expected to reflect the low δ15N of newly fixed ammonium (NH4 +; −2 to 0‰) (Carpenter et al., 1997; Knapp et al., 2018; Minagawa & Wada, 1986). N from atmospheric deposition is associated with a similarly low δ15N (Altieri et al., 2016 and references therein). Conversely, the δ15N−DON released by phytoplankton is generally more elevated, reflecting the δ15N of the incident inorganic N substrates. The δ15N of nitrate varies regionally, posting a global ocean average of ∼5‰, but the δ15N of Pacific waters of western Arctic basins is distinctly higher at 8‰ due to benthic coupled nitrification‐denitrification processes (Granger et al., 2018). DON and NO3 − produced through newly regenerated N, typically NH4 +, will often have a lower δ15N compared to ambient substate pools as lighter isotopes are preferentially excreted (Fawcett et al., 2011).
In turn the δ18O of NO3 − is distinct among N sources and also sensitive to NO3 − production and consumption terms. The δ18O of NO3 − in atmospheric deposition is exceptionally high (e.g., Hastings et al., 2003). Nitrate produced by the nitrification of ammonium empirically converges on δ18O of water (Buchwald et al., 2012; Casciotti et al., 2010). Assimilatory and dissimilatory NO3 − consumption results in a parallel increase of both δ18O and δ15N in a 1:1 ratio (Granger et al., 2004, 2008) in proportion to nitrate consumed. This relationship can be used to determine the influence of processes other than assimilation on the NO3 − pool.
The goals of this study are to determine, using an isotopic approach, (a) the dominant sources of NO3 − and DON in the ECAA and Baffin Bay and (b) the transformations affecting these pools. This study focuses on the Nares Strait, Jones Sound, Lancaster Sound, and Baffin Bay, whose coastal regions are more extensively bordered by glaciers compared to other Arctic regions and experience a large amount of glacial runoff (Rogalla et al., 2023). Nutrient input from the numerous small glacially fed rivers in this region is not as well characterized as that from the large terrestrial rivers in other Arctic regions such as the Laptev Sea and the western Arctic (Letscher, Hansell, Kadko, & Bates, 2013; Thibodeau et al., 2017). This region of the Arctic also experiences high continuous permafrost coverage, the degradation of which could be a source of labile nitrogen in local rivers (Francis et al., 2023; Frey et al., 2007). Eastern Baffin Bay is also a highly productive area important for fisheries in Greenland. Determining the sources of nutrient inputs to coastal waters is essential to predict the effect of climate change on primary productivity in these economically important regions. Furthermore, Baffin Bay (via the Davis Strait) accounts for roughly a third of all freshwater input from the Arctic to the North Atlantic, including both liquid freshwater and sea ice (Haine et al., 2015). Shifts in the transport and cycling of nitrogen within the ECAA due to changes in glacial coverage, permafrost melt, or other climate change effects could translate to broader impacts outside the Arctic.
2. Materials and Methods
2.1. Regional Setting and Sample Collection
Samples were collected during Legs 2a and 2b of the ArcticNet expedition aboard the Canadian Coast Guard Ship (CCGS) Amundsen from July 5th to August 15th of 2019. The expedition took place in the ECAA including Baffin Bay, the Nares Strait, Lancaster Sound, and Jones Sound (Figure 1). In this region, prevailing currents flow southward through Nares Strait and into Kane Basin, Smith Sound, and finally Baffin Bay. In northwestern Baffin Bay, currents flow eastward from Lancaster Sound into Baffin Bay. At the surface, these water masses are Pacific‐derived and circulate in the Canadian and Makarov Basins before entering the ECAA through the Nares Strait and Lancaster Sound, respectively. In Baffin Bay, Atlantic water enters along the southwestern coast of Greenland and travels north until it converges with water flowing south from the Smith Sound and circulates southwards on the eastern side of Baffin Island (Lehmann et al., 2019, 2022; Tang et al., 2004). Atlantic water from the Eurasian Basin of the Arctic Ocean can be found at depth in Baffin Bay (Alkire et al., 2010). Ice cover in this region varies by season and along an east‐west gradient, with the lowest coverage in the summer and ice persisting much longer on the western side of Baffin Bay in spring (Tang et al., 2004). Some sea ice in Baffin Bay is formed locally, while some is formed in the Nares Strait and transported south. Ice formed in other channels is typically blocked by landfast ice and does not enter Baffin Bay (Tang et al., 2004).
Figure 1.
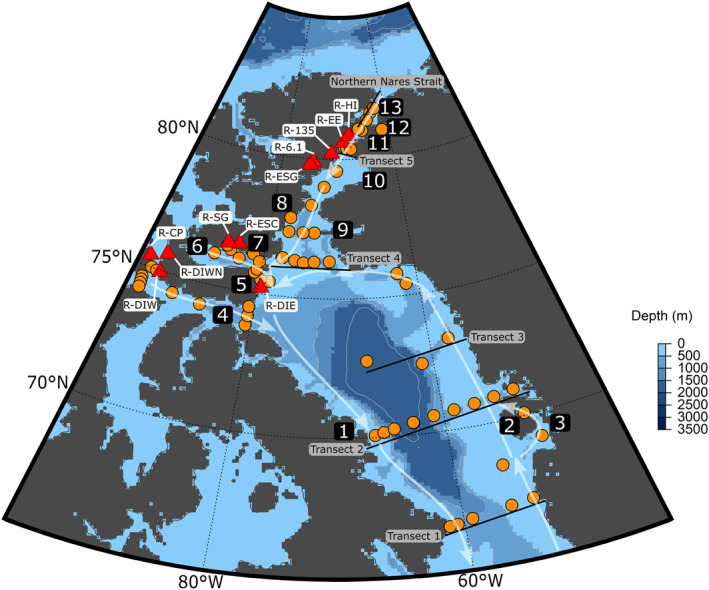
Map of the sampling locations. CTD casts are marked with orange circles. Red triangles represent river locations, which are also labeled by name. Transects 1–5, and the Northern Nares Strait are labeled with solid black lines. The directions of surface currents are shown by the white arrows. Important regions and features are as follows: 1: Clyde River, 2: Disko Island, 3: Jakobshavn Glacier, 4: Lancaster Sound, 5: Devon Island, 6: Jones Sound, 7: Manson Icefield, 8: Talbot Inlet, 9: Smith Sound, 10: Kane Basin, 11: Kennedy Channel, 12: Petermann Glacier, 13: Hall Basin. 9–13 are all considered part of the Nares Strait.
Samples for the analysis of DON concentration and isotopic composition were collected using 12 L Niskin bottles at depths of 100 and 80 m, and then upwards to the surface in 10 m intervals. Samples at 100 and 80 m were collected in 15 mL centrifuge tubes, and samples taken above 80 m were collected in 60 mL high‐density polyethylene (HDPE) plastic bottles. All centrifuge tubes and bottles were acid‐washed and rinsed three times with sample water prior to collection. All samples were frozen with a headspace to allow water expansion upon freezing until analysis. Samples taken during Leg 2b were also filtered with GF/F filters before freezing. Leg 2a samples were filtered prior to analysis with Supor™ 0.45 micron polyethersulfone filters. A comparison was performed on the effect of the different filters on [DON] and δ15N−DON and no significant difference was observed. Riverine samples were collected from the surface waters of 11 rivers, filtered with GF/F filters, and frozen. Due to methodological limitations, not all samples collected were analyzed for [DON] and δ15N of DON; this is expanded upon in Section 2.2. Water samples were also collected for δ18O of H2O analysis; samples were collected without bubbles in 2 mL glass vials and stored at 4°C.
2.2. Concentration and Isotopic Analysis of DON, NO3 −, and δ18O of H2O
[NO3 −], [NO2 −] and [PO4 3−] were measured at sea using a Bran and Luebbe Autoanalyzer III. Working standards were prepared at each station and checked against reference standard material (KANSO CRM, lots CI and CG to cover the range of Atlantic‐ and Pacific‐derived waters in the region). Ammonium concentration in riverine samples was measured using a SEAL AQ300 Discrete Analyzer following the U.S. Environmental Protection Agency (EPA) salicylate method EPA‐148‐D.
The concentration and δ15N of DON were measured as in Knapp et al. (2005). Briefly, total dissolved nitrogen (TDN) was oxidized to NO3 − using recrystallized persulfate followed by measurement on a NOx analyzer by chemiluminescent detection (Braman & Hendrix, 1989). The δ15N of TDN (oxidized to NO3 −) was then analyzed using the denitrifier method (Sigman et al., 2001; Weigand et al., 2016). As there is currently no efficient means to remove DIN, the combined concentration and isotopic composition of NO3 −, NO2 −, and NH4 + (if present) must be analyzed to calculate the δ15N of DON by isotopic mass balance. [NO3 − + NO2 −] was thus also measured by chemiluminescence prior to persulfate oxidation and the δ15N−NO3 − was determined using the denitrifier method (Casciotti et al., 2002; Sigman et al., 2001; Weigand et al., 2016). Only samples in which DON represents over 50% of TDN were analyzed for isotopic composition. This is because as the ratio of DON to DIN decreases, the error associated with the δ15N of DON calculated by isotopic mass balance increases.
To prepare samples for isotopic analysis of DON, 1 mL of freshly prepared persulfate oxidizing reagent (POR) was added to 6 mL of sample in 12 mL threaded test tubes with Teflon‐lined phenolic screw caps (Corning 99447‐161). These samples were then autoclaved for 1 hr and analyzed for [TDN]. The POR blank was typically <0.4 μmol L−1and [TDN] was corrected for blank contribution. The international standards USGS40, USGS64, and USGS65 and an internal standard of 6‐aminocaproic acid (5 μmol L−1) were analyzed within each run to verify oxidation efficiency and that no fractionation occurred during the persulfate oxidation step. The average percent yield of standard concentrations was >95%, and the standard deviation from known isotopic composition was ±0.4‰.
Prior to isotopic analysis, the pH of the autoclaved samples, standards, and blanks was adjusted to 3–4 with 6N HCl. Neutralized samples were injected into 2 mL of Pseudomonas chlororaphis suspended in media. When analyzing NO3 − alone, Pseudomonas aureofaciens was instead used to measure the δ18O of NO3 −. In the samples in which NO2 − accumulated, NO2 − was removed using sulfamic acid, as in Granger and Sigman (2009). The target sample size was 20 nmol. The product N2O was purified and analyzed using a continuous flow isotope ratio mass spectrometer (Elementar Americas PrecisION) equipped with a custom on‐line gas extraction and purge‐trap system and PAL autosampler. Samples were standardized using a two‐point correction with the international standards IAEA‐NO‐3 (δ15N = 4.7‰ vs. air) and USGS34 (δ15N = −1.8‰ vs. air). The δ15N of DON was determined by isotopic mass balance taking into consideration the concentration and δ15N of the POR blank as well as sample NO3 − and TDN. The average standard deviation for duplicate δ15N‐DON analysis was generally lower than ±0.5‰. Error propagation was determined using a Monte Carlo method, as in Knapp et al. (2018).
The δ18O of H2O was measured using an integrated off‐axis cavity absorption spectrometer (Los Gatos Research, LGR, Triple Liquid Water Isotope Analyzer, model 912‐0032) at the University of Calgary as described in Ahmed et al. (2020). Chlorophyll‐a was measured using High Performance Liquid Chromatography (HPLC) at the University of British Columbia as described in Burt et al. (2018). δ18O of H2O and salinity were used to calculate fractions of freshwater and sea ice melt, as described in Supporting Information S1. Plots of surface data and cross‐sections were generated with the Ocean Data View software (Schlitzer, 2021).
2.3. Model for Riverine NO3 − Cycling
A simple steady‐state isotopic model was used to apportion the sources and sinks of NO3 − in ECAA and Baffin Bay rivers based on previously established understandings of riverine nitrogen cycling and the isotopic composition of nitrogen and oxygen sources in Arctic riverine ecosystems. We then compared NO3 − values to our model to explore potential influences on NO3 − in rivers. This model included two sources of NO3 − supplied to rivers: (a) NO3 − from the nitrification of NH4 +, sourced from permafrost, atmospheric NH4 +, or in‐river mineralization (e.g., Alves et al., 2013; Fouché et al., 2020; Wagner et al., 2002) and (b) uncycled NO3 − from atmospheric deposition (e.g., Hastings et al., 2004).
We assumed that complete nitrification of NH4 + results in NO3 − with a δ15N of ∼1.2‰ akin to atmospheric and permafrost end members (range: −6–10‰ (Ansari et al., 2013; Arendt et al., 2016; Clark et al., 2020; Heikoop et al., 2015; Louiseize et al., 2014; Wynn et al., 2007)). The δ18O produced during the nitrification of NH4 + was estimated to be ∼−14.2‰ (range: −8.9 to −19.5‰), assuming that at least 2/3 of the O atoms are derived from water during nitrification (Boshers et al., 2019; Casciotti et al., 2010; Heikoop et al., 2015). For this estimation, we assume the δ18O of water ranged from −12‰ to −22‰ (Arendt et al., 2016; Wynn et al., 2007) and that the δ18O of dissolved oxygen ranged from 23.5‰ to 24.2‰ based on the δ18O of air (Kiddon et al., 1993; Wang & Veizer, 2000; Wynn et al., 2007 and references therein). We assumed isotope effects of the δ18O of NO3 − and exchange with H2O during bacterial nitrification, as in Casciotti et al. (2010) and Buchwald et al. (2012). We also assumed that NH4 + was completely oxidized to NO3 − in rivers, as NH4 + was absent or very low in all our samples, with the highest concentration being 0.56 μmol L−1 at R‐ESC. We assumed that atmospheric deposition added NO3 − with a δ15N of −3.5‰ and a δ18O of 72.1‰ (Ansari et al., 2013; Hastings et al., 2004; Heikoop et al., 2015; Louiseize et al., 2014).
We assumed a kinetic N isotope effect (ε) of 6.0‰ for NO3 − assimilation (Rafter & Sigman, 2016) and a corresponding 18ε:15ε of 1:1 (Granger et al., 2004). The isotope effect is defined as , where 14 k and 15 k are the rate coefficients of the reactions for the light and heavy isotopes, respectively. We excluded denitrification, the canonical conversion of NO3 − to the nitrogen gases N2O and N2 under anaerobic conditions, due to the high O2 concentrations in the rivers (Dalsgaard et al., 2014). We considered two main scenarios for the extent of N recycling and its impact on primary production within the rivers: (a) 50% recycled production, and (b) 25% recycled production. These parameters were chosen as plausible scenarios based on estimates of nutrient recycling in the Arctic (Tank et al., 2012; Whalen & Cornwell, 1985). More details about the model are included in Supporting Information S1.
We used this model to reproduce the deviation from the 18ε:15ε ratio of ∼1 observed for pure NO3 − assimilation (Granger et al., 2004) of our riverine samples. We refer to this deviation as the Δ(15,18), which is the difference between NO3 − δ15N and δ18O (Rafter et al., 2013).
2.4. Isotope Effect of DON Consumption
In areas where chlorophyll‐a was the highest, we estimated the 15ε of surface mixed layer DON consumption using a closed system Rayleigh distillation model (δ15N of DON vs. ln[DON]) as in Knapp et al. (2018). A low [DON] concomitant with elevated δ15N indicates consumption, as kinetic isotope fractionation during consumption increases the δ15N of the residual DON pool.
3. Results
3.1. Physical Characteristics of the ECAA and Baffin Bay
Near shore waters were influenced by freshwater input from rivers and/or glacial meltwater, as evidenced by fresher surface waters with distinctly low δ18O H2O values (−6.05 to −0.54‰). Freshwater influx was evident throughout the study region, particularly near Talbot Inlet, the Petermann Glacier, the Jakobshavn Glacier, in Lancaster Sound, and near the riverine stations R‐SG and R‐ESC in Jones Sound (Figure S1A in Supporting Information S1).
Within the Nares Strait, Jones Sound, and Lancaster Sound, water at depth was predominantly Pacific derived. Along Eastern Baffin Bay, the input of Atlantic waters was observed at depth, due to the West Greenland current, which carries North Atlantic water into Baffin Bay. Finally, in Western Baffin Bay, there was an initial increase in Pacific derived water at depth and then a decrease as the waters mixed with Atlantic derived water (Figure S2 in Supporting Information S1). This was consistent with previous observations of water masses in the area (Lehmann et al., 2022, and references therein).
Many of the rivers sampled in this study were glacially fed, particularly those on Ellesmere Island, such as R‐ESG and R‐6.1. Others were not located near glaciers, for example, R‐DIW and R‐DIW‐N on western Devon Island, and R‐CP on Cornwallis. Rivers ranged from 0.5 to 3 m deep and ∼1 to ∼30 m wide. δ18O of H2O in rivers ranged from −18.4 to −28.4‰ (Brown et al., 2022). Satellite imagery of the river sampling locations is provided in Figures S3–S6 in Supporting Information S1.
3.2. Chlorophyll‐a
Chlorophyll‐a concentrations at the surface (4 m) were low throughout the study region (typically <2 μg L−1) except in the northern Nares Strait, where a particularly large bloom near Petermann Glacier was observed, as well as in Jones Sound, and the western side of Transect 1 (Figure 2a).
Figure 2.
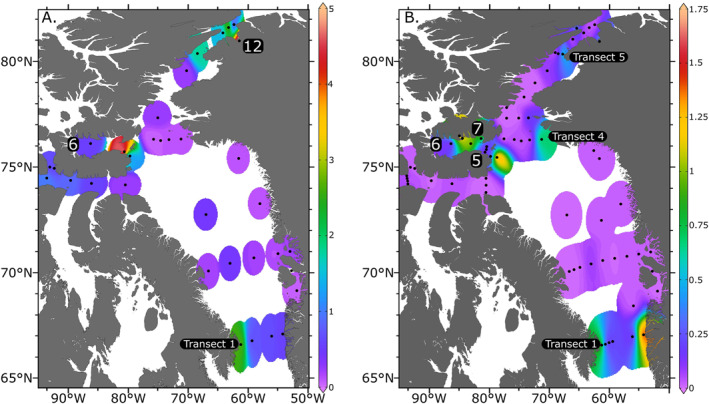
(a) Surface chlorophyll‐a (μg L−1). (b) [NO3 −] (μmol L−1) distribution in surface waters of the study area. 1—Clyde River, 3—Jakobshavn Glacier, 5—Devon Island, 6—Jones Sound, 8—Talbot Inlet, 12—Petermann Glacier.
3.3. Nitrate Concentration and Isotopic Composition in the ECAA and Baffin Bay
Nitrate concentration was near zero in all surface waters, except in the Jones Sound, as well as the eastern and western ends of the Davis Strait (Transect 1 in Figures 1 and 2b). At Transect 1, the δ15N−NO3 − was lower in the east (6.5‰), and higher in the west (9.8‰), where a higher chlorophyll‐a concentration was also observed (Figure 2a). δ18O of NO3 − across this transect was similar in the east (3.3‰) and west (3.4‰). At the mouth of the Jones Sound, a δ15N and δ18O of NO3 − of 14‰ and 50‰ were observed. Nitrate was generally completely consumed in the mixed layer (upper 10 m) within the study area and increased with depth to up to ∼15 μmol N L−1 at 100 m depth (Figure 3).
Figure 3.
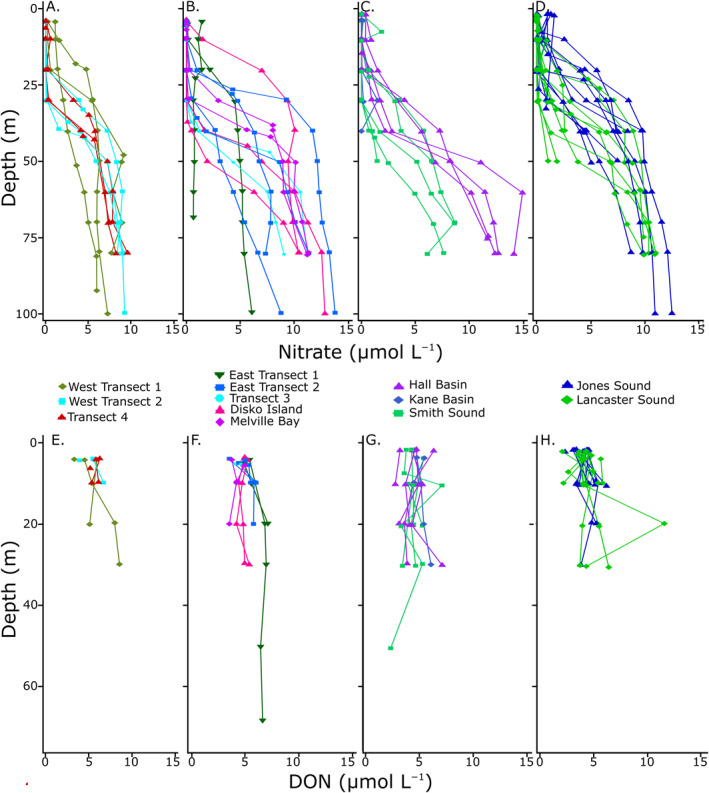
NO3 − (top) and DON (bottom) concentration depth profiles for (a) and (e) Western Baffin Bay; (b) and (f) Eastern Baffin Bay; (c) and (g) Nares Strait; and (d) and (h) Jones and Lancaster Sounds.
Nitrate concentration and isotopic composition of river samples are provided in Table 1. The concentration of NO3 − among riverine samples covered a broad range, from 0.44 to 47 μmol L−1 (Table 1). NO2 − concentrations were <0.2 μmol L−1 in all samples, both riverine and marine, expect for R‐6.1, which had 1.4 μmol L−1 NO2 −. High variability was observed even in rivers adjacent to one another, such as R‐6.1 and R‐ESG. The isotopic composition of NO3 − was highly variable between rivers, indicating variability in NO3 − sources and/or transformations. R‐SG had the lowest δ15N−NO3 − (0.71‰) and a [NO3 −] of 2.7 μmol L−1. R‐ESG had the highest δ15N−NO3 − (10.3‰) and a [NO3 −] of 0.44 μmol N L−1. The adjacent river R‐6.1, which was about 20–30 m wide, had a similarly elevated δ15N−NO3 − of 10‰ but much higher [NO3 −] of 11 μmol L−1. In contrast, R‐ESG was a river running through a crevasse adjacent to Eugenie Glacier and was only ∼1 m wide. R‐ESG and R‐6.1 were drastically different with respect to their δ18O of NO3 −, which was −2.7‰ in R‐6.1 and 49‰ in R‐ESG. There was no correlation between the δ15N−NO3 − and ln([NO3 −]) in rivers (P = 0.72, R 2 = 0.05), but δ18O of NO3 − was strongly negatively correlated with ln([NO3 −]) (P = 0.002, R 2 = 0.63), as shown in Figure 4.
Table 1.
Concentration and δ15N and δ18O of NO3 − and DON, As Well As Δ(15,18) of NO3 − (Calculated as in Rafter et al., 2013) and Notable Features of River Samples
| Station | [NO3 −] | δ15N−NO3 − | δ18O−NO3 − | Δ(15,18) | [DON] | δ15N−DON | Notable features and location |
|---|---|---|---|---|---|---|---|
| Unit | μmol L−1 | ‰ | ‰ | ‰ | μmol L−1 | ‰ | |
| Glacial | |||||||
| R‐DIE | 1.30 | 3.40 | 18 | −14 | 0.54 | NA | Drains Devon Ice Cap, rocks and sand; Devon Island |
| R‐ESC | 3.30 | 2.60 | −6.3 | 8.9 | 1.80 | NA | Compact sand, rocks; Ellesmere Island |
| R‐SG | 2.70 | 0.71 | −1.4 | 2.1 | 3.10 | 5.8 | Multiple glaciers, rocks and sand; Ellesmere Island |
| R‐6.1 | 11.00 | 10.00 | −2.7 | 13 | 0.00 | NA | Adjacent to glacial moraine, silt, mud and clay; Ellesmere Island |
| R‐ESG | 0.44 | 10.00 | 49 | −38 | 4.90 | 7.2 | Within glacier, silt, clay, pebbles; Ellesmere Island |
| R‐135 | 3.50 | 6.60 | 7.3 | −0.68 | 0.16 | NA | Rocks, sand and silt; Ellesmere Island |
| R‐EE | 2.70 | 4.80 | 10 | −5.4 | 1.50 | NA | Rocks, some sand and silt; Ellesmere Island |
| R‐HI | 4.70 | 5.30 | −0.80 | 6.1 | 1.40 | NA | Rocks, sand, mud; Ellesmere Island |
| Terrestrial | |||||||
| R‐CP | 4.10 | 2.90 | −7.5 | 10 | 2.20 | NA | Downstream of lake, icy at edge, medium and large flat rocks, possibly shale; Cornwallis Island |
| R‐DIW | 20.00 | 4.40 | −9.3 | 14 | 1.60 | NA | Medium and large flat rocks and sandstone; Devon Island |
| R‐DIW‐N | 47.00 | 4.80 | −9.6 | 14 | 0.99 | NA | Greenery at mouth, medium rocks and mud; Devon Island |
Note. “R” denotes the rivers sampled. Rivers were named based on proximity to geographic features or CTD stations: Devon Island East (R‐DIE), Eastern Sydkap Icecap (R‐ESC), Sydkap Glacier (R‐SG), Station 6.1 (R‐6.1), Eugenie's Sister Glacier (R‐ESG), Station 135 (R‐135), Ellesmere Island East (R‐EE), Hans Island (R‐HI), Copland Point (R‐CP), Devon Island West (R‐DIW), and Devon Island West North (R‐DIW‐N). Rivers are divided by their dominant landscape.
Figure 4.
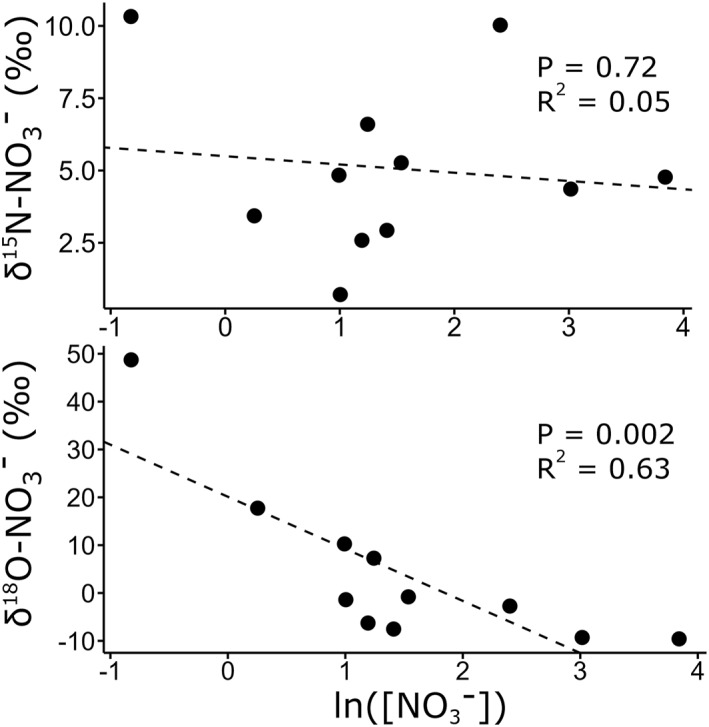
The δ15N (top) and δ18O (bottom) of NO3 − against ln([NO3 −]) in all rivers sampled. Pearson's correlation coefficient and P are also included.
High variation was also observed between R‐ESC and R‐SG, both of which are west of Grise Fjord. R‐SG was around 5 m wide and ∼0.5 m deep, while R‐ESC was roughly 20 m wide and between 1 and 2 m deep. R‐ESC was more inland than R‐SG. R‐SG and R‐ESC had similar NO3 − concentrations, but the δ15N−NO3 − was almost 2‰ higher at R‐ESC than R‐SG. Furthermore, the δ18O of NO3 − at R‐ESC was nearly 4.5 times lower than that at R‐SG. Conversely, two other stations in similar areas, R‐DIW and R‐DIW‐N, both located on the west side of Devon Island had significantly different NO3 − concentrations (20 and 47 μmol L−1, respectively), but similar isotopic signatures (e.g., 4.4‰ and 4.8‰ for δ15N of NO3 −, respectively).
We considered that the isotopic composition of riverine NO3 − was derived from two end‐member sources, namely NO3 − produced proximately by nitrification and uncycled atmospheric NO3 −. However, the NO3 − isotopic composition of our samples did not fall along the mixing line for the two end members. The isotope values were explained by also invoking the partial assimilation of NO3 − and associated isotopic enrichment of residual NO3 − (Figure 5). This indicates that atmospheric depositions, assimilation and nitrification contributed, to some extent, to the δ15N and δ18O of NO3 − signatures of the river samples.
Figure 5.
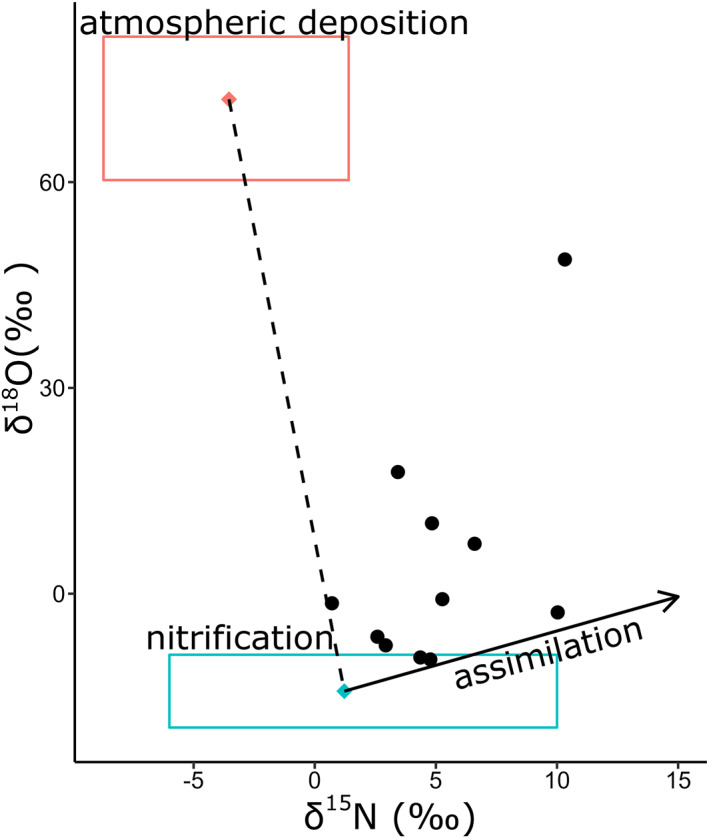
δ18O−NO3 − versus δ15N−NO3 − for riverine samples, with mixing between nitrification of NH4 + (blue box) and atmospheric deposition (pink box) marked by the dashed line. The ε18: ε15 of assimilation (∼1) is noted with the solid black arrow.
We used a steady‐state isotopic model to confirm that these three processes (nitrification, atmospheric depositions and NO3 − assimilation) could produce a deviation from the 18ε:15 ε ratio of ∼1 observed for pure NO3 − assimilation (Granger et al., 2004) for our riverine samples. We define the deviation from the 8ε:15 ε ratio of ∼1 as the difference between NO3 − δ15N and δ18O, referred to as Δ(15,18) (Rafter et al., 2013). Assuming that 25%–50% of the dissolved nitrogen was recycled within the rivers, we were able to reproduce the full range of observed Δ(15,18) (Figure 6).
Figure 6.
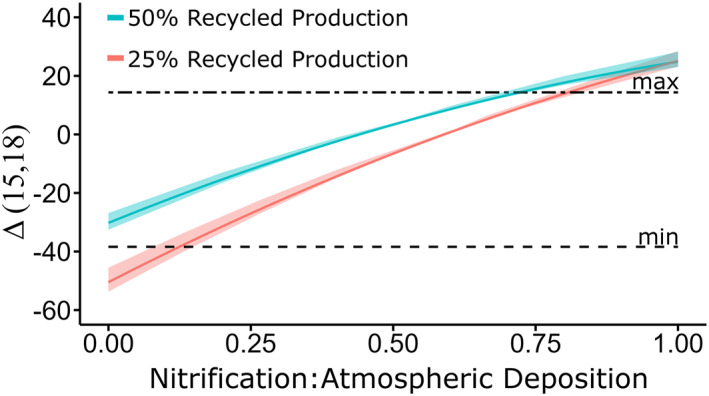
Results from simple box model simulations evaluating the capacity of different sources of NO3 − in glacial rivers of the ECAA to generate δ15N and δ18O and Δ(15,18) signatures. The ratio of nitrified NH4 + to atmospheric deposition is on the x axis, and Δ(15–18) on the y‐axis. The blue line represents 50% recycled production, and the pink line represents 25% recycled production. The shaded colors represent model outputs when using the outer ranges of the end members. The black dashed lines represent the minimum and maximum Δ(15,18) observed in the riverine samples.
3.4. DON Distribution and δ15N in the ECAA and Baffin Bay
The surface distribution of [DON] and δ15N of DON are shown in Figure 7. DON concentrations were highly variable but had similar values between regions, ranging from 3.2 to about 6.0 μmol L−1 in Baffin Bay and the Nares Strait. Lancaster Sound and Jones Sound had lower DON concentrations, ranging from 2.1 to 5.7 μmol L−1. In the mixed layer (upper 10 m) of the water column, [DON] increased with salinity (Figure 8). [DON] in riverine samples were moderate‐to‐low (0–4.9 μmol L−1), even when [NO3 −] was high. Due to the presence of NO3 −, the δ15N of DON could only be measured at R‐SG and R‐ESG (5.8 and 7.2‰ respectively).
Figure 7.
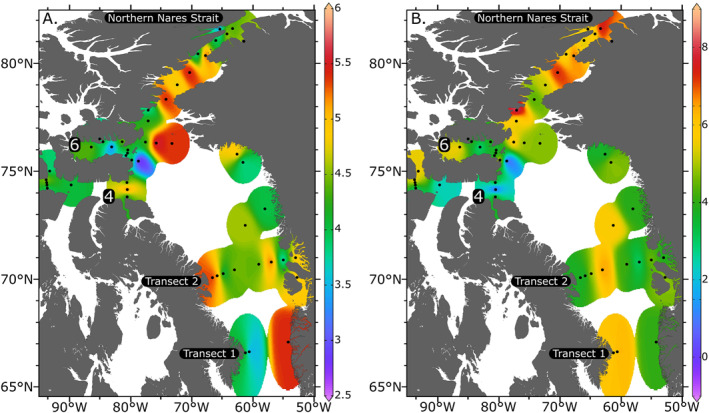
(a) [DON] (μmol L−1) and (b) δ15N of DON (‰) in the surface water. Transects 1, 2, and Northern Nares Strait are denoted with black boxes. Lancaster (4) and Jones (6) Sounds are also labeled.
Figure 8.
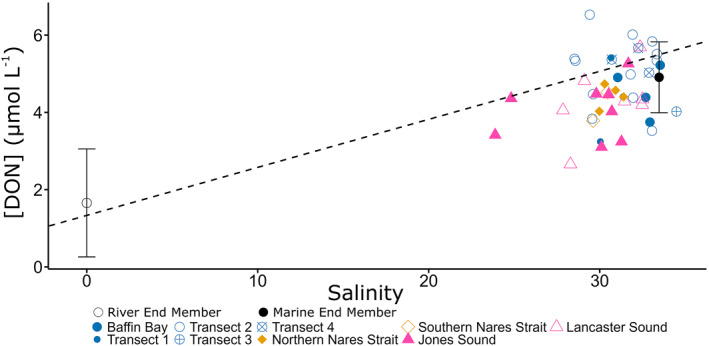
[DON] (μmol L−1) in the mixed layer (upper 10 m) of the water column versus salinity. Blue dots represent samples located within Baffin Bay, orange diamonds represent samples in Nares Strait, and pink triangles represent samples located in sounds to the east of Baffin Bay. Riverine (hollow) and marine (solid) end members are marked with black dots with solid lines representing the standard deviation for end member estimates. The dashed line represents mixing between the two end members.
However, no significant relationship between [DON] and the fraction of freshwater input was observed in Jones Sound, where the highest freshwater fraction was calculated (Figure 2a). [DON] did not fall directly along the pure mixing line at most stations, suggesting surface ocean DON production and consumption processes (Figure 8). The relationship between DON concentration and δ15N with depth was variable and often only characterized within the upper 40 m because NO3 − concentrations below 40 m depth were often too high to allow calculating the δ15N of DON from isotopic mass balance. Depth profiles of [DON] are depicted in the bottom panel of Figure 3. Concentration and δ15N of DON increased with depth in Transects 1 and 2 of Lancaster Sound, and in some areas near Disko Island. The depth relationship between [DON] and δ15N was more variable at some of the more southernmost stations, ranging from an increase in [DON] and a slight decrease in δ15N, a slight increase in both δ15N and [DON], or no change in isotopic composition.
In two areas where highest chlorophyll‐a concentrations were measured, the Northern Nares Strait and Western Transect 1, chlorophyll‐a and DON concentrations in the upper 10 m were inversely correlated (Pearson R 2 = 0.75, p‐value = 0.04; Spearman ρ = −1; Figure 9) and ln([DON]) and δ15N‐DON were also inversely correlated (Pearson R 2 = 0.54, p‐value = 0.10; Spearman ρ = −1 and p‐value = 0.02; Figure 10). These trends were not observed in Jones Sound, where relatively high chlorophyll‐a concentrations were also observed. Furthermore, no relationships were observed between the freshwater fraction and [DON] or freshwater fraction and δ15N of DON in the Northern Nares Strait and Western Transect 1, precluding a significant source of low [DON] and elevated δ15N−DON from rivers at these locations.
Figure 9.
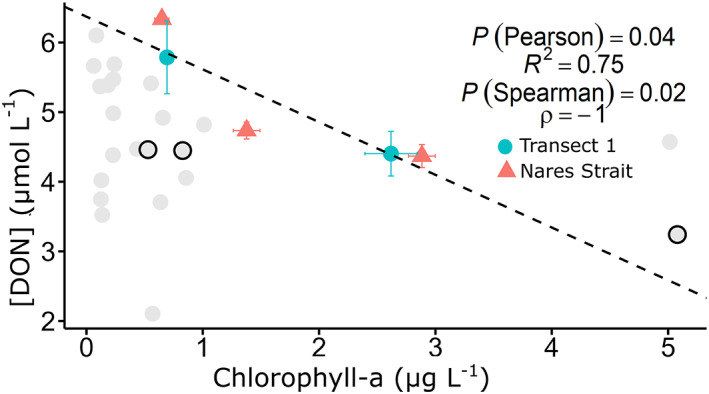
[DON] (μmol L−1) in the northern Nares Strait (north of 80°N) (comprising Hall Basin and Kennedy Channel) and western Transect 1, against chlorophyll‐a concentration (μg L−1). Data are from the upper 10 m. The Pearson and Spearman's rank correlation coefficients and p‐values (P) are shown. Data from all other stations are shown with light gray dots, with Jones Sound outlined in black.
Figure 10.
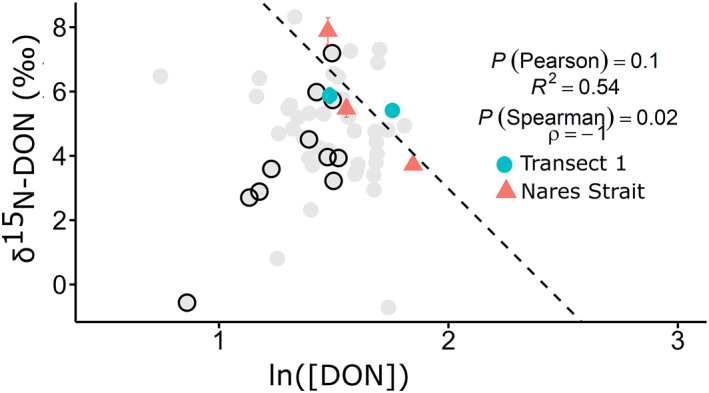
Ln(DON) versus δ15N of DON in the northern Nares Strait (north of 80°N) and western Transect 1. Data are from the upper 10 m of the water column. The Pearson and Spearman's rank correlation coefficients and p‐values (P) are shown. Data from all other stations are shown with light gray dots, with Jones Sound outlined in black.
4. Discussion
4.1. Nitrogen Cycling in ECAA Rivers
Baffin Bay, through the Davis Strait, accounts for one third of all Arctic riverine discharge into the North Atlantic; therefore, major shifts in river discharges are expected to significantly impact nutrient cycling in the region (Haine et al., 2015). River discharges from Arctic watersheds are steadily increasing in response to changes in the North Atlantic Oscillation and global mean surface air temperature (McClelland et al., 2016; Peterson et al., 2002; Rood et al., 2017). In fact, small watersheds feeding minor rivers have been warming at faster rates than large ones (such as the Lena and Mackenzie river watersheds). They also cover areas that are at a high risk of permafrost degradation and contain large amounts of soil‐bound carbon (Speetjens et al., 2023). Arctic rivers can transport massive quantities of dissolved and particulate inorganic and organic nitrogen, sustaining primary productivity in coastal waters (Letscher, Hansell, Kadko, & Bates, 2013; McClelland et al., 2016; Thibodeau et al., 2017). Conversely, increased river discharge and coastal erosion can lead to unfavorable nearshore light conditions, which could negatively impact primary productivity (Terhaar et al., 2021). Our data suggest that the nutrient composition of the small but numerous rivers in the ECAA is not comparable to major terrestrial rivers in other Arctic regions.
The concentration and isotopic composition of riverine NO3 − in this study were highly spatially distinctive, indicating variable contributions of end‐member sources as well as production and consumption terms. We considered two main sources of NO3 − in rivers: atmospheric deposition or the proximate nitrification of NH4 + from either permafrost or atmospheric deposition (and internal recycling). Some rivers with low [NO3 −] had particularly high δ18O, up to 48‰, suggesting a significant fraction of uncycled atmospheric NO3 −. The δ18O of NO3 − from atmospheric deposition in the summer reflects the δ18O of the O sources, which are ozone (O3) and hydroxyl radicals (OH). O3 has a higher δ18O (90–122‰; Johnston & Thiemens, 1997; Krankowsky et al., 1995), while OH usually has δ18O < 0 (Hastings et al., 2004). NO3 − receives two O atoms from O3, and one from OH, significantly increasing the δ18O of NO3 − from atmospheric deposition (65.2–79.6‰; Hastings et al., 2004).
Other rivers had a low δ18O of NO3 − (7 out of 11 rivers with δ18O of NO3 − < 0‰), which portends the nitrification of exogenous and recycled NH4 +. During nitrification, most of the O atoms originate from water, hence the δ18O of newly nitrified NO3 − approaches the O isotopic signature of its H2O source (Boshers et al., 2019). Since freshwater in high latitude systems has a lower δ18O of H2O (∼−22‰; Arendt et al., 2016; Louiseize et al., 2014), the low δ18O of NO3 − observed in some ECAA river waters could indicate a significant input from nitrification. Nitrification within the rivers is supported by the observed negative correlation between δ18O–NO3 − and ln[NO3 −] (Figure 4).
The NO3 − isotopic signatures (both δ15N and δ18O) suggested that mixing between atmospheric NO3 − and nitrified NH4 + alone could not account for the observed δ15N and δ18O. To determine the contribution of these end members, we used a steady‐state isotopic model described in Section 2.3 and Supporting Information S1, and were able to reproduce the range of observed δ15N, δ18O as well as Δ(15,18) in riverine samples (Figure 6). Including NO3 − assimilation helped explain the observed dual NO3 − isotopic values (solid arrow in Figure 5). This requirement for NO3 − removal by assimilation is corroborated by previous studies, which have observed the uptake of NO3 − by phytoplankton in Arctic rivers (Beaton et al., 2017; Snyder & Bowden, 2014). Our isotopic model also suggests that the majority of our data appear to be more influenced by the input of microbially derived (nitrified) NO3 − rather than uncycled atmospheric NO3 −. The ratio of nitrification to atmospheric deposition was between 0.12 and 0.82, with the ratio of most samples over 0.5 (Figure 6). This indicates that nitrification is putatively an important process to provide bioavailable N to Arctic rivers. This influence is likely seasonally dependent. Rivers in this study were sampled after the early melt season (early June to mid−July), during which remineralization takes over as the dominant source of NO3 − as opposed to atmospheric NO3 − deposition (Louiseize et al., 2014).
Two major potential sources of nitrified NH4 + could be permafrost or atmospheric deposition. Some Canadian Arctic permafrost is known to contain a large quantity of NH4 + (Fouché et al., 2020) and contact with permafrost can influence the chemical composition of rivers (Frey & McClelland, 2009; Frey et al., 2007; Heikoop et al., 2015; Vonk et al., 2015). However, rivers in direct contact with organic matter sources such as permafrost and debris are typically DON‐rich (Fouché et al., 2020; Frey & McClelland, 2009; Frey et al., 2007; Wadham et al., 2016), and most of the rivers in this study were DON‐depleted (Table 1). NH4 + can also be found in atmospheric deposition in similar proportions to atmospheric NO3 − (Clark et al., 2020; Fouché et al., 2020). This could indicate that the nitrified NO3 − in these rivers was sourced from the atmospheric deposition of NH4 +.
This is not to rule out any influence of permafrost derived NO3 − on these rivers. While we did not see the characteristic high [DON] observed in rivers with increased permafrost coverage, we were unable to analyze δ15N‐DON due to analytical limitations in all but two of our riverine samples (R‐SG and R‐ESG) as [DON] was less than 50% of the TDN pool. As a result, we do not have much insight into DON cycling within these rivers. Future studies should better constrain the isotopic composition (δ15N) and lability of permafrost DON as well as soil conditions, which can affect nitrogen cycling. Frey et al. (2007), for example, suggest that organic nitrogen in the permafrost of Alaskan watersheds was more easily remineralized compared to West Siberian watersheds, as the Siberian watersheds have high water saturation, which can limit remineralization of DON and facilitate denitrification. Improvements to the analytical methods for measuring stable isotopes of DON (e.g., the capability to remove DIN prior to DON analysis) could elucidate the contribution of permafrost in delivering DON to glacially fed rivers.
Prior studies have observed low δ15N‐DON in rivers and streams, at around −4 to 2‰ (Thibodeau et al., 2017; Ye et al., 2018). These values have been attributed to N sources from atmospheric deposition, aquatic and/or terrestrial N2 fixation, or plant litter decomposition. Assuming that DON sources in these rivers would have similar δ15N to this range, the δ15N‐DON in R‐SG and R‐ESG was slightly elevated (5.8‰ and 7.2‰, respectively). This may indicate kinetic isotope fractionation during consumption or a source of recalcitrant DON with a particularly high δ15N. DON consumption will preferentially utilize the lighter isotopes, elevating the δ15N‐DON of the remaining pool (Bourbonnais et al., 2009; Knapp et al., 2005, 2018).
R‐ESG also had a very distinct δ18O‐NO3 − signature of atmospheric NO3 −, and a Δ(15,18) that could indicate NO3 − assimilation based on our model results (Figure 6). Coupled low [NO3 −] and relatively higher [DON] (Table 1) could indicate the consumption of atmospherically derived NO3 − producing DON. R‐ESG's low [NO3 −] (0.44 μmol L−1) and high δ18O–NO3 − (49‰) directly contrasts with those of the adjacent river R‐6.1 (11 μmol L−1and −2.7‰). We attribute this extreme variability to different landscape features associated with these rivers. R‐ESG was running directly through a glacier, while R‐6.1 was next to a glacial moraine containing significant terrestrial material. Therefore, R‐ESG was likely in more direct contact with the glacial end member and thus might have a relatively higher atmospherically derived NO3 − fraction. R‐6.1, on the other hand, has a Δ(15,18) that could indicate a predominantly nitrified source of nitrate. R‐6.1 was devoid of DON, which could also indicate limited interaction with the permafrost at this location. We posit that the sources of nitrogen in these rivers greatly vary depending on watershed characteristics and microbial metabolisms as suggested by Kaiser et al. (2017), even within small spatial scales.
DON concentrations previously reported for glacial rivers are greatly variable depending on the characteristics of the glacier it is fed by and the interactions of the melt water with the surrounding features. Studies on the Leverett Glacier, a land terminating glacier on the Greenland Ice Sheet adjacent to eastern Transect 1, reported a range from 5.1 to 14.0 μmol L−1 for DON in the surface ice, while DON in basal ice and summer ice melt was on average about 3.0 and 12.0 μmol L−1, respectively (Holland et al., 2019; Wadham et al., 2016). Higher DON values were attributed to the presence of debris or microbial production of DON. However, other studies reported near zero DON concentration in supraglacial streams, cryoconite melt water, snow, and short ice cores (Holland et al., 2019; Telling et al., 2012; Wadham et al., 2016). Runoff from the Leverett Glacier, which feeds into the Watson River, also had a relatively low average DON concentration of 1.7 μmol L−1, with a maximum of 6.3 μmol L−1 (Wadham et al., 2016). Thus, the low [DON] seen in river samples in this study could be characteristic of glacial melt that has not significantly mixed with high DON basal ice or debris.
The type of soil in this study region also likely influences the input of DON in small Arctic rivers. The sediment in most of the rivers sampled mostly consisted of sand and rocks, even those that were not adjacent to glaciers. The fact that the terrestrial rivers sampled in Western Lancaster Sound (R‐CP, R‐DIW, and R‐DIW‐N) all had relatively low [DON] (0.99–2.2 μmol L−1) compared to large terrestrial rivers in other regions indicates that the sediments at these locations are likely low in organic matter. High dissolved organic carbon (DOC) concentrations have been observed in Arctic regions with fine‐grained watersheds, whereas DOC tended to be lower when the watershed consisted of coarser grains and shallow bedrock (O’Donnell et al., 2016). However, this region also contains high continuous permafrost coverage, and as permafrost degrades, it is expected to significantly release organic matter into the surrounding water bodies (Francis et al., 2023; Frey et al., 2007). When considering the nutrient forcings in this region, it is thus important to consider the influence of both glacially fed rivers, and small terrestrial rivers with low organic matter concentrations.
Particulate nitrogen (PN) can also significantly impact the N pool in riverine and marine ecosystems. In larger Arctic rivers, the relationship between PN and DON can vary greatly. One study, for instance, found that the annual PN yield in the Yenisey is much lower than that of DON, but in the Kolyma and Mackenzie PN has a greater annual yield (McClelland et al., 2016). PN can be an important source of N, particularly nearshore, whereas DON can be transported further offshore in addition to supporting nearshore production. There is some indication that a low δ15N of PN in Arctic rivers could be linked to glacial retreat. Nitrogen fixing plants, which are dominant in areas of glacial retreat, impart a characteristically low δ15N into the terrigenous nitrogen pool (McCLelland et al., 2016 and references therein). While we can surmise that the δ15N of PN could be imparted onto that of DON or NO3 − through decomposition or remineralization, because all samples in this study were filtered prior to analysis, we cannot estimate what the δ15N of PN may be. Future studies would benefit from exploring this source of nitrogen, as PN input is expected to increase with increased riverine discharge and coastal erosion.
Our findings fill important research gaps regarding the greater influence of small rivers in the ECAA on nutrient supply to the Arctic and circulation into the Atlantic. For large terrestrial Arctic rivers, much research has been conducted to weigh the relative influence of factors such as increased nutrient delivery (both organic and inorganic), increased stratification, and changes in light penetration (i.e., due to increased particulate matter input) on primary productivity (Le Fouest et al., 2013; Tank et al., 2012; Terhaar et al., 2021; Thibodeau et al., 2017; Treat et al., 2016; Tremblay et al., 2015). However, our study of smaller Arctic rivers, including those glacially fed, indicates that increased DON delivery may not be a ubiquitous consequence of increased riverine discharge in the Arctic, particularly in the ECAA where these small rivers and tributaries are dominant.
4.2. NO3 − and DON Cycling in the ECAA and Baffin Bay
Several factors control primary productivity in the Arctic, including nitrogen, iron and light availability (Tremblay et al., 2015). Previous studies suggest that rivers can greatly influence localized nitrogen availability and primary production, though this localized production only represents a relatively minor fraction of net marine primary productivity in the Arctic (Tremblay et al., 2015).
Nitrate concentrations were close to zero in the surface mixed layer at most stations (<10 m depth), though concentration at some surface stations exceeded 1 μmol L−1 (Figure 4). This is consistent with N being the limiting nutrient in Arctic regions (Figure 2b). [NO3 −] increased with depth as expected. The [NO3 −] found at depth is sourced from the dominant water mass or produced in situ via remineralization. The water in this region is predominantly Pacific‐derived (Figure S2 in Supporting Information S1), apart from Eastern Baffin Bay, where the Western Greenland Current brings North Atlantic water to the subsurface. Western Baffin Bay also sees an increase in Atlantic water contribution at depth due to mixing.
Two of the rivers sampled in Western Lancaster Sound (R‐DIW, and R‐DIW‐N) had elevated [NO3 −] (20 and 47 μmol L−1 respectively), which was not reflected in the nearby coastal waters. This suggests the rapid uptake of riverine NO3 −, or dilution by mixing with the marine end member. This corroborates prior studies wherein riverine supplied NO3 − is rapidly consumed nearshore (Emmerton et al., 2008; Tremblay et al., 2014, 2015). Discrete chlorophyll‐a measurements showed relatively small increases in chlorophyll‐a in areas which also had increased NO3 − (e.g., near Devon Island), and satellite data for July and August 2019 in this region showed higher chlorophyll‐a nearshore (Figure S7 in Supporting Information S1). This could indicate localized NO3 − consumption.
Rivers have been a significant source of bioavailable DON in neighboring coastal waters (Letscher, Hansell, Kadko, & Bates, 2013; Lobbes et al., 2000; Thibodeau et al., 2017). For instance, Lobbes et al. (2000) reported an average [DON] of 12.0 μmol L−1 for several Russian rivers, and Thibodeau et al. (2017) measured [DON] ranging between 13.9 and 21.8 μmol L−1 in the Siberian Arctic. Some of these studies found that up to 70% of the terrigenous DON delivered by Arctic rivers was consumed within the shelf waters of the western and Eurasian Arctic (Letscher, Hansell, Kadko, & Bates, 2013; Thibodeau et al., 2017).
DON accumulated to concentrations up to 6.1 μmol L−1 in surface waters near Davis Strait (Figure 7a) and could thus sustain primary productivity if a significant fraction of that DON is labile. However, the labile versus recalcitrant DON fractions were not analyzed in this study. DON showed no distinct or consistent trends with depth, and because [NO3 −] increased rapidly with depth, δ15N–DON was not measured in a significant number of samples beyond the surface mixed layer (Figures S8E–S8H in Supporting Information S1).
Notably, data from this study contrast with findings from other Arctic regions, where [DON] increased with the fraction of river water (Thibodeau et al., 2017) and where terrestrial DON was subsequently consumed in Arctic surface waters (Letscher, Hansell, Kadko, & Bates, 2013; Thibodeau et al., 2017). Instead, [DON] seemed to increase with higher salinity in the mixed surface layer (upper 10 m), though this correlation was overall not significant (Figure 8). This suggests that small but abundant rivers could dilute the marine surface DON pool in the ECAA and Baffin Bay. Deviations from a pure mixing line in Figure 8 suggest that competing sources or transformations are affecting the DON pool, for example, in situ production/consumption by phytoplankton assemblages in the mixed surface waters, inputs from freshwater and buoyancy, or Ekman‐driven upwellings (Bhatia et al., 2021; Cape et al., 2019; Thibodeau et al., 2017). The DON pool would also be influenced by the lability of the localized sources. Increased freshwater inputs with low DON concentrations could further stratify the water column in coastal ECAA and Baffin Bay waters, inhibiting vertical exchange with nutrient‐rich deep waters, potentially decreasing primary productivity in the region if DIN is concomitantly low.
There was noticeable variation in both DON concentrations and isotopic composition in the surface waters (Figure 7). This could be due to local variations in DON sources. Previous studies have found that the δ15N of DON can reflect the δ15N of the new N source (Knapp et al., 2018). DON produced from a low δ15N source, such as newly nitrified NO3 − from N2 fixation, can have a similarly low δ15N−DON. In samples where we were able to measure both δ15N–NO3 − and DON, the δ15N−DON was on average ∼5‰ lower than that of NO3 −, with a negative correlation between δ15N−DON and δ15N–NO3 − (Figure S9 in Supporting Information S1, Pearson R 2 = 0.23, p‐value = 0.003). This could indicate DON production from NO3 −. Knapp et al. (2018) observed a similar trend in the Eastern Tropical South Pacific surface waters, where subsurface DON bracketed the δ15N−NO3 − by ±3‰. Conversely, the lack of spatial variation in DON isotopic composition and concentration observed in some areas, such as Transect 4, where surface [DON] ranged from 5.7 to 6.1 μmol L−1 and δ15N−DON mostly remained constant (4.8–4.9‰), could suggest recalcitrant DON (Bourbonnais et al., 2009; Knapp et al., 2005). This would be in line with observations in the Siberian Arctic where DON in brackish mixing zones was predominantly recalcitrant (Dittmar et al., 2001).
Potential DON consumption was identified only at two main regions in the study area where chlorophyll‐a was elevated. We observed a slight negative relationship between chlorophyll‐a and DON concentrations in the surface mixed layer in both the northern segment of the Nares Strait and the western half of Transect 1 (Pearson R 2 = 0.75, p‐value = 0.04, Spearman ρ = −1, p‐value = 0.02; Figure 9). This mirrors the relationship between chlorophyll‐a and inorganic nutrients in incubation studies (Buapet et al., 2008; Lafarga‐De la Cruz et al., 2006), and a similar inverse relationship has been observed between DON and chlorophyll‐a in lakes (Berman, 1997). [DON] in the surface mixed layer was also negatively correlated with δ15N (Pearson R 2 = 0.54, p‐value = 0.10, Spearman ρ = −1, p‐value = 0.02, n = 4) (Figure 10) which is indicative of consumption as 14N is preferentially taken up, enriching the remaining DON pool (Knapp et al., 2018). Our isotope effect of 6.9‰, estimated using a closed‐system Rayleigh model, is comparable to previously measured isotope effects of DON consumption of 5.5‰ (Knapp et al., 2018). Based on this relationship between [DON] and chlorophyll‐a, we posit the occurrence of net DON uptake by phytoplankton, a process which has been proposed in other nearshore Arctic regions (Tank et al., 2012; Thibodeau et al., 2017).
Though Jones Sound also had high chlorophyll, we excluded it in this analysis as we suspect substantial riverine discharge obscured the relationship between chlorophyll‐a, DON, and δ15N of DON (Figure S1 in Supporting Information S1). A recalcitrant DON pool could also explain why we did not observe a distinct consumption signal at most stations. Furthermore, any correlation between [DON] and chlorophyll‐a is likely hindered by the low spatial resolution of available chlorophyll‐a measurements, though satellite data corroborates our observations of mostly low chlorophyll‐a within Baffin Bay (Figure S7 in Supporting Information S1). Furthermore, DON cycling is likely complex and highly dynamic with simultaneous consumption and production decoupled in space and time. These processes could complicate the relationship between chlorophyll‐a and DON.
Sea ice melt represents another potential source of DON in the ECAA and Baffin Bay. Similar to surface ice in some glaciers, sea ice can have a higher concentration of DON, as observed in the Antarctic (Fripiat et al., 2014; Dall’Osto et al., 2017). Fripiat et al. (2014) suggested that high DON was released by microbial communities within the sea ice following NO3 − assimilation. However, no significant correlation was observed between sea ice melt and DON in this study.
5. Conclusions
NO3 − was the dominant dissolved nitrogen species in the ECAA rivers. We used a steady‐state isotopic box model to apportion the sources of NO3 − in rivers. Our model suggested that NO3 − assimilation, in addition to mixing between inputs from atmospheric deposition and nitrified NH4 +, is needed to explain the observed δ15N and δ18O of NO3 − in rivers. NO3 − putatively derived from the nitrification of permafrost or atmospheric NH4 + was found to be a main source of NO3 − in most rivers, while only a few rivers (e.g., R‐ESG) had significant input of NO3 − from atmospheric deposition. Our stable isotopic data suggest that a significant fraction of this NO3 − was assimilated in the river and/or rapidly consumed near the shore.
DON concentrations were relatively low in rivers (less than 4.9 μmol L−1). Unlike relationships observed for coastal waters adjacent to major Arctic rivers (Letscher, Hansell, Kadko, & Bates, 2013; Thibodeau et al., 2017; Tremblay et al., 2014), we observed that DON concentrations generally increased with salinity. We posit that increased riverine discharge in the ECAA and Baffin Bay may result in increased stratification of the surface ocean and dilution of the ambient DON pool. However, it is important to note that geochemical signatures of Arctic rivers can vary seasonally (Alkire et al., 2017; Manning et al., 2020), and our river measurements were collected during a relatively narrow period (July 8th–14 August 2019) occurring after the peak annual discharge.
We observed evidence for DON consumption in the northern Nares Strait as well as the western Transect 1. This indicates that DON could be utilized by phytoplankton in the ECAA and Baffin Bay, although the source of this DON as well as its composition and lability require further investigation. We estimated an isotope effect for DON consumption of 6.9‰, which is in line with previous studies (5.5‰; Knapp et al., 2018). Our findings are summarized in Figure 11. This study provides a baseline for DON cycling in the ECAA and Baffin Bay and highlights further areas of research needed to better understand N‐cycling in this dynamic region. Due to the high volume of riverine flux from the CAA to the North Atlantic, it is important to better characterize N‐cycling within the region and consider the significance and uniqueness of the numerous small rivers and tributaries.
Figure 11.
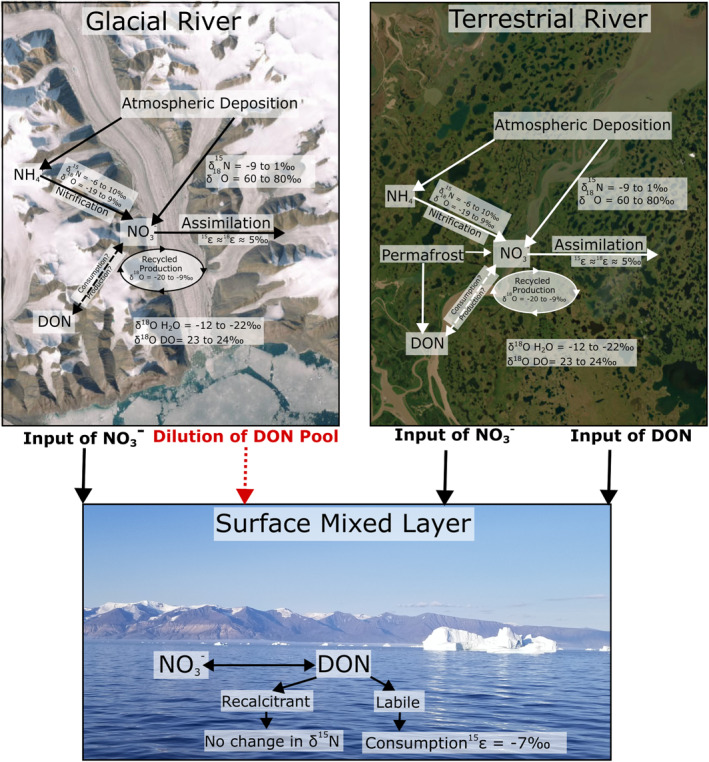
Schematic overview of the processes and findings in the study area. The satellite image of glacial river pictured is of R‐ESG, the terrestrial river is the Mackenzie River (Esri, 2023). Surface mixed layer photo was taken off the coast of Greenland during Transect 2.
Supporting information
Supporting Information S1
Acknowledgments
This work was funded by the National Science Foundation (NSF) (award 1927755 to Bourbonnais). Research on the CCGS Amundsen was funded by ArcticNet, a Network of Centers of Excellence of Canada and the Amundsen Science program, which is supported through Université Laval by the Canada Foundation for Innovation. We also thank Dr. Nadine Lehmann for providing the R code used for Figure 1. Sample collection took place off the shores and within the rivers of Inuit, Inughuit Nunaat, and Kalaallit Nunaat land, and samples were analyzed on Congaree and Tsalaguwetiyi land. We thank the crew of the CCGS Amundsen for making this research possible. We also thank Darcy Perin, Maggie Gaspar, Elise Lumsden, Adrianna Webb, Jaquan High, and Miles Hampton for assistance in sample analysis.
Westbrook, H. C. , Bourbonnais, A. , Manning, C. C. M. , Tremblay, J.‐É. , Ahmed, M. M. M. , Else, B. , & Granger, J. (2024). Dissolved nitrogen cycling in the Eastern Canadian Arctic Archipelago and Baffin Bay from stable isotopic data. Global Biogeochemical Cycles, 38, e2023GB007926. 10.1029/2023GB007926
Data Availability Statement
Data are available at the NSF Arctic Data Center (Bourbonnais & Westbrook, 2023).
References
- Ahmed, M. M. M. , Else, B. G. T. , Capelle, D. , Miller, L. A. , & Papakyriakou, T. (2020). Underestimation of surface pCO2 and air‐sea CO2 fluxes due to freshwater stratification in an Arctic shelf sea, Hudson Bay. Elementa: Science of the Anthropocene, 8(1). 10.1525/elementa.084 [DOI] [Google Scholar]
- Alkire, M. B. , Falkner, K. K. , Boyd, T. , & Macdonald, R. W. (2010). Sea ice melt and meteoric water distributions in Nares Strait, Baffin Bay, and the Canadian Arctic Archipelago. Journal of Marine Research, 68(6), 767–798. 10.1357/002224010796673867 [DOI] [Google Scholar]
- Alkire, M. B. , Jacobson, A. D. , Lehn, G. O. , Macdonald, R. W. , & Rossi, M. W. (2017). On the geochemical heterogeneity of rivers draining into the straits and channels of the Canadian Arctic Archipelago. Journal of Geophysical Research: Biogeosciences, 122(10), 2527–2547. 10.1002/2016jg003723 [DOI] [Google Scholar]
- Altieri, K. E. , Fawcett, S. E. , Peters, A. J. , Sigman, D. M. , & Hastings, M. G. (2016). Marine biogenic source of atmospheric organic nitrogen in the subtropical North Atlantic. Proceedings of the National Academy of Sciences of the United States of America, 113(4), 925–930. 10.1073/pnas.1516847113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, R. J. E. , Wanek, W. , Zappe, A. , Richter, A. , Svenning, M. M. , Schleper, C. , & Urich, T. (2013). Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia‐oxidizing archaea. The ISME Journal, 7(8), 1620–1631. 10.1038/ismej.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari, A. H. , Hodson, A. J. , Heaton, T. H. E. , Kaiser, J. , & Marca‐Bell, A. (2013). Stable isotopic evidence for nitrification and denitrification in a High Arctic glacial ecosystem. Biogeochemistry, 113(1–3), 341–357. 10.1007/s10533-012-9761-9 [DOI] [Google Scholar]
- Arendt, C. A. , Heikoop, J. M. , Newman, B. D. , Wilson, C. J. , Graham, D. E. , Dafflon, B. , et al. (2016). Isotopic and chemical identification of hydrological pathways in a watershed underlain by shallow discontinuous permafrost. In Conference Presentation Abstract. American Geophysical Union Fall Meeting. [Google Scholar]
- Arrigo, K. , & van Dijken, G. L. (2015). Continued increases in Arctic Ocean primary production. Progress in Oceanography, 136, 60–70. 10.1016/j.pocean.2015.05.002 [DOI] [Google Scholar]
- Beaton, A. D. , Wadham, J. L. , Hawkings, J. , Bagshaw, E. A. , Lamarche‐Gagnon, G. , Mowlem, M. C. , & Tranter, M. (2017). High‐Resolution in situ measurement of nitrate in runoff from the Greenland Ice Sheet. Environmental Science and Technology, 51(21), 12518–12527. 10.1021/acs.est.7b03121 [DOI] [PubMed] [Google Scholar]
- Berman, T. (1997). Dissolved organic nitrogen utilization by an Aphanizomenon bloom in Lake Kinneret. Journal of Plankton Research, 19(5), 577–586. 10.1093/plankt/19.5.577 [DOI] [Google Scholar]
- Bhatia, M. P. , Waterman, S. , Burgess, D. O. , Williams, P. L. , Bundy, R. M. , Mellett, T. , et al. (2021). Glaciers and nutrients in the Canadian Arctic Archipelago marine system. Global Biogeochemical Cycles, 35(8). 10.1029/2021GB006976 [DOI] [Google Scholar]
- Bif, M. B. , Bourbonnais, A. , Hansell, D. A. , Granger, J. , Westbrook, H. C. , & Altabet, M. A. (2022). Controls on surface distributions of dissolved organic carbon and nitrogen in the southeast Pacific Ocean. Marine Chemistry, 244, 104136. 10.1016/j.marchem.2022.104136 [DOI] [Google Scholar]
- Bintanja, R. , & Selten, F. M. (2014). Future increases in Arctic precipitation linked to local evaporation and sea‐ice retreat. Nature, 509(7501), 479–482. 10.1038/nature13259 [DOI] [PubMed] [Google Scholar]
- Boshers, D. S. , Granger, J. , Tobias, C. R. , Böhlke, J. K. , & Smith, R. L. (2019). Constraining the oxygen isotopic composition of nitrate produced by nitrification. Environmental Science and Technology, 53(3), 1206–1216. 10.1021/acs.est.8b03386 [DOI] [PubMed] [Google Scholar]
- Bourbonnais, A. , Lehmann, M. F. , Waniek, J. J. , & Schulz‐Bull, D. E. (2009). Nitrate isotope anomalies reflect N2 fixation in the Azores Front region (subtropical NE Atlantic). Journal of Geophysical Research, 114(C3). 10.1029/2007jc004617 [DOI] [Google Scholar]
- Bourbonnais, A. , & Westbrook, H. C. (2023). Dissolved nitrogen concentrations and stable isotopic composition in the eastern Canadian Arctic Archipelago and Baffin Bay, Legs 2a and 2b of the ArcticNet expedition, CCGS Amundsen, July 5th to August 15th 2019 [Dataset]. Arctic Data Center. 10.18739/A2JM23H6Z [DOI]
- Braman, R. S. , & Hendrix, S. A. (1989). Nanogram nitrite and nitrate determination in environmental and biological materials by Vanadium(III) reduction with chemiluminescence detection. Analytical Chemistry, 61(24), 2715–2718. 10.1021/ac00199a007 [DOI] [PubMed] [Google Scholar]
- Bronk, D. A. , Glibert, P. , Malone, T. , Banahan, S. , & Sahlsten, E. (1998). Inorganic and organic nitrogen cycling in Chesapeake Bay: Autotrophic versus heterotrophic processes and relationships to carbon flux. Aquatic Microbial Ecology, 15, 177–189. 10.3354/ame015177 [DOI] [Google Scholar]
- Bronk, D. A. , See, J. H. , Bradley, P. , & Killberg, L. (2007). DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences, 4(3), 283–296. 10.5194/bg-4-283-2007 [DOI] [Google Scholar]
- Brown, K. A. , Manning, C. C. M. , Jones, S. F. , Izett, R. W. , Capelle, D. W. , Else, B. G. T. , et al. (2022). Canadian Arctic Archipelago rivers program: Nutrient, dissolved organic carbon, and water isotope data 2016–2019. PANGAEA. 10.1594/PANGAEA.945702 [DOI] [Google Scholar]
- Buapet, P. , Hiranpan, R. , Ritchie, R. J. , & Prathep, A. (2008). Effect of nutrient inputs on growth, chlorophyll, and tissue nutrient concentration of Ulva reticulata from a tropical habitat. ScienceAsia, 34(2), 245. 10.2306/scienceasia1513-1874.2008.34.245 [DOI] [Google Scholar]
- Buchwald, C. , Santoro, A. E. , McIlvin, M. R. , & Casciotti, K. L. (2012). Oxygen isotopic composition of nitrate and nitrite produced by nitrifying cocultures and natural marine assemblages. Limnology & Oceanography, 57(5), 1361–1375. 10.4319/lo.2012.57.5.1361 [DOI] [Google Scholar]
- Burt, W. J. , Westberry, T. K. , Behrenfeld, M. J. , Zeng, C. , Izett, R. W. , & Tortell, P. D. (2018). Carbon: Chlorophyll ratios and net primary productivity of subarctic Pacific surface waters derived from autonomous shipboard sensors. Global Biogeochemical Cycles, 32(2), 267–288. 10.1002/2017gb005783 [DOI] [Google Scholar]
- Cape, M. R. , Straneo, F. , Beaird, N. , Bundy, R. M. , & Charette, M. A. (2019). Nutrient release to oceans from buoyancy‐driven upwelling at Greenland tidewater glaciers. Nature Geoscience, 12(1), 34–39. 10.1038/s41561-018-0268-4 [DOI] [Google Scholar]
- Carpenter, E. J. , Harvey, H. R. , Fry, B. , & Capone, D. G. (1997). Biogeochemical tracers of the marine cyanobacterium Trichodesmium . Deep Sea Research Part I: Oceanographic Research Papers, 44(1), 27–38. 10.1016/S0967-0637(96)00091-X [DOI] [Google Scholar]
- Casciotti, K. L. , McIlvin, M. , & Buchwald, C. (2010). Oxygen isotopic exchange and fractionation during bacterial ammonia oxidation. Limnology & Oceanography, 55(2), 753–762. 10.4319/lo.2010.55.2.0753 [DOI] [Google Scholar]
- Casciotti, K. L. , Sigman, D. M. , Hastings, M. G. , Böhlke, J. K. , & Hilkert, A. (2002). Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Analytical Chemistry, 74(19), 4905–4912. 10.1021/ac020113w [DOI] [PubMed] [Google Scholar]
- Clark, S. C. , Granger, J. , Mastorakis, A. , Aguilar‐Islas, A. , & Hastings, M. G. (2020). An investigation into the origin of nitrate in Arctic Sea Ice. Global Biogeochemical Cycles, 34(2). 10.1029/2019gb006279 [DOI] [Google Scholar]
- Dall’Osto, M. , Ovadnevaite, J. , Paglione, M. , Beddows, D. C. S. , Ceburnis, D. , Cree, C. , et al. (2017). Antarctic sea ice region as a source of biogenic organic nitrogen in aerosols. Scientific Reports, 7(1), 6047. 10.1038/s41598-017-06188-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard, T. , Stewart, F. J. , Thamdrup, B. , Brabandere, L. D. , Revsbech, N. P. , Ulloa, O. , et al. (2014). Oxygen at nanomolar levels reversibly suppresses process rates and gene expression in anammox and denitrification in the oxygen minimum zone off northern Chile. mBio, 5(6). 10.1128/mbio.01966-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar, T. , Fitznar, H. P. , & Kattner, G. (2001). Origin and biogeochemical cycling of organic nitrogen in the eastern Arctic Ocean as evident from D‐ and L‐amino acids. Geochimica et Cosmochimica Acta, 65(22), 4103–4114. 10.1016/s0016-7037(01)00688-3 [DOI] [Google Scholar]
- Emmerton, C. A. , Lesack, L. F. W. , & Vincent, W. F. (2008). Nutrient and organic matter patterns across the Mackenzie River, estuary and shelf during the seasonal recession of sea‐ice. Journal of Marine Systems, 74(3–4), 741–755. 10.1016/j.jmarsys.2007.10.001 [DOI] [Google Scholar]
- Esri . (2023). World imagery basemap [Software]. Esri and Maxar. Retrieved from https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9
- Fawcett, S. E. , Lomas, M. W. , Casey, J. R. , Ward, B. B. , & Sigman, D. M. (2011). Assimilation of upwelled nitrate by small eukaryotes in the Sargasso Sea. Nature Geoscience, 4(10), 717–722. 10.1038/ngeo1265 [DOI] [Google Scholar]
- Feng, D. , Gleason, C. J. , Lin, P. , Yang, X. , Pan, M. , & Ishitsuka, Y. (2021). Recent changes to Arctic river discharge. Nature Communications, 12(1), 6917. 10.1038/s41467-021-27228-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouché, J. , Christiansen, C. T. , Lafrenière, M. J. , Grogan, P. , & Lamoureux, S. F. (2020). Canadian permafrost stores large pools of ammonium and optically distinct dissolved organic matter. Nature Communications, 11(1), 4500. 10.1038/s41467-020-18331-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, A. , Ganeshram, R. S. , Tuerena, R. E. , Spencer, R. G. M. , Holmes, R. M. , Rogers, J. A. , & Mahaffey, C. (2023). Permafrost degradation and nitrogen cycling in Arctic Rivers: Insights from stable nitrogen isotope studies. Biogeosciences, 20(2), 365–382. 10.5194/bg-20-365-2023 [DOI] [Google Scholar]
- Frey, K. E. , & McClelland, J. W. (2009). Impacts of permafrost degradation on Arctic River biogeochemistry. Hydrological Processes, 23(1), 169–182. 10.1002/hyp.7196 [DOI] [Google Scholar]
- Frey, K. E. , McClelland, J. W. , Holmes, R. M. , & Smith, L. C. (2007). Impacts of climate warming and permafrost thaw on the riverine transport of nitrogen and phosphorus to the Kara Sea. Journal of Geophysical Research, 112(G4), 112. 10.1029/2006jg000369 [DOI] [Google Scholar]
- Fripiat, F. , Sigman, D. M. , Fawcett, S. E. , Rafter, P. A. , Weigand, M. A. , & Tison, J. L. (2014). New insights into sea ice nitrogen biogeochemical dynamics from the nitrogen isotopes. Global Biogeochemical Cycles, 28(2), 115–130. 10.1002/2013gb004729 [DOI] [Google Scholar]
- Fuhrman, J. (1987). Close coupling between release and uptake of dissolved free amino acids in seawater studied by an isotope dilution approach. Marine Ecology Progress Series, 37(1), 45–52. 10.3354/meps037045 [DOI] [Google Scholar]
- Granger, J. , & Sigman, D. M. (2009). Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method. Rapid Communications in Mass Spectrometry, 23(23), 3753–3762. 10.1002/rcm.4307 [DOI] [PubMed] [Google Scholar]
- Granger, J. , Sigman, D. M. , Gagnon, J. , Tremblay, J. , & Mucci, A. (2018). On the properties of the Arctic halocline and deep water masses of the Canada Basin from nitrate isotope ratios. Journal of Geophysical Research: Oceans, 123(8), 5443–5458. 10.1029/2018jc014110 [DOI] [Google Scholar]
- Granger, J. , Sigman, D. M. , Lehmann, M. F. , & Tortell, P. D. (2008). Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria. Limnology & Oceanography, 53(6), 2533–2545. 10.4319/lo.2008.53.6.2533 [DOI] [Google Scholar]
- Granger, J. , Sigman, D. M. , Needoba, J. A. , & Harrison, P. J. (2004). Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnology & Oceanography, 49(5), 1763–1773. 10.4319/lo.2004.49.5.1763 [DOI] [Google Scholar]
- Haine, T. W. N. , Cury, B. , Gerdes, R. , Hansen, E. , Karcher, M. , Lee, C. , et al. (2015). Arctic freshwater export: Status, mechanisms, and prospects. Global and Planetary Change, 124, 13–35. 10.1016/j.gloplacha.2014.11.013 [DOI] [Google Scholar]
- Hastings, M. G. , Sigman, D. M. , & Lipschultz, F. (2003). Isotopic evidence for source changes of nitrate in rain at Bermuda. Journal of Geophysical Research, 108(D24). 10.1029/2003jd003789 [DOI] [Google Scholar]
- Hastings, M. G. , Steig, E. J. , & Sigman, D. M. (2004). Seasonal variations in N and O isotopes of nitrate in snow at Summit, Greenland: Implications for the study of nitrate in snow and ice cores. Journal of Geophysical Research, 109(D20), 109. 10.1029/2004jd004991 [DOI] [Google Scholar]
- Heikoop, J. M. , Throckmorton, H. M. , Newman, B. D. , Perkins, G. B. , Iversen, C. M. , Chowdhury, T. R. , et al. (2015). Isotopic identification of soil and permafrost nitrate sources in an Arctic tundra ecosystem. Journal of Geophysical Research: Biogeosciences, 120(6), 1000–1017. 10.1002/2014jg002883 [DOI] [Google Scholar]
- Holland, A. T. , Williamson, C. J. , Sgouridis, F. , Tedstone, A. J. , McCutcheon, J. , Cook, J. M. , et al. (2019). Dissolved organic nutrients dominate melting surface ice of the Dark Zone (Greenland Ice Sheet). Biogeosciences, 16(16), 3283–3296. 10.5194/bg-16-3283-2019 [DOI] [Google Scholar]
- Holmes, R. M. , McClelland, J. W. , Peterson, B. J. , Tank, S. E. , Bulygina, E. , Eglinton, T. I. , et al. (2012). Seasonal and annual fluxes of nutrients and organic matter from large rivers to the Arctic Ocean and surrounding seas. Estuaries and Coasts, 35(2), 369–382. 10.1007/s12237-011-9386-6 [DOI] [Google Scholar]
- Johnston, J. C. , & Thiemens, M. H. (1997). The isotopic composition of tropospheric ozone in three environments. Journal of Geophysical Research, 102(D21), 25395–25404. 10.1029/97jd02075 [DOI] [Google Scholar]
- Juranek, L. W. (2022). Changing biogeochemistry of the Arctic Ocean: Surface nutrient and CO2 cycling in a warming, melting north. Oceanography, 35(3–4), 144–155. 10.5670/oceanog.2022.120 [DOI] [Google Scholar]
- Kaiser, K. , Canedo‐Oropeza, M. , McMahon, R. , & Amon, R. M. W. (2017). Origins and transformations of dissolved organic matter in large Arctic Rivers. Scientific Reports, 7(1), 13064. 10.1038/s41598-017-12729-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddon, J. , Bender, M. L. , Orchardo, J. , Caron, D. A. , Goldman, J. C. , & Dennett, M. (1993). Isotopic fractionation of oxygen by respiring marine organisms. Global Biogeochemical Cycles, 7(3), 679–694. 10.1029/93gb01444 [DOI] [Google Scholar]
- Knapp, A. N. , Casciotti, K. L. , & Prokopenko, M. G. (2018). Dissolved organic nitrogen production and consumption in Eastern tropical South Pacific surface waters. Global Biogeochemical Cycles, 32(5), 769–783. 10.1029/2017gb005875 [DOI] [Google Scholar]
- Knapp, A. N. , Sigman, D. M. , & Lipschultz, F. (2005). N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic time‐series study site. Global Biogeochemical Cycles, 19(1). 10.1029/2004gb002320 [DOI] [Google Scholar]
- Krankowsky, D. , Bartecki, F. , Klees, G. G. , Mauersberger, K. , Schellenbach, K. , & Stehr, J. (1995). Measurement of heavy isotope enrichment in tropospheric ozone. Geophysical Research Letters, 22(13), 1713–1716. 10.1029/95gl01436 [DOI] [Google Scholar]
- Lafarga‐De la Cruz, F. , Valenzuela‐Espinoza, E. , Millán‐Núñez, R. , Trees, C. C. , Santamaría‐del‐Ángel, E. , & Núñez‐Cebrero, F. (2006). Nutrient uptake, chlorophyll‐a and carbon fixation by Rhodomonas sp. (Cryptophyceae) cultured at different irradiance and nutrient concentrations. Aquacultural Engineering, 35(1), 51–60. 10.1016/j.aquaeng.2005.08.004 [DOI] [Google Scholar]
- Le Fouest, V. , Babin, M. , & Tremblay, J.‐É. (2013). The fate of riverine nutrients on Arctic shelves. Biogeosciences, 10(6), 3661–3677. 10.5194/bg-10-3661-2013 [DOI] [Google Scholar]
- Lehmann, N. , Kienast, M. , Granger, J. , Bourbonnais, A. , Altabet, M. A. , & Tremblay, J.‐É. (2019). Remote western Arctic nutrients fuel remineralization in deep Baffin Bay. Global Biogeochemical Cycles, 33(6), 649–667. 10.1029/2018gb006134 [DOI] [Google Scholar]
- Lehmann, N. , Kienast, M. , Granger, J. , & Tremblay, J.‐É. (2022). Physical and biogeochemical influences on nutrients through the Canadian Arctic Archipelago: Insights from nitrate isotope ratios. Journal of Geophysical Research: Oceans, 127(3). 10.1029/2021jc018179 [DOI] [Google Scholar]
- Letscher, R. T. , Hansell, D. A. , Carlson, C. A. , Lumpkin, R. , & Knapp, A. N. (2013). Dissolved organic nitrogen in the global surface ocean: Distribution and fate. Global Biogeochemical Cycles, 27(1), 141–153. 10.1029/2012gb004449 [DOI] [Google Scholar]
- Letscher, R. T. , Hansell, D. A. , Kadko, D. , & Bates, N. R. (2013). Dissolved organic nitrogen dynamics in the Arctic Ocean. Marine Chemistry, 148, 1–9. 10.1016/j.marchem.2012.10.002 [DOI] [Google Scholar]
- Lewis, K. M. , van Dijken, G. L. , & Arrigo, K. R. (2020). Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science, 369(6500), 198–202. 10.1126/science.aay8380 [DOI] [PubMed] [Google Scholar]
- Link, P. M. , & Tol, R. S. J. (2009). Economic impacts on key Barents Sea fisheries arising from changes in the strength of the Atlantic thermohaline circulation. Global Environmental Change, 19(4), 422–433. 10.1016/j.gloenvcha.2009.07.007 [DOI] [Google Scholar]
- Lobbes, J. M. , Fitznar, H. P. , & Kattner, G. (2000). Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean. Geochimica et Cosmochimica Acta, 26(17), 2973–2983. 10.1016/s0016-7037(00)00409-9 [DOI] [Google Scholar]
- Louiseize, N. L. , Lafrenière, M. J. , & Hastings, M. G. (2014). Stable isotopic evidence of enhanced export of microbially derived NO3 − following active layer slope disturbance in the Canadian High Arctic. Biogeochemistry, 121(3), 565–580. 10.1007/s10533-014-0023-x [DOI] [Google Scholar]
- Manning, C. C. M. , Preston, V. L. , Jones, S. F. , Michel, A. P. M. , Nicholson, D. P. , Duke, P. J. , et al. (2020). River inflow dominates methane emissions in an Arctic coastal system. Geophysical Research Letters, 47(10). 10.1029/2020gl087669 [DOI] [Google Scholar]
- McClelland, J. W. , Holmes, R. M. , Peterson, B. J. , Raymond, P. A. , Striegl, R. G. , Zhulidov, A. V. , et al. (2016). Particulate organic carbon and nitrogen export from major Arctic rivers. Global Biogeochemical Cycles, 30(5), 629–643. 10.1002/2015gb005351 [DOI] [Google Scholar]
- McLaughlin, F. A. , & Carmack, E. C. (2010). Deepening of the nutricline and chlorophyll maximum in the Canada Basin interior, 2003–2009. Geophysical Research Letters, 37(24). 10.1029/2010gl045459 [DOI] [Google Scholar]
- Minagawa, M. , & Wada, E. (1986). Nitrogen isotope ratios of red tide organisms in the East China Sea: A characterization of biological nitrogen fixation. Marine Chemistry, 19(3), 245–259. 10.1016/0304-4203(86)90026-5 [DOI] [Google Scholar]
- Moschonas, G. , Gowen, R. J. , Paterson, R. F. , Mitchell, E. , Stewart, B. M. , McNeill, S. , et al. (2017). Nitrogen dynamics and phytoplankton community structure: The role of organic nutrients. Biogeochemistry, 134(1), 125–145. 10.1007/s10533-017-0351-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OˈDonnell, J. A. , Aiken, G. R. , Swanson, D. K. , Panda, S. , Butler, K. D. , & Baltensperger, A. P. (2016). Dissolved organic matter composition of Arctic rivers: Linking permafrost and parent material to riverine carbon. Global Biogeochemical Cycles, 30(12), 1811–1826. 10.1002/2016GB005482 [DOI] [Google Scholar]
- Peterson, B. J. , Holmes, R. M. , McClelland, J. W. , Vörösmarty, C. J. , Lammers, R. B. , Shiklomanov, A. I. , et al. (2002). Increasing river discharge to the Arctic Ocean. Science, 298(5601), 2171–2173. 10.1126/science.1077445 [DOI] [PubMed] [Google Scholar]
- Rafter, P. A. , DiFiore, P. J. , & Sigman, D. M. (2013). Coupled nitrate nitrogen and oxygen isotopes and organic matter remineralization in the Southern and Pacific Oceans. Journal of Geophysical Research: Oceans, 118(10), 4781–4794. 10.1002/jgrc.20316 [DOI] [Google Scholar]
- Rafter, P. A. , & Sigman, D. M. (2016). Spatial distribution and temporal variation of nitrate nitrogen and oxygen isotopes in the upper equatorial Pacific Ocean. Limnology & Oceanography, 61(1), 14–31. 10.1002/lno.10152 [DOI] [Google Scholar]
- Rogalla, B. , Allen, S. E. , Colombo, M. , Myers, P. G. , & Orians, K. J. (2023). Continental and glacial runoff fingerprints in the Canadian Arctic Archipelago, the Inuit Nunangat Ocean. Journal of Geophysical Research: Biogeosciences, 128(5). 10.1029/2022JG007072 [DOI] [Google Scholar]
- Rood, S. B. , Kaluthota, S. , Philipsen, L. J. , Rood, N. J. , & Zanewich, K. P. (2017). Increasing discharge from the Mackenzie River system to the Arctic Ocean. Hydrological Processes, 31(1), 150–160. 10.1002/hyp.10986 [DOI] [Google Scholar]
- Sanders, T. , Fiencke, C. , Fuchs, M. , Haugk, C. , Juhls, B. , Mollenhauer, G. , et al. (2022). Seasonal nitrogen fluxes of the Lena River Delta. Ambio, 51(2), 423–438. 10.1007/s13280-021-01665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlitzer, R. (2021). Ocean data viewer [Software]. Retrieved from https://odv.awi.de
- Sigman, D. M. , Casciotti, K. L. , Andreani, M. , Barford, C. , Galanter, M. B. J. K. , & Böhlke, J. K. (2001). A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analytical Chemistry, 73(17), 4145–4153. 10.1021/ac010088e [DOI] [PubMed] [Google Scholar]
- Sipler, R. , & Bronk, D. A. (2014). Dynamics of dissolved organic nitrogen. In Hansel D. A. & Carlson C. A. (Eds.), Biogeochemistry of dissolved organic matter (2nd ed., pp. 127–232). 10.1016/b978-0-12-405940-5.00004-2 [DOI] [Google Scholar]
- Snyder, L. , & Bowden, W. B. (2014). Nutrient dynamics in an oligotrophic Arctic stream monitored in situ by wet chemistry methods. Water Resources Research, 50(3), 2039–2049. 10.1002/2013wr014317 [DOI] [Google Scholar]
- Speetjens, N. J. , Hugelius, G. , Gumbricht, T. , Lantuit, H. , Berghuijs, W. R. , Pika, P. A. , et al. (2023). The pan‐Arctic catchment database (ARCADE). Earth System Science Data, 15(2), 541–554. 10.5194/essd-15-541-2023 [DOI] [Google Scholar]
- Tang, C. C. L. , Ross, C. K. , Yao, T. , Petrie, B. , DeTracey, B. M. , & Dunlap, E. (2004). The circulation, water masses and sea‐ice of Baffin Bay. Progress in Oceanography, 63(4), 183–228. 10.1016/j.pocean.2004.09.005 [DOI] [Google Scholar]
- Tank, S. E. , Manizza, M. , Holmes, R. M. , McClelland, J. W. , & Peterson, B. J. (2012). The processing and impact of dissolved riverine nitrogen in the Arctic Ocean. Estuaries and Coasts, 35(2), 401–415. 10.1007/s12237-011-9417-3 [DOI] [Google Scholar]
- Telling, J. , Stibal, M. , Anesio, A. M. , Tranter, M. , Nias, I. , Cook, J. , et al. (2012). Microbial nitrogen cycling on the Greenland Ice Sheet. Biogeosciences, 9(7), 2431–2442. 10.5194/bg-9-2431-2012 [DOI] [Google Scholar]
- Terhaar, J. , Lauerwald, R. , Regnier, P. , Gruber, N. , & Bopp, L. (2021). Around one third of current Arctic Ocean primary production sustained by rivers and coastal erosion. Nature Communications, 12(1), 169. 10.1038/s41467-020-20470-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau, B. , Bauch, D. , & Voss, M. (2017). Nitrogen dynamic in Eurasian coastal Arctic ecosystem: Insight from nitrogen isotope. Global Biogeochemical Cycles, 31(5), 836–849. 10.1002/2016gb005593 [DOI] [Google Scholar]
- Treat, C. C. , Wollheim, W. M. , Varner, R. K. , & Bowden, W. B. (2016). Longer thaw seasons increase nitrogen availability for leaching during fall in tundra soils. Environmental Research Letters, 11(6), 064013. 10.1088/1748-9326/11/6/064013 [DOI] [Google Scholar]
- Tremblay, J.‐É. , Anderson, L. G. , Matrai, P. , Coupel, P. , Bélanger, S. , Michel, C. , & Reigstad, M. (2015). Global and regional drivers of nutrient supply, primary production and CO2 drawdown in the changing Arctic Ocean. Progress in Oceanography, 139, 171–196. 10.1016/j.pocean.2015.08.009 [DOI] [Google Scholar]
- Tremblay, J.‐É. , Michel, C. , Hobson, K. A. , Gosselin, M. , & Price, N. M. (2006). Bloom dynamics in early opening waters of the Arctic Ocean. Limnology & Oceanography, 51(2), 900–912. 10.4319/lo.2006.51.2.0900 [DOI] [Google Scholar]
- Tremblay, J.‐É. , Raimbault, P. , Garcia, N. , Lansard, B. , Babin, M. , & Gagnon, J. (2014). Impact of river discharge, upwelling and vertical mixing on the nutrient loading and productivity of the Canadian Beaufort Shelf. Biogeosciences, 11(17), 4853–4868. 10.5194/bg-11-4853-2014 [DOI] [Google Scholar]
- Vonk, J. E. , Tank, S. E. , Bowden, W. B. , Laurion, I. , Vincent, W. F. , Alekseychik, P. , et al. (2015). Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems. Biogeosciences, 12(23), 7129–7167. 10.5194/bg-12-7129-2015 [DOI] [Google Scholar]
- Wadham, J. L. , Hawkings, J. , Telling, J. , Chandler, D. , Alcock, J. , O’Donnell, E. , et al. (2016). Sources, cycling and export of nitrogen on the Greenland Ice Sheet. Biogeosciences, 13(22), 6339–6352. 10.5194/bg-13-6339-2016 [DOI] [Google Scholar]
- Wagner, D. , Spieck, E. , Bock, E. , & Pfeiffer, E.‐M. (2002). Microbial life in terrestrial permafrost: Methanogenesis and nitrification in gelisols as potentials for exobiological process. In Horneck G. & Baumstark‐Khan C. (Eds.), Astrobiology (pp. 143–159). 10.1007/978-3-642-59381-9_10 [DOI] [Google Scholar]
- Wang, X. , & Veizer, J. (2000). Respiration–photosynthesis balance of terrestrial aquatic ecosystems, Ottawa area, Canada. Geochimica et Cosmochimica Acta, 64(22), 3775–3786. 10.1016/s0016-7037(00)00477-4 [DOI] [Google Scholar]
- Wassmann, P. , Duarte, C. M. , Agusti, S. , & Sejr, M. K. (2011). Footprints of climate change in the Arctic marine ecosystem. Global Change Biology, 17(2), 1235–1249. 10.1111/j.1365-2486.2010.02311.x [DOI] [Google Scholar]
- Weigand, M. A. , Foriel, J. , Barnett, B. , Oleynik, S. , & Sigman, D. M. (2016). Updates to instrumentation and protocols for isotopic analysis of nitrate by the denitrifier method. Rapid Communications in Mass Spectrometry, 30(12), 1365–1383. 10.1002/rcm.7570 [DOI] [PubMed] [Google Scholar]
- Whalen, S. C. , & Cornwell, J. C. (1985). Nitrogen, phosphorus, and organic carbon cycling in an Arctic Lake. Canadian Journal of Fisheries and Aquatic Sciences, 42(4), 797–808. 10.1139/f85-102 [DOI] [Google Scholar]
- Wu, P. , Wood, R. , & Stott, P. (2005). Human influence on increasing Arctic river discharges. Geophysical Research Letters, 32(2). 10.1029/2004gl021570 [DOI] [Google Scholar]
- Wynn, P. M. , Hodson, A. J. , Heaton, T. H. E. , & Chenery, S. R. (2007). Nitrate production beneath a high Arctic glacier, Svalbard. Chemical Geology, 244(1–2), 88–102. 10.1016/j.chemgeo.2007.06.008 [DOI] [Google Scholar]
- Yamamoto‐Kawai, M. , Carmack, E. , & McLaughlin, F. (2006). Nitrogen balance and Arctic throughflow. Nature, 443(7107), 43. 10.1038/443043a [DOI] [PubMed] [Google Scholar]
- Ye, F. , Guo, W. , Wei, G. , & Jia, G. (2018). The sources and transformations of dissolved organic matter in the Pearl River Estuary, China, as revealed by stable isotopes. Journal of Geophysical Research: Oceans, 123(9), 6893–6908. 10.1029/2018jc014004 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bourbonnais, A. , & Westbrook, H. C. (2023). Dissolved nitrogen concentrations and stable isotopic composition in the eastern Canadian Arctic Archipelago and Baffin Bay, Legs 2a and 2b of the ArcticNet expedition, CCGS Amundsen, July 5th to August 15th 2019 [Dataset]. Arctic Data Center. 10.18739/A2JM23H6Z [DOI]
- Esri . (2023). World imagery basemap [Software]. Esri and Maxar. Retrieved from https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9
- Schlitzer, R. (2021). Ocean data viewer [Software]. Retrieved from https://odv.awi.de
Supplementary Materials
Supporting Information S1
Data Availability Statement
Data are available at the NSF Arctic Data Center (Bourbonnais & Westbrook, 2023).


