ABSTRACT
Background
Percutaneous coronary intervention (PCI) using drug‐eluting stents is an established strategy for the treatment of significant obstructive coronary artery disease. Evidence supports that intravascular imaging‐guided PCI offers advantages over conventional angiography‐guided PCI, though its use is limited, likely due to high costs. Angiography‐guided PCI relies on visual estimation, leading to inter‐ and intra‐observer variability and suboptimal outcomes. Quantitative coronary angiography (QCA) provides reliable information about vascular dimensions, overcoming these limitations. Poststenting postdilation with appropriately sized noncompliant balloons improves outcomes by increasing lumen area and reducing stent malapposition.
Aims
We investigated the procedural details of each modality used to guide PCI and assessed the utility of QCA‐guided PCI with routine postdilation when intravascular imaging is unavailable.
Methods and Results
A systematic search was conducted from inception to May 31, 2024, identifying nine randomized controlled trials (with over 500 patients) that compared outcomes of PCI guided by intravascular imaging versus conventional angiography or QCA. The findings indicate that intravascular imaging guidance significantly improves clinical outcomes compared to angiography guidance. Notably, QCA‐guided PCI with routine postdilation yielded outcomes comparable to those achieved with intravascular imaging‐guided PCI.
Conclusions
QCA‐guided PCI with routine postdilation may be a viable alternative for improving PCI outcomes, especially in settings where intravascular imaging is unavailable.
Keywords: intravascular imaging, percutaneous coronary intervention, quantitative coronary angiography
Abbreviations
- DES
drug‐eluting stent
- Guide‐DES
quantitative coronary angiography versus intravascular ultrasound GUIDancE for drug‐eluting stent implantation
- IVUS
intravascular ultrasound
- LAD
left anterior descending coronary artery
- LM
left main coronary artery
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
- QCA
quantitative coronary angiography
1. Introduction
Percutaneous coronary intervention (PCI) using drug‐eluting stents (DESs) is an accepted therapeutic modality for the management of significant obstructive coronary artery disease [1, 2]. It improves the prognosis of acute coronary syndrome and ameliorates symptoms associated with chronic coronary syndrome. The clinical course following stent placement is variable, as it is influenced by procedural results and individual patient characteristics. Numerous efforts have been made to improve the outcomes after DES implantation. Intravascular imaging offers advantages over conventional angiography in evaluating preintervention lesion characteristics, guiding stent deployment and optimization, and identifying postintervention complications [3]. Mounting evidence supports the benefits of intravascular imaging‐guided PCI over conventional angiography‐guided PCI [4]. However, its global implementation remains limited due to affordability constraints for many catheterization laboratories and patients [5, 6]. Hence, there may be a need for alternative practical approaches to improve PCI outcomes in settings where intravascular imaging is not available.
To address this, a systematic search of the MEDLINE, Embase, and Cochrane databases was conducted from inception until May 31, 2024. The search aimed to identify randomized controlled trials comparing outcomes between PCI guided by intravascular imaging and PCI guided by conventional angiography or quantitative coronary angiography (QCA). Eligible studies were those that randomly assigned patients undergoing PCI with drug‐eluting stents to either an intravascular imaging‐guided group or an angiography/QCA‐guided group and reported clinical outcomes with a sufficient sample size ( > 500 patients). The study identification process is illustrated in the PRISMA flow diagram (Figure 1). Twenty‐one randomized trials met the eligibility criteria; however, twelve trials with small sample sizes or surrogate marker endpoints were excluded from the analysis. Ultimately, nine randomized clinical trials were included. Additionally, a comprehensive review of articles related to QCA was conducted, focusing on QCA‐guided PCI as an alternative approach to improving PCI outcomes when intravascular imaging is unavailable.
Figure 1.
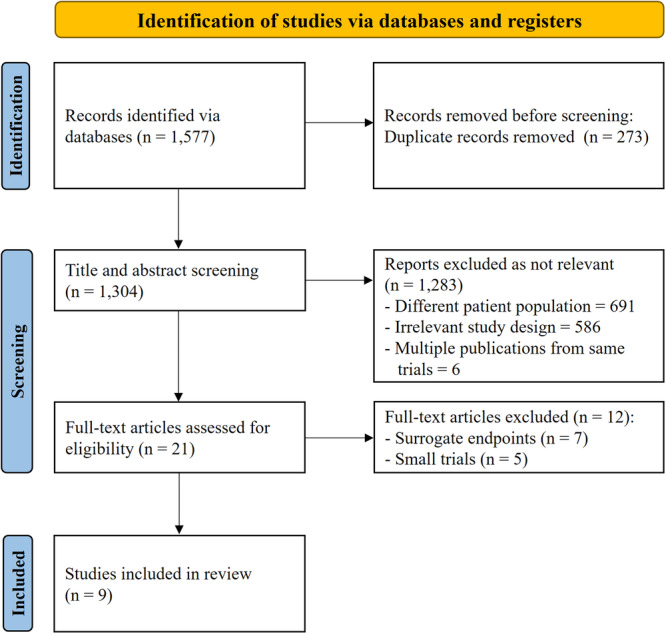
PRISMA flow diagram.
1.1. Angiography‐Guided PCI
Coronary angiography remains the gold standard for evaluating the suitability of the lesion for PCI and guiding the PCI procedure. Angiographic assessment of intermediate stenosis may not reliably predict whether the stenosis can induce ischemia, necessitating the acquisition of additional evidence of physiological significance [1, 2, 7]. However, once PCI is planned for an ischemia‐producing lesion, it was traditionally performed based on visual estimation, commonly referred to as angiography‐guided PCI.
In brief, the reference artery diameter can be estimated using the guiding catheter size and predilated balloon sizes, while lesion length may be determined using the balloon length (15 or 20 mm) [8, 9]. A balloon‐to‐artery diameter ratio of 1.1 is recommended for stent size selection and implantation [8, 10, 11]. Although a larger size aligns with the concept of “bigger is better,” a balloon‐to‐artery diameter ratio exceeding 1.2 is associated with a higher risk of coronary perforation or stent edge dissection [12, 13, 14]. Therefore, meticulous attention to stent sizing with a balloon‐to‐artery diameter ratio of 1.1 is crucial for achieving optimal results without procedural complications. Procedural success is conventionally defined as successful placement of the stent at the target location with diameter stenosis < 20%, normal antegrade flow, no flow‐limiting dissection, and absence of major side branch occlusion based on visual evaluation. Numerous studies have designated this methodology as the standard PCI technique since the introduction of coronary stents. However, angiography‐guided PCI through visual estimation requires a substantial learning curve, with large interobserver and intra‐observer variabilities [9, 15], which may constitute inherent limitations contributing to suboptimal outcomes, particularly for inexperienced operators [16, 17].
1.2. Intravascular Imaging‐Guided PCI
Intravascular imaging modalities, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) serve as both diagnostic and guidance tools for PCI [3]. Accurate dimension measurements, including the reference artery diameter and lesion length, are crucial for selecting the appropriate stent and optimizing implantation. However, to obtain a true cross‐sectional image, the imaging catheter should be parallel to the arterial wall because the beam direction for images is perpendicular to the centerline; otherwise, the measured value may not accurately represent the true diameter. Intravascular imaging may overestimate the reference artery diameter, especially near the curved portion of the artery due to oblique sectional images, necessitating caution against using excessively larger stents. Similarly, the longitudinal to‐and‐fro movement of the imaging catheter with each heartbeat can lead to false elongation of the lesion length. These limitations might potentially introduce errors and warrant thoughtful consideration in clinical or research applications [18, 19].
The processes of stent selection, implantation, and optimization using intravascular imaging are well‐summarized in an expert consensus document [3], and a distal lumen reference‐based approach may be safe and straightforward with subsequent optimization (Table 1). Adjunctive postdilation is recommended to achieve the absolute or relative criteria for optimal stent expansion [20, 21, 22], while avoiding major stent malapposition (axial distance ≥ 0.4 mm with longitudinal extension ≥ 1 mm) or large edge dissection (media dissection, dissection angle ≥ 60°, or dissection length > 2 mm). Despite the use of imaging guidance, optimal stent deployment is observed in approximately 50% of patients [3, 4], emphasizing the need for improvement.
Table 1.
Intravascular imaging and QCA for PCI guidance and optimization.
| Intravascular imaging‐guided PCI | QCA‐guided PCI | |
|---|---|---|
| References selection (landing zones) | The largest lumen areas proximal and distal to the stenosis with plaque burden < 50% on intravascular imaging (IVUS, OCT). | Normal or normal‐looking areas proximal and distal to the stenosis on angiograms. |
| Reference diameters | Measured by intravascular imaging. | Measured by QCA. |
| Stent diameter | Mean distal lumen diameter with up rounding stent (0−0.25 mm), or mean distal EEM diameter with rounding down to the nearest 0.25 mm stent size. | Stent size to reach a distal reference target diameter (distal reference diameter by QCA + 5−10% of distal reference diameter by QCA). |
| Stent length | Estimated by measuring the distance between landing zones using intravascular imaging. | Estimated using balloons (15 mm and 20 mm) or the radiopaque tip of the guidewire (30 mm). |
| Stent optimization | Postdilation is optional and depends on intravascular imaging findings, guided by optimal imaging criteria. | Intensive actual postdilation with noncompliant balloons is mandatory to minimize residual stenosis. |
| Ideal final results (optimal criteria) | MSA > 5.5mm2 by IVUS (MSA > 4.5 mm2 by OCT)*, MSA > 90% (or 100%) of distal reference lumen area or MSA > 80% of average reference lumen area in non‐LM lesions, and complete stent apposition, and no major edge dissection on intravascular imaging. | In‐stent minimum diameter stenosis (< 10% by visual estimation), and a smooth transition at the stent edges (a horizontal shape for the proximal edge and a smooth tapering‐off shape for the distal edge), and no major edge dissection on angiography. |
Abbreviations: EEM, external elastic membrane; IVUS, intravascular ultrasound; LM, left main coronary artery; MSA, minimum stent area; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography.
For LM lesions, distal segment > 7 mm² and proximal segment > 8 mm² as measured by IVUS.
Numerous randomized trials comparing intravascular imaging to angiographic guidance for DES implantation have yielded conflicting results for the primary outcome, with the majority favouring imaging‐guided PCI over angiography‐guided PCI [4, 23, 24, 25, 26, 27, 28, 29, 30, 31]. However, in these clinical trials, angiography guidance relied on visual estimation without a standardized protocol, whereas imaging guidance adhered to strict study protocols (Table 2). It is important to clarify systematic, step‐by‐step procedural methodological details for both modalities to ensure a fair comparison between the two procedures. Additionally, high‐pressure postdilation after stenting was performed less frequently in angiography‐guided PCI. The significance of high‐pressure postdilation to minimize residual stenosis after stenting was initially identified through IVUS examination during the bare‐metal stent era [32]. Under‐expansion of the stent delivery balloon is common during stent deployment, necessitating postdilation with noncompliant balloons to enhance stent expansion, particularly in challenging and resistant lesions [33, 34, 35, 36]. While postdilation tends to be less frequently performed in ST‐segment elevation myocardial infarction due to concerns about no‐reflow, it effectively reduces residual stenosis and stent malapposition, ultimately contributing to improved long‐term outcomes [37, 38].
Table 2.
Major trials comparing intravascular imaging‐guided and angiography‐guided PCI.
| Trials | Number | Lesions | Guidance | Postdilation | Primary endpoint | Results | p value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging | Angio | Imaging | Angio | Imaging | Angio | Imaging | Angio | ||||
| Reset [23] | 269 | 274 | Long | IVUS | Visual | 55% | 45% | MACE | HR 0.59 | 0.16 | |
| IVUS‐XPL [24] | 700 | 700 | Long | IVUS | Visual | 76% | 57% | TLF | HR 0.48 | 0.007 | |
| Ultimate [25] | 724 | 724 | All comers | IVUS | Visual | 96.6% | 94.9% | TVF | HR 0.53 | 0.029 | |
| Flavor [26] | 838 | 844 | Intermed | IVUS | Visual(FFR) | NA | D/MI/R | 8.5% | 8.1% | Noninferior | |
| Renovate [27] | 1092 | 547 | Complex | IVUS/OCT | Visual | 60.4% | 45.5% | TLF | HR 0.64 | 0.008 | |
| Ilumien 4 [28] | 1233 | 1254 | Complex | OCT | Visual | 95.5% | 83% | TLF | HR 0.90 | 0.45 | |
| October [29] | 600 | 601 | Bifurcation | OCT | Visual | NA | TLF | HR 0.70 | 0.035 | ||
| Guide‐DES [30] | 765 | 763 | All comers | IVUS | QCA | 97.7% | 97.3% | TLF | 3.8% | 3.81% | Noninferior |
| IVUS‐ACS [31] | 1753 | 1752 | ACS | IVUS | ST | 96.9% | 93.2% | TVF | HR 0.55 | < 0.001 | |
Abbreviations: ACS, acute coronary syndrome; Angio, angiography; D/MI/R, death/myocardial infarction/revascularization; FFR, fractional flow reserve; HR, hazard ratio; Intermed, intermediate; IVUS, intravascular ultrasound; MACE, major adverse cardiac events; NA, not available; OCT, optical coherence tomography; QCA, quantitative coronary angiography; ST, standard technique; TLF, target lesion failure; TVF, target vessel failure.
1.3. Quantitative Coronary Angiography‐Guided PCI
Over the past 30 years, numerous imaging studies have highlighted the visual underestimation of reference segment diameters, the tendency to select smaller DES, and the oversight of high‐pressure postdilation with a noncompliant balloon, all leading to notable residual stenosis and less favorable outcomes. Poststenting residual stenosis consistently correlates with an elevated risk of target lesion failure. It is recommended to target minimal residual diameter stenosis of less than 10% visually (or 20% by QCA) following stent implantation [39, 40]. Moreover, contemporary thin‐strut DESs may necessitate more vigorous high‐pressure postdilation for optimal stent expansion due to their reduced radial strength and increased recoil [41]. Fortunately, the robust polymer of DESs can withstand aggressive postdilation [42]. Therefore, adopting proper DES sizing through on‐site QCA and systematically incorporating high‐pressure postdilation to ensure optimal stent apposition and expansion may overcome the limitations associated with angiography‐guided PCI. Although QCA‐measured values may not precisely reflect the true diameters of the reference segments, starting PCI with approximate values can result in optimal stent deployment through meticulous postdilation (Near values approach: start with near values, finish with optimal results).
The subsequent sections delineate the detailed and updated PCI methodologies employed in the quantitative coronary angiography versus intravascular ultrasound GUIDancE for Drug‐Eluting Stent implantation (Guide‐DES) trial, revealing comparable outcomes between IVUS‐guided PCI and QCA‐guided PCI (Figures 2, 3, 4) [30, 43]. However, additional comparative studies between imaging‐guided PCI and QCA‐guided PCI are crucial for promoting the real‐world application of QCA‐guided PCI. Ongoing studies (NCT04111770, NCT05388357, NCT05529459) will aid in understanding the utility of QCA in daily PCI practice and contribute to overcoming clinical inertia.
Figure 2.
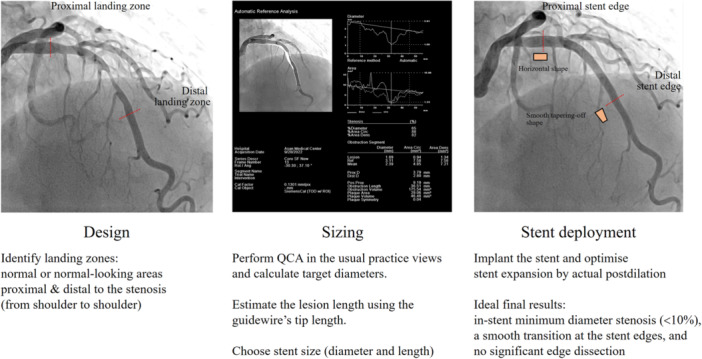
Outline of QCA‐guided PCI. PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography.
Figure 3.
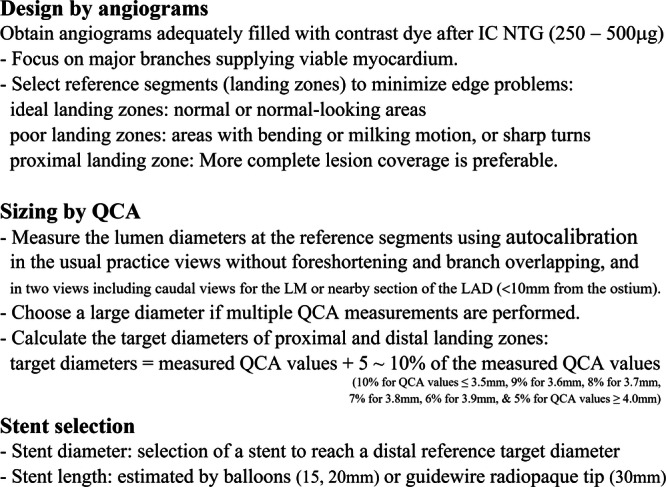
Design, QCA, and stent selection. IC NTG, intracoronary nitroglycerine; LAD, left anterior descending coronary artery; LM, left main coronary artery; QCA, quantitative coronary angiography.
Figure 4.
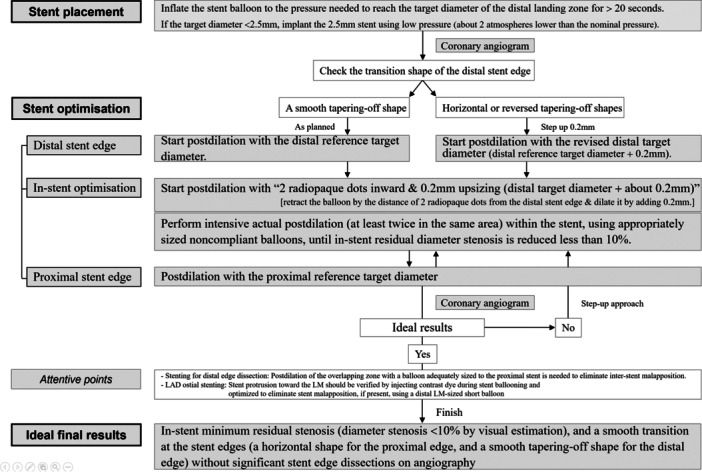
Algorithm for QCA‐guided PCI. LAD, left anterior descending coronary artery; LM, left main coronary artery; PCI, percutaneous coronary intervention; QCA, quantitative coronary angiography.
1.3.1. Brief History
The advent of coronary angiography in 1958 fundamentally altered the diagnosis and treatment of coronary artery disease [44]. However, intra‐ and interobserver variabilities in visual assessment have been significant concerns since the early period, necessitating an objective and reproducible approach to accurately describe coronary artery dimensions. The first validation study of QCA using digital computation was conducted in 1977 [45]. In the 1980s, the development of the Digital Imaging and Communications in Medicine system, coupled with an innovative contour detection algorithm [46, 47], facilitated the measurement of vessels with intricate contours, resulting in significant advancements in QCA. Offline QCA analysis has greatly contributed to the development of coronary stents by enabling accurate measurements of late lumen loss and stenosis diameter.
1.3.2. QCA Measurements
Calibration is a crucial step in QCA analysis that ensures precise quantitative measurements. Initially, catheter calibration was employed for this purpose, but on‐site implementation proved inconvenient. In contemporary practice, user‐friendly QCA software with automatic calibration has gained popularity, eliminating the need for manual catheter calibration. The system's automatic calibration of the isocentre involves calculating the calibration factor based on image geometry, requiring the evaluated organ to be in the isocentre during image acquisition [48, 49]. Simultaneously, automatic calibration of the table‐to‐object distance involves calculating the calibration factor based on the distance between the table and the centre of the evaluated organ. Measurements obtained with automatic isocentre calibration closely align with those derived from automatic table‐to‐object distance calibration (rs = 0.93, p < 0.01) [50].
1.3.2.1. Pitfalls of QCA
In radiographic imaging, where the anatomy is projected onto a plane using a point source and large area detector, accurate determination of vessel magnification is pivotal for converting image pixel measurements to precise physical vessel dimensions [45, 46, 47]. Employing an object of known size within the field of view as a reference aids in establishing the angiogram's magnification. However, the validity of calibration is confined to the region around the reference object, and vessels that are not parallel to the image plane may appear foreshortened due to the projection process.
In general, IVUS tends to overestimate dimensions; OCT serves as the accurate standard, while QCA tends to underestimate vessel dimensions [19, 51, 52]. A large co‐registration study has demonstrated significant concordance in diameter measurements between IVUS and QCA (r = 0.89, p < 0.001) [52]. QCA tends to underestimate the diameter of small vessels and overestimate that of large vessels compared to IVUS. The intersection diameter is 3.8 mm, designating vessels with diameters < 3.8 mm as smaller and those > 3.8 mm as larger. Despite the pioneering work on on‐site QCA guidance [53, 54, 55] and its availability in every catheterization laboratory, it has not been commonly utilized in routine PCI procedures. QCA values can vary based on the calibration point, angiographic view, and vessel size, as described above. This variability might make interventionists hesitant to adopt it, emphasizing the need for practical and reliable approaches to overcome its limitations.
1.3.2.2. Design and QCA
As outlined in Figure 2, we propose a straightforward algorithm designed to mitigate the shortcomings inherent in current embedded QCA programs. High‐quality coronary angiograms, adequately filled with contrast dye by manual or automatic injection, should be obtained after the administration of intracoronary nitroglycerine (250–500 µg). Meticulous planning is of paramount importance for the success of PCI (Design everything: careful design, clean outcomes ‐ Figures 3, and 4). When selecting arteries for stenting, priority should be given to major branches supplying viable myocardium [56]. Conversely, a “keep it open” approach is advisable, without stenting, for smaller side branches. The landing zones should be carefully determined based on various angiographic views, including those that provide a comprehensive visualization of the entire coronary artery. The ideal reference segments, both proximal and distal to the stenosis, should exhibit a visually normal or normal‐looking appearance to cover the entire stenosis. It is preferable to ensure more comprehensive coverage of the proximal reference zone to minimize the risk of proximal edge restenosis.
In routine practice, it is recommended to perform QCA measurement of the reference vessel (landing zone) diameters and lesions in the usual practice view. Care should be taken to select the best images without vessel foreshortening and/or branch overlapping for accurate assessment [57]. While a single QCA measurement at the usual practice view is generally acceptable, obtaining QCA measurements at two different views, including caudal views, may be preferable for the left main coronary artery (LM) and proximal portion (within 10 mm from the ostium) of the left anterior descending coronary artery (LAD). Additionally, repeated QCA is recommended when the vessel size increases after predilation of a severely stenotic coronary artery or chronic total occlusion. Optimal target diameter calculation entails selecting the largest value among multiple measurements when different views are acquired. The following formula presents the target diameters outlined in the Guide DES trial to guide stent selection, deployment, and optimization [30, 43]: Target diameter = measured QCA value + 5%–10% of the measured QCA value. Specifically, the percentage for adjustment varies based on the measured QCA value (Supporting Information S1: Table S1): 10% for QCA values ≤ 3.5 mm, 9% for 3.6 mm, 8% for 3.7 mm, 7% for 3.8 mm, 6% for 3.9 mm, and 5% for QCA values ≥ 4.0 mm.
1.3.3. Stenting Procedures
Oversized stenting carries the risk of procedural complications [12, 13, 14], while undersized stenting may yield suboptimal results. Therefore, precise stent sizing is crucial for the success of PCI.
While QCA measurements may not offer the accuracy of intravascular imaging in determining the reference vessel diameter, they are more reliable than visual estimation. Initiating PCI can be safely guided by the target diameter of the distal reference vessel obtained via on‐site QCA, with the aim of achieving optimal results through routine high‐pressure postdilation (Figure 4, and Supplementary Figure 1).
1.3.3.1. Stent Selection and Implantation
Stent size selection is based on the calculated target diameter of the distal reference segment [30, 43]. For longer lesions, the stent length is visually estimated using a radiopaque guidewire tip (30 mm), while an uninflated balloon (15 or 20 mm) is utilized for shorter lesions. Predilation is necessary for lesions deemed uncrossable by the stent; otherwise, direct stenting is preferable. For the treatment of aorto‐ostial or LAD ostial diseases, it is important to position the proximal stent edge carefully at the ostium to prevent excessive protrusion into the aorta or LM, respectively (Supporting Information S1: Figure S2).
Usually, a stent that is smaller than the distal target diameter yet adequate to reach it is chosen (Supporting Information S1: Figure S1A–E). During stent deployment, the stent balloon is inflated to the pressure necessary to reach the target diameter of the distal reference segment for a minimum of 20 s, ensuring adequate stent expansion at the distal part. However, when a substantial diameter discrepancy exists between the proximal and distal references, it is crucial to carefully consider the maximum expansion limit of the chosen stent to avoid malapposition at the proximal part due to the stent's expansion limit. Deploying a larger stent beyond the distal target diameter at low pressure (about two atmosphere lower than nominal pressure) is recommended under these circumstances, followed by postdilation using a proximal‐sized balloon to achieve complete apposition of the proximal stent segment. Similarly, if the target diameter is below 2.5 mm in stentable small arteries, implanting a 2.5‐mm stent at low pressure (approximately two atmospheres lower than the nominal pressure) for at least 20 s is recommended (Supporting Information S1: Figure S1F). A balloon inflation time of at least 20 s may facilitate adequate stent expansion at the distal part without causing the hemodynamic effects of transient left ventricular dysfunction [58, 59].
Coronary angiography is recommended immediately following stent placement, after intracoronary nitroglycerine (250–500 µg) administration, to assess the transitional shape of the distal stent edge (Figure 4). In the presence of a smooth tapering‐off shape, optimization of the distal edge begins with the original target diameter (Supporting Information S1: Figure S1A–D). If a horizontal or reversed tapering‐off shape is identified, distal edge optimization is initiated with the revised distal target diameter (distal target diameter + approximately 0.2 mm) (Supporting Information S1: Figure S1E). The latter may occasionally be observed after stenting at the mid to distal LAD, probably attributable to underestimation of stent sizing by QCA or vessel dilation following stent placement. The value of 0.2 mm is chosen arbitrarily based on healthy normal intimal thickness, the mean difference between IVUS‐ and QCA‐measured diameter, and the investigator's experience [52, 60].
1.3.3.2. Stent Optimization
Poststenting optimization utilizing a noncompliant balloon of appropriate size, considering the target diameter of the proximal and distal reference segments, is mandatory. Proximal and distal edge optimization entails placing the radiopaque marker of the noncompliant balloon over the stent edge and dilating the balloon up to the target diameters (Supporting Information S1: Figure S3). Optimization of the distal in‐stent portion begins with “two dots inward and 0.2 mm upsizing (distal target diameter + approximately 0.2 mm),” which means retracting the balloon by a distance of two radiopaque dots (2 × 1 mm in length) from the distal stent edge and then dilating it by adding 0.2 mm. This yields a minimum lumen area greater than the distal reference lumen area without the risk of edge dissection, considering that the minimum lumen area is commonly observed at the distal part of the stent. Thereafter, multiple actual postdilations, performed at least twice in the same area within the stent using appropriately sized balloons, are recommended to ensure adequate stent expansion and eliminate stent malapposition. Preferably, assessment should be performed using stent boost subtract imaging, which enhances angiographic visualization of the stent and ensures accurate positioning of the balloon catheter. If the result is deemed suboptimal, a step‐up approach with upsizing postdilations (previous ballooning size + approximately 0.2 mm) is recommended (Figure 4).
For LAD ostial stenting, it is essential to confirm stent protrusion towards the LM by injecting contrast dye during stent ballooning. If significant stent protrusion is present, it should be dilated to eliminate stent malapposition using a distal LM‐sized short balloon (Supporting Information S1: Figure S4A). In cases where additional stent implantation is required to address dissection at the distal stent edge, postdilation of the overlapping zone with a balloon appropriately sized to the proximal stent is necessary to eliminate inter‐stent malapposition at the overlapping site (Supporting Information S1: Figure S4B). For calcified and nondilatable lesions, the pre‐stenting lesion preparation using scoring balloons, cutting balloons, super high‐pressure balloons, rotablation, orbital atherectomy or intravascular lithotripsy facilitates stent expansion. However, achieving poststenting dilation for such challenging lesions can be accomplished via intravascular lithotripsy or super high‐pressure balloons [61, 62]. Finally, this QCA‐based PCI algorithm is applicable to both main epicardial arteries and side branches, including the two‐stent technique (Supporting Information S1: Figure S1D).
1.3.3.3. Ideal Final Results
The desired final outcome should feature a harmonious appearance on angiography, including a horizontally shaped proximal edge, a smooth tapering‐off shape for the distal edge between the reference segment and the stent, free from dissection, with minimum residual in‐stent stenosis (diameter stenosis < 10% by visual estimation). The optimal percentage of diameter taper between the distal stent edge and the 5‐mm distal reference, allowing sufficient stent expansion without edge complications, remains uncertain. Studies suggest a natural reduction in coronary artery diameter at a rate of approximately 0.5% per millimeter [63, 64]. While a taper of approximately 5% between the distal stent edge and the 5‐mm distal reference seems reasonable [14, 65], further research is needed to determine the ideal diameter taper index for improving PCI outcomes.
1.4. Future Directions
The development of three‐dimensional and artificial intelligence‐assisted QCA shows promise in improving accuracy by enabling the three‐dimensional reconstruction of the coronary artery. This facilitates the evaluation of vessel segment length, diameter, and volume [66, 67, 68, 69]. Furthermore, the sophisticated physiological parameters derived from QCA, such as quantitative flow ratio and pressure pullback gradient index, have the potential to guide and enhance PCI outcomes [70, 71]. Ongoing trials will further delineate their role in the future [72, 73]. In the Guide‐DES trial, which utilized the conventional QCA programs built into the angiography machine, the degrees of adjustment in the measured QCA values to the target diameters were chosen based on published data, experience from IVUS studies, and safety considerations, necessitating further refinement. If the QCA values measured by emerging QCA programs prove to be consistent and accurate across various angiographic views and vessel sizes, 10% oversizing (balloon‐to‐artery diameter ratio of 1.1) of the measured values overall may be applicable in stent selection and optimization.
PCI methods using imaging guidance are well established and popular, while QCA guidance is not as widely adopted (Table 1). Achieving effective utilization of QCA in guiding PCI demands comprehensive training and education, mirroring the crucial role of education in intravascular imaging. Proficiency in both imaging‐guided and QCA‐guided PCI is imperative, requiring initial hands‐on experience to ensure outcomes comparable to those of imaging‐guided PCI when employing QCA. Various resources, both in‐person and online, can be employed to seamlessly integrate QCA‐guided PCI alongside imaging‐guided PCI, providing valuable assistance for PCI procedures, particularly in scenarios where intravascular imaging is inaccessible.
2. Conclusions
Although intravascular imaging is the preferred modality for PCI guidance, QCA‐guided PCI can serve as a viable alternative in circumstances where intravascular imaging is not readily available. The limitations of angiography‐guided PCI, which relies on visual estimation, may result in suboptimal outcomes. QCA, with its cost‐effectiveness and easy availability in every catheterization laboratory, justifies the transition from angiography‐guided to QCA‐guided PCI. Continuous innovation is warranted to further enhance the efficacy of current in‐built conventional QCA programs and overcome existing barriers.
Author Contributions
Cheol Whan Lee: conceptualization, data curation, formal analysis, methodology, project administration, writing–original draft, writing–review and editing. Pil Hyung Lee: formal analysis, methodology, writing–review and editing. Seung‐whan lee: conceptualization, writing–review and editing. Patrick W. Serruys: formal analysis, methodology, writing–review and editing.
Ethics Statement
The authors have nothing to report. This study did not collect data from human or animal subjects but from an open research repository.
Conflicts of Interest
Dr. C.W. Lee received a grant from Biotronik. Dr. S.W. Lee is receiving grants from Biotronik and Boston Scientific. Dr. P.H. Lee is receiving grants from Abbott Cardiovascular, Boston Scientific, and Medtronic. Dr. P.W. Serruys reports consultancy fees for SMT, Novartis, Meril Life Sciences, Xeltis, and Philips.
Supporting information
Supporting information.
Acknowledgments
We express our gratitude to research nurses Jiwon Baek and Juhee Kim for their efforts in preparing the angiographic images, and to the participating investigators for their valuable contributions to the Guide‐DES trial. The authors have nothing to report.
Data Availability Statement
The authors have nothing to report.
References
- 1. Neumann F. J., Sousa‐Uva M., Ahlsson A., et al., “2018 ESC/EACTS Guidelines on Myocardial Revascularization,” European Heart Journal 40 (2019): 87–165. [DOI] [PubMed] [Google Scholar]
- 2. Lawton J. S., Tamis‐Holland J. E., Bangalore S., et al., “2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization,” Journal of the American College of Cardiology 79 (2022): e21–e129. [DOI] [PubMed] [Google Scholar]
- 3. Räber L., Mintz G. S., Koskinas K. C., et al., “Clinical use of Intracoronary Imaging. Part 1: Guidance and Optimization of Coronary Interventions. An Expert Consensus Document of the European Association of Percutaneous Cardiovascular Interventions,” European Heart Journal 39, no. 35 (2018): 3281–3300. [DOI] [PubMed] [Google Scholar]
- 4. Stone G. W., Christiansen E. H., Ali Z. A., et al., “Intravascular Imaging‐Guided Coronary Drug‐Eluting Stent Implantation: An Updated Network Meta‐Analysis,” The Lancet 403 (2024): 824–837. [DOI] [PubMed] [Google Scholar]
- 5. Fazel R., Yeh R. W., Cohen D. J., et al., “Intravascular Imaging During Percutaneous Coronary Intervention: Temporal Trends and Clinical Outcomes in the USA,” European Heart Journal 44, no. 38 (2023): 3845–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mentias A., Sarrazin M. V., Saad M., et al., “Long‐Term Outcomes of Coronary Stenting With and Without use of Intravascular Ultrasound,” JACC: Cardiovascular Interventions 13 (2020): 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park H., Kang D. Y., and Lee C. W., “Functional Angioplasty: Definitions, Historical Overview, and Future Perspectives,” Korean Circulation Journal 52 (2022): 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schatz R. A., Baim D. S., Leon M., et al., “Clinical Experience With the Palmaz‐Schatz Coronary Stent. Initial Results of a Multicenter Study,” Circulation 83 (1991): 148–161. [DOI] [PubMed] [Google Scholar]
- 9. Campbell P. T., Mahmud E., and Marshall J. J., “Interoperator and Intraoperator (In)Accuracy of Stent Selection Based on Visual Estimation,” Catheterization and Cardiovascular Interventions 86 (2015): 1177–1183. [DOI] [PubMed] [Google Scholar]
- 10. Schatz R. A., Palmaz J. C., Tio F. O., Garcia F., Garcia O., and Reuter S. R., “Balloon‐Expandable Intracoronary Stents in the Adult Dog,” Circulation 76 (1987): 450–457. [DOI] [PubMed] [Google Scholar]
- 11. De Benedetti E. and Urban P., “Coronary Stenting: Why Size Matters,” Heart 93 (2006): 1500–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis S. G., Ajluni S., Arnold A. Z., et al., “Increased Coronary Perforation in the New Device Era. Incidence, Classification, Management, and Outcome,” Circulation 90 (1994): 2725–2730. [DOI] [PubMed] [Google Scholar]
- 13. Stankovic G., Orlic D., Corvaja N., et al., “Incidence, Predictors, In‐Hospital, and Late Outcomes of Coronary Artery Perforations,” The American Journal of Cardiology 93 (2004): 213–216. [DOI] [PubMed] [Google Scholar]
- 14. Chamié D., Bezerra H. G., Attizzani G. F., et al., “Incidence, Predictors, Morphological Characteristics, and Clinical Outcomes of Stent Edge Dissections Detected by Optical Coherence Tomography,” JACC: Cardiovascular Interventions 6 (2013): 800–813. [DOI] [PubMed] [Google Scholar]
- 15. Nallamothu B. K., Spertus J. A., Lansky A. J., et al., “Comparison of Clinical Interpretation With Visual Assessment and Quantitative Coronary Angiography in Patients Undergoing Percutaneous Coronary Intervention in Contemporary Practice: The Assessing Angiography (A2) Project,” Circulation 127 (2013): 1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ng A. K. Y., Ng P. Y., Ip A., Lam L. T., and Siu C. W., “Survivals of Angiography‐Guided Percutaneous Coronary Intervention and Proportion of Intracoronary Imaging at Population Level: The Imaging Paradox,” Frontiers in Cardiovascular Medicine 9 (2022): 792837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi K. H., Lee S. Y., Song Y. B., et al., “Prognostic Impact of Operator Experience and IVUS Guidance on Long‐Term Clinical Outcomes After Complex PCI,” JACC: Cardiovascular Interventions 16 (2023): 1746–1758. [DOI] [PubMed] [Google Scholar]
- 18. Gutiérrez‐Chico J. L., Serruys P. W., Girasis C., et al., “Quantitative Multi‐Modality Imaging Analysis of a Fully Bioresorbable Stent: A Head‐To‐Head Comparison between QCA, IVUS and OCT,” The International Journal of Cardiovascular Imaging 28 (2012): 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsuchida K., Serruys P. W., Bruining N., et al., “Two‐Year Serial Coronary Angiographic and Intravascular Ultrasound Analysis of In‐Stent Angiographic Late Lumen Loss and Ultrasonic Neointimal Volume From the Taxus II Trial,” The American Journal of Cardiology 99 (2007): 607–615. [DOI] [PubMed] [Google Scholar]
- 20. Fujii K., Carlier S. G., Mintz G. S., et al., “Stent Underexpansion and Residual Reference Segment Stenosis Are Related to Stent Thrombosis after Sirolimus‐Eluting Stent Implantation,” Journal of the American College of Cardiology 45 (2005): 995–998. [DOI] [PubMed] [Google Scholar]
- 21. Song H. G., Kang S. J., Ahn J. M., et al., “Intravascular Ultrasound Assessment of Optimal Stent Area to Prevent In‐Stent Restenosis After Zotarolimus‐, Everolimus‐, and Sirolimus‐Eluting Stent Implantation,” Catheterization and Cardiovascular Interventions 83 (2014): 873–878. [DOI] [PubMed] [Google Scholar]
- 22. Lee Y. J., Zhang J. J., Mintz G. S., et al., “Impact of Intravascular Ultrasound‐Guided Optimal Stent Expansion on 3‐year Hard Clinical Outcomes,” Circulation: Cardiovascular Interventions 14 (2021): e011124. [DOI] [PubMed] [Google Scholar]
- 23. Kim J. S., Kang T. S., Mintz G. S., et al., “Randomized Comparison of Clinical Outcomes Between Intravascular Ultrasound and Angiography‐Guided Drug‐Eluting Stent Implantation for Long Coronary Artery Stenoses,” JACC: Cardiovascular Interventions 6 (2013): 369–376. [DOI] [PubMed] [Google Scholar]
- 24. Hong S. J., Kim B. K., Shin D. H., et al., “Effect of Intravascular Ultrasound–Guided vs Angiography‐Guided Everolimus‐Eluting Stent Implantation: The IVUS‐XPL Randomized Clinical Trial,” Journal of the American Medical Association 314 (2015): 2155–2163. [DOI] [PubMed] [Google Scholar]
- 25. Zhang J., Gao X., Kan J., et al., “Intravascular Ultrasound Versus Angiography‐Guided Drug‐Eluting Stent Implantation,” Journal of the American College of Cardiology 72 (2018): 3126–3137. [DOI] [PubMed] [Google Scholar]
- 26. Koo B. K., Hu X., Kang J., et al., “Fractional Flow Reserve or Intravascular Ultrasonography to Guide PCI,” New England Journal of Medicine 387 (2022): 779–789. [DOI] [PubMed] [Google Scholar]
- 27. Lee J. M., Choi K. H., Song Y. B., et al., “Intravascular Imaging–Guided or Angiography‐Guided Complex PCI,” New England Journal of Medicine 388 (2023): 1668–1679. [DOI] [PubMed] [Google Scholar]
- 28. Ali Z. A., Landmesser U., Maehara A., et al., “Optical Coherence Tomography–Guided Versus Angiography‐Guided PCI,” New England Journal of Medicine 389 (2023): 1466–1476. [DOI] [PubMed] [Google Scholar]
- 29. Holm N. R., Andreasen L. N., Neghabat O., et al., “OCT or Angiography Guidance for PCI in Complex Bifurcation Lesions,” New England Journal of Medicine 389 (2023): 1477–1487. [DOI] [PubMed] [Google Scholar]
- 30. Lee P. H., Hong S. J., Kim H. S., et al., “Quantitative Coronary Angiography vs Intravascular Ultrasonography to Guide Drug‐Eluting Stent Implantation: A Randomized Clinical Trial,” JAMA Cardiology 9, no. 5 (2024): 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li X., Ge Z., Kan J., et al., “Intravascular Ultrasound‐Guided Versus Angiography‐Guided Percutaneous Coronary Intervention in Acute Coronary Syndromes (IVUS‐ACS): A Two‐Stage, Multicentre, Randomised Trial,” The Lancet 403 (2024): 1855–1865. [DOI] [PubMed] [Google Scholar]
- 32. Colombo A., Hall P., Nakamura S., et al., “Intracoronary Stenting Without Anticoagulation Accomplished With Intravascular Ultrasound Guidance,” Circulation 91 (1995): 1676–1688. [DOI] [PubMed] [Google Scholar]
- 33. Brodie B. R., Cooper C., Jones M., Fitzgerald P., and Cummins F., “Is Adjunctive Balloon Postdilatation Necessary After Coronary Stent Deployment? Final Results From the Postit Trial,” Catheterization and Cardiovascular Interventions 59 (2003): 184–192. [DOI] [PubMed] [Google Scholar]
- 34. Aziz S., Morris J. L., Perry R. A., and Stables R. H., “Stent Expansion: A Combination of Delivery Balloon Underexpansion and Acute Stent Recoil Reduces Predicted Stent Diameter Irrespective of Reference Vessel Size,” Heart 93 (2006): 1562–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muraoka Y., Sonoda S., Tsuda Y., Tanaka S., Okazaki M., and Otsuji Y., “Effect of Intravascular Ultrasound‐Guided Adjuvant High‐Pressure Non‐Compliant Balloon Postdilation After Drug‐Eluting Stent Implantation,” Heart and Vessels 26 (2011): 565–571. [DOI] [PubMed] [Google Scholar]
- 36. Biswas S., Soon K., and Lim Y. L., “Adjunctive Balloon Dilatation After Stent Deployment: Beneficial or Deleterious?,” International Journal of Cardiology 157 (2012): 3–7. [DOI] [PubMed] [Google Scholar]
- 37. Van der Hoeven B. L., Liem S. S., Dijkstra J., et al., “Stent Malapposition after Sirolimus‐Eluting and Bare‐Metal Stent Implantation in Patients With ST‐Segment Elevation Myocardial Infarction: Acute and 9‐month Intravascular Ultrasound Results of the MISSION! Intervention Study,” JACC Cardiovasc Interv 1 (2008): 192–201. [DOI] [PubMed] [Google Scholar]
- 38. Park H., Ahn J. M., Kang D. Y., et al., “Optimal Stenting Technique for Complex Coronary Lesions: Intracoronary Imaging‐Guided Pre‐Dilation, Stent Sizing, and Post‐Dilation,” JACC Cardiovasc Interv 13 (2020): 1403–1413. [DOI] [PubMed] [Google Scholar]
- 39. Levine G. N., Bates E. R., Blankenship J. C., et al., “2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention,” Journal of the American College of Cardiology 58 (2011): e44–e122. [DOI] [PubMed] [Google Scholar]
- 40. Chang C. C., Kogame N., Onuma Y., et al., “Defining Device Success for Percutaneous Coronary Intervention Trials: A Position Statement From the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology,” EuroIntervention 15 (2020): 1190–1198. [DOI] [PubMed] [Google Scholar]
- 41. de Ribamar Costa J., Mintz G. S., Carlier S. G., et al., “Intravascular Ultrasound Assessment of Drug‐Eluting Stent Expansion,” American Heart Journal 153 (2007): 297–303. [DOI] [PubMed] [Google Scholar]
- 42. Basalus M. W. Z., Tandjung K., Van Apeldoorn A. A., Ankone M. J. K., and Von Birgelen C., “Effect of Oversized Partial Postdilatation on Coatings of Contemporary Durable Polymer‐Based Drug‐Eluting Stents: A Scanning Electron Microscopy Study,” Journal of Interventional Cardiology 24 (2011): 149–161. [DOI] [PubMed] [Google Scholar]
- 43. Lee P. H., Hong S. J., Kim H. S., et al., “Quantitative Coronary Angiography Versus Intravascular Ultrasound Guidance for Drug‐Eluting Stent Implantation (Guide‐Des): Study Protocol for a Randomised Controlled Non‐Inferiority Trial,” BMJ Open 12 (2022): e052215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. West J. W. and Guzman S. V., “Coronary Dilatation and Constriction Visualized by Selective Arteriography,” Circulation Research 7 (1959): 527–536. [DOI] [PubMed] [Google Scholar]
- 45. Brown B. G., Bolson E., Frimer M., and Dodge H. T., “Quantitative Coronary Arteriography: Estimation of Dimensions, Hemodynamic Resistance, and Atheroma Mass of Coronary Artery Lesions Using the Arteriogram and Digital Computation,” Circulation 55 (1977): 329–337. [DOI] [PubMed] [Google Scholar]
- 46. Serruys P. W., Reiber J. H. C., Wijns W., et al., “Assessment of Percutaneous Transluminal Coronary Angioplasty by Quantitative Coronary Angiography: Diameter Versus Densitometric Area Measurements,” The American Journal of Cardiology 54 (1984): 482–488. [DOI] [PubMed] [Google Scholar]
- 47. Mario C. D., Haase J., Boer A., Reiber J. H. C., and Serruys P. W., “Edge Detection Versus Densitometry in the Quantitative Assessment of Stenosis Phantoms: An in Vivo Comparison in Porcine Coronary Arteries,” American Heart Journal 124 (1992): 1181–1189. [DOI] [PubMed] [Google Scholar]
- 48. Wunderlich W., Fischer F., Linderer T., and Kirkeeide R. L., “Analytic Isocenter Calibration. A New Approach for Accurate X‐Ray Gantries,” Angiology 46 (1995): 577–582. [DOI] [PubMed] [Google Scholar]
- 49. Tomkowiak M. T., Speidel M. A., Raval A. N., and Van Lysel M. S., “Calibration‐Free Device Sizing Using an Inverse Geometry X‐Ray System,” Medical Physics 38 (2011): 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pinton F. A., Falcão B. A. A., Mariani J., Kajita L. J., Filho A. E., and Neto P. A. L., “Accuracy and Precision of Online Quantitative Coronary Angiography With Automatic Calibration: A Pilot Study,” Revista Brasileira de Cardiologia Invasiva (English Edition) 23 (2015): 58–60. [Google Scholar]
- 51. Kubo T., Akasaka T., Shite J., et al., “OCT Compared With Ivus in a Coronary Lesion Assessment: The OPUS‐Class Study,” JACC Cardiovasc Imaging 6 (2013): 1095–1104. [DOI] [PubMed] [Google Scholar]
- 52. Goto K., Mintz G. S., Litherland C., et al., “Lumen Measurements From Quantitative Coronary Angiography and IVUS: A Prospect Substudy,” JACC: Cardiovascular Imaging 9 (2016): 1011–1013. [DOI] [PubMed] [Google Scholar]
- 53. Serruys P. W., de Bruyne B., Carlier S., et al., “Randomized Comparison of Primary Stenting and Provisional Balloon Angioplasty Guided by Flow Velocity Measurement,” Circulation 102 (2000): 2930–2937. [DOI] [PubMed] [Google Scholar]
- 54. Di Mario C., Moses J. W., Anderson T. J., et al., “Randomized Comparison of Elective Stent Implantation and Coronary Balloon Angioplasty Guided by Online Quantitative Angiography and Intracoronary Doppler,” Circulation 102 (2000): 2938–2944. [DOI] [PubMed] [Google Scholar]
- 55. Ishibashi Y., Nakatani S., Sotomi Y., et al., “Relation Between Bioresorbable Scaffold Sizing Using QCA‐Dmax and Clinical Outcomes at 1 Year in 1,232 Patients From 3 Study Cohorts (Absorb Cohort B, Absorb Extend, and Absorb II),” JACC: Cardiovascular Interventions 8 (2015): 1715–1726. [DOI] [PubMed] [Google Scholar]
- 56. Lunardi M., Louvard Y., Lefèvre T., et al., “Definitions and Standardized Endpoints for Treatment of Coronary Bifurcations,” EuroIntervention 19 (2023): e807–e831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kotoku N., Ninomiya K., Ding D., et al., “Murray Law‐Based Quantitative Flow Ratio to Assess Left Main Bifurcation Stenosis: Selecting the Angiographic Projection Matters,” The International Journal of Cardiovascular Imaging 40 (2024): 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sorrentino S., De Rosa S., Ambrosio G., et al., “The Duration of Balloon Inflation Affects the Luminal Diameter of Coronary Segments After Bioresorbable Vascular Scaffolds Deployment,” BMC Cardiovascular Disorders 15 (2015): 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kraitchman D. L., Sampath S., Castillo E., et al., “Quantitative Ischemia Detection During Cardiac Magnetic Resonance Stress Testing by use of Fastharp,” Circulation 107 (2003): 2025–2030. [DOI] [PubMed] [Google Scholar]
- 60. St Goar F. G., Pinto F. J., Alderman E. L., et al., “Detection of Coronary Atherosclerosis in Young Adult Hearts Using Intravascular Ultrasound,” Circulation 86 (1992): 756–763. [DOI] [PubMed] [Google Scholar]
- 61. Rheude T., Rai H., Richardt G., et al., “Super High‐Pressure Balloon Versus Scoring Balloon to Prepare Severely Calcified Coronary Lesions: The ISAR‐CALC Randomised Trial,” EuroIntervention 17 (2021): 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wańha W., Tomaniak M., Wańczura P., et al., “Intravascular Lithotripsy for the Treatment of Stent Underexpansion: The Multicenter IVL‐Dragon Registry,” Journal of Clinical Medicine 11 (2022): 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zubaid M., Buller C., and Mancini G. B., “Normal Angiographic Tapering of the Coronary Arteries,” The Canadian Journal of Cardiology 18 (2002): 973–980. [PubMed] [Google Scholar]
- 64. Javier S. P., Mintz G. S., Popma J. J., et al., “Intravascular Ultrasound Assessment of the Magnitude and Mechanism of Coronary Artery and Lumen Tapering,” The American Journal of Cardiology 75 (1995): 177–180. [DOI] [PubMed] [Google Scholar]
- 65. Sakurai R., Ako J., Morino Y., et al., “Predictors of Edge Stenosis Following Sirolimus‐Eluting Stent Deployment (A Quantitative Intravascular Ultrasound Analysis From the SIRIUS Trial),” The American Journal of Cardiology 96 (2005): 1251–1253. [DOI] [PubMed] [Google Scholar]
- 66. Schuurbiers J. C. H., Lopez N. G., Ligthart J., et al., “In Vivo Validation of CAAS QCA‐3D Coronary Reconstruction Using Fusion of Angiography and Intravascular Ultrasound (Angus),” Catheterization and Cardiovascular Interventions 73 (2009): 620–626. [DOI] [PubMed] [Google Scholar]
- 67. Tu S., Xu L., Ligthart J., et al., “In Vivo Comparison of Arterial Lumen Dimensions Assessed by Co‐Registered Three‐Dimensional (3D) Quantitative Coronary Angiography, Intravascular Ultrasound and Optical Coherence Tomography,” The International Journal of Cardiovascular Imaging 28 (2012): 1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim Y., Bray J. J. H., Waterhouse B., et al., “Quantitative Evaluation and Comparison of Coronary Artery Characteristics by 3D Coronary Volume Reconstruction,” Scientific Reports 11 (2021): 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu X., Wang X., Chen D., and Zhang H., “Automatic Quantitative Coronary Analysis Based on Deep Learning,” Applied Sciences 13 (2023): 2975. [Google Scholar]
- 70. Kogame N., Takahashi K., Tomaniak M., et al., “Clinical Implication of Quantitative Flow Ratio After Percutaneous Coronary Intervention for 3‐Vessel Disease,” JACC: Cardiovascular Interventions 12 (2019): 2064–2075. [DOI] [PubMed] [Google Scholar]
- 71. Dai N., Hwang D., Lee J. M., et al., “Feasibility of Quantitative Flow Ratio‐Derived Pullback Pressure Gradient Index and Its Impact on Diagnostic Performance,” JACC: Cardiovascular Interventions 14 (2021): 353–355. [DOI] [PubMed] [Google Scholar]
- 72. Hara H., Gao C., Kogame N., et al., “A Randomised Controlled Trial of the Sirolimus‐Eluting Biodegradable Polymer Ultra‐Thin Supraflex Stent Versus the Everolimus‐Eluting Biodegradable Polymer Synergy Stent for Three‐Vessel Coronary Artery Disease: Rationale and Design of the Multivessel TALENT Trial,” EuroIntervention 16 (2020): 997. [DOI] [PubMed] [Google Scholar]
- 73. Hara H., Serruys P. W., O'Leary N., et al., “Angiography‐Derived Physiology Guidance vs Usual Care in an All‐Comers PCI Population Treated With the Healing‐Targeted Supreme Stent and Ticagrelor Monotherapy: Pioneer IV Trial Design,” American Heart Journal 246 (2022): 32–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors have nothing to report.


