Abstract
Vagus nerve stimulation (VNS) has gained enormous traction as a promising bioelectronic therapy. In particular, the delivery of VNS paired with training to promote neural changes has demonstrated clinical success for stroke recovery and found far-reaching application in other domains, from autism to psychiatric disorders to normal learning. The success of paired VNS has been extensively documented. Here, we consider a more unusual question: why does VNS have such broad utility, and perhaps more importantly, when does VNS not work? We present a discussion of the concepts that underlie VNS therapy and an anthology of studies that describe conditions in which these concepts are violated and VNS fails. We focus specifically on the mechanisms engaged by implanted VNS, and how the parameters of stimulation, stimulation method, pharmacological manipulations, accompanying comorbidities, and specifics of concurrent training interact with these mechanisms to impact the efficacy of VNS therapy. As paired VNS therapy is increasing translated to clinical implementation, a clear understanding of the conditions in which it does, and critically, does not work is fundamental to the success of this approach.
Keywords: vagal nerve stimulation, vagus nerve stimulation, rehabilitation, parameters, recovery
Main Text
Vagus nerve stimulation (VNS) therapy has emerged as a promising bioelectronic intervention for a panoply of neurological disorders, from stroke to posttraumatic stress disorder to autism [1–4]. Generally, this approach is based on delivering bursts of VNS that coincide with various forms of training, ranging from upper limb rehabilitation to sensory retraining to cognitive therapy. Some applications of VNS therapy have found clinical success, and many other implementations are in various stages of development and translation. The broad applicability of VNS for such a wide spectrum of disorders raises an important question: when does VNS not work?
Here, we provide a discussion of the concepts that underlie VNS therapy and an anthology of studies that describe conditions in which these concepts are violated and VNS fails. Many other forms of VNS have been developed that are premised on non-contingent stimulation, such as for epilepsy, modulation of the immune system, and neuroprotection. We have constrained the scope of this review to studies that explicitly utilize VNS delivered with an implanted stimulator and paired with training to promote synaptic plasticity and target treatment of neurological disorders.
Conceptual basis of VNS therapy
The application of paired VNS therapy is based on the principles of reinforcement, a foundational concept in neuroscience [1,3]. Generally, reinforcement arises from neuromodulatory feedback that shapes future responses based on antecedent neural activity. Stimulation of the vagus nerve drives rapid, phasic activation of multiple neuromodulatory networks, a key component in reinforcement. For example, VNS triggers action potentials in the noradrenergic locus coeruleus (LC) within approximately 100 msec of the onset of stimulation, and spiking continues for roughly the duration of stimulation [5]. Consequently, VNS drives activation of noradrenergic fibers throughout the brain, resulting in a rapid, widespread release of norepinephrine [6]. The cholinergic system exhibits similar rapid activation by VNS [6–8]. This engagement of neuromodulatory networks is critical to VNS actions on the central nervous system, as evidenced in studies that inhibit noradrenergic and cholinergic transmission. Depletion of norepinephrine, as well as other neuromodulatory networks including acetylcholine and serotonin, blocks the effects of VNS [9–11]. Taken together, these findings implicate activation of neuromodulatory networks as one component of VNS therapy.
Despite the global release of neuromodulators evoked by stimulation, the effects of VNS are quite specific. For instance, preclinical studies show that VNS delivered during performance of forelimb motor task reshapes the motor networks that control the forelimb without changing the motor networks that control other movements not targeted by training [9,12,13]. Similar robust plasticity is observed in specific networks in auditory cortex when VNS is paired with various auditory stimuli [14,15].
This specificity reveals the other necessary component of VNS therapy: the concurrent training. Training, whether it consists of presentation of sensory stimuli or performance of a motor task or cognitive exercises, is subserved by training-specific activity in glutamatergic networks. In the case of motor training, movement of the forelimb is driven in part by spiking in corticospinal networks that send movement commands to muscles in the forelimb. Delivery of VNS concurrent with forelimb movement thus drives neuromodulator release to facilitate plasticity in the active corticospinal networks that produce the movement. Conversely, corticospinal neurons that are not activated by training, such as those that control the lower limb, are exposed to norepinephrine released in response to VNS but fail to be potentiated [9,12,13]. Thus, the neural activity that comprises the concurrent training gives rise to the specificity of plasticity and defines the second component of VNS therapy (Fig. 1).
Figure 1. Components of Paired VNS.
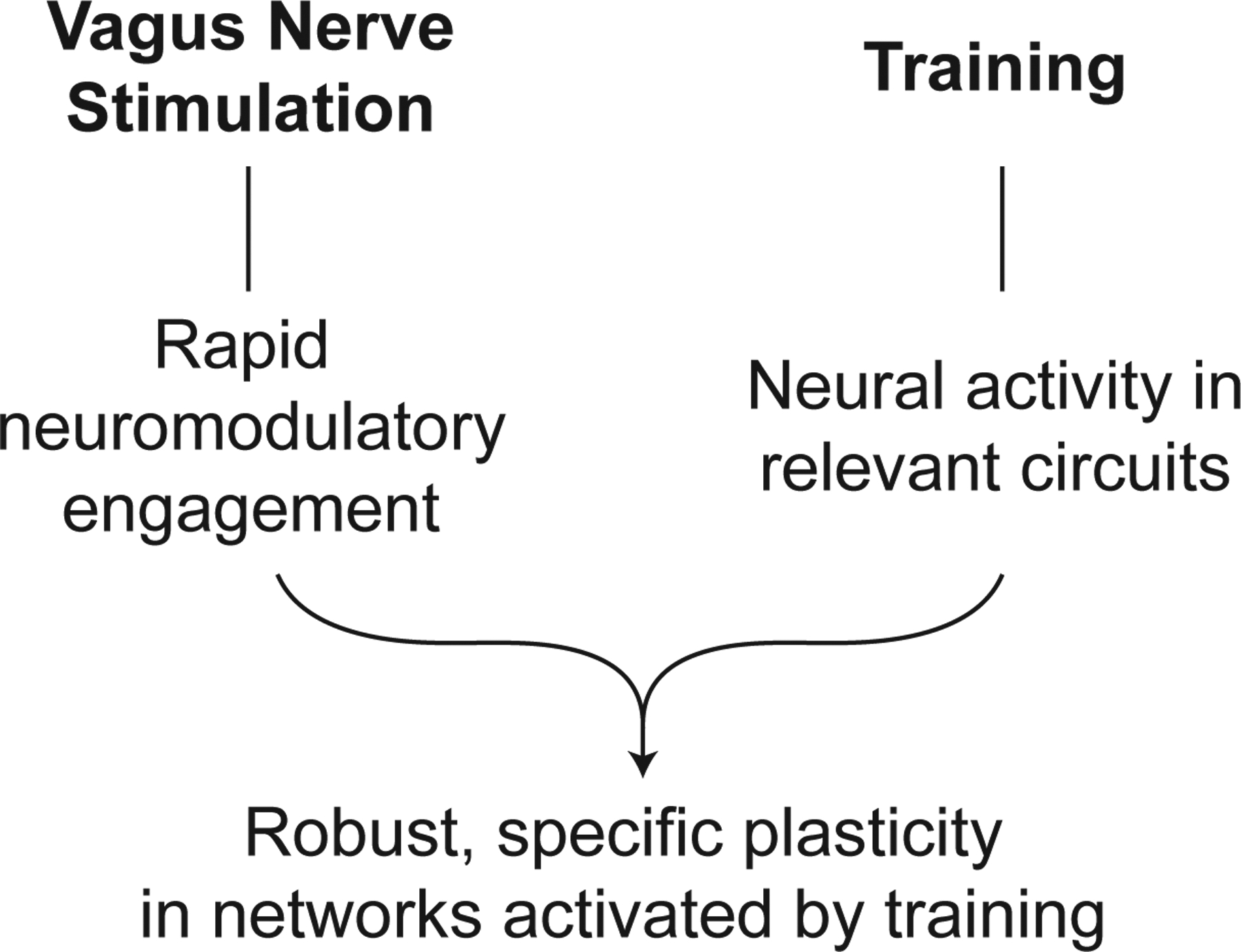
Paired VNS is based on the combination of two components: VNS and training. VNS drives rapid, phasic activation of neuromodulatory networks. Coupling this neuromodulator release with training, which engenders neural activity in relevant circuits, enhances synaptic plasticity specific to the paired training.
Applying VNS therapy to treat disease
This combination of VNS and training to promote synaptic plasticity has been successfully employed in a number of contexts. A number of animal studies show that pairing VNS with rehabilitative training of the forelimb enhances recovery of motor function compared to equivalent training without VNS in models of stroke [16–20]. Consistent with the presumed action on synaptic plasticity, VNS-dependent recovery drives synaptic reorganization in corticospinal networks that control the forelimb [20]. Related preclinical studies show that pairing VNS with rehabilitative training similarly enhances plasticity and promotes recovery in models of spinal cord injury, traumatic brain injury, and peripheral nerve damage [21–25]. The common theme in these applications is the paired delivery of VNS with targeted rehabilitative training.
Beyond preclinical models, this approach has found clinical success. A recent large pivotal study employing a similar approach demonstrates that VNS paired with upper limb rehabilitation significantly enhances recovery of arm and hand function compared to equivalent training without stimulation in individuals with chronic stroke [26]. This led to FDA approval of VNS therapy as the first neuromodulation therapy for chronic stroke. Congruent approaches using paired VNS are now being developed for a range of other neurological disorders. For example, VNS has been paired with rehabilitation for spinal cord injury, auditory stimuli for treatment of tinnitus, and cognitive behavioral therapy for PTSD [2,3,27–30].
The etiology and the accompanying therapy for each condition is distinct, but the use of VNS is common. How can electrical stimulation via an implanted device on the cervical trunk of the vagus nerve, which has relatively limited first order connectivity to targets in the central nervous system, exert an effect on motor control of the hand, processing of sound, and fear response? Or reaching even further, if VNS can effect these disparate systems, what does VNS not treat? Below, we explore the overlapping answer to these questions and discuss how an understanding of the conditions in which a therapy is not effective are as or more informative as conditions in which it is.
Deliver VNS at the wrong level
Since VNS is based on the timed release of neuromodulators during training, manipulations that influence the activation of neuromodulatory networks could be reasonably expected to impact the effects of VNS. The main determinants of neuromodulator network activation in response to VNS are the parameters of stimulation. Generally, an individual train of VNS is comprised of biphasic electrical pulses of a particular intensity and width delivered at a particular rate for a particular duration. Changing any of these stimulation parameters has a conveniently straightforward effect on the activation of neuromodulatory networks: higher stimulation intensities drive greater spiking activity in the noradrenergic LC, indicative of greater neuromodulatory engagement [5]. Similarly, faster frequencies evoke a greater rate of neural activity in the LC, and longer trains engender a greater duration of spiking [5].
This reliance on stimulation parameters presents a simple opportunity: if paired VNS is based on the activation of neuromodulatory networks, perhaps more activation would result in greater VNS-dependent effects. This concept has been extensively explored in animal models of VNS-directed plasticity. The most well-characterized parameter is stimulation intensity [13,31,32]. As expected, low intensity VNS generates insufficient activation and fails to promote plasticity. Moderate intensity VNS results in sufficient activation and enhances plasticity relevant to the paired training. Paradoxically, high intensity stimulation also fails to promote plasticity, resulting in an inverted-U relationship between stimulation intensity and VNS-dependent plasticity (Fig. 2). Other parameters follow a similar pattern, though the amount of experimental evidence is more limited. Moderate stimulation frequencies enhance VNS-dependent plasticity, whereas lower and higher frequencies fail to do so [33]. Train duration and number of pulses per stimulation train also exhibit an inverted-U relationship with plasticity [34]. Similar principles hold for the effect of VNS connectivity, BOLD, and pupil response in individuals with epilepsy [35–37]. These findings are apparent across a range of different training paradigms, indicating that they represent a core feature of VNS itself.
Figure 2. Inverted-U relationship between VNS actions and stimulation parameters.
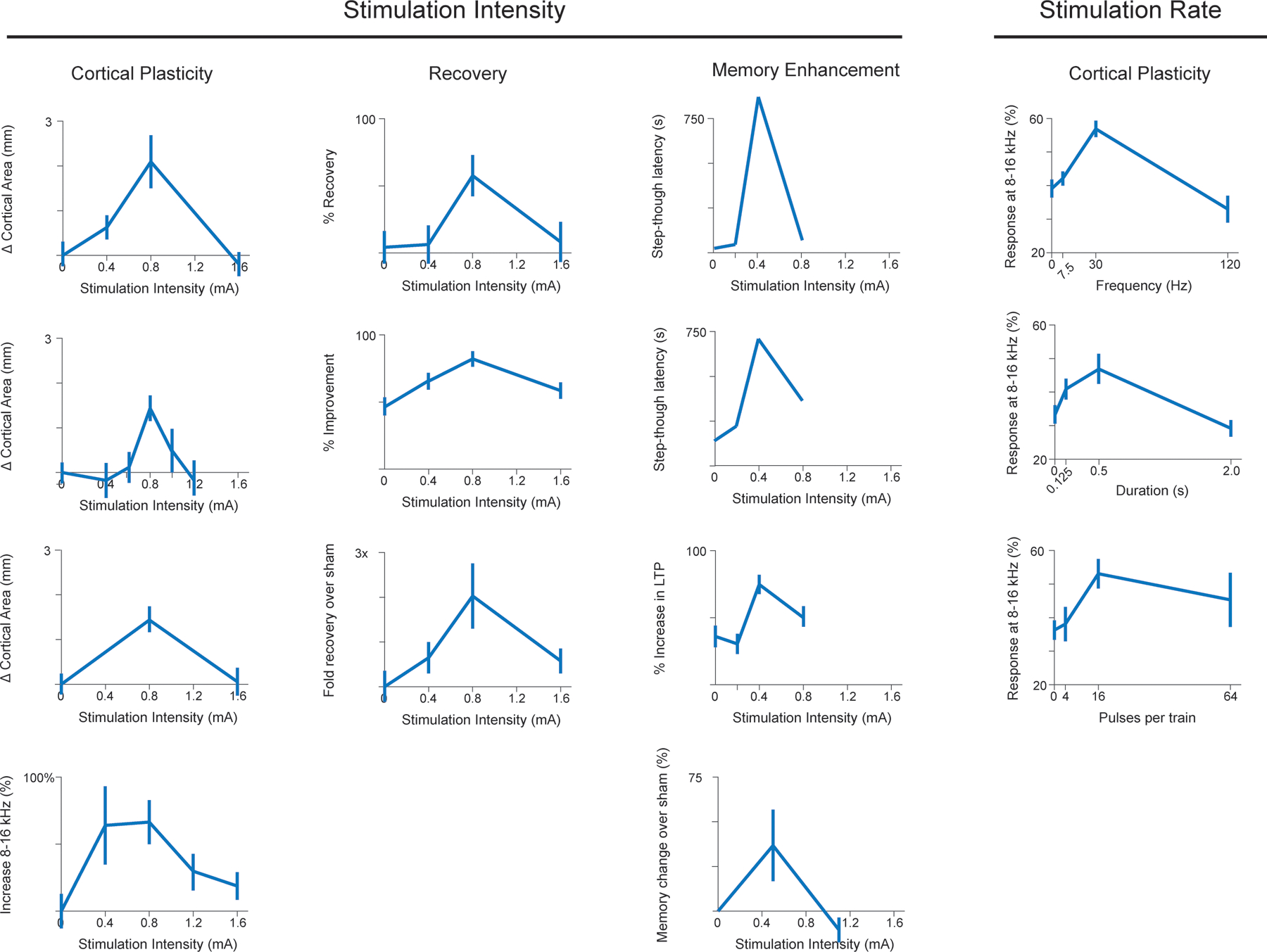
Across a wide range of models, applications, and measures, VNS effects exhibit an inverted-U relationship with the parameters of stimulation. Moderate stimulation intensity, frequency, and duration reliably produce the largest effects, whereas both lower and higher parameters are less effective. Data from, beginning in the top left and reading across, Morrison et al., Brain Stimulation, 2019; Pruitt et al., Translational Stroke Research, 2021; Clark et al., Neurobiology of Learning and Memory, 1995; Buell et al., Brain Stimulation, 2018; Morrison et al, Behavioural Brain Research, 2020; Souza, Experimental Neurology, 2021; Clark et al., Neurobiology of Learning and Memory, 1998; Buell et al., Neuroscience, 2019; Morrison et al., Brain Research, 2021; Unpublished; Zuo et al., Physiology & Behavior, 2007; Buell et al., Neuroscience, 2019; Borland et al., Brain Stimulation, 2016; Clark et al., Nature Neuroscience, 1999.
Nonetheless, these studies focus on VNS-dependent actions on neural plasticity, which could be only superficially relevant to the use of VNS as a therapy. The true test is whether VNS therapy exhibits a similar reliance on parameters in the context of recovery. Indeed, a number of studies reinforce this concept. In a preclinical model of ischemic stroke, moderate intensity VNS paired with rehabilitative training enhances recovery of forelimb function, while low and high intensity VNS both fail to provide any additional benefits over rehabilitative training without stimulation [38]. Highlighting the commonality of this effect across disparate applications and training regimens, moderate intensity VNS is more effective than low and high intensity VNS when paired with fear extinction training and tactile rehabilitation [39]. Landmark early studies observed similar effects on VNS-dependent enhancement of memory, both in rats and humans [40,41]. Thus, given that the inverted-U appears to extend beyond neurophysiology and indeed directly impact the intended therapeutic effects, a clearer understanding of this phenomenon is desired.
What is responsible for the inverted-U? A number of potential explanations could account for this relationship. The fact that greater intensity, frequency, and duration all drive greater activity in neuromodulatory networks and all exhibit a similar inverted-U response indicates that the amount of neuromodulatory activation, and consequently the amount of neuromodulator release, is the primary driving factor. The few studies that have evaluated the effect of VNS parameters directly on neuromodulatory release or indirectly on neuromodulator fiber activation report that higher intensities result in greater levels of activation [8,42]. This places the level of neuromodulator release evoked by VNS squarely at the center of candidates to mediate the inverted-U.
A simple model that could give rise to the inverted-U is single desensitizing system (Fig. 3A). In this scenario, receptors sensitive to neuromodulators released by VNS, such as norepinephrine-sensing G-protein-coupled adrenergic receptors, would fail to be activated at low stimulation levels that produce minimal neuromodulator release. Moderate stimulation levels would provide sufficient neuromodulator release to activate receptors and generate a response. High levels of stimulation would engender oversaturating levels of neuromodulator concentration, resulting in receptor desensitization and subsequent abrogation of a response to later stimulations. This desensitization phenomenon (or really, set of related phenomena) is common amongst the G-protein-coupled receptors that bind neuromodulators released in response to VNS [43]. Although difficult to directly probe in vivo, some experimental evidence supports this model. Interleaving high intensity stimulation (which would desensitize receptors) with moderate intensity stimulation prevents the VNS-dependent enhancement of plasticity that would normally be induced by moderate intensity stimulation [44]. This active inhibition of plasticity by high intensity stimulation is consistent with notion that receptor desensitization could undergird the inverted-U.
Figure 3. Potential models underlying the inverted-U effect of VNS.
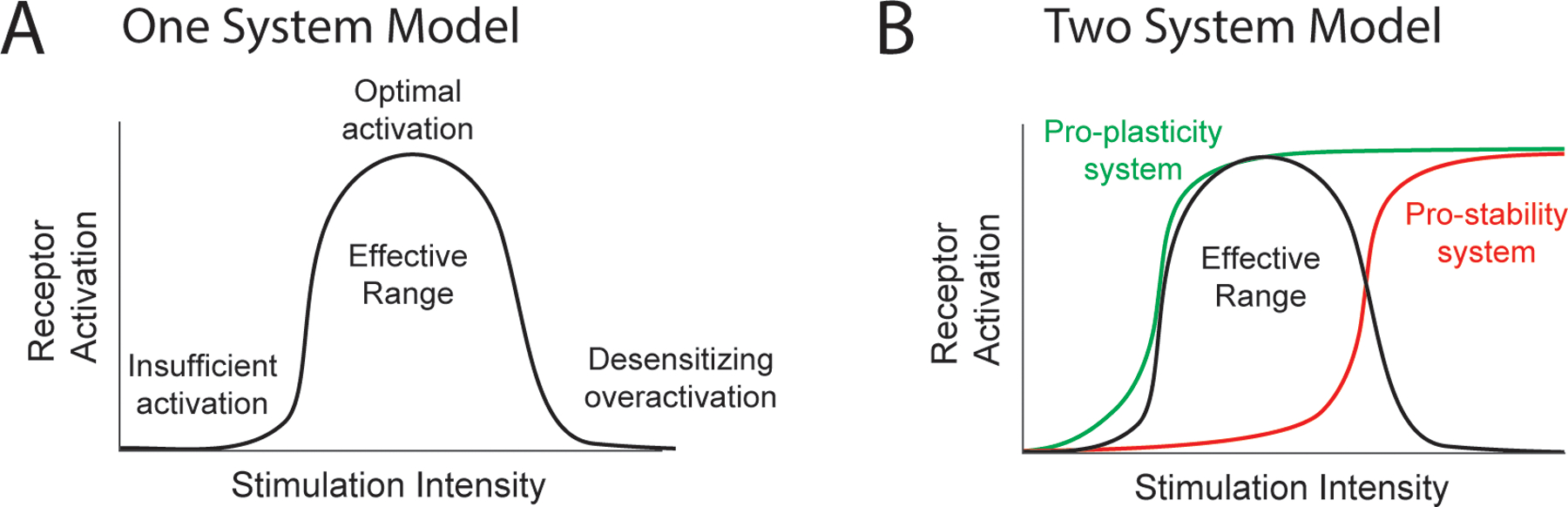
(A) In the one system model, low intensities fail to sufficiently activate the system, moderate intensities produce optimal activation and consequent efficacy, and high intensities overactive and desensitize the system, thus limiting effects. (B) In the two system model, moderate intensities engage the low-threshold system and generate efficacy. Higher intensities activate the overriding high-threshold system and limit efficacy. Together, these systems produce an inverted-U response. As shown in this example, a combination of α- and β-adrenergic receptors could exhibit this behavior.
A second model comprised of a low-threshold pro-plasticity system and an overriding high-threshold pro-stability system could also account for the inverted-U phenomenon (Fig. 3B). In this case, moderate stimulation levels would provide sufficient activation of the low-threshold system to promote plasticity. At higher stimulation levels, activation of the higher-threshold overriding system would promote stability and thus prevent plasticity. Although this explanation is less parsimonious than a single system, the noradrenergic system recognized to be central to the effects of VNS has several features that could give rise to this configuration. First, different classes of adrenergic receptors are activated at different norepinephrine levels [45,46]. Cortical α2-adrenergic receptors, which have a high affinity for norepinephrine and thus would be activated at moderate levels of stimulation, are necessary for VNS-dependent plasticity [47]. Alternatively, β-adrenergic receptors have a lower affinity for norepinephrine and thus would solely be activated at higher levels of stimulation. Second, α- and β-adrenergic receptors can cause functionally opposing effects on synaptic plasticity, where the concentration of norepinephrine dictates the synaptic potentiation or depression [48].
Irrespective of the underlying mechanism, the inverted-U relationship poses real challenges for the application of VNS therapy. The effective stimulation parameters are defined by a relatively narrow range, and at present, there are no simple, universally-accepted biomarkers that report the necessary level of nerve activation for paired VNS. The conventional approach for selecting parameters for a bioelectronic intervention, such as VNS for epilepsy or spinal cord stimulation for chronic pain, is to start at a relatively low level and ramp stimulation until efficacy is achieved and side effects are minimized. Unfortunately, this approach is not feasible for paired VNS, as continued escalation risks exceeding the peak of the inverted-U and blocking the effects of stimulation. The current clinical application of paired VNS for stroke uses the parameters that have been demonstrated most effective across a range of studies [26,38]. Validation of a simple, accessible, rapid biomarker of the desired effect target of VNS, for instance activation of the LC, has the potential for significant importance. The ability to individualize dosing and selected stimulation at the peak of the inverted-U may be a strategy to increase the number of responders to VNS therapy and maximize the magnitude of benefits.
Deliver VNS at the wrong time
The efficacy of VNS is predicated not simply on stimulation, but rather on stimulation concurrent with training. As a result, manipulations that interfere with the temporal association between these elements would be expected to reduce the effect of VNS therapy. Indeed, this has been observed across a wide range of time scales. In a number of preclinical studies, a matched amount of VNS delivered hours after training sessions fails to enhance plasticity or promote recovery [19,24,49,50]. Variance on the order of seconds may even abrogate the effects of paired VNS [11,21,49,51].
The need for close temporal association between stimulation and training is described by the synaptic eligibility trace, a prevailing concept in the action of neuromodulators on synaptic plasticity [52–54]. In this model, glutamatergic plasticity following Hebbian learning rules over the millisecond timescale is instantiated by the arrival of neuromodulator reinforcement within seconds [54]. In the simplest sense, this is a synaptic expression of classical conditioning, in which the conditioned stimulus is reinforced by the timely arrival of an unconditioned stimulus. In the context of VNS therapy, training provides the glutamatergic activation, such as motor command activity in corticospinal networks, and VNS provides the neuromodulatory reinforcement. As in classical conditioning, failure to provide a consistent reinforcing unconditioned stimulus after the conditioned stimulus does not engender learning. Similarly, failing to deliver VNS following the activation of the target circuits by training does not enhance plasticity and consequently fails to improve recovery.
The notion that the timing of stimulation with training is central to VNS efficacy has clear clinical implications. In the context of stroke therapy, the conventional implementation of VNS is comprised of a therapist pressing a button to trigger stimulation coincident with an individual’s arm movement during rehabilitative exercises [1,26]. This approach is in line with the importance of temporal pairing and has been demonstrably successful in enhancing recovery of upper limb function in individuals with stroke. Interestingly, these studies also led to a natural experiment that may provide some insight into the impact of timing. A subset of individuals that underwent VNS therapy received an additional year-long course of VNS at home [55]. Unlike the in-office VNS therapy which relied on a therapist to trigger stimulation during movement, this at-home study did not explicitly deliver stimulation coincident with movement. Over the course of years of therapy, VNS generally maintained prior gains over the very long term and produced continuing improvements, both critical elements for stroke patients, but the rate of these gains was lower than that driven by therapist-triggered VNS [55,56]. We note that many elements differed between the in-office and at-home phases of the study which could explain differences in rate of recovery, including an effect of the order of treatment, elapsing time since stroke, and the absence of a therapist guiding exercises. Despite these caveats, these findings provide a basis for continued evaluation and optimization of VNS timing to enhance recovery. Even with the appropriate stimulation parameters and an effective training regimen, a mis-timed combination of these components may reduce efficacy.
Deliver VNS with the wrong concurrent training
Since the concurrent training is a critical element of VNS therapy, it stands to reason that selection of the wrong or a suboptimal therapy would produce limited effects. From an experimental standpoint, it is generally sensible to select the best training for pairing with VNS; thus, few studies directly examine the impact of variably effective training regimens. However, a few studies illuminate the importance of this consideration. In a preclinical model of chronic sensory loss, pairing VNS with a simplified tactile training regimen produced partial somatosensory recovery, whereas pairing with a rich, complex sensory retraining regimen generated full restoration of somatosensory function [57]. Moreover, it is important that the concurrent training be directed at the functions that are the intended target of the intervention. Pairing VNS with motor rehabilitation produces a nearly complete restoration of motor function and a partial restoration of somatosensation [24]. Correspondingly, pairing VNS with somatosensory retraining fully restores somatosensory function and partly restores motor function [25].
These findings illustrate two fundamental points, one scientific and one practical: The notion that VNS effect is largely restricted to the function targeted by rehabilitation is in alignment with the neural mechanisms engaged by VNS. Reinforcement learning progressively reshapes behavior toward the trained paradigm without substantively impacting behaviors that are irrelevant to the training. Consequently, VNS enhances this training-specific effect and does not influence other behaviors. Practically speaking, this informs the types of rehabilitation that should be paired with VNS to maximize efficacy. In the context of stroke, the most effective rehabilitative paradigm would presumably incorporate a rich set of exercises in the domains that represent the rehabilitative goals of the individual. In other words, it may be most useful to pair VNS with motor exercises, tactile retraining exercises, and even gait or speech therapy [58]. On the other hand, suboptimal training regimens that do not address the underlying impairment would fail to be efficacious. Consequently, clever construction and individualized selection of the concurrent training represents a means to optimize VNS therapy.
Deliver VNS therapy in the wrong context
Because VNS therapy requires engagement of neuromodulator systems, pharmacological perturbation of these systems can block the effects of VNS. A number of preclinical studies demonstrate the necessity of intact neuromodulatory signaling, encompassing norepinephrine, acetylcholine, and serotonin, in the brain for the effects of VNS on plasticity and recovery [9–11,24,47]. A clinical study provides some preliminary corroborating evidence. In a pilot study exploring the use of VNS paired with tones for the treatment of tinnitus, individuals taking flupentixol/melitracen, a combination drug that exerts an impact on both cholinergic and noradrenergic transmission (as well as many other systems), failed to demonstrate VNS-dependent improvements in measures of tinnitus severity [27].
Other conditions for which paired VNS therapy is being developed are associated with comorbidities that are managed by drugs that influence neuromodulatory transmission. For example, VNS therapy is in clinical evaluation to improve upper limb recovery in individuals with incomplete cervical spinal cord injury (SCI), a condition which is commonly associated with urinary retention. To treat this symptom, individuals with SCI are often prescribed oxybutynin, a cholinergic antagonist [59]. As acetylcholine signaling in the central nervous system is required for the effects of VNS on plasticity and recovery, treatment with oxybutynin could potentially block the actions of VNS paired with rehabilitation to target improvements in arm and hand function. Similarly, prazosin, an adrenergic antagonist, is commonly used for reducing nightmares related to PTSD and could limit VNS-dependent enhancement of fear extinction. Fortunately, not all pharmacological approaches that influence neuromodulators are doomed to prevent the actions of VNS. Preclinical studies provide evidence that clinically-relevant levels of oxybutynin and prazosin, typically orders of magnitude less than those utilized in preclinical studies to entirely block transmission, do not inhibit VNS-dependent plasticity [60]. Together, these findings highlight the need to consider the class and dose of drugs in the translation of paired VNS therapies.
Beyond pharmacology, other clinical conditions that themselves diminish neuromodulatory transmission may limit VNS effects. VNS combined with behavioral interventions has been proposed for the treatment of neurodevelopmental disorders [4]. Initial evidence from a transgenic rat model of Rett syndrome demonstrates the potential for directing synaptic plasticity with VNS [61]. However, Rett syndrome is associated with pathophysiological changes in LC neuron activity, which may restrict VNS effectiveness [62,63]. Similarly, the core pathologies of Alzheimer’s disease and Parkinson’s disease relate to loss of cholinergic and noradrenergic neurons, which may limit utility of VNS in these conditions [64,65]. Indirect action on neuromodulatory transmission may also exert an impact. Indeed, a recent study shows that chronic pain, which is associated with alterations in noradrenergic signaling and LC activity, blocks VNS-dependent recovery of motor and sensory function [66].
The clinical impact of these studies is clear: consider whether co-occurring conditions or accompanying pharmacological treatments may block neuromodulatory networks engaged by VNS. However, beyond simply identifying particular characteristics that may limit VNS efficacy, it may be possible to build on this understanding and rationally alter VNS delivery to facilitate recovery. One yet untested but straightforward approach is to select stimulation parameters to offset baseline changes in neuromodulatory transmission. For example, conditions that cause some loss of neurons in the LC or that partially block adrenergic receptors may necessitate greater stimulation intensities to induce sufficient adrenergic transmission.
How to make the most of VNS therapy
Rather than looking grim, cataloging the many instances in which paired VNS fails to work provides insight into why it does work and may help guide development directed toward maximizing effectiveness. Enhancing efficacy is both timely and clinically important. The recent FDA approval of VNS therapy for chronic stroke portends an expansion in the clinical use of this intervention in the near term. While the therapy demonstrably provides benefits in a large share of individuals, it leaves ample room for improvement [67,68].
The two components that comprise VNS therapy - stimulation and concurrent training - both represent opportunities for optimization. The current clinical applications of paired VNS largely employ 0.5 second trains of 0.8 mA pulses delivered at 30 Hz, in line with much preclinical data described above. While this approach has been effective on balance, it is conceivable that individuals may benefit from personalized stimulation parameters. Given the inverted-U relationship, scaling stimulation intensity up or down to match the optimal level of activation has the potential to increase effectiveness within individuals and to convert to responders individuals that may otherwise not have benefitted at conventional stimulation parameters. While conceptually simple, this idea is limited by the absence of a simple, clear biomarker of VNS efficacy. A number of measures have been examined, but to date, none alone or in combination provide an unambiguous report of the inverted-U. Continued effort towards this goal has potential to substantively impact the utility of VNS therapy.
Concurrent training, the second ingredient of VNS therapy, is also ripe for optimization. Although the nature of the training varies widely depending on the intended target of VNS therapy, we will predominantly focus our discussion on the implementation of VNS paired with upper limb rehabilitation. The nature of the activity that is paired with VNS strongly influences the magnitude of recovery [57]. Consequently, selection of the types of exercises performed during rehabilitation is likely a key consideration. Historically, the rehabilitative exercises delivered with VNS for stroke were largely standardized across individuals, a necessary approach to ensure practical conduct of the clinical trials. However, a more individualized approach that is focused on the specific deficits of each patient is common practice for post-stroke rehabilitation and may result in larger improvements. Other strategies that allow more intensive or longer courses of training, such as VNS during telerehabilitation or self-directed game-based rehabilitative exercises, also hold promise to yield greater recovery [69].
VNS therapy represents a means to direct synaptic plasticity and thereby potentially treat a wide range of neurological conditions. The recent success of VNS therapy for stroke highlights the clinical utility of this approach and paves the way for future applications. However, effectiveness relies on a number of factors that demand attention. The stimulation, the accompanying training, and the temporal combination of these elements merit careful consideration in the course of conceptualizing, developing, and translating novel VNS therapies. The future prospects of VNS therapy are contingent upon good design choices during development that maximize effectiveness, and we should learn as much as possible from all the conditions in which it fails.
Acknowledgements
This work was supported by in part by National Institutes of Health UH3 NS109497 (SAH) and R01 NS103803 (MPK and RLR).
Footnotes
Declaration of Interest
MPK has a financial interest in MicroTransponder, Inc., which is developing VNS for stroke. RLR is founder and CEO of Xnerve, Inc., which is developing neurostimulation technologies. SAH has no conflicts of interest to disclose.
References
- [1].Engineer ND, Kimberley TJ, Prudente CN, Dawson J, Tarver WB, Hays SA. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci 2019;13. 10.3389/fnins.2019.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Noble LJ, Souza RR, McIntyre CK. Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies. Psychopharmacology (Berl) 2019;236:355–67. 10.1007/s00213-018-4994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hays SA, Rennaker RL, Kilgard MP. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res 2013;207:275–99. 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord 2017;9:1–8. 10.1186/s11689-017-9203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2016;298:21. 10.1016/j.expneurol.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Collins L, Boddington L, Steffan PJ, McCormick D. Vagus nerve stimulation induces widespread cortical and behavioral activation. Curr Biol 2021;31:2088–2098.e3. 10.1016/j.cub.2021.02.049. [DOI] [PubMed] [Google Scholar]
- [7].Nichols JA, Nichols AR, Smirnakis SM, Engineer ND, Kilgard MP, Atzori M. Vagus nerve stimulation modulates cortical synchrony and excitability through the activation of muscarinic receptors. Neuroscience 2011;189:207–14. [DOI] [PubMed] [Google Scholar]
- [8].Mridha Z, de Gee JW, Shi Y, Alkashgari R, Williams J, Suminski A, et al. Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat Commun 2021 121 2021;12:1–14. 10.1038/s41467-021-21730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hulsey DR, Hays SA, Khodaparast N, Ruiz A, Das P, Rennaker RL, et al. Reorganization of Motor Cortex by Vagus Nerve Stimulation Requires Cholinergic Innervation. Brain Stimul 2016;9:174–81. 10.1016/j.brs.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hulsey DR, Shedd CM, Sarker SF, Kilgard MP, Hays SA. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol 2019;320:112975. 10.1016/j.expneurol.2019.112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bowles S, Hickman J, Peng X, Williamson WR, Huang R, Washington K, et al. Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 2022;110:2867–2885.e7. 10.1016/J.NEURON.2022.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex 2011;22:2365–74. 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- [13].Morrison RA, Hulsey DR, Adcock KS, Rennaker RL, Kilgard MP, Hays SA. Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul 2019;12:256–62. 10.1016/j.brs.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature 2011;470:101–4. 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain Stimul 2015;8(3):637–44. 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Khodaparast N, Hays SA, Sloan AM, Hulsey DR, Ruiz A, Pantoja M, et al. Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol Dis 2013;60. 10.1016/j.nbd.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [17].Hays SA, Khodaparast N, Hulsey DR, Ruiz A, Sloan AM, Ii RLR, et al. Training Improves Functional Recovery After Intracerebral Hemorrhage. Stroke 2014;45:97–100. 10.1161/STROKEAHA.114.006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hays SA, Ruiz A, Bethea T, Khodaparast N, Carmel JB, Rennaker RL, et al. Vagus nerve stimulation during rehabilitative training enhances recovery of forelimb function after ischemic stroke in aged rats. Neurobiol Aging 2016;43:111–8. 10.1016/j.neurobiolaging.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Khodaparast N, Kilgard MP, Casavant R, Ruiz A, Qureshi I, Ganzer PD, et al. Vagus Nerve Stimulation During Rehabilitative Training Improves Forelimb Recovery After Chronic Ischemic Stroke in Rats. Neurorehabil Neural Repair 2016;30:676–84. 10.1177/1545968315616494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Meyers EC, Solorzano BR, James J, Ganzer PD, Lai ES, Rennaker RL, et al. Vagus Nerve Stimulation Enhances Stable Plasticity and Generalization of Stroke Recovery. Stroke 2018;49. 10.1161/STROKEAHA.117.019202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ganzer PD, Darrow MJ, Meyers EC, Solorzano BR, Ruiz AD, Robertson NM, et al. Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife 2018;7:1–19. 10.7554/eLife.32058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Darrow MJ, Torres M, Sosa MJ, Danaphongse TT, Haider Z, Rennaker RL, et al. Vagus Nerve Stimulation Paired With Rehabilitative Training Enhances Motor Recovery After Bilateral Spinal Cord Injury to Cervical Forelimb Motor Pools. Neurorehabil Neural Repair 2020;34:200–9. 10.1177/1545968319895480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pruitt DT, Schmid AN, Kim LJ, Abe CM, Trieu JL, Choua C, et al. Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma 2016;33:871–9. 10.1089/neu.2015.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Meyers EC, Kasliwal N, Solorzano BR, Lai E, Bendale G, Berry A, et al. Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun 2019;10:1–14. 10.1038/s41467-019-13695-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Darrow MJ, Mian TM, Torres M, Haider Z, Danaphongse T, Rennaker RL, et al. Restoration of Somatosensory Function by Pairing Vagus Nerve Stimulation with Tactile Rehabilitation. Ann Neurol 2020;87:194–205. 10.1002/ana.25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dawson J, Liu CY, Francisco GE, Cramer SC, Wolf SL, Dixit A, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet 2021;397:1545–53. 10.1016/S0140-6736(21)00475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ridder D De, Vanneste S, Engineer ND, Kilgard MPMP. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: a case series. Neuromodulation Technol Neural Interface 2013;17(2):170–9. [DOI] [PubMed] [Google Scholar]
- [28].Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, et al. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci Rep 2017;7:11960. 10.1038/s41598-017-12178-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].NCT04064762. Targeted Plasticity Therapy for Posttraumatic Stress Disorder. Natl Clin Trials Regist n.d https://clinicaltrials.gov/ct2/show/NCT04064762. [Google Scholar]
- [30].NCT04288245. Targeted Plasticity Therapy for Upper Limb Rehabilitation in Spinal Cord Injuries. ClinicalTrialsGov n.d https://www.clinicaltrials.gov/ct2/show/NCT04288245. [Google Scholar]
- [31].Borland MS, Vrana WA, Moreno NA, Fogarty EA, Buell EP, Sharma P, et al. Cortical Map Plasticity as a Function of Vagus Nerve Stimulation Intensity. Brain Stimul 2016;9:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morrison RA, Danaphongse TT, Pruitt DT, Adcock KS, Mathew JK, Abe ST, et al. A limited range of vagus nerve stimulation intensities produce motor cortex reorganization when delivered during training. Behav Brain Res 2020;391:112705. 10.1016/J.BBR.2020.112705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Buell EP, Loerwald KW, Engineer CT, Borland MS, Buell JM, Kelly CA, et al. Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul 2018;11:1218–24. 10.1016/j.brs.2018.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Buell EP, Borland MS, Loerwald KW, Chandler C, Hays SA, Engineer CT, et al. Vagus Nerve Stimulation Rate and Duration Determine whether Sensory Pairing Produces Neural Plasticity. Neuroscience 2019;406:290–9. 10.1016/j.neuroscience.2019.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bartolomei F, Bonini F, Vidal E, Trébuchon A, Lagarde S, Lambert I, et al. How does vagal nerve stimulation (VNS) change EEG brain functional connectivity? Epilepsy Res 2016;126:141–6. 10.1016/J.EPLEPSYRES.2016.06.008. [DOI] [PubMed] [Google Scholar]
- [36].Verner R, Szaflarski JP, Allendorfer JB, Vonck K, Giannicola G. Modulation of the thalamus by microburst vagus nerve stimulation: a feasibility study protocol. Front Neurol 2023;14:1169161. 10.3389/FNEUR.2023.1169161/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vespa S, Stumpp L, Liberati G, Delbeke J, Nonclercq A, Mouraux A, et al. Characterization of vagus nerve stimulation-induced pupillary responses in epileptic patients. Brain Stimul 2022;15:1498–507. 10.1016/J.BRS.2022.11.002. [DOI] [PubMed] [Google Scholar]
- [38].Pruitt DT, Danaphongse TT, Lutchman M, Patel N, Reddy P, Wang V, et al. Optimizing Dosing of Vagus Nerve Stimulation for Stroke Recovery. Transl Stroke Res 2021;12:65–71. 10.1007/s12975-020-00829-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Souza RR, Robertson NM, McIntyre CK, Rennaker RL, Hays SA, Kilgard MP. Vagus nerve stimulation enhances fear extinction as an inverted-U function of stimulation intensity. Exp Neurol 2021;341:113718. 10.1016/j.expneurol.2021.113718. [DOI] [PubMed] [Google Scholar]
- [40].Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem 1995;63:213–6. [DOI] [PubMed] [Google Scholar]
- [41].Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci 1999;2:94–8. [DOI] [PubMed] [Google Scholar]
- [42].Roosevelt RW, Smith DC, Clough RW, Jensen RA, Browning RA. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res 2006;1119:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rajagopal S, Shenoy SK. GPCR desensitization: Acute and prolonged phases. Cell Signal 2018;41:9–16. 10.1016/J.CELLSIG.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Morrison RA, Danaphongse TT, Abe ST, Stevens ME, Ezhil V, Seyedahmadi A, et al. High intensity VNS disrupts VNS-mediated plasticity in motor cortex. Brain Res 2021;1756. 10.1016/j.brainres.2021.147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, et al. Locus ceruleus norepinephrine release: A central regulator of cns spatio-temporal activation? Front Synaptic Neurosci 2016;8:25. 10.3389/fnsyn.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Liggett SB, Raymond JR. Pharmacology and molecular biology of adrenergic receptors. Baillieres Clin Endocrinol Metab 1993;7:279–306. 10.1016/S0950-351X(05)80178-8. [DOI] [PubMed] [Google Scholar]
- [47].Tseng CT, Gaulding SJ, Dancel CLE, Thorn CA. Local activation of α2 adrenergic receptors is required for vagus nerve stimulation induced motor cortical plasticity. Sci Reports 2021 111 2021;11:1–13. 10.1038/s41598-021-00976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Salgado H, Köhr G, Treviño M. Noradrenergic ‘Tone’Determines Dichotomous Control of Cortical Spike-Timing-Dependent Plasticity. Sci Rep 2012;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hays SA, Khodaparast N, Ruiz A, Sloan AM, Hulsey DR, Rennaker RL, et al. The timing and amount of vagus nerve stimulation during rehabilitative training affect post-stroke recovery of forelimb strength. Neuroreport 2014;25(9):676–82. 10.1097/WNR.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peña DF, Engineer ND, McIntyre CK. Rapid Remission of Conditioned Fear Expression with Extinction Training Paired with Vagus Nerve Stimulation. Biol Psychiatry 2012;73:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Souza RR, Powers MB, Rennaker RL, McIntyre CK, Hays SA, Kilgard MP. Timing of vagus nerve stimulation during fear extinction determines efficacy in a rat model of PTSD. Sci Reports 2022 121 2022;12:1–11. 10.1038/s41598-022-20301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Izhikevich EM. Solving the Distal Reward Problem through Linkage of STDP and Dopamine Signaling. Cereb Cortex 2007;17:2443–52. 10.1093/CERCOR/BHL152. [DOI] [PubMed] [Google Scholar]
- [53].Yagishita S, Hayashi-Takagi A, Ellis-Davies GCR, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science (80-) 2014;345:1616–20. 10.1126/SCIENCE.1255514/SUPPL_FILE/YAGASHITA-SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, et al. Distinct Eligibility Traces for LTP and LTD in Cortical Synapses. Neuron 2015;88:528–38. https://doi.org/ 10.1016/j.neuron.2015.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Dawson J, Engineer ND, Prudente CN, Pierce D, Francisco G, Yozbatiran N, et al. Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Stroke: One-Year Follow-up. Neurorehabil Neural Repair 2020;34:609–15. 10.1177/1545968320924361. [DOI] [PubMed] [Google Scholar]
- [56].Francisco GE, Engineer ND, Dawson J, Kimberley TJ, Cramer SC, Prudente CN, et al. Vagus Nerve Stimulation Paired With Upper-Limb Rehabilitation After Stroke: 2- and 3-Year Follow-up From the Pilot Study. Arch Phys Med Rehabil 2023. 10.1016/J.APMR.2023.02.012. [DOI] [PubMed] [Google Scholar]
- [57].Darrow MJ, Mian TM, Torres M, Haider Z, Danaphongse T, Seyedahmadi A, et al. The tactile experience paired with vagus nerve stimulation determines the degree of sensory recovery after chronic nerve damage. Behav Brain Res 2021;396. 10.1016/j.bbr.2020.112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Morrison RA, Hays SA, Kilgard MP. Vagus Nerve Stimulation as a Potential Adjuvant to Rehabilitation for Post-stroke Motor Speech Disorders. Front Neurosci 2021;15:1059. 10.3389/FNINS.2021.715928/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Taweel W Al, Seyam R. Neurogenic bladder in spinal cord injury patients. Res Reports Urol 2015;7:85–99. 10.2147/RRU.S29644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Morrison RA, Abe ST, Danaphongse T, Ezhil V, Somaney A, Adcock KS, et al. Common Cholinergic, Noradrenergic, and Serotonergic Drugs Do Not Block VNS-Mediated Plasticity. Front Neurosci 2022;0:211. 10.3389/FNINS.2022.849291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Adcock KS, Chandler C, Buell EP, Solorzano BR, Loerwald KW, Borland MS, et al. Vagus nerve stimulation paired with tones restores auditory processing in a rat model of Rett syndrome. Brain Stimul 2020;13:1494–503. 10.1016/j.brs.2020.08.006. [DOI] [PubMed] [Google Scholar]
- [62].Wu Y, Zhong W, Cui N, Johnson CM, Xing H, Zhang S, et al. Characterization of Rett Syndrome-like phenotypes in Mecp2-knockout rats. J Neurodev Disord 2016;8. 10.1186/S11689-016-9156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Taneja P, Ogier M, Brooks-Harris G, Schmid DA, Katz DM, Nelson SB. Pathophysiology of Locus Ceruleus Neurons in a Mouse Model of Rett Syndrome. J Neurosci 2009;29:12187–95. 10.1523/JNEUROSCI.3156-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Http://DxDoiOrg/101586/147371758111703 2014;8:1703–18. 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Paredes-Rodriguez E, Vegas-Suarez S, Morera-Herreras T, De Deurwaerdere P, Miguelez C. The Noradrenergic System in Parkinson’s Disease. Front Pharmacol 2020;11:435. 10.3389/FPHAR.2020.00435/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Adcock KS, Hulsey DR, Danaphongse T, Haider Z, Morrison RA, Kilgard MP, et al. Radial nerve injury causes long-lasting forelimb sensory impairment and motor dysfunction in rats. PAIN Reports 2021;6:e957. 10.1097/pr9.0000000000000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kwakkel G, Dobkin BH. Vagus Nerve Stimulation for Upper Limb Function: Significant Difference, but Clinically Important? Stroke 2021:3407–9. 10.1161/STROKEAHA.121.035648. [DOI] [PubMed] [Google Scholar]
- [68].Putrino D, Krakauer JW. Neurotechnology’s Prospects for Bringing About Meaningful Reductions in Neurological Impairment. Https://DoiOrg/101177/15459683221137341 2022:154596832211373. 10.1177/15459683221137341. [DOI] [PubMed] [Google Scholar]
- [69].Pruitt DT, Duong-Nguyen Y-N, Meyers EC, Epperson JD, Wright JM, Hudson RA, et al. Usage of RePlay as a Take-Home System to Support High-Repetition Motor Rehabilitation After Neurological Injury. Https://HomeLiebertpubCom/G4h 2023;12:73–85. 10.1089/G4H.2022.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]


