Abstract
Background
Associations between childhood trauma, neurodevelopment, alcohol use disorder (AUD), and posttraumatic stress disorder (PTSD) are understudied during adolescence.
Methods
Using 1652 participants (51.75% female, baseline Mage = 14.3) from the Collaborative Study of the Genetics of Alcoholism, we employed latent growth curve models to (1) examine associations of childhood physical, sexual, and non-assaultive trauma (CPAT, CSAT, and CNAT) with repeated measures of alpha band EEG coherence (EEGc), and (2) assess whether EEGc trajectories were associated with AUD and PTSD symptoms. Sex-specific models accommodated sex differences in trauma exposure, AUD prevalence, and neural development.
Results
In females, CSAT was associated with higher mean levels of EEGc in left frontocentral (LFC, ß = 0.13, p = 0.01) and interhemispheric prefrontal (PFI, ß = 0.16, p < 0.01) regions, but diminished growth in LFC (ß = −0.07, p = 0.02) and PFI (ß = −0.07, p = 0.02). In males, CPAT was associated with lower mean levels (ß = −0.17, p = 0.01) and increased growth (ß = 0.11, p = 0.01) of LFC EEGc. Slope of LFC EEGc was inversely associated with AUD symptoms in females (ß = −1.81, p = 0.01). Intercept of right frontocentral and PFI EEGc were associated with AUD symptoms in males, but in opposite directions. Significant associations between EEGc and PTSD symptoms were also observed in trauma-exposed individuals.
Conclusions
Childhood assaultive trauma is associated with changes in frontal alpha EEGc and subsequent AUD and PTSD symptoms, though patterns differ by sex and trauma type. EEGc findings may inform emerging treatments for PTSD and AUD.
Keywords: alcohol use disorder, childhood trauma, EEG coherence, neurodevelopment, posttraumatic stress disorder
Introduction
Childhood trauma is common, with an estimated ~20–66% of individuals in the United States experiencing at least one traumatic event before adulthood (Blaustein, 2013; Finkelhor, Turner, Ormrod, & Hamby, 2009; Read, Ouimette, White, Colder, & Farrow, 2011). Childhood trauma encompasses interpersonal victimization (e.g. physical, sexual abuse/violence) as well as non-interpersonal events (e.g. accidents, illness, loss; Briggs-Gowan, Carter, & Ford, 2012; Mongillo, Briggs-Gowan, Ford, & Carter, 2009). Exposure to childhood trauma is thought to disrupt ‘normative’ stages of childhood development, including cognitive, emotional, and social skills development, and predisposes children to psychiatric sequelae (D'Andrea, Ford, Stolbach, Spinazzola, & van der Kolk, 2012; Mongillo et al., 2009; Teicher & Samson, 2013), including posttraumatic stress disorder (PTSD) (Duncan, Saunders, Kilpatrick, Hanson, & Resnick, 1996; Khoury, Tang, Bradley, Cubells, & Ressler, 2010) and alcohol use disorder (AUD) (Schückher, Sellin, Fahlke, & Engström, 2018). Experiencing childhood trauma yields PTSD and AUD hazard ratios of ~1.4–3.5 (Sartor et al., 2011, 2012) thus, it is important to improve our understanding of the mechanisms by which exposure to trauma may impact development and psychiatric outcomes.
Epidemiologic studies report high co-occurrence between PTSD and both problematic alcohol use and AUD (Brown, Stout, & Mueller, 1999; Debell et al., 2014; Kessler, Chiu, Demler, Walters, & Walters, 2005; Mills, Teesson, Ross, & Peters, 2006; Pietrzak, Goldstein, Southwick, & Grant, 2011). One model for understanding this comorbidity is the shared liability model, which suggests that shared, common factors (e.g. trauma, genetics, physiological, and psychological traits) contribute to increased risk for alcohol use behaviors, AUD, and PTSD (Danovitch, 2016). Indeed, twin and molecular studies suggest correlated genetic risk (Bountress et al., 2022; Sartor et al., 2011; Sheerin et al., 2020; Xian et al., 2000). Growing research has begun to explore the role of shared neurobiological mechanisms (Brady & Sinha, 2005; Gilpin & Weiner, 2017). Neurophysiological and neuropsychological measurement can further our understanding of shared risk beyond self-report data (Davis et al., 2013). Some work has focused on the role of early stress in producing a cascade of neurobiological changes, such as reduced functional activity and/or structural alterations in key brain areas, that may act to increase risk for psychiatric disorders (Teicher et al., 2003). More research is needed to uncover possible mechanisms by which early trauma may influence neuropsychological development.
Electroencephalogram (EEG) has long been used to examine individual differences in brain function and neuropsychiatric health (Smit et al., 2021). EEG coherence (EEGc) is a heritable measure of neural functional connectivity, which measures the degree of synchrony in brain oscillatory activity between two regions (Chorlian, Rangaswamy, & Porjesz, 2009; Chorlian et al., 2007; Markovska-Simoska, Pop-Jordanova, & Pop-Jordanov, 2018). The human central nervous system has a prolonged developmental course, with critical periods in early life and adolescence, especially in prefrontal regions (Larsen & Luna, 2018; Silbereis, Pochareddy, Zhu, Li, & Sestan, 2016). Childhood stress can harm neural development during these sensitive periods (Andersen et al., 2008). Increased neural functional connectivity, measured via EEG, has been observed in cross-sectional studies of childhood trauma (Cook, Ciorciari, Varker, & Devilly, 2009), and adults with AUD and PTSD (Almli et al., 2018; Dunkley et al., 2015; Huang, Mohan, De Ridder, Sunaert, & Vanneste, 2018; Park et al., 2017), suggesting that increased functional connectivity may be a shared pathway of risk for AUD and PTSD. Associations between EEGc and neuropsychiatric conditions are complex, but better characterized in schizophrenia (Maran, Grent-; -Jong, & Uhlhaas, 2016), where greater connectivity in lower frequency bands (delta and theta) corresponds to lower cognitive performance and abnormal cortical organization (Di Lorenzo et al., 2015; Lehmann et al., 2014). However, increased coherence is not always adverse – frontal alpha connectivity, involved in information processing and attention (Foxe & Snyder, 2011), is lower in schizophrenia compared to controls (e.g. Di Lorenzo et al., 2015; Lehmann et al. 2014; Tauscher, Fischer, Neumeister, Rappelsberger, & Kasper, 1998). Prior research from the Collaborative Study on the Genetics of Alcoholism (COGA) indicates that AUD manifests as increased resting EEG interhemispheric theta and alpha coherence in fronto-central, fronto-temporal, temporo-parietal, centroparietal and parietal-occipital regions (Meyers et al., 2021; Porjesz & Rangaswamy, 2007; Rangaswamy & Porjesz, 2008). Another COGA study indicated that AD polygenic scores were associated with increased fronto-central, temporo-parietal, centroparietal, and parietal-occipital interhemispheric theta and alpha connectivity in males (Meyers et al., 2019a). This research on EEGc could be useful in investigating a possible shared pathway of risk for AUD and PTSD.
Research on the relationship between trauma and neural functional connectivity has focused almost exclusively on adults, ignoring periods of rapid EEGc development in adolescence and young adulthood (Cook et al., 2009; Thatcher, North, & Biver, 2008). Normative developmental trajectories of EEGc in adolescence have not been comprehensively characterized to our knowledge (Segalowitz, Santesso, & Jetha, 2010) as there are only a few studies with smaller samples; however, emerging literature from COGA suggests alpha coherence tends to increase throughout adolescence, flatten around mid-twenties, and then slowly decline (Chorlian et al., 2024). Disrupted alpha rhythm, power, and frontal asymmetry have also been detected in individuals with a range of psychiatric conditions (Eidelman-Rothman, Levy, & Feldman, 2016; Ippolito et al., 2022; Périard et al., 2024), including PTSD in adults (Badura-Brack et al., 2015; Huang et al., 2014; Kemp et al., 2010; Meyer et al., 2015; Popescu et al., 2019). Alpha frequency EEGc is of particular interest as alpha activity has been implicated in studies of impaired memory and cognitive decline in older adults (Babiloni et al., 2018; Blinowska et al., 2017; Hogan, Swanwick, Kaiser, Rowan, & Lawlor, 2003; Zhang et al., 2021), and is hypothesized to be related to acetylcholine levels in the brain (Babiloni et al., 2013; Sharma & Nadkarni, 2020). Childhood trauma is associated with increased right alpha EEG asymmetry (Meiers, Nooner, De Bellis, Debnath, & Tang, 2020), which has also been linked to low mood and social withdrawal in adolescence (Stewart, Towers, Coan, & Allen, 2011). Yet, only a single, small study of EEG functional connectivity and trauma in adolescence has been published (Cook et al., 2009). Correlations of brain connectivity measures with attention and psychopathology suggest that increased EEG functional connectivity contributes to deficits in cognitive functioning and psychopathology (Canuet et al., 2011; Imperatori et al., 2015; Kamarajan et al., 2020; Zinn, Zinn, & Jason, 2016), but without longitudinal data, cannot be definitively tested. To the best of our knowledge, EEGc specifically has not yet been examined longitudinally as a biological marker that may link trauma exposure to alcohol-related outcomes and PTSD in adolescents.
The purpose of this study is to investigate the relations among childhood trauma, trajectories of EEG functional connectivity, and risk for AUD and PTSD in adolescence and young adulthood. Using data from COGA, a multi-site study of extended families densely affected with alcohol-related problems (Agrawal et al., 2023; Meyers et al., 2023), we examined associations between childhood trauma, longitudinal EEGc trajectories, and downstream associations between EEGc and alcohol and trauma-related outcomes (i.e. PTSD symptoms) in COGA's prospective study of adolescent and young adult offspring. We hypothesized that childhood trauma would be associated with differences in frontal alpha EEGc, with the most robust effects observed for those individuals with history of childhood sexual assault. We also expected that these differences in functional connectivity would be associated with increased AUD and PTSD symptoms. We hope findings increase understanding of long-term effects of childhood trauma on neural functioning and psychiatric and behavioral outcomes, enhance knowledge of biological mechanisms by which trauma influences outcomes, and advance work towards novel opportunities for intervention targets.
Methods
Sample
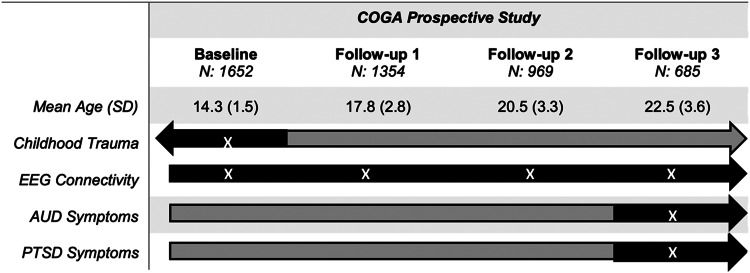
The Collaborative Study on the Genetics of Alcoholism (COGA)'s prospective study began its multi-site data collection in 2004 and ended in 2019. Details on data collection and procedures have been published previously (Dick et al., 2023). Briefly, 3715 offspring (14 495 total assessments) from families densely affected with AUD and community comparison families who had at least one parent interviewed in an earlier phase of the COGA study, were enrolled when they were between the ages of 12–22, with new participants added as they reached the age of 12. The COGA study re-assessed participants approximately every two years. A comprehensive battery was administered that included the adult Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) or the age-appropriate adolescent SSAGA (cSSAGA-A) and parent version (cSSAGA-P) if participants were under age 18. The SSAGA and cSSAGA-A covered substance use problems as well as other psychiatric disorders, including PTSD. As previously detailed (Meyers et al., 2023), a brain function battery was administered at each assessment. These included neurophysiological measures during resting state. Figure 1 shows available data and which assessments are used in the present study. Due to differences in EEG functional connectivity during adolescence compared to adulthood (Segalowitz et al., 2010), and to account for the mean age of onset of regular drinking for the sample (17.48 for males and 17.85 for females), the analytic sample was limited to adolescents age 12–16 at baseline with three or more EEG assessments (n = 1652).
Figure 1.
This flowchart illustrates the COGA assessments used in the present study. Gray bars represent data collected in COGA, and black bars and white ‘X’ represent assessment waves used in the current study. The analytic sample was limited to individuals aged 12–16 at baseline.
Assessment of primary constructs
Lifetime history of 21 potentially traumatic events (DSM-IV Criterion A) were based on cSSAGA-A baseline assessments. Although the SSAGA or cSSAGA-A was given at all assessment timepoints, participants were asked to report lifetime maximum DSM-5 AUD symptom count (AUDsx; range 0–11) and, for those who endorsed a Criterion A event, lifetime maximum DSM-IV PTSD Criterion B-D symptom count scores (PTSDsx; range 0–20) at each wave. Therefore, in this study we used lifetime symptom counts ascertained at follow-up 3 (Mage = 22) to allow sufficient opportunity for AUDsx to present in late adolescence/young adulthood. Based on evidence that interpersonal assaultive events are more ‘potent’ than non-assaultive events, that traumatic events cluster together, and to remain consistent with prior studies (Meyers et al., 2019b; Subbie-Saenz de Viteri et al., 2020), we constructed three non-mutually exclusive variables representing report of (1) one or more childhood physical assaultive traumas (CPAT; stabbed, shot, mugged, threatened with a weapon, robbed, kidnapped, held captive), (2) childhood sexual assaultive traumas (CSAT; rape or molestation), and (3) childhood non-assaultive traumas (CNAT; life-threatening accident, disaster, witnessing someone seriously injured or killed, unexpectedly finding a dead body), experienced prior to age 13, to ensure that traumatic exposure occurred prior to longitudinal EEGc measures. CPAT, CSAT, and CNAT were endorsed by 8.65%, 43.93%, and 25.24% of the sample, respectively. Based on literature that suggests socioeconomic status (SES) can impact brain development (Hackman, Farah, & Meaney, 2010), we covaried for family SES as indexed by parental report of highest education in COGA Phase 1–3.
EEG Recording and Data Processing procedures have been detailed previously (Meyers et al., 2023) and are summarized in the Supplementary materials.
Statistical analyses
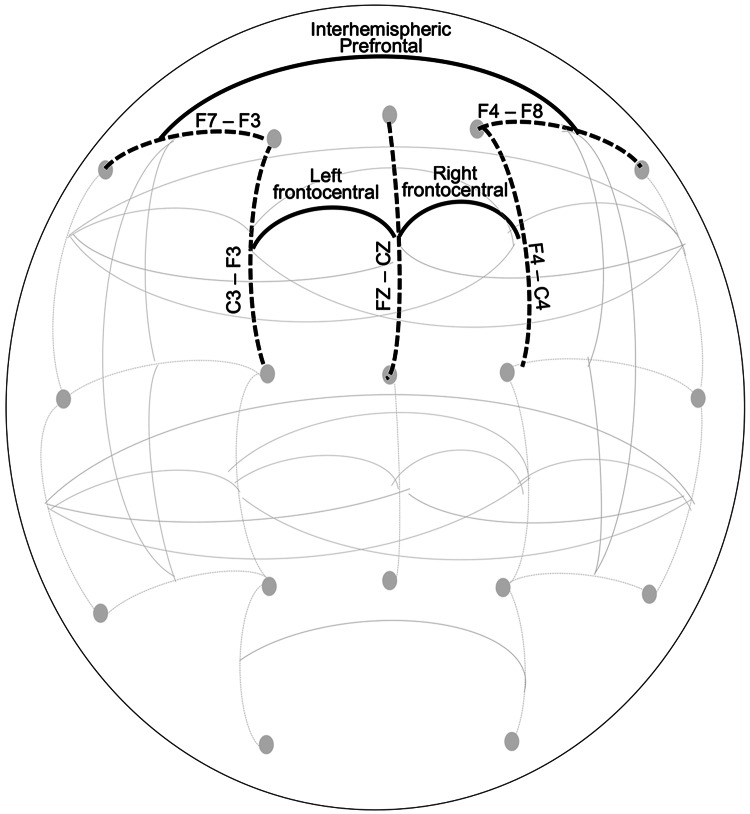
All analyses were stratified by sex and clustered by family to account for relatedness. Primary analyses examined discrete childhood trauma variables (CPAT, CSAT, CNAT) as predictors of intercept and slope of EEGc (alpha EEG inter- and intra-hemispheric coherence) during adolescence and young adulthood using latent growth curve models (LGCM) for those with three or more EEG assessments. We ran the LGCM models in Mplus version 8.9 (Muthén & Muthén, 1998-2017), which employs full information maximum likelihood to account for missing data. A non-response analysis indicated that individuals who did not return for follow-up were younger (OR: 2.1, p < 0.001) and less likely to report a prior non-assaultive trauma (OR: 1.3, p < 0.001). No significant differences regarding sex, race/ethnicity, sexual and assaultive trauma exposure, or EEGc were observed. EEGc from bipolar pairs at three frontal sites (Fig. 2), left intra-hemispheric frontal-central (LFC: FZ-CZ--F3-C3), right intra-hemispheric frontal-central (RFC: FZ-CZ--F4-C4), and prefrontal inter-hemispheric (PFI: F8-F4--F7-F3) were examined in separate models. We modeled both linear and quadratic growth components for EEGc. The models that included the quadratic term did not converge, thus we moved ahead with a linear slope term. Any mention of slope in the following sections refer to linear slope.
Figure 2.
This figure displays a schematic of bipolar electrode pairs (indicated by black dotted lines) and coherence pairs (indicated by black solid lines) derived between bipolar electrode pairs.
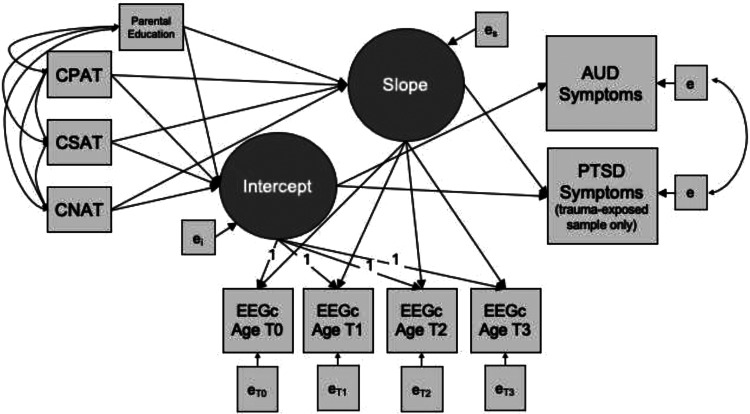
Next, we examined the association between childhood trauma exposure and the intercepts and slopes of EEGc. The intercept estimates individual variation in starting values, which may reflect different types of exposures prior to the measurement period, as well as other trait differences. The slope reflects how change in EEGc occurs over time, on average. Given the known co-occurrence of different types of trauma exposures, the models allowed for simultaneous testing of associations between trauma exposure types and EEGc intercept and slope. The models also evaluated whether intercept and growth parameters in EEGc were associated with AUDsx in the full sample. In a subset of the sample that endorsed exposure to a potentially traumatic event (i.e. met Criterion A for PTSD diagnosis) at any point from baseline to follow-up 3, we added PTSDsx as an additional dependent variable to account for the co-occurrence of AUD and PTSD. Figure 3 is an illustration of the path diagram for the analytic model.
Figure 3.
This figure displays a path diagram of the latent growth curve model used to evaluate the association between childhood trauma and intercept and slope of EEG coherence, as well as associations between EEG coherence intercept and slope with AUD symptoms. In the trauma-exposed subsample, PTSD symptoms were also examined as a dependent variable, with the correlation between AUD and PTSD symptoms accounted for by the model. Growth curves were estimated based on individually varying age at the time of each EEG coherence observation. Parental education was included as a covariate. Note: CNAT, Childhood non-assaultive trauma; CSAT, Childhood sexual assaultive trauma; CPAT, Childhood physical assaultive trauma; EEGc, EEG coherence.
Results
Descriptive characteristics of the sample, stratified by sex
Demographic, alcohol and trauma-related characteristics of the sex-stratified sample are provided in Table 1. The overall sample (N = 1652) was 51.75% female. There were no sex differences among race/ethnic groups or participation rates. At baseline, females were significantly younger than males, but mean age did not significantly differ at the three follow-up assessments. Male participants reported significantly more lifetime maximum drinks, and AUD symptoms. Although male and female participants did not differ on rates of childhood trauma exposure, male participants were more likely to report CPAT, whereas female participants were more likely to report CSAT. Among participants exposed to any traumatic event prior to follow-up 3, mean PTSD symptoms 0.75 in males and 1.56 in females, and the modal response for both males and females was zero symptoms. Female participants reported significantly more PTSD symptoms and significantly fewer AUD symptoms compared to male participants.
Table 1.
Descriptive information for demographic, alcohol-related, and trauma-related characteristics of the sample
| Mean (s.d.) or N (%) | Male baseline N = 797 | Female baseline N = 855 | z/t/χ2 | p |
|---|---|---|---|---|
| Race/ethnicity | ||||
| White | 468 (58.72) | 506 (63.49) | −0.19 | 0.849 |
| Black/African American | 251 (31.49) | 277 (32.40) | −0.39 | 0.697 |
| Asian | 3 (0.38) | 4 (0.47) | −0.29 | 0.771 |
| Hispanic | 104 (13.05) | 99 (11.58) | 0.91 | 0.363 |
| N at follow-up 1 | 653 (81.93) | 701 (81.99) | −0.03 | 0.976 |
| N at follow-up 2 | 461 (57.84) | 508 (59.42) | −0.65 | 0.516 |
| N at follow-up 3 | 319 (40.02) | 366 (42.81) | −1.15 | 0.250 |
| Age at baseline | 14.44 (1.54) | 14.16 (1.49) | −3.76 | <0.001 |
| Age at follow-up 1 | 17.82 (2.85) | 17.74 (2.70) | −0.59 | 0.558 |
| Age at follow-up 2 | 20.41 (3.18) | 20.51 (3.40) | 0.62 | 0.538 |
| Age at follow-up 3 | 22.74 (3.31) | 22.39 (3.85) | −1.98 | 0.050 |
| Parental education (years) | 13.16 (2.21) | 13.04 (2.14) | −1.03 | 0.304 |
| Ever drink | 660 (82.81) | 707 (82.69) | 0.07 | 0.952 |
| Regular drinker | 542 (68.01) | 566 (66.20) | 0.78 | 0.435 |
| Maximum drinks | 14.90 (12.71) | 9.85 (9.79) | −9.08 | <0.001 |
| Onset age of regular drinking | 17.48 (2.49) | 17.85 (2.43) | 3.06 | 0.002 |
| AUD symptoms (full sample) | 1.45 (2.09) | 1.03 (1.87) | −4.31 | <0.001 |
| Any childhood trauma | 258 (32.37) | 249 (29.12) | 1.43 | 0.153 |
| Non-assaultive trauma | 216 (27.10) | 201 (23.51) | 1.68 | 0.093 |
| Physical-assaultive trauma | 99 (12.42) | 44 (5.15) | 5.25 | <0.001 |
| Sexual-assaultive trauma | 11 (1.38) | 54 (6.32) | −5.16 | <0.001 |
| Any trauma by follow-up 3 | 408 (51.19) | 459 (53.68) | −5.06 | <0.001 |
| PTSD symptoms* | 0.75 (2.60) | 1.56 (3.84) | 3.59 | <0.001 |
| AUD symptoms* | 1.20 (1.91) | 0.95 (1.72) | −2.03 | 0.043 |
Note: AUD, alcohol use disorder, and PTSD, post-traumatic stress disorder. PTSD symptoms were assessed only in those who reported a history of trauma. Maximum drinks, AUD symptoms, PTSD symptoms were lifetime measures assessed at follow-up 3. *These items (PTSD and AUD symptoms) were calculated among individuals who reported any trauma exposure by follow-up 3. Bold text indicates p-value less than 0.05. Bold italic text indicates p-value less than 0.01.
EEG functional connectivity patterns and associations with childhood trauma
Results of LGCMs estimating the unconditional mean intercept and linear slope of EEGc over time are displayed in Table 2. There was significant positive mean intercept and slope of all three frontal alpha EEGc pairs, indicating that overall, coherence tended to increase over time. The association of CPAT, CSAT, and CNAT with intercept and slope of frontal alpha EEGc, and the association between slope and intercept with AUD symptoms in male and female participants are displayed in Table 3. In the male-only model, we observed a significant association between CPAT and intercept (ß = −0.17, p = 0.010) and slope (ß = 0.11, p = 0.006) of LFC alpha EEGc (FZ-CZ--F3-C3). This suggests that males who reported CPAT had lower values at baseline, but a faster rate of change in EEGc over time. This association was not observed in male RFC or PFI alpha EEGc (FZ-CZ--F4-C4 and F8-F4--F7-F3, respectively). In contrast, in female participants, we observed a significant association between CSAT and intercept (ß = 0.13, p = 0.008) and slope (ß = −0.07, p = 0.016) of LFC alpha EEGc (FZ-CZ--F3-C3), as well as the intercept (ß = 0.16, p = 0.002) and slope (ß = −0.07, p = 0.024) of PFI alpha EEGc (F8-F4--F7-F3). This indicates that female participants who reported CSAT had higher initial values of LFC and PFI alpha EEGc, and a decreased rate of change in LFC and PFI alpha EEGc over time. We observed no significant associations between CNAT and EEGc, and no associations between CSAT or CPAT on our measure of RFC alpha EEGc (FZ-CZ--F4-C4). Greater parental education was associated with decreased intercept (ß = −0.02, p = 0.003) of LFC alpha EEGc in males but had no association with intercept or slope in females.
Table 2.
Results of the unconditional linear growth model for repeated measures of three frontal alpha EEG coherence Pairs in adolescent male and female COGA participants
| Left frontal-central (LFC) EEG coherence FZ-CZ--F3-C3 |
Right frontal-central (RFC) EEG coherence FZ-CZ--F4-C4 |
Prefrontal interhemispheric (PFI) EEG coherence F8-F4--F7-F3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||
| Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | |
| Unconditional linear growth model | ||||||||||||
| Intercept Mean | 0.41 (0.02) | <0.001 | 0.46 (0.02) | <0.001 | 0.43 (0.02) | <0.001 | 0.46 (0.02) | <0.001 | 0.22 (0.02) | <0.001 | 0.26 (0.02) | <0.001 |
| Slope mean | 0.01 (0.00) | <0.001 | 0.01 (0.00) | <0.001 | 0.01 (0.00) | <0.001 | 0.01 (0.00) | <0.001 | 0.00 (0.00) | 0.001 | 0.00 (0.00) | 0.003 |
| Intercept Variance | 0.04 (0.01) | 0.005 | 0.01 (0.00) | 0.001 | 0.02 (0.01) | 0.131 | 0.03 (0.01) | 0.001 | 0.01 (0.01) | 0.024 | 0.03 (0.00) | <0.001 |
| slope Variance | 0.00 (0.00) | 0.004 | 0.00 (0.00) | <0.001 | 0.00 (0.00) | 0.009 | 0.00 (0.00) | <0.001 | 0.00 (0.00) | <0.001 | 0.00 (0.00) | <0.001 |
| Intercept-slope Correlation | −0.00 (0.00) | 0.012 | 0.00 (0.00) | 0.001 | −0.00 (0.00) | 0.132 | −0.00 (0.00) | 0.001 | 0.00 (0.00) | 0.028 | −0.00 (0.00) | <0.001 |
| Observations | 797 | – | 855 | – | 797 | – | 855 | – | 797 | – | 855 | – |
| AIC | −1872.95 | – | 1268.54 | – | −1861.41 | – | −1259.75 | – | −2135.71 | – | −1876.17 | – |
| BIC | −1830.82 | – | −1225.78 | – | −1819.28 | – | −1216.99 | – | −2093.58 | – | −1833.41 | – |
| H0 log-likelihood | 945.48 | – | 643.27 | – | 939.70 | – | 638.88 | – | 1076.86 | – | 947.09 | – |
| H1 maximum log-likelihood | 952.62 | – | 642.37 | – | 954.66 | – | 637.40 | – | 1091.47 | – | 949.60 | – |
Note: Bold text indicates p-value less than 0.05. Bold italic text indicates p-value less than 0.01.
Table 3.
Linear growth model results for the effect of childhood trauma exposure on slope and intercept of three frontal alpha EEG coherence Pairs and subsequent AUD symptoms in adolescent male and female COGA participants
| Left frontal-central (LFC) EEG coherence FZ-CZ--F3-C3 |
Right frontal-central (RFC) EEG coherence FZ-CZ--F4-C4 |
Prefrontal interhemispheric (PFI) EEG coherence F8-F4--F7-F3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||
| Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | |
| Associations with intercept and slope of alpha EEG coherence | ||||||||||||
| CNAT on intercept | 0.00 (0.05) | 0.960 | −0.04 (0.03) | 0.240 | 0.01 (0.04) | 0.727 | −0.02 (0.02) | 0.382 | −0.03 (0.02) | 0.058 | −0.02 (0.03) | 0.552 |
| CSAT on intercept | 0.16 (0.12) | 0.184 | 0.13 (0.05) | 0.008 | 0.21 (0.13) | 0.094 | 0.07 (0.12) | 0.558 | −0.01 (0.05) | 0.830 | 0.16 (0.05) | 0.002 |
| CPAT on intercept | −0.17 (0.07) | 0.010 | −0.06 (0.08) | 0.494 | −0.05 (0.06) | 0.381 | −0.01 (0.06) | 0.857 | −0.00 (0.02) | 0.972 | −0.05 (0.08) | 0.52 |
| Parental education on intercept | −0.02 (0.01) | 0.003 | −0.02 (0.01) | 0.069 | −0.01 (0.01) | 0.078 | −0.01 (0.06) | 0.149 | −0.00 (0.00) | 0.575 | −0.01 (0.01) | 0.079 |
| CNAT on slope | 0.00 (0.03) | 0.886 | 0.01 (0.02) | 0.771 | −0.01 (0.02) | 0.820 | 0.00 (0.01) | 0.908 | 0.02 (0.01) | 0.102 | −0.00 (0.02) | 0.937 |
| CSAT on slope | −0.05 (0.06) | 0.394 | −0.07 (0.03) | 0.016 | −0.09 (0.07) | 0.183 | −0.02 (0.06) | 0.719 | 0.05 (0.03) | 0.064 | −0.07 (0.03) | 0.024 |
| CPAT on slope | 0.11 (0.04) | 0.006 | 0.04 (0.05) | 0.450 | 0.04 (0.03) | 0.236 | 0.00 (0.03) | 0.933 | −0.01 (0.01) | 0.592 | 0.05 (0.05) | 0.310 |
| Parental education on slope | 0.01 (0.01) | 0.057 | 0.01 (0.01) | 0.449 | 0.00 (0.00) | 0.402 | 0.00 (0.00) | 0.897 | −0.00 (0.00) | 0.422 | 0.00 (0.00) | 0.910 |
| Associations with AUD symptoms | ||||||||||||
| Intercept on AUD symptoms | −0.14 (0.43) | 0.745 | −0.21 (0.70) | 0.770 | 0.36 (0.18) | 0.039 | 0.23 (1.79) | 0.897 | −3.33 (0.05) | <0.001 | 0.26 (0.47) | 0.589 |
| Slope on AUD symptoms | −0.60 (0.51) | 0.238 | −1.81 (0.73) | 0.013 | −1.35 (1.12) | 0.229 | −5.94 (13.33) | 0.656 | 1.89 (1.61) | 0.42 | −1.13 (1.06) | 0.288 |
| Observations | 797 | – | 855 | – | 797 | – | 855 | – | 797 | – | 855 | |
| AIC | 4729.54 | – | 5802.46 | – | 4748.93 | – | 5829.92 | – | 4480.00 | – | 5196.12 | |
| BIC | 4898.05 | – | 5973.50 | – | 4917.44 | – | 6000.96 | – | 4648.51 | – | 5367.16 | |
| H0 log-likelihood | −2328.77 | – | −2865.23 | −2338.46 | – | −2878.96 | −2204.00 | – | −2562.06 | |||
| H1 maximum log-likelihood | −1819.08 | – | −2310.33 | −1829.38 | – | −2321.86 | −1696.35 | – | −2004.76 | |||
Note: CNAT, Childhood non-assaultive trauma; CSAT, Childhood sexual assaultive trauma; CPAT, Childhood physical assaultive trauma. Bold text indicates p-value less than 0.05. Bold italic text indicates p-value less than 0.01.
Associations between EEG functional connectivity and AUD symptoms
Results of LGCMs estimating the association between intercept and slope of frontal alpha EEGc at three frontal bipolar pairs with AUDsx in male and female participants are displayed in the lower half of Table 3. In males, we observed a significant positive association between intercept and AUDsx in RFC alpha EEGc (ß = 0.36, p = 0.04), and a negative association between intercept and AUDsx in PFI alpha EEGc (ß = −3.33, p < 0.001). EEGc These results suggest that both higher initial values in LFC alpha EEGc, but greater initial values PFI alpha EEGc were associated with increased AUDsx. In female participants, we observed a significant negative association with slope of LFC alpha EEGc (ß = −1.81, p = 0.013), which suggests steeper decline in LFC alpha EEGc over time was associated with increased AUDsx. We observed no associations between intercept and EEGc on AUDsx in females, and no association between slope and AUDsx in males.
Association between EEG functional connectivity and AUD and PTSD symptoms in trauma-exposed participants
Results of LGCMs estimating the associations between intercept and slope of alpha EEGc and subsequent AUDsx and PTSDsx in male and female trauma-exposed participants are displayed in Table 4. We found that intercept of LFC alpha EEGc (FZ-CZ--F3-C3) was positively associated with both AUDsx (ß = 0.53, p < 0.001) and PTSDsx (ß = 2.65, p < 0.001) in trauma-exposed females, suggesting that greater initial values of EEGc were associated with higher levels of PTSD and AUD symptoms. There were no FZ-CZ--F3-C3 intercept or slope associations with AUDsx or PTSDsx observed in trauma-exposed males. For our measure of RFC alpha EEGc (FZ-CZ--F4-C4), we found that intercept was significantly positively associated with both AUDsx (ß = 4.10, p < 0.001) and PTSDsx (ß = 2.47, p < 0.001) in males. In females, slope of RFC alpha EEGc (FZ-CZ--F4-C4) was negatively associated with AUDsx (ß = −3.16, p = 0.002) and PTSDsx (ß = −5.19, p = 0.001), suggesting that diminished growth of EEGc was associated with greater AUDsx and PTSDsx. For PFI alpha EEGc (F8-F4--F7-F3), intercept was significantly positively associated with PTSD symptoms in males (ß = 1.05, p < 0.001), and females (ß = 1.69, p < 0.001). Slope of the same coherence pair (F8-F4--F7-F3), was negatively associated with PTSDsx (ß = −2.02, p = 0.0231) in trauma-exposed males, suggesting that higher initial values and diminished growth of PFI alpha EEGc associated with increased PTSDsx.
Table 4.
Linear growth model results measuring associations between slope and intercept of three frontal alpha EEG coherence pairs and subsequent AUD symptoms in the trauma-exposed subsample of adolescent male and female COGA participants
| Left frontal-central (LFC) EEG coherence FZ-CZ--F3-C3 |
Right frontal-central (RFC) EEG coherence FZ-CZ--F4-C4 |
Prefrontal interhemispheric (PFI) EEG coherence F8-F4--F7-F3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |||||||
| Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | Beta (s.e.) | p | |
| Associations with AUD and PTSD symptoms | ||||||||||||
| Intercept on AUD symptoms | 0.08 (0.45) | 0.862 | 0.53 (0.12) | <0.001 | 4.10 (0.19) | <0.001 | −0.58 (0.66) | 0.377 | 0.41 (0.60) | 0.494 | 0.53 (1.10) | 0.633 |
| Slope on AUD symptoms | −0.91 (0.77) | 0.237 | −0.79 (0.77) | 0.305 | −1.55 (1.26) | 0.218 | −3.16 (1.03) | 0.002 | −1.39 (0.83) | 0.094 | −0.22 (0.58) | 0.700 |
| Intercept on PTSD symptoms | 0.28 (0.15) | 0.066 | 2.65 (0.12) | <0.001 | 2.47 (0.09) | <0.001 | 0.15 (0.18) | 0.419 | 1.05 (0.13) | <0.001 | 1.69 (0.08) | <0.001 |
| Slope on PTSD symptoms | 0.01 (0.48) | 0.992 | −0.48 (0.94) | 0.612 | −0.22 (0.56) | 0.699 | −5.19 (1.52) | 0.001 | −2.02 (0.89) | 0.023 | −0.82 (0.55) | 0.137 |
| Correlation between AUD and PTSD symptoms | 0.01 (0.01) | 0.312 | 0.01 (0.01) | 0.552 | 0.00 (0.01) | 0.863 | 0.01 (0.01) | 0.494 | −0.00 (0.01) | 0.887 | 0.00 (0.01) | 0.718 |
| Observations | 408 | – | 459 | – | 408 | – | 459 | – | 408 | – | 459 | – |
| AIC | 3034.34 | – | 3950.75 | – | 3061.07 | – | 3934.99 | – | 2900.58 | – | 3497.02 | – |
| BIC | 3198.81 | – | 4120.04 | – | 3225.54 | – | 4104.28 | – | 3065.04 | – | 3666.31 | – |
| H0 log-likelihood | −1476.17 | – | −1934.37 | – | −1489.537 | – | −1926.49 | −1409.29 | – | −1707.51 | – | |
| H1 maximum log-likelihood | −1037.24 | – | −1495.84 | – | −1057.53 | – | −1489.05 | −970.62 | – | −1261.84 | – | |
Note: Analyses were limited to individuals who reported at least one traumatic experience during any assessment from baseline to the third follow-up. PTSD symptoms and AUD symptoms represent the lifetime maximum symptoms as reported at the third follow-up assessment. Models account for childhood trauma variables and parental education associations with intercept and slope of EEG coherence. Bold text indicates p-value less than 0.05. Bold italic text indicates p-value less than 0.01.
Discussion
This is the first study, to our knowledge, to examine the association between childhood trauma and longitudinal EEG coherence (EEGc) across adolescence and its role as a potential biological mechanism linking trauma exposure to AUD and PTSD symptoms in young adulthood. We observed differences in trajectories of left frontocentral alpha EEGc related to childhood sexual and physical assaultive trauma in females and childhood physical assaultive trauma in males. The left frontotemporal region of the brain is associated with language, learning, and memory, and has been implicated in studies of the effect of traumatic stress on the brain (Bremner, 2006; Carrion, Weems, Richert, Hoffman, & Reiss, 2010; Carrion & Wong, 2012; Stark et al., 2015). EEG interhemispheric alpha coherence in the prefrontal region also showed increased intercept but diminished growth in females with history of childhood sexual assaultive trauma, suggesting differences in executive function in these individuals. EEGc was associated with subsequent AUD and PTSD symptoms in trauma-exposed participants, with patterns indicating that trauma exposure was associated with worse AUD/PTSD outcomes.
As hypothesized, we found different patterns of EEGc over time related to childhood trauma exposure before age 13. Results demonstrating differences in both intercept and slope of EEGc are associated with childhood assaultive trauma and psychopathology extend the largely cross-sectional, adult-focused literature (Cardenas, Price, & Fein, 2018; Cook et al., 2009; Park et al., 2017; Tcheslavski & Gonen, 2012). Our models indicated higher baseline left frontocentral EEGc in females with CSAT, consistent with a small (n = 30) cross-sectional study by Ito, Teicher, Glod, and Ackerman (1998), which observed higher alpha EEGc in the left hemisphere of children with severe sexual or physical abuse histories. The present study extends this literature by demonstrating nuanced trajectories: decreased alpha EEGc growth in females with CSAT and increased growth in males with CPAT. One explanation for these findings is that childhood trauma may lead to extended hyperarousal and mesolimbic system overactivation, disrupting typical neural network development (Teicher et al., 1997; Teicher & Samson, 2016). Notably, no associations between CNAT and EEGc were observed, consistent with literature indicating assaultive trauma poses higher risk for PTSD (Breslau et al., 1998), depression (McCutcheon et al., 2009), and more pronounced changes in brain development (De Bellis & Zisk, 2014; Meyers et al., 2019b). Additionally, this study extends literature on functional connectivity and shared liability for AUD and PTSD. Differences in frontal functional connectivity were associated with AUD and PTSD symptoms, but to varying degrees across sexes. Importantly, findings demonstrated associations with both slope and intercept, offering insights potentially missed in prior cross-sectional studies. Together, these findings on trauma, AUD, and EEGc offer some of the first longitudinal evidence of associations between childhood trauma and frontal neural connectivity, with implications for understanding development of psychopathology.
The present study observed significant associations between childhood trauma and alpha EEGc in only the left hemisphere for both males and females, regardless of trauma type. Previous studies have reported differences between hemispheres in individuals with childhood trauma, such as smaller left dorsolateral prefrontal cortex volume in those with childhood trauma (Carballedo et al., 2012; Lu et al., 2019; Paquola, Bennett, & Lagopoulos, 2016). While our study investigated connectivity in the frontal and central regions of the brain, other studies have seen lateral differences in the temporal region, such as smaller left hippocampal volume compared to controls (Bremner et al., 1997; Shu et al., 2013; Stein, Koverola, Hanna, Torchia, & McCLARTY, 1997). Further, less hippocampal activation is associated with increased PTSD symptoms (van Rooij et al., 2018). Another study observed abnormal connectivity between the left PFC and anterior hippocampus in children with PTSD (Heyn et al., 2019). Given the findings from the present study and previous connectivity and imaging studies, future studies should investigate the associations between sex differences and trauma type with connectivity between the PFC and hippocampus in individuals reporting childhood trauma.
Sex differences in trauma type, PTSD, and AUD, are well documented by previous research (Beals et al., 2013; Boudoukha, Ouagazzal, & Goutaudier, 2017; Chung & Breslau, 2008; Erol & Karpyak, 2015; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Men are more likely to experience trauma, but less likely to be diagnosed with PTSD compared to women (Beals et al., 2013; Kessler et al., 1995). Women are more likely to have co-morbid PTSD and AUD (Peltier et al., 2022), potentially due to higher risk of recurring, high impact trauma (Beals et al., 2013; Hien, Cohen, & Campbell, 2005). In our sample, CSAT was more prevalent in females, and CPAT more prevalent in males. Females also reported significantly higher PTSD symptoms, which may be related to their higher rates of CSAT. Moreover, sex may play also influence the relationship between trauma and neurocognitive development (Helpman et al., 2017). Prior research in COGA (Meyers et al., 2019b) found that females reporting sexual assault had a decreased rate of change in frontal theta oscillations during response inhibition. Structural and functional neuroimaging studies also demonstrate interactions among sex, type, and timing of trauma exposure (for review, see Helpman et al., 2017). In the present study, we observed sex differences dependent on trauma type: in males, CPAT was linked to lower baseline left frontocentral alpha EEGc and a faster rate of change, while in females, CSAT was linked to higher starting values and a slower rate of change. While the observed sex differences may relate to the variation in physical v. assaultive trauma prevalence, the differences in direction of these associations may also suggest true sex-specific effects.
The findings from this study have important implications. First, these results suggest that traumatic experiences in childhood have lasting impacts on brain function, as evidenced by the fact that sexual/physical abuse was associated with both concurrent influences on EEGc and on changes across development. Additionally, type of trauma experienced may have differential associations with EEGc across sexes. Females with childhood sexual assault exposure and males with childhood physical assault exposure may be particularly high-risk groups on whom prevention and intervention efforts should be prioritized. Although links between trauma and PTSD and AUD have been well-established, these study results begin to suggest that atypical changes in alpha EEGc across development may be one factor by which certain traumatic experiences confer risk for AUD and PTSD. This study joins other emerging work (e.g. Kummar, Correia, and Fujiyama, 2019) in suggesting that there may be some clinical value in integrating EEG measures into treatment for trauma-exposed individuals, as such biological markers may enable earlier diagnoses and/or an avenue through which to provide neurofeedback over the course of treatment.
Limitations and future directions
Limitations
Missing data – LGCM requires at least three timepoints, which were present in ~85% of COGA's prospective study sample overall, but only 59% of the analytic sample in the present analyses. Childhood Trauma Measures – While some traumatic events would have occurred during the study period (baseline through follow-up 3), many of the childhood traumatic events assessed relied on retrospective self-reports. Retrospective reports may be unstable over time, typically underestimating trauma exposure prevalence, potentially biasing this study's findings towards the null, especially for the individuals who were older at baseline. This may also account in part for the relatively small effect sizes observed for the effect of trauma on EEGc. Additionally, participants’ perceptions of how stressful they found the traumatic event, experiences of prolonged/repeated traumas, and childhood maltreatment (e.g. neglect) were not measured. Although our models accounted for the effect of different types of trauma, we did not present the effect of multiple types of trauma exposure (e.g. CPAT*CSAT) due to power limitations, despite evidence that children often experience multiple types of childhood abuse (Chiu et al., 2013). Future analyses that account for ‘dose’ effects (e.g. an individual who has been stabbed, shot, and mugged would have a CPAT dose of 3) and interactive effects would add further insight into our understanding of the effects of complex trauma on the developing brain. Additionally, as symptom counts reflected lifetime problems, it is unknown whether the symptoms reported reflect participants’ current state or whether they had recovered by the time of assessment. Confounding variables – This was an observational study, so we caution against any causal inferences based on the significant associations in our findings, as these associations could be attributed to third variables. There are several factors unaccounted for in the present analyses that may impact the neurodevelopmental processes examined, such as parental factors, sociodemographic factors, fetal alcohol exposure, and individual factors, including genetics and family history of alcohol problems (Pandey et al., 2020). Additional research that more comprehensively integrates the range of important factors is needed.
Future directions
This study focused on alpha EEGc, which is just one of several potentially informative EEG phenotypes. The number of EEG phenotypes available is so vast that future research would likely benefit from integrating machine learning and other methods for the management of big data (Golmohammadi, Harati Nejad Torbati, Lopez de Diego, Obeid, & Picone, 2019; Pawan & Dhiman, 2023) while maintaining a focus on clinical relevance when executing these techniques. In addition, studies that more comprehensively assess childhood trauma (e.g. repeated/prolonged trauma), maltreatment (e.g. neglect), and other stressful life experiences (e.g. discrimination, neighborhood violence) are needed to better understand the way that adverse experiences in childhood may impact neural connectivity and subsequent psychopathology. Another important future direction for this research is the integration of genetic and other biological factors (e.g. family history) associated with trauma, PTSD, and alcohol-related outcomes (e.g. consumption, problems, AUD) and EEG connectivity. Including these informative measures may shed further light on the shared neurobiological mechanisms of risk between PTSD and AUD, as well as increase our ability to better identify individuals who may benefit most from intervention.
Conclusions
This novel study explored associations between childhood trauma and longitudinal alpha EEG coherence in frontal brain regions in children and adolescents, and their association with subsequent AUD and PTSD in young adulthood. Our findings suggest that childhood assaultive trauma is associated with changes in neural connectivity patterns, and the nature of these associations differs by sex and trauma exposure type. Further, trajectories of neural connectivity were associated with subsequent AUD and PTSD symptoms. These findings contribute valuable insights into the neurodevelopmental consequences of childhood trauma, extending beyond the predominantly cross-sectional adult literature. Importantly, sex-specific variations in neural connectivity underscore the need for a comprehensive understanding of trauma's impact on brain functioning. The relevance of EEG coherence as a potential biomarker for early diagnosis and targeted interventions is also emphasized. Moving forward, integrating machine learning techniques and exploring other genetic and biological factors could enhance our understanding of the shared neurobiological mechanisms underlying PTSD, AUD, and trauma-related EEG coherence, with the goal to guide targeted interventions for high-risk groups.
Supporting information
Neale et al. supplementary material
Acknowledgements
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes ten different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, D. Dick, R. Hart, J. Salvatore); The Children's Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); J. Nurnberger Jr., L. Wetherill, X., Xuei, D. Lai, S. O'Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, Z. Neale, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, F. Aliev, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children's Hospital of Philadelphia and University of Pennsylvania); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting- Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Contributor Information
COGA Investigators:
B. Porjesz, V. Hesselbrock, T. Foroud, A. Agrawal, D. Dick, V. Hesselbrock, H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki, S. Kuperman, J. Kramer, J. Meyers, C. Kamarajan, A. Pandey, L. Bierut, J. Rice, K. Bucholz, A. Agrawal, M. Schuckit, J. Tischfield, R. Hart, J. Salvatore, L. Almasy, A. Goate, P. Slesinger, D. Scott, L. Bauer, J. Nurnberger, Jr., L. Wetherill, X. Xuei, D. Lai, S. O'Connor, G. Chan, D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, Z. Neale, G. Pandey, N. Mullins, A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone, J. Moore, F. Aliev, Z. Pang, S. Kuo, A. Merikangas, H. Chin, A. Parsian, Ting-Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724002599.
Funding statement
This research was funded by the National Institute on Alcohol Abuse and Alcoholism (U10AA008401 to BP, and R01AA030010 to JM and AA).
Competing interests
The authors declare that they have no competing interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Agrawal, A., Brislin, S. J., Bucholz, K. K., Dick, D., Hart, R. P., Johnson, E. C., … Porjesz, B. (2023). The collaborative study on the genetics of alcoholism: Overview. Genes, Brain and Behavior, 22(5), e12864. 10.1111/gbb.12864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli, L. M., Lori, A., Meyers, J. L., Shin, J., Fani, N., Maihofer, A. X., … Ressler, K. J. (2018). Problematic alcohol use associates with sodium channel and clathrin linker 1 (SCLT1) in trauma-exposed populations. Addiction Biology, 23(5), 1145–1159. 10.1111/adb.12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, S. L., Tomada, A., Vincow, E. S., Valente, E., Polcari, A., & Teicher, M. H. (2008). Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences, 20(3), 292–301. 10.1176/jnp.2008.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni, C., Del Percio, C., Bordet, R., Bourriez, J.-L., Bentivoglio, M., Payoux, P., … Rossini, P. M. (2013). Effects of acetylcholinesterase inhibitors and memantine on resting-state electroencephalographic rhythms in Alzheimer's disease patients. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 124(5), 837–850. 10.1016/j.clinph.2012.09.017 [DOI] [PubMed] [Google Scholar]
- Babiloni, C., Del Percio, C., Lizio, R., Noce, G., Lopez, S., Soricelli, A., … Stocchi, F. (2018). Functional cortical source connectivity of resting state electroencephalographic alpha rhythms shows similar abnormalities in patients with mild cognitive impairment due to Alzheimer's and Parkinson's diseases. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 129(4), 766–782. 10.1016/j.clinph.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Badura-Brack, A. S., Becker, K. M., McDermott, T. J., Ryan, T. J., Becker, M. M., Hearley, A. R., … Wilson, T. W. (2015). Decreased somatosensory activity to non-threatening touch in combat veterans with posttraumatic stress disorder. Psychiatry Research, 233(2), 194–200. 10.1016/j.pscychresns.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals, J., Belcourt-Dittloff, A., Garroutte, E. M., Croy, C., Jervis, L. L., Whitesell, N. R., … The AI-SUPERPFP team. (2013). Trauma and conditional risk of posttraumatic stress disorder in two American Indian reservation communities. Social Psychiatry and Psychiatric Epidemiology, 48(6), 895–905. 10.1007/s00127-012-0615-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein, M. E. (2013). Childhood trauma and a framework for intervention. In Rossen E. A. & Hull R. V. (Eds.), Supporting and educating traumatized students: A guide for school-based professionals (pp. 3–21). USA: OUP. [Google Scholar]
- Blinowska, K. J., Rakowski, F., Kaminski, M., De Vico Fallani, F., Del Percio, C., Lizio, R., & Babiloni, C. (2017). Functional and effective brain connectivity for discrimination between Alzheimer's patients and healthy individuals: A study on resting state EEG rhythms. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(4), 667–680. 10.1016/j.clinph.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Boudoukha, A. H., Ouagazzal, O., & Goutaudier, N. (2017). When traumatic event exposure characteristics matter: Impact of traumatic event exposure characteristics on posttraumatic and dissociative symptoms. Psychological Trauma: Theory, Research, Practice, and Policy, 9(5), 561–566. 10.1037/tra0000243 [DOI] [PubMed] [Google Scholar]
- Bountress, K. E., Bustamante, D., Subbie-Saenz de Viteri, S., Chatzinakos, C., Sheerin, C., Daskalakis, N. P., … Amstadter, A. (2022). Differences in genetic correlations between posttraumatic stress disorder and alcohol-related problems phenotypes compared to alcohol consumption-related phenotypes. Psychological Medicine, 53(12), 5767–5777. 10.1017/S0033291722002999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, K. T., & Sinha, R. (2005). Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. The American Journal of Psychiatry, 162(8), 1483–1493. 10.1176/appi.ajp.162.8.1483 [DOI] [PubMed] [Google Scholar]
- Bremner, J. D. (2006). Traumatic stress: Effects on the brain. Dialogues in Clinical Neuroscience, 8(4), 445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., … Charney, D. S. (1997). Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—A preliminary report. Biological Psychiatry, 41(1), 23–32. 10.1016/S0006-3223(96)00162-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau, N., Kessler, R. C., Chilcoat, H. D., Schultz, L. R., Davis, G. C., & Andreski, P. (1998). Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Archives of General Psychiatry, 55(7), 626–632. 10.1001/archpsyc.55.7.626 [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan, M. J., Carter, A. S., & Ford, J. D. (2012). Parsing the effects violence exposure in early childhood: Modeling developmental pathways. Journal of Pediatric Psychology, 37(1), 11–22. 10.1093/jpepsy/jsr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, P. J., Stout, R. L., & Mueller, T. (1999). Substance use disorder and posttraumatic stress disorder comorbidity: Addiction and psychiatric treatment rates. Psychology of Addictive Behaviors, 13(2), 115–122. 10.1037/0893-164X.13.2.115 [DOI] [Google Scholar]
- Canuet, L., Ishii, R., Pascual-Marqui, R. D., Iwase, M., Kurimoto, R., Aoki, Y., … Takeda, M. (2011). Resting-state EEG source localization and functional connectivity in schizophrenia-like psychosis of epilepsy. PloS One, 6(11), e27863. 10.1371/journal.pone.0027863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballedo, A., Lisiecka, D., Fagan, A., Saleh, K., Ferguson, Y., Connolly, G., … Frodl, T. (2012). Early life adversity is associated with brain changes in subjects at family risk for depression. The World Journal of Biological Psychiatry, 13(8), 569–578. 10.3109/15622975.2012.661079 [DOI] [PubMed] [Google Scholar]
- Cardenas, V. A., Price, M., & Fein, G. (2018). EEG coherence related to fMRI resting state synchrony in long-term abstinent alcoholics. NeuroImage. Clinical, 17, 481–490. 10.1016/j.nicl.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion, V. G., Weems, C. F., Richert, K., Hoffman, B. C., & Reiss, A. L. (2010). Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biological Psychiatry, 68(5), 491–493. 10.1016/j.biopsych.2010.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion, V. G., & Wong, S. S. (2012). Can traumatic stress alter the brain? Understanding the implications of early trauma on brain development and learning. Journal of Adolescent Health, 51(2, Supplement), S23–S28. 10.1016/j.jadohealth.2012.04.010 [DOI] [PubMed] [Google Scholar]
- Chiu, G. R., Lutfey, K. E., Litman, H. J., Link, C. L., Hall, S. A., & McKinlay, J. B. (2013). Prevalence and overlap of childhood and adult physical, sexual, and emotional abuse: A descriptive analysis of results from the Boston area community health (BACH) survey. Violence and Victims, 28(3), 381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorlian, D. B., Kamarajan, C., Meyers, J. L., Pandey, A. K., Zhang, J., Kinreich, S., & Porjesz, B. (2024). Non-linear development of EEG coherence in adolescents and young adults shown by the analysis of neurophysiological trajectories and their covariance. bioRxiv, 2024.03.13.584867. 10.1101/2024.03.13.584867 [DOI] [Google Scholar]
- Chorlian, D. B., Rangaswamy, M., & Porjesz, B. (2009). EEG coherence: Topography and frequency structure. Experimental Brain Research, 198(1), 59–83. 10.1007/s00221-009-1936-9 [DOI] [PubMed] [Google Scholar]
- Chorlian, D. B., Tang, Y., Rangaswamy, M., O'Connor, S., Rohrbaugh, J., Taylor, R., & Porjesz, B. (2007). Heritability of EEG coherence in a large sib-pair population. Biological Psychology, 75(3), 260–266. 10.1016/j.biopsycho.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, H., & Breslau, N. (2008). The latent structure of post-traumatic stress disorder: Tests of invariance by gender and trauma type. Psychological Medicine, 38(4), 563–573. 10.1017/S0033291707002589 [DOI] [PubMed] [Google Scholar]
- Cook, F., Ciorciari, J., Varker, T., & Devilly, G. J. (2009). Changes in long term neural connectivity following psychological trauma. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 120(2), 309–314. 10.1016/j.clinph.2008.11.021 [DOI] [PubMed] [Google Scholar]
- D'Andrea, W., Ford, J., Stolbach, B., Spinazzola, J., & van der Kolk, B. A. (2012). Understanding interpersonal trauma in children: Why we need a developmentally appropriate trauma diagnosis. The American Journal of Orthopsychiatry, 82(2), 187–200. 10.1111/j.1939-0025.2012.01154.x [DOI] [PubMed] [Google Scholar]
- Danovitch, I. (2016). Post-traumatic stress disorder and opioid use disorder: A narrative review of conceptual models. Journal of Addictive Diseases, 35(3), 169–179. 10.1080/10550887.2016.1168212 [DOI] [PubMed] [Google Scholar]
- Davis, T. A., Jovanovic, T., Norrholm, S. D., Glover, E. M., Swanson, M., Spann, S., & Bradley, B. (2013). Substance use attenuates physiological responses associated with PTSD among individuals with co-morbid PTSD and SUDs. Journal of Psychology & Psychotherapy, Suppl 7, 006. 10.4172/2161-0487.S7-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debell, F., Fear, N. T., Head, M., Batt-Rawden, S., Greenberg, N., Wessely, S., & Goodwin, L. (2014). A systematic review of the comorbidity between PTSD and alcohol misuse. Social Psychiatry and Psychiatric Epidemiology, 49(9), 1401–1425. 10.1007/s00127-014-0855-7 [DOI] [PubMed] [Google Scholar]
- De Bellis, M. D., & Zisk, A. (2014). The biological effects of childhood trauma. Child and Adolescent Psychiatric Clinics of North America, 23(2), 185–222. 10.1016/j.chc.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo, G., Daverio, A., Ferrentino, F., Santarnecchi, E., Ciabattini, F., Monaco, L., … Siracusano, A. (2015). Altered resting-state EEG source functional connectivity in schizophrenia: The effect of illness duration. Frontiers in Human Neuroscience, 9, 234. 10.3389/fnhum.2015.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, D. M., Balcke, E., McCutcheon, V., Francis, M., Kuo, S., Salvatore, J., … Bucholz, K. (2023). The collaborative study on the genetics of alcoholism: Sample and clinical data. Genes, Brain and Behavior, 22(5), e12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, R. D., Saunders, B. E., Kilpatrick, D. G., Hanson, R. F., & Resnick, H. S. (1996). Childhood physical assault as a risk factor for PTSD, depression, and substance abuse: Findings from a national survey. The American Journal of Orthopsychiatry, 66(3), 437–448. 10.1037/h0080194 [DOI] [PubMed] [Google Scholar]
- Dunkley, B. T., Sedge, P. A., Doesburg, S. M., Grodecki, R. J., Jetly, R., Shek, P. N., … Pang, E. W. (2015). Theta, mental flexibility, and post-traumatic stress disorder: Connecting in the parietal cortex. PloS One, 10(4), e0123541. 10.1371/journal.pone.0123541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman-Rothman, M., Levy, J., & Feldman, R. (2016). Alpha oscillations and their impairment in affective and post-traumatic stress disorders. Neuroscience & Biobehavioral Reviews, 68, 794–815. 10.1016/j.neubiorev.2016.07.005 [DOI] [PubMed] [Google Scholar]
- Erol, A., & Karpyak, V. M. (2015). Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence, 156, 1–13. 10.1016/j.drugalcdep.2015.08.023 [DOI] [PubMed] [Google Scholar]
- Finkelhor, D., Turner, H., Ormrod, R., & Hamby, S. L. (2009). Violence, abuse, and crime exposure in a national sample of children and youth. Pediatrics, 124(5), 1411–1423. 10.1542/peds.2009-0467 [DOI] [PubMed] [Google Scholar]
- Foxe, J. J., & Snyder, A. C. (2011). The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Frontiers in Psychology, 2, 154. 10.3389/fpsyg.2011.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin, N. W., & Weiner, J. L. (2017). Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes, Brain, and Behavior, 16(1), 15–43. 10.1111/gbb.12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmohammadi, M., Harati Nejad Torbati, A. H., Lopez de Diego, S., Obeid, I., & Picone, J. (2019). Automatic analysis of EEGs using big data and hybrid deep learning architectures. Frontiers in Human Neuroscience, 13, 76. Retrieved from https://www.frontiersin.org/articles/10.3389/fnhum.2019.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman, D. A., Farah, M. J., & Meaney, M. J. (2010). Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews. Neuroscience, 11(9), 651–659. 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helpman, L., Zhu, X., Suarez-Jimenez, B., Lazarov, A., Monk, C., & Neria, Y. (2017). Sex differences in trauma-related psychopathology: A critical review of neuroimaging literature (2014–2017). Current Psychiatry Reports, 19(12), 104. 10.1007/s11920-017-0854-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn, S. A., Keding, T. J., Ross, M. C., Cisler, J. M., Mumford, J. A., & Herringa, R. J. (2019). Abnormal prefrontal development in pediatric posttraumatic stress disorder: A longitudinal structural and functional magnetic resonance imaging study. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 4(2), 171–179. 10.1016/j.bpsc.2018.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien, D., Cohen, L., & Campbell, A. (2005). Is traumatic stress a vulnerability factor for women with substance use disorders? Clinical Psychology Review, 25(6), 813–823. 10.1016/j.cpr.2005.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, M. J., Swanwick, G. R. J., Kaiser, J., Rowan, M., & Lawlor, B. (2003). Memory-related EEG power and coherence reductions in mild Alzheimer's disease. International Journal of Psychophysiology, 49(2), 147–163. 10.1016/S0167-8760(03)00118-1 [DOI] [PubMed] [Google Scholar]
- Huang, M.-X., Yurgil, K. A., Robb, A., Angeles, A., Diwakar, M., Risbrough, V. B., … Baker, D. G. (2014). Voxel-wise resting-state MEG source magnitude imaging study reveals neurocircuitry abnormality in active-duty service members and veterans with PTSD. NeuroImag : Clinical, 5, 408–419. 10.1016/j.nicl.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Mohan, A., De Ridder, D., Sunaert, S., & Vanneste, S. (2018). The neural correlates of the unified percept of alcohol-related craving: A fMRI and EEG study. Scientific Reports, 8(1), 923. 10.1038/s41598-017-18471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperatori, C., Fabbricatore, M., Innamorati, M., Farina, B., Quintiliani, M. I., Lamis, D. A., … Della Marca, G. (2015). Modification of EEG functional connectivity and EEG power spectra in overweight and obese patients with food addiction: An eLORETA study. Brain Imaging and Behavior, 9(4), 703–716. 10.1007/s11682-014-9324-x [DOI] [PubMed] [Google Scholar]
- Ippolito, G., Bertaccini, R., Tarasi, L., Di Gregorio, F., Trajkovic, J., Battaglia, S., & Romei, V. (2022). The role of alpha oscillations among the main neuropsychiatric disorders in the adult and developing human brain: Evidence from the last 10 years of research. Biomedicines, 10(12), 3189. 10.3390/biomedicines10123189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y., Teicher, M. H., Glod, C. A., & Ackerman, E. (1998). Preliminary evidence for aberrant cortical development in abused children. The Journal of Neuropsychiatry and Clinical Neurosciences, 10(3), 298–307. 10.1176/jnp.10.3.298 [DOI] [PubMed] [Google Scholar]
- Kamarajan, C., Ardekani, B. A., Pandey, A. K., Chorlian, D. B., Kinreich, S., Pandey, G., … Porjesz, B. (2020). Random forest classification of alcohol use disorder using EEG source functional connectivity, neuropsychological functioning, and impulsivity measures. Behavioral Sciences (Basel, Switzerland), 10(3), 62. 10.3390/bs10030062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp, A. H., Griffiths, K., Felmingham, K. L., Shankman, S. A., Drinkenburg, W., Arns, M., … Bryant, R. A. (2010). Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology, 85(2), 350–354. 10.1016/j.biopsycho.2010.08.001 [DOI] [PubMed] [Google Scholar]
- Kessler, R. C., Chiu, W. T., Demler, O., Walters, E. E., & Walters, E. E. (2005). Prevalence, severity, and comorbidity of 12–month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry, 62(6), 617. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., & Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52(12), 1048–1060. 10.1001/archpsyc.1995.03950240066012 [DOI] [PubMed] [Google Scholar]
- Khoury, L., Tang, Y. L., Bradley, B., Cubells, J. F., & Ressler, K. J. (2010). Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depression and Anxiety, 27(12), 1077–1086. 10.1002/da.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummar, A. S., Correia, H., & Fujiyama, H. (2019). A brief review of the EEG literature on mindfulness and fear extinction and its potential implications for posttraumatic stress symptoms (PTSS). Brain Sciences, 9(10), 258. 10.3390/brainsci9100258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, B., & Luna, B. (2018). Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience and Biobehavioral Reviews, 94, 179–195. 10.1016/j.neubiorev.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, D., Faber, P. L., Pascual-Marqui, R. D., Milz, P., Herrmann, W. M., Koukkou, M., … Kochi, K. (2014). Functionally aberrant electrophysiological cortical connectivities in first episode medication-naive schizophrenics from three psychiatry centers. Frontiers in Human Neuroscience, 8, 635. 10.3389/fnhum.2014.00635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S., Xu, R., Cao, J., Yin, Y., Gao, W., Wang, D., … Xu, Y. (2019). The left dorsolateral prefrontal cortex volume is reduced in adults reporting childhood trauma independent of depression diagnosis. Journal of Psychiatric Research, 112, 12–17. 10.1016/j.jpsychires.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Maran, M., Grent-’t-Jong, T., & Uhlhaas, P. J. (2016). Electrophysiological insights into connectivity anomalies in schizophrenia: A systematic review. Neuropsychiatric Electrophysiology, 2(1), 6. 10.1186/s40810-016-0020-5 [DOI] [Google Scholar]
- Markovska-Simoska, S., Pop-Jordanova, N., & Pop-Jordanov, J. (2018). Inter- and intra-hemispheric EEG coherence study in adults with neuropsychiatric disorders. Prilozi (Makedonska Akademija Na Naukite I Umetnostite. Oddelenie Za Medicinski Nauki), 39(2–3), 5–19. 10.2478/prilozi-2018-0037 [DOI] [PubMed] [Google Scholar]
- McCutcheon, V. V., Heath, A. C., Nelson, E. C., Bucholz, K. K., Madden, P. a. F., & Martin, N. G. (2009). Accumulation of trauma over time and risk for depression in a twin sample. Psychological Medicine, 39(3), 431–441. 10.1017/S0033291708003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiers, G., Nooner, K., De Bellis, M. D., Debnath, R., & Tang, A. (2020). Alpha EEG asymmetry, childhood maltreatment, and problem behaviors: A pilot home-based study. Child Abuse & Neglect, 101, 104358. 10.1016/j.chiabu.2020.104358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, T., Smeets, T., Giesbrecht, T., Quaedflieg, C. W. E. M., Smulders, F. T. Y., Meijer, E. H., & Merckelbach, H. L. G. J. (2015). The role of frontal EEG asymmetry in post-traumatic stress disorder. Biological Psychology, 108, 62–77. 10.1016/j.biopsycho.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Meyers, J. L., Brislin, S. J., Kamarajan, C., Plawecki, M. H., Chorlian, D., Anohkin, A., … Porjesz, B. (2023). The collaborative study on the genetics of alcoholism: Brain function. Genes, Brain and Behavior, 22(5), e12862. 10.1111/gbb.12862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, J. L., Chorlian, D. B., Johnson, E. C., Pandey, A. K., Kamarajan, C., Salvatore, J. E., … Porjesz, B. (2019a). Association of polygenic liability for alcohol dependence and EEG connectivity in adolescence and young adulthood. Brain Sciences, 9(10), 280. 10.3390/brainsci9100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, J. L., McCutcheon, V. V., Pandey, A. K., Kamarajan, C., Subbie, S., Chorlian, D., … Porjesz, B. (2019b). Early sexual trauma exposure and neural response inhibition in adolescence and young adults: Trajectories of frontal theta oscillations during a go/no-go task. Journal of the American Academy of Child and Adolescent Psychiatry, 58(2), 242–255.e2. 10.1016/j.jaac.2018.07.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, J. L., Zhang, J., Chorlian, D. B., Pandey, A. K., Kamarajan, C., Wang, J.-C., … Porjesz, B. (2021). A genome-wide association study of interhemispheric theta EEG coherence: Implications for neural connectivity and alcohol use behavior. Molecular Psychiatry, 26(9), 5040–5052. 10.1038/s41380-020-0777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills, K. L., Teesson, M., Ross, J., & Peters, L. (2006). Trauma, PTSD, and substance use disorders: Findings from the Australian national survey of mental health and well-being. American Journal of Psychiatry, 163(4), 652–658. 10.1176/ajp.2006.163.4.652 [DOI] [PubMed] [Google Scholar]
- Mongillo, E. A., Briggs-Gowan, M., Ford, J. D., & Carter, A. S. (2009). Impact of traumatic life events in a community sample of toddlers. Journal of Abnormal Child Psychology, 37(4), 455–468. 10.1007/s10802-008-9283-z [DOI] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (1998). Mplus user's guide. Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Pandey, G., Seay, M. J., Meyers, J. L., Chorlian, D. B., Pandey, A. K., Kamarajan, C., … Porjesz, B. (2020). Density and dichotomous family history measures of alcohol use disorder as predictors of behavioral and neural phenotypes: A comparative study across gender and race/ethnicity. Alcoholism, Clinical and Experimental Research, 44(3), 697–710. 10.1111/acer.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola, C., Bennett, M. R., & Lagopoulos, J. (2016). Understanding heterogeneity in grey matter research of adults with childhood maltreatment—A meta-analysis and review. Neuroscience & Biobehavioral Reviews, 69, 299–312. 10.1016/j.neubiorev.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Park, S. M., Lee, J. Y., Kim, Y. J., Lee, J.-Y., Jung, H. Y., Sohn, B. K., … Choi, J.-S. (2017). Neural connectivity in internet gaming disorder and alcohol use disorder: A resting-state EEG coherence study. Scientific Reports, 7(1), 1333. 10.1038/s41598-017-01419-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawan, & Dhiman, R. (2023). Machine learning techniques for electroencephalogram based brain-computer interface: A systematic literature review. Measurement: Sensors, 28, 100823. 10.1016/j.measen.2023.100823 [DOI] [Google Scholar]
- Peltier, M. R., Roberts, W., Verplaetse, T. L., Zakiniaeiz, Y., Burke, C., Moore, K. E., & McKee, S. A. (2022). Sex differences across retrospective transitions in posttraumatic stress and substance use disorders. Journal of Dual Diagnosis, 18(1), 11–20. 10.1080/15504263.2021.2016027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périard, I. A.-C., Dierolf, A. M., Lutz, A., Vögele, C., Voderholzer, U., Koch, S., … Schulz, A. (2024). Frontal alpha asymmetry is associated with chronic stress and depression, but not with somatoform disorders. International Journal of Psychophysiology, 200, 112342. 10.1016/j.ijpsycho.2024.112342 [DOI] [PubMed] [Google Scholar]
- Pietrzak, R. H., Goldstein, R. B., Southwick, S. M., & Grant, B. F. (2011). Prevalence and axis I comorbidity of full and partial posttraumatic stress disorder in the United States: Results from wave 2 of the national epidemiologic survey on alcohol and related conditions. Journal of Anxiety Disorders, 25(3), 456–465. 10.1016/j.janxdis.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu, M., Popescu, E.-A., DeGraba, T. J., Fernandez-Fidalgo, D. J., Riedy, G., & Hughes, J. D. (2019). Post-traumatic stress disorder is associated with altered modulation of prefrontal alpha band oscillations during working memory. Clinical Neurophysiology, 130(10), 1869–1881. 10.1016/j.clinph.2019.06.227 [DOI] [PubMed] [Google Scholar]
- Porjesz, B., & Rangaswamy, M. (2007). Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. TheScientificWorldJournal, 7, 131–141. 10.1100/tsw.2007.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy, M., & Porjesz, B. (2008). Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: A review of human brain oscillations as effective endophenotypes. Brain Research, 1235, 153–171. 10.1016/j.brainres.2008.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read, J. P., Ouimette, P., White, J., Colder, C., & Farrow, S. (2011). Rates of DSM-IV-TR trauma exposure and posttraumatic stress disorder among newly matriculated college students. Psychological Trauma: Theory, Research, Practice and Policy, 3(2), 148–156. 10.1037/a0021260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor, C. E., McCutcheon, V. V., Nelson, E. C., Duncan, A. E., Bucholz, K. K., & Heath, A. C. (2012). Investigating the association between childhood sexual abuse and alcohol use disorders in women: Does it matter how we ask about sexual abuse? Journal of Studies on Alcohol and Drugs, 73(5), 740–748. 10.15288/jsad.2012.73.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor, C. E., McCutcheon, V. V., Pommer, N. E., Nelson, E. C., Grant, J. D., Duncan, A. E., … Heath, A. C. (2011). Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychological Medicine, 41(7), 1497–1505. 10.1017/S0033291710002072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schückher, F., Sellin, T., Fahlke, C., & Engström, I. (2018). The impact of childhood maltreatment on age of onset of alcohol use disorder in women. European Addiction Research, 24(6), 278–285. 10.1159/000494766 [DOI] [PubMed] [Google Scholar]
- Segalowitz, S. J., Santesso, D. L., & Jetha, M. K. (2010). Electrophysiological changes during adolescence: A review. Brain and Cognition, 72(1), 86–100. 10.1016/j.bandc.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Sharma, R., & Nadkarni, S. (2020). Biophysical basis of alpha rhythm disruption in Alzheimer's disease. eNeuro, 7(2), ENEURO.0293-19.2020. 10.1523/ENEURO.0293-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheerin, C. M., Bountress, K. E., Meyers, J. L., Saenz de Viteri, S. S., Shen, H., Maihofer, A. X., … Amstadter, A. B. (2020). Shared molecular genetic risk of alcohol dependence and posttraumatic stress disorder (PTSD). Psychology of Addictive Behaviors, 34(5), 613–619. 10.1037/adb0000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, X.-J., Xue, L., Liu, W., Chen, F.-Y., Zhu, C., Sun, X.-H., … Zhao, H. (2013). More vulnerability of left than right hippocampal damage in right-handed patients with post-traumatic stress disorder. Psychiatry Research: Neuroimaging, 212(3), 237–244. 10.1016/j.pscychresns.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M., & Sestan, N. (2016). The cellular and molecular landscapes of the developing human central nervous system. Neuron, 89(2), 248–268. 10.1016/j.neuron.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit, D. J. A., Andreassen, O. A., Boomsma, D. I., Burwell, S. J., Chorlian, D. B., de Geus, E. J. C., … Wright, M. J. (2021). Large-scale collaboration in ENIGMA-EEG: A perspective on the meta-analytic approach to link neurological and psychiatric liability genes to electrophysiological brain activity. Brain and Behavior, 11(8), e02188. 10.1002/brb3.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, E. A., Parsons, C. E., Van Hartevelt, T. J., Charquero-Ballester, M., McManners, H., Ehlers, A., … Kringelbach, M. L. (2015). Post-traumatic stress influences the brain even in the absence of symptoms: A systematic, quantitative meta-analysis of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 56, 207–221. 10.1016/j.neubiorev.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Stein, M. B., Koverola, C., Hanna, C., Torchia, M. G., & McCLARTY, B. (1997). Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine, 27(4), 951–959. 10.1017/S0033291797005242 [DOI] [PubMed] [Google Scholar]
- Stewart, J. L., Towers, D. N., Coan, J. A., & Allen, J. J. B. (2011). The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology, 48(1), 82–95. 10.1111/j.1469-8986.2010.01035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbie-Saenz de Viteri, S., Pandey, A., Pandey, G., Kamarajan, C., Smith, R., Anokhin, A., … Meyers, J. L. (2020). Pathways to post-traumatic stress disorder and alcohol dependence: Trauma, executive functioning, and family history of alcoholism in adolescents and young adults. Brain and Behavior, 10(11), e01789. 10.1002/brb3.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher, J., Fischer, P., Neumeister, A., Rappelsberger, P., & Kasper, S. (1998). Low frontal electroencephalographic coherence in neuroleptic-free schizophrenic patients. Biological Psychiatry, 44(6), 438–447. 10.1016/s0006-3223(97)00428-9 [DOI] [PubMed] [Google Scholar]
- Tcheslavski, G. V., & Gonen, F. F. (2012). Alcoholism-related alterations in spectrum, coherence, and phase synchrony of topical electroencephalogram. Computers in Biology and Medicine, 42(4), 394–401. 10.1016/j.compbiomed.2011.12.006 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., Andersen, S. L., Polcari, A., Anderson, C. M., Navalta, C. P., & Kim, D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews, 27(1–2), 33–44. 10.1016/s0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., Ito, Y., Glod, C. A., Andersen, S. L., Dumont, N., & Ackerman, E. (1997). Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRIa. Annals of the New York Academy of Sciences, 821(1), 160–175. 10.1111/j.1749-6632.1997.tb48277.x [DOI] [PubMed] [Google Scholar]
- Teicher, M. H., & Samson, J. A. (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American Journal of Psychiatry, 170(10), 1114–1133. 10.1176/appi.ajp.2013.12070957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H., & Samson, J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 57(3), 241–266. 10.1111/jcpp.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher, R. W., North, D. M., & Biver, C. J. (2008). Development of cortical connections as measured by EEG coherence and phase delays. Human Brain Mapping, 29(12), 1400–1415. 10.1002/hbm.20474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij, S. J. H., Stevens, J. S., Ely, T. D., Hinrichs, R. C., Michopoulos, V., Winters, S. J., … Jovanovic, T. (2018). The role of the hippocampus in predicting future PTSD symptoms in recently traumatized civilians. Biological Psychiatry, 84(2), 106–115. 10.1016/j.biopsych.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian, H., Chantarujikapong, S. I., Scherrer, J. F., Eisen, S. A., Lyons, M. J., Goldberg, J., … True, W. R. (2000). Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and Alcohol Dependence, 61(1), 95–102. 10.1016/S0376-8716(00)00127-7 [DOI] [PubMed] [Google Scholar]
- Zhang, H., Geng, X., Wang, Y., Guo, Y., Gao, Y., Zhang, S., … Wang, L. (2021). The significance of EEG alpha oscillation spectral power and beta oscillation phase synchronization for diagnosing probable Alzheimer disease. Frontiers in Aging Neuroscience, 13, 631587. 10.3389/fnagi.2021.631587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn, M. L., Zinn, M. A., & Jason, L. A. (2016). Intrinsic functional hypoconnectivity in core neurocognitive networks suggests central nervous system pathology in patients with myalgic encephalomyelitis: A pilot study. Applied Psychophysiology and Biofeedback, 41(3), 283–300. 10.1007/s10484-016-9331-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neale et al. supplementary material