Abstract
The metabolism of [4-14C]oestrone and of [6,7-3H2]oestrone sulphate was studied during cyclic perfusion and once-through perfusion of the isolated rat liver. The following results were obtained. 1. As shown by once-through perfusion, the two steroids are metabolized differently during the first passage through the organ. [4-14C]Oestrone was taken up by the liver and partly delivered as oestradiol-17β and oestriol into the medium. After uptake of [6,7-3H2]oestrone sulphate, only oestrone, liberated by hydrolysis, was delivered into the medium; no oestradiol-17β or oestriol could be detected in the medium after one passage through the organ. This indicates that intracellular oestrone, which was taken up as such, and oestrone, which derived from intracellular hydrolysis, may be metabolized in different compartments of the liver cell. 2. The results of the cyclic perfusion showed that intracellular oestrone is preferentially conjugated with glucuronic acid, and subsequently excreted into the bile. Intracellular oestrone sulphate is preferably reduced to oestradiol sulphate, thus indicating that oestrone sulphate is a better substrate for the 17β-hydroxy steroid oxidoreductase than is oestrone. 3. Albumin-bound oestrone sulphate acts as a large reservoir, and in contrast with free oestrone is protected from enzyme attack by its strong binding to albumin. 4. Oestrone sulphate is partly converted into the hormonally active oestrone by liver tissue. This suggests that liver not only inactivates oestrogens, but also provides the organism with oestrone, which is subsequently readily taken up by other organs.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradlow H. L. Extraction of steroid conjugates with a neutral resin. Steroids. 1968 Mar;11(3):265–272. doi: 10.1016/s0039-128x(68)80139-4. [DOI] [PubMed] [Google Scholar]
- Brooks S. C., Leithauser G., De Loecker W. C., De Wever F. In vitro stimulation of protein synthesis in uterine microsomal supernatant by estrone sulfate. Endocrinology. 1969 Apr;84(4):901–907. doi: 10.1210/endo-84-4-901. [DOI] [PubMed] [Google Scholar]
- Brown J. B., Smyth B. J. Oestrone sulphate--the major circulating oestrogen in the normal menstrual cycle? J Reprod Fertil. 1971 Jan;24(1):142–142. doi: 10.1530/jrf.0.0240142. [DOI] [PubMed] [Google Scholar]
- Dahm K., Breuer H. Vergleichende Untersuchungen über den Stoffwechsel von Oestronsulfat und Oestron in Zellfraktionen der Rattenleber. Biochim Biophys Acta. 1967 Feb 14;137(1):196–198. [PubMed] [Google Scholar]
- Fishman J., Hellman L. Comparative fate of estrone and estrone sulfate in man. J Clin Endocrinol Metab. 1973 Jan;36(1):160–164. doi: 10.1210/jcem-36-1-160. [DOI] [PubMed] [Google Scholar]
- Gelbke H. P., Knuppen R. A new method for preventing oxidative decomposition of catechol estrogens during chromatography. J Chromatogr. 1972 Sep 20;71(3):465–471. doi: 10.1016/s0021-9673(01)91900-4. [DOI] [PubMed] [Google Scholar]
- Hellström K., Sjövall J., Vihko R. Plasma levels and excretion of solvolyzable steroids. Acta Endocrinol (Copenh) 1969 Mar;60(3):501–511. doi: 10.1530/acta.0.0600501. [DOI] [PubMed] [Google Scholar]
- Höller M., Breuer H. Eine optimale Methode zur hämoglobinfreien Perfusion der isolierten Rattenleber. Z Klin Chem Klin Biochem. 1974 Sep;12(9):398–402. [PubMed] [Google Scholar]
- Höller M., Breuer H. The effect of fluorocarbon FC 43 on the metabolism of steroids during perfusion of the isolated rat liver. Z Klin Chem Klin Biochem. 1975 Jul;13(7):319–323. doi: 10.1515/cclm.1975.13.7.319. [DOI] [PubMed] [Google Scholar]
- Höller M., Chalybäus C., Breuer H. Effect of oxygen shortage on the metabolism of oestrone in the hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem. 1976 Sep;357(9):1215–1221. doi: 10.1515/bchm2.1976.357.2.1215. [DOI] [PubMed] [Google Scholar]
- Longcope C. The metabolism of estrone sulfate in normal males. J Clin Endocrinol Metab. 1972 Jan;34(1):113–122. doi: 10.1210/jcem-34-1-113. [DOI] [PubMed] [Google Scholar]
- Loriaux D. L., Ruder H. J., Lipsett M. B. The measurement of estrone sulfate in plasma. Steroids. 1971 Oct;18(4):463–472. doi: 10.1016/0039-128x(71)90059-6. [DOI] [PubMed] [Google Scholar]
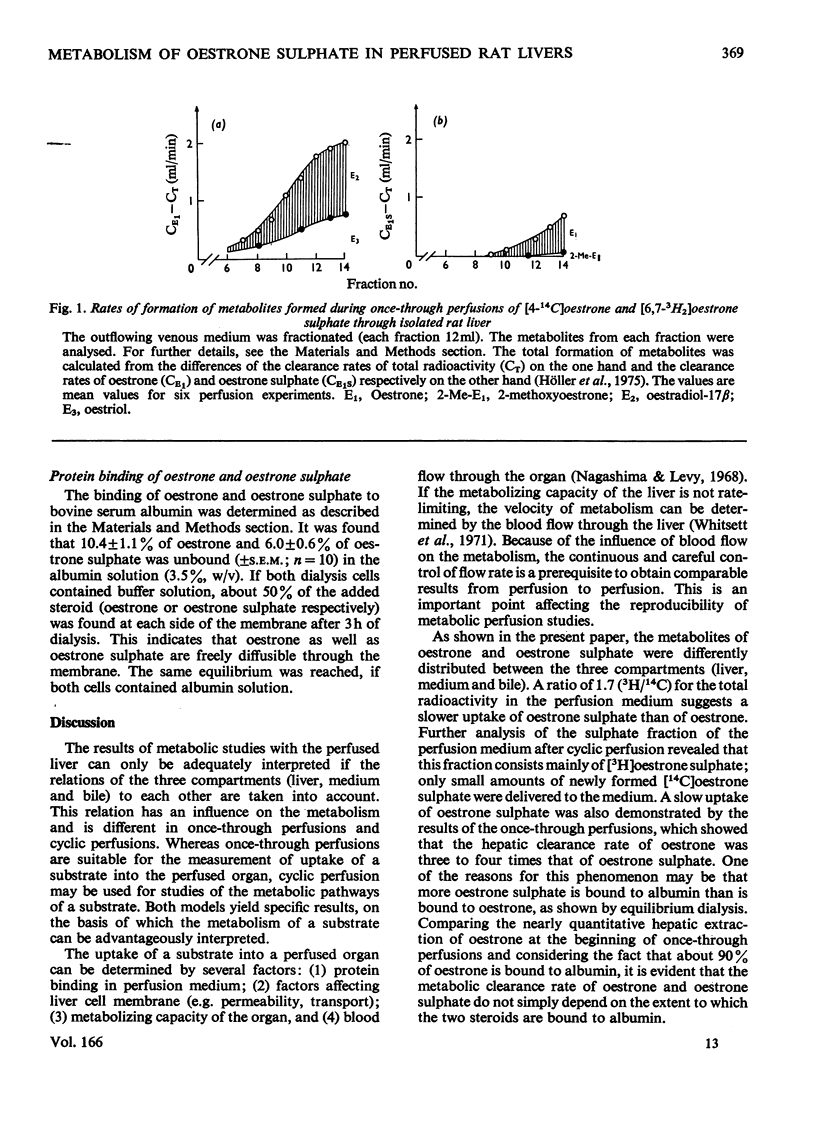
- Nagashima R., Levy G. Effect of flow rate on the distribution kinetics of a drug from perfusate to a perfused organ. J Pharm Sci. 1968 Nov;57(11):2000–2002. doi: 10.1002/jps.2600571140. [DOI] [PubMed] [Google Scholar]
- Osawa Y., Slaunwhite W. R., Jr Studies on phenolic steroids in human subjects. 13. A rapid assay of urinary estrogen conjugates in pregnancy. Steroids. 1970 Jan;15(1):73–88. doi: 10.1016/s0039-128x(70)80005-8. [DOI] [PubMed] [Google Scholar]
- PURDY R. H., ENGEL L. L., ONCLEY J. L. The characterization of estrone sulfate from human plasma. J Biol Chem. 1961 Apr;236:1043–1050. [PubMed] [Google Scholar]
- Rosenthal H. E., Pietrzak E., Slaunwhite W. R., Jr, Sandberg A. A. Binding of estrone sulfate in human plasma. J Clin Endocrinol Metab. 1972 May;34(5):805–813. doi: 10.1210/jcem-34-5-805. [DOI] [PubMed] [Google Scholar]
- Ruder H. J., Loriaux L., Lipsett M. B. Estrone sulfate: production rate and metabolism in man. J Clin Invest. 1972 Apr;51(4):1020–1033. doi: 10.1172/JCI106862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. A., Kirdani R. Y., Back N., Weyman P., Slaunwhite W. R., Jr Biliary excretion and enterohepatic circulation of estrone and estriol in rodents. Am J Physiol. 1967 Nov;213(5):1138–1142. doi: 10.1152/ajplegacy.1967.213.5.1138. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Sjövall K., Vihko R. Steroid sulfates in human pregnancy plasma. Steroids. 1968 May;11(5):703–715. doi: 10.1016/s0039-128x(68)80017-0. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Whitsett T. L., Dayton P. G., McNay J. L. The effect of hepatic blood flow on the hepatic removal rate of oxyphenbutazone in the dog. J Pharmacol Exp Ther. 1971 Apr;177(1):246–255. [PubMed] [Google Scholar]


