Abstract
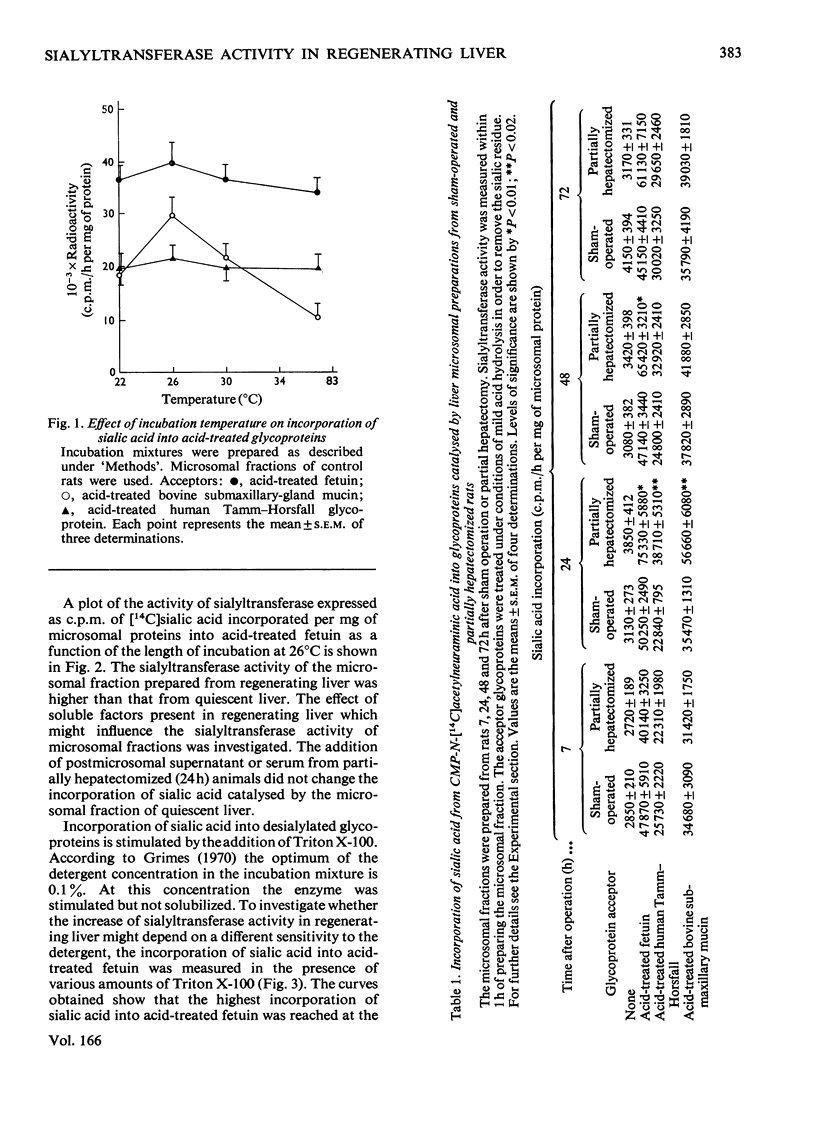
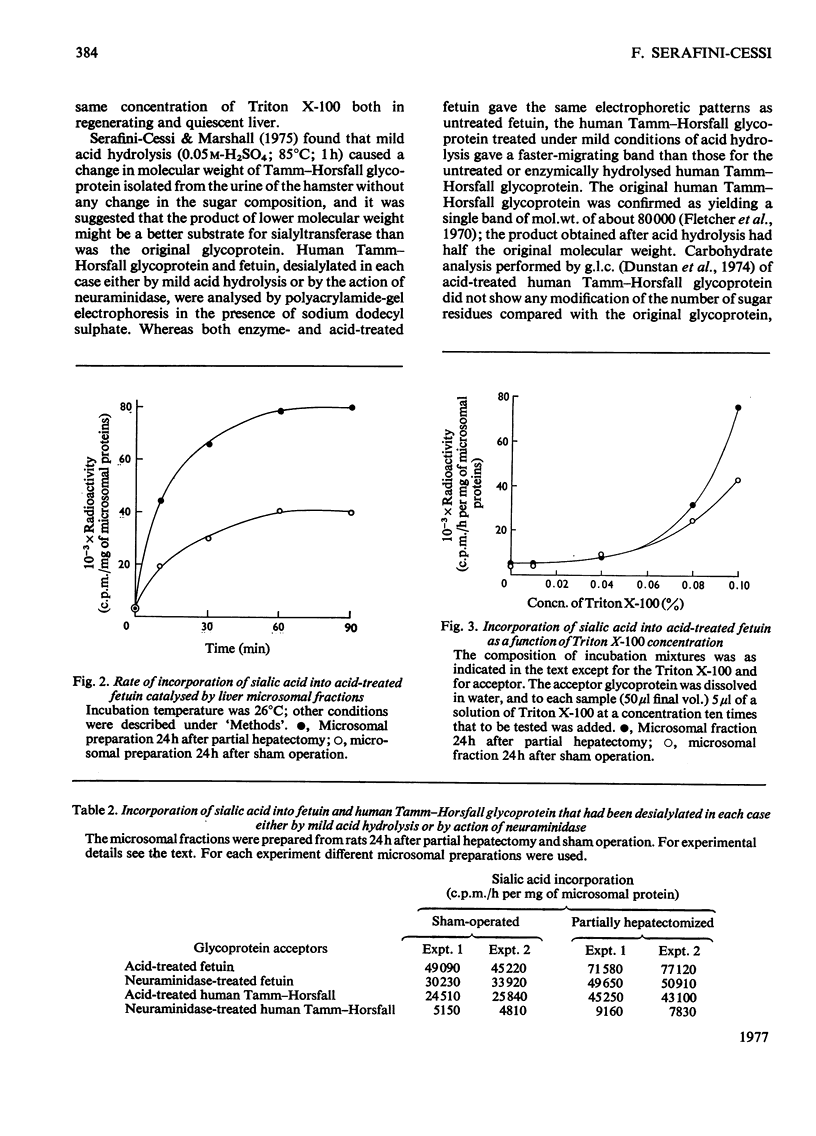
Liver microsomal fractions catalyse the transfer of sialic acid from CMP-N-acetyl-neuraminic acid to various exogenous acceptors such as desialylated fetuin, desialylated human Tamm–Horsfall glycoprotein and desialylated bovine submaxillary-gland mucin. An increase in the rate of incorporation of sialic acid into desialylated glycoproteins was found after a lag period (7h) in regenerating liver. The increase was maximum 24h after partial hepatectomy for all acceptors tested. At later times after operation the sialyltransferase activity remained high only for desialylated fetuin. No soluble factors from liver or serum of partially hepatectomized animals influenced the activity of the sialyltransferases bound to the microsomal fraction. The sensitivity of sialyltransferases to activation by Triton X-100, added to the incubation medium, was unchanged in the microsomal preparation from animals 24h after sham operation or partial hepatectomy. The full activity of sialyltransferases towards the various desialylated acceptors showed some differences. Human Tamm–Horsfall glycoprotein was a good acceptor of sialic acid only when desialylated by mild acid hydrolysis. After this treatment, but not after enzymic hydrolysis, a decrease in molecular weight of human Tamm–Horsfall glycoprotein was observed. Further, the sialyltransferase activity as a function of incubation temperature gave different curves according to the acceptor used. The relationship between the biosynthesis of glycoproteins by regenerating liver and the sialyltransferase activity of microsomal fraction after partial hepatectomy is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu N., Maeda H. R. Formation of glucosamine 6-phosphate in regenerating rat liver. Biochim Biophys Acta. 1971 Aug 19;244(2):311–317. doi: 10.1016/0304-4165(71)90231-5. [DOI] [PubMed] [Google Scholar]
- BRAUN G. A., MARSH J. B., DRABKIN D. L. Synthesis of plasma albumin and tissue proteins in regenerating liver. Metabolism. 1962 Sep;11:957–966. [PubMed] [Google Scholar]
- BUCHER N. L. REGENERATION OF MAMMALIAN LIVER. Int Rev Cytol. 1963;15:245–300. doi: 10.1016/s0074-7696(08)61119-5. [DOI] [PubMed] [Google Scholar]
- Bauer C. H., Hassels B. F., Reutter W. G. Galactose metabolism in regenerating rat liver. Biochem J. 1976 Jan 15;154(1):141–147. doi: 10.1042/bj1540141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B. Membrane glycoprotein transferases: time courses of activity changes in a synchronized cell population and enzyme activity half-lives in an asynchronous population. Arch Biochem Biophys. 1971 Jul;145(1):310–318. doi: 10.1016/0003-9861(71)90041-5. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Sialyl transferase activity in normal and RNA- and DNA-virus transformed cells utilizing desialyzed, trypsinized cell plasma membrane external surface glycoproteins as exogenous acceptors. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1256–1262. doi: 10.1016/0006-291x(72)90603-1. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Glick M. C., Warren L. Glycopeptides from the surface of control and virus-transformed cells. Science. 1971 Apr 9;172(3979):169–171. doi: 10.1126/science.172.3979.169. [DOI] [PubMed] [Google Scholar]
- Carlson D. M., David J., Rutter W. J. Galactosyltransferase activities in pancreas, liver and gut of the developing rat. Arch Biochem Biophys. 1973 Aug;157(2):605–612. doi: 10.1016/0003-9861(73)90680-2. [DOI] [PubMed] [Google Scholar]
- Dunstan D. R., Grant A. M., Marshall R. D., Neuberger A. A protein, immunologically similar to Tamm-Horsfall glycoprotein, produced by cultured baby hamster kidney cells. Proc R Soc Lond B Biol Sci. 1974 Jul 30;186(1085):297–316. doi: 10.1098/rspb.1974.0051. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The chemical composition. Biochem J. 1970 Nov;120(2):417–424. doi: 10.1042/bj1200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes W. J. Glycosyltransferase and sialic acid levels of normal and transformed cells. Biochemistry. 1973 Feb 27;12(5):990–996. doi: 10.1021/bi00729a031. [DOI] [PubMed] [Google Scholar]
- Grimes W. J. Sialic acid transferases and sialic acid levels in normal and transformed cells. Biochemistry. 1970 Dec 22;9(26):5083–5092. doi: 10.1021/bi00828a007. [DOI] [PubMed] [Google Scholar]
- Jato-Rodriguez J. J., Mookerjea S. UDP-galactose:glycoprotein galactosyltransferase activity in tissues of developing rat. Arch Biochem Biophys. 1974 May;162(1):281–292. doi: 10.1016/0003-9861(74)90127-1. [DOI] [PubMed] [Google Scholar]
- KELLER E. B., ZAMECNIK P. C. The effect of guanosine diphosphate and triphosphate on the incorporation of labeled amino acids into proteins. J Biol Chem. 1956 Jul;221(1):45–59. [PubMed] [Google Scholar]
- Khalkhali Z., Serafini-Cessi F., Marshall R. D. The UDP-N-acetylglucosamine-asparagine-sequon N-acetyl-beta-D-glucosaminyltransferase activity in preparations of rough endoplasmic reticulum from regenerating rat liver. Biochem J. 1977 Apr 15;164(1):257–261. doi: 10.1042/bj1640257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macbeth R. A., Bekesi J. G., Sugden E., Bice S. The metabolism of plasma glycoproteins. I. Studies on the rate of incorporation of glucosamine-1-14C into protein-bound hexosamine and N-acetylneuraminic acid in the normal rat. J Biol Chem. 1965 Oct;240(10):3707–3713. [PubMed] [Google Scholar]
- Marshall R. D., Zamecnik P. C. Some physical properties of lysyl and arginyl-transfer RNA synthetases of Escherichia coli B. Biochim Biophys Acta. 1969 Jul 1;181(2):454–464. doi: 10.1016/0005-2795(69)90279-7. [DOI] [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Van den Hamer C. J., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. IV. Preparation of radioactive, sialic acid-free ceruloplasmin labeled with tritium on terminal D-galactose residues. J Biol Chem. 1966 Aug 25;241(16):3745–3749. [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- ROBINSON G. B., MOLNAR J., WINZLER R. J. BIOSYNTHESIS OF GLYCOPROTEINS. I. INCORPORATION OF GLUCOSAMINE-14C INTO LIVER AND PLASMA PROTEINS OF THE RAT. J Biol Chem. 1964 Apr;239:1134–1141. [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- Serafini-Cessi F. Serum glycoprotein synthesis after partial hepatectomy in the rat. Biochem J. 1976 Jul 15;158(1):153–155. doi: 10.1042/bj1580153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950 May;74(1):106–108. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Warren L., Fuhrer J. P., Buck C. A. Surface glycoproteins of normal and transformed cells: a difference determined by sialic acid and a growth-dependent sialyl transferase. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1838–1842. doi: 10.1073/pnas.69.7.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]


