Abstract
Aquaporins (AQPs) are integral membrane proteins responsible for facilitating the transmembrane transport of water and small solutes. Their involvement in diverse physiological functions extends to pathological conditions, including cancer, positioning them as promising targets for anticancer therapy. Tumor cells, particularly those with high metastatic potential, exhibit elevated AQP expression, reinforcing their critical role in tumor biology. Emerging evidence highlights AQPs' involvement in key oncogenic processes such as cell migration, proliferation, and tumor-associated edema, suggesting their potential as novel therapeutic targets. Despite this, the development of selective and potent AQP inhibitors has proven challenging. Efforts to produce small-molecule AQP inhibitors have largely been unsuccessful. However, recent advancements include monoclonal human IgG antibodies targeting extracellular domains of aquaporin-4, offering new therapeutic strategies, particularly in glioblastoma, where AQP-4 is overexpressed. However, recent advancements include monoclonal human IgG antibodies targeting extracellular domains of aquaporin-4, offering new therapeutic strategies, particularly in glioblastoma, where AQP-4 is over expressed. These antibodies hold promise for selectively targeting and eradicating AQP-4-expressing cells in malignant brain tumors. This review discusses the critical role AQPs play in cancer, including their contributions to tumor cell proliferation, migration, angiogenesis, and edema formation. Additionally, we explore innovative therapeutic approaches, such as antibody-based interventions, and outline potential future research directions in AQP-targeted cancer therapies.
Aquaporins (AQPs) are integral membrane proteins responsible for facilitating the transmembrane transport of water and small solutes.
1. Introduction
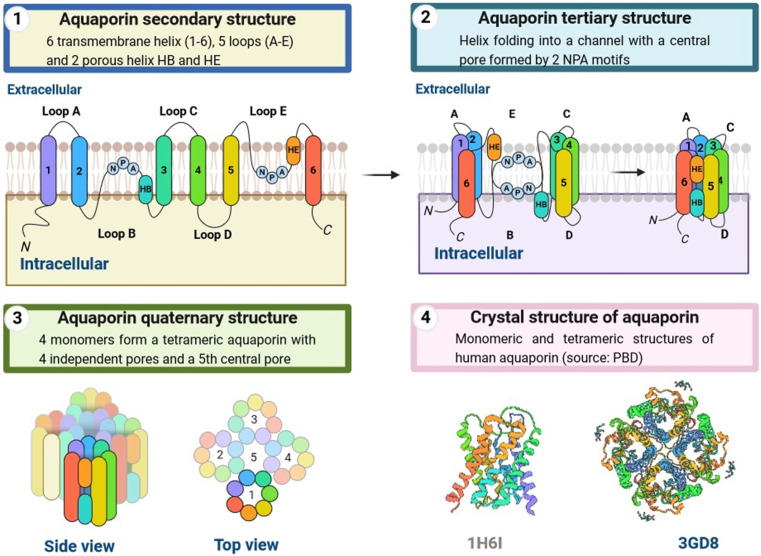
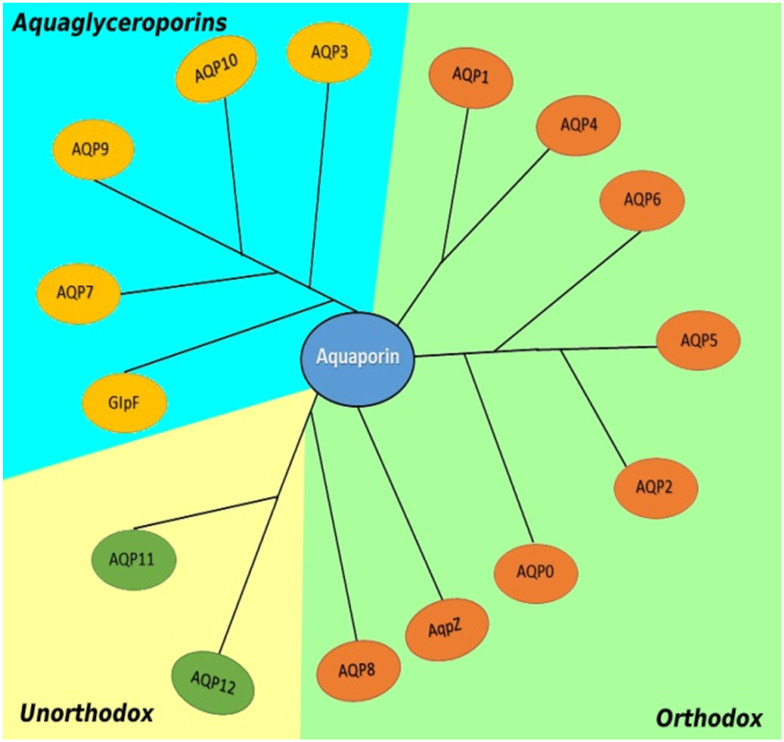
Aquaporins (AQPs) are water-channel proteins found in all life forms and are essential to life.1,2 Aquaporins are found in secondary, tertiary, and quaternary structures. The secondary form is composed of 6 transmembrane alpha helices and 5 loops. These helices fold into a channel with an NPA (amino acids) motif at its center, forming the pore. Four subunits form the tetrameric quaternary form of the aquaporin as shown in Fig. 1. AQPs are glycosylated, widely-expressed, integral membrane proteins found in bacteria,3 mammals,4 plants,5 sand yeast.6 The first AQP detected, originally designated CHIP28 and later renamed aquaporin-1, was found in human erythrocytes6 and renal proximal tubule membrane. Mammalian AQP isoforms have been identified7 and are expressed in multiple organs and tissues, including the brain,8 liver, lungs, kidneys, skin, eyes, secretary glands,9 and sweat glands.9 The 13 AQPs (AQP0-12) present in mammals have been divided into orthodox AQPs (AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP8) and aquaglyceroporins (AQP3, AQP7, AQP9, AQP10), based on their ability to conduct only water or water and acidic solutes, respectively, in specific glycerol, as shown in Fig. 1. Because of their distinct developmental mechanisms and transport properties, a new group called unorthodox AQPs has been identified,10 which includes AQP11 and AQP12.11 Many AQPs also transport ammonia (AQP3, AQP4, AQP6, AQP8, and AQP9) and/or hydrogen peroxide (AQP1, AQP3, AQP5, AQP8, and AQP9) and are also referred to as ammoniaporins (or aquaammoniaporins)12 or peroxiporins because of their biophysical properties.13 Some AQPs allow physiologically important gases, including CO2, NO, and O2, to permeate membranes. AQP expression, transportation properties, and pharmacological gating are being intensely investigated, with several important roles having been elucidated both in healthy and diseased conditions.14
Fig. 1. Aquaporins' secondary, tertiary, and quaternary structures are described. The secondary form is made up of six transmembrane alpha helices and five loops. These helices fold into a channel with an NPA (amino acids) motif in the centre, establishing the pore. The aquaporin has four subunits that create a tetrameric quaternary structure.
A growing amount of data has addressed AQP involvement in inflammation; AQPs take part as regulators of innate host defenses at the cell membrane.9 AQPs may be involved in the inflammatory activation that regulates the volume of cells.6 These last proteins are less known, and their composition and subcellular distribution vary in part from those of other AQP classes.7 The widespread distribution of AQPs in cells and tissues has increased the scientific interest in their structural and functional characterization, helping to reinforce the idea that water permeability is necessary for a variety of physiological processes.
AQP-dependent gas transport is faster than free diffusion and plays an important role in biological functions.9 Phenotypic analyses have identified physiological and pathological implications of AQP function (reviewed in, e.g. in brain swelling and glandular fluid secretion).9,11 The therapeutic opportunities for AQP water channel blockers in cerebral edema, water retention, and intraocular pressure control correlated with glaucoma and other disorders have also been addressed. Increasing numbers of studies over the past 10 years suggest that increased water movement across AQP channels, caused by local osmotic gradients, is responsible for the development of membrane protrusions during cell migration, a general phenomenon observed for several cell types and specific AQPs., e.g., AQP1, AQP3, AQP4, or AQP9.11,12 Several AQPs are upregulated in many types of cancer, and their expression is correlated with higher tumor grades.14
Increasing numbers of studies over the past 10 years suggest that increased water movement across AQP channels, caused by local osmotic gradients, is responsible for the development of membrane protrusions during cell migration,15 a general phenomenon observed for several cell types and specific AQPs., e.g., AQP1,16 AQP3,17 AQP4,18 or AQP9.19 Several AQPs are upregulated in many types of cancer, and their expression is correlated with higher tumor grades.
2. AQP structure
AQPs are relatively simple proteins and are similar to ion channels and solute transporters in terms of their structure and function.20 For several mammalian AQPs, high-resolution X-ray crystal structures have been calculated. Every ∼30 kDa AQP monomer consists of six membrane-spanning helical domains and two small helical segments (HB and HE) containing cytoplasmic and extracellular vestige channels, respectively. Such vestibules are linked in length by a narrow aqueous pore of ∼25 Å (1 Å = 0.1 nm).21 The TM domains have two “loops” entering the membrane and facing each other through a preserved asparagine–proline–alanine (NPA) motif, with a cytoplasmic orientation of both N- and C-terminal tails.22 The structure is formed by a single chain with about 270 amino acid residues, with the amino (N) and carboxyl (C) termini are located in the cytoplasm.23 Two highly conserved sequence motifs, NPA motifs with a short helix, are located on opposite sides of the monomer.21 NPA motifs bend into the molecule to pair with each other and form the water channel.22 A cysteine residue (Cys 189) can be found in most AQPs near the channel in an extracellular orientation, which can block AQPs with functional sensitivity to mercury. Each monomer in the tetramer works as an independent water channel. Several studies have shown that the central pores in AQP1, AQP4, and AQP5 are permeable to O2, CO2, or NO, respectively.21–23
3. AQP family member
Aquaporin-0 (AQP0) is expressed primarily in the eye lens fibers and is known as the main intrinsic protein (MIP) of the lens.24 It was named MIP 26, as it migrated to the same extent as 26 kDa proteins on gels.10 Researchers have investigated MIP for the last 30 years, and various approaches have suggested possible functions without conclusive results. More recent studies, however, have unraveled its structure and function, and it is now also known as aquaporin-0 (AQP0).25 Mutations in the AQP0 gene in humans and mice lead to genetic cataracts; deleting the Aqp0 gene in mice leads to a lack of formation of sutures required to maintain the architecture of the lens fiber, leading to a disturbed accommodation and focus of the eye lens. MIP/AQP0 functions as both a water channel and an adhesive molecule in lens fibers to help reduce the intercellular space required for lens transparency and accommodation.26
AQP1 is expressed abnormally in endothelial cells of tumors as shown in Fig. 2. There is a mouse knockout of Aqp1 the metastatic potential of AQP1-expressing versus non-expressing tumors has been described. Knockdown of AQP1 with siRNA reversed tumor growth in a mucous melanoma model.27 Without specific, powerful, and non-toxic water channel inhibitors of AQPs, their precise role in cell migration is still elusive.28 However, the possible clinical effects of HAQP1 inhibitors may naturally supplement existing treatments for cancer, such as anti-VEGF therapy in cases where resistance to anti-VEGF treatment has been identified.29 AQP1 is found in endothelial cells in the large microvessels outside the central nervous system, such as the kidney, AQP1 expression in the proximal and narrow descending tubes of Henle is high, which correlates with extremely high permeability in these structures.30 In contrast, AQPs seem to be expressed in renal tubular epithelia that have very low water permeability (thin upward limb, thick upward limb, and distal convoluted tubules). In the last part of the renal tubule, the connecting tubules.28 AQP1 is highly expressed in the proximal tubule and thin descending limbs of the loop of Henle, which are both very water permeable at all times. AQP1 is the red-cell water channel.26–28 Some sections of the tubules reabsorb 90% of the filtered water. The first “structures” of AQP1, the projection structures and the 3D reconstruction obtained from two-dimensional crystal electron microscopy at 12–16 Å resolution, show that the AQP1 operating unit is a tetramer with the helices in each monomer normally oriented toward the membrane plane.28,29 The path followed by water in AQP1 has been clearly defined in the first atomic structure (3.8 Å resolution) reported in the year 2000.27 The water pathway consists of extracellular vestibules and cytoplasm connected by a narrow pore, which acts as a selectivity filter.26 The concept of emergency medical technician is related to a complex molecular and cellular procedure by which epithelial cells lose certain characteristics (such as cell–cell adhesion, planar and apical–basal polarity, and lack of motility) and acquire mesenchymal features (motility, invasiveness, and resistance to apoptosis), and the fact that AQP1 serves a significant part during this stage offers a significant indicator for the role of AQP1 during mammalian carcinogenesis.29
Fig. 2. In mammals, aquaporin isoforms are often organised into subfamilies. Humans have paralogs from tandem duplications, resulting in four extra AQP7 pseudogenes and a second copy of AQP12. The majority of them have a distinct tissue-specific expression pattern. Most mammalian AQPs may be dynamically controlled, making them prospective targets for the development of novel therapies to treat disorders related with water homeostasis disruptions.
AQP2 is a VP-regulated water channel. In the presence of VP, AQP2 builds to the surface of the main cell and the liquid is distributed across the epithelium through osmotic gradients (Fig. 2). In the lumen of the tubule, the base component is hypertonic and water flows down the osmotic gradient in the presence of VP.31 The basolateral membrane of primary cells is always permeable to water due to the presence of AQP3 or AQP4.32 Therefore, AQP2 is the rate-limiting factor in the apical membrane that controls the water reabsorption in the kidney collection channel.31 The cell biology underlying this mechanism is understood in part, with a signal cascade triggered by the interaction of VP with its receptor, activation of adenylyl cyclase to increase intracellular cAMP, activation of protein kinase A, and phosphorylation of AQP2.32 Phosphorylation is a critical event that causes the accumulation of AQP2 membranes, and several laboratories33,34 are engaged in research to further understand this process.35 AQP2 is a 29 kDa hydrophobic glycoprotein.35 Vasopressin withdrawal is linked to endocytic recovery into a unique nonacidic endosomal body of functional water channels.36 Two major forms of hereditary nephrogenic diabetes insipidus (NDI) have been identified: a more common recessive X-linked form caused by V2 receptor mutations and a rare non-X-linked form caused by AQP2 mutations.37 There have been multiple mutations associated with human NDI in the AQP2 coding sequence.35 Expression of the mutants T126M, R187C, S116P, and A147T in Xenopus oocytes caused impaired plasma membrane trafficking.38 Nonetheless, the pathways used to induce NDIs in mammalian cells by AQP2 mutations remain unknown. Aquaporin 2 in cancer has received a lot of attention because of its probable function in regulating tumour growth and metastasis. Recent research suggests that changes in the expression of this water channel protein may affect cell proliferation and death in a variety of cancer forms.31,32,35,36
AQP3 is found in plasma membranes in basal epidermis keratinocytes in normal skin. In response to skin pressure in diseases such as atopic eczema, and skin carcinomas, AQP3 expression in human skin is increased. The stratum corneum water content and elasticity of AQP3-knockout mice were decreased relative to wild-type (WT) mice and compromised wound healing and epidermal biosynthesis.14 Recent evidence shows the role of AQPs in angiogenesis, cell proliferation, and metastasis. AQP3 is reportedly involved in skin tumorigenesis. Aqp3 null mice are resistant to skin tumor growth, while skin squamous cell carcinomas.36 Human and mouse skin express AQP3 in the plasma membranes of the basal layer of keratinocytes. AQP3 is overexpressed in colon cancer, lung cancer, oral squamous cell carcinoma, and esophageal cancer.14 In addition, AQP3 is overexpressed with aquaporin-5 (AQP5) in hepatocellular carcinoma and pancreatic ductal adenocarcinomas.37 In a clinical study, 65 of 89 gastric adenocarcinomas overexpressed, which was correlated with histological classification, lymph node metastasis, and lymphovascular invasion that decreased patient survival relative to tumors with lower AQP3 expression.39 In breast cancer, CXCL12/CXCR4-dependent cell migration is key in progression, and AQP3 is required for H2O2 transport via AQP3 channel activation. CXCL12/CXCR4-dependent cell migration plays a vital role in breast cancer progression.14 AQP3 is expressed in multiple human epithelial plasma basolateral membranes. AQP3 is involved in the transport of water and glycerol in the gastric tract, including the ileum39 and distal colon.40 AQP3 is also found in the upper and lower airways where osmotic water transportation is facilitated via airway epithelial cells.41 The plasma membrane of keratinocytes in the basal and spinal layers of the epidermis has high levels of AQP3.42 In this case, AQP3 serves as water and glycerol transporter to support skin moisture and can also contribute to cell movement during wound healing. In corneal epithelia, AQP3 facilitates the transfer of water and glycerol, similar to its role during wound healing.43
AQP4 is slightly permeable to water. In the mammalian brain, isoforms 1, 4, and 9 are expressed.8 The most abundant brain, spinal, and optic nerve isoform is AQP4, which regulates water homeostasis.44 Verkman's group initially named this aquaporin the mercury-insensitive water channel (MIWC), which was later reclassified as AQP4.44 AQP4 is enriched in astrocytes and ependymal cells fastened to perivascular astrocytes in the astrocyte region nearest to the vessel, the concentration of AQP4 is high. AQP4 can become mislocalized, with widespread distribution of AQP4 in the astrocyte instead of in the blood vessel.8 AQP4 regulates bidirectional fluid exchange due to its particularly high expression in the blood–brain (BBB) and blood–cerebrospinal fluid (CSF) barriers. An alteration of AQP4 transcription or translation has been correlated with an increasing number of neurological conditions.44 Imbalances of blood fluid homeostasis have been linked with conditions such as traumatic brain injuries and stroke. Increasing evidence implicate AQP4 in brain inflammation, removal of lymph fluids, synaptic plasticity, and extracellular space control.44,45 AQP4 functions are largely based on results of post mortems, in vitro studies, and the use of deficient mouse models in several pathogenic conditions.37 BBB failures can occur in many brain injuries, due to the loss of AQP4 polarization in astrocytologically perivascular endfeet. This can be especially relevant in Alzheimer's disease and for brain aging.46 AQP4 has been reported as the main antigen in neuromyelitis spectral syndrome (NMOSD), a neurological autoimmune disease.44 AQP4 NMOSD is an astrocytopathy characterized by astrocyte impairment, optical nerve and spinal cord demyelination, and brain demyelination.45 Patients usually have demyelinating lesions that extend over three or more vertebral sections. Around 80% of the patients who have NMOSD have AQP4 (AQP4-IgG) autoantibodie.46 Many disorders, including cytotoxic brain edema, may be treated through inhibition of AQP4. In a rat stroke model, it was reported that TGN-020 (2-(nicotinamide)-1,3,4-thiadiazole) acts as a strong AQP4 blocker.47 Aquaporins play functions in tumour development, oedema, angiogenesis, and cell migration. Aquaporin inhibitors or downregulators could be new anti-cancer medications.46
AQP5 has been found to be highly regulated in multiple tumors and is known to be involved in carcinogenesis in multiple organs and systems in humans.48 AQP5 can increase extracellular H2O2 inflows with dual effects on the cellular oxidative response.49 AQP5 induces higher sensitivity initially in acute oxidative stress conditions, whereas cells expressing AQP5 show better cell survival and resilience in chronic stress.50 AQP5 mRNA and protein are expressed in human breast cancer cell lines MCF7 and MDA-MB-231, both of which are derived from epithelial duct cells.48 Since most tumorigenesis begins in ductal epithelial cells, and some originate in lobular breast cells, the presence of AQP5 suggests an important role.49 AQP5 was found to play a major role in the secretion by salivary and lacrimal glands. Sjogren's syndrome, a chronic autoimmune condition, has been associated with an AQP5 transport disorder.48 Recently, AQP5 has garnered attention for its potential carcinogenic functions in multiple organs and systems. AQP5 was found to be upregulated in cancer cells and tumor tissues, strongly suggesting a role in tumor development involving multiple signal transduction pathways.51 The ARS/mitogen-activated protein kinase pathway is regulated by cAMP-dependent PKA phosphorylation of AQP5.52 Phosphorylation regulates the abundance of AQP5 in the plasma membrane. Indeed, the contrasting phosphorylation status of AQP5 between cancer and normal tissues indicates that its role is associated with phosphorylation.53 Moreover, pH and phosphorylation are a shorter-term regulatory mechanism commonly used by several members of the AQP family, which in turn affects the transport activities of the AQP channel.54 AQP5 appears to function following stress insult in the form of cellular oxidation, probably via a dual mechanism in which AQP5–H2O2 activates signal transduction of water cascades, which then stimulates genetic expression of antioxidants.49
AQP6 is abundant in acid-secreting type-A intracellular vesicles of intercalated cells in the renal aggregation duct.55 In comparison, rat AQP6 is only present in intracellular sites with no noticeable expression in the plasma membrane when expressed in mammalian cell lines. AQP6 is found in membranes of acid-secreting intercalated cells in the renal set conduit, primarily in the intracellular vesicles, colocalizing with H+-ATPase. In rat models of persistent alkalosis or lithium-induced NDI, expression of AQP6 is increased but its distribution is not modified.55 However, the distribution and function of AQP6 are less clearly defined, but they may be important because of the low water permeability and high anion permeability of this isoform (both may by a low pH). AQP6 might control anion movement instead of water movement, which may influence the activity of outer hair cells, which are highly sensitive to chloride modulation.56 Earlier studies indicate that AQP6 is found in human and rat inner ears but its localization remains controversial, likely because of the presence of other AQPs. In the apical portion of supporting cells, AQP6 was located in the vestibular epithelia both in humans and rats.55 AQP6 mRNA was found to be expressed in the cochlea, endolymphatic sac, and vestibule by RT-PCR of microdissected tissues. The RT-PCR products had an estimated size of 208 bp. AQP6 was highly expressed in the cochlea but less so in the organs of Corti in any form of vascular stria cells (basal, intermediate, and marginal).55 AQP6 also was found in the endolymphatic sac's epithelial layer. In the vestibule, AQP6 was also found in the endolymphatic sac's epithelial layer. In the vestibule, AQP6 has been widely observed in the sensory epithelia of the saccule, utricle, and pair.56
Aquaglyceroporin or AQP7, promotes the permeation of water and glycerol across cell membranes and is essential for lipid and energy homeostasis.57 Regulating the permeability of glycerol by AQP7 has been proposed as a potential therapeutic solution for metabolic fat complications.57 In the mouse adipocyte line model 3T3-L1, increased expression of AQP7 mRNA and release of glycerol during the differentiation of cells were associated with possible fat accumulation in adipocytes through plasma membrane glycerol permeability.58 AQP7 expression is lower in the white adipose tissue of obese humans than in normal controls but unchanged in patients with type 2 diabetes.57 However, the relationship between human AQPs and obesity is contradictory; aquaglyceroporins have multiple functions in energy metabolism and homeostasis in adipose tissue. Some have been implicated in disorders related to obesity and metabolism, such as metabolic syndrome.58 While fasting, AQP7 filters the intracellular glycerol into the bloodstream to be processed by AQP9 in the liver where it is used for gluconeogenesis. Therefore, the fine-tuning of the production of AQP7 for glycerol release from adipose tissue and its absorption by the liver could be the rate-limiting stage for preserving acceptable blood glucose levels.57 This indicates that there should be synchronized regulation of glycerol fluxes to ensure fine-tuning of energy homeostasis by AQP7 in the kidney, endocrine pancreas, small intestine, and muscles, which also express other aquaglyceroporins. AQP7 expression has been documented in the testis and human sperm cells and is associated with sperm motility and viability, indicating a role in sperm metabolism.58 Moreover, glycerol is a possible sperm substrate during transit through the epididymis and may be used as an energy substrate during spermatogenesis, which is likely to be channeled through AQP7.58
The functions of AQP8 are currently disputed. First, it has been proposed that water transport mediated by AQP8 may be particularly important for the rapid expansion of mitochondrial volume.59 In contrast, a different group has hypothesized that rapid volume equilibrium in mitochondria in reaction to an osmotic gradient was attributable to its large surface-to-volume ratio rather than the strong membrane water permeability induced by AQP.60 In comparison, only minor variations in phenotype were identified between WT and Aqp8-deficient mice. AQP8 was expected to participate in the transport of ammonia, as its expression has been able to rescue ammonium absorption in mutant yeast, which suggests that the protein is used for the transport of NH3 in humans.59 The increased acidification of the ammonium-containing oocyte medium corresponded to NH3 protein diffusion. Given the indirect effect of AQP8 expression on growth complementation, light-scattering experiments with reconstituting vesicles were performed. AQP8 was shown to be permeable to formamide, which indicated that the protein could carry ammonium in vivo and contribute physiologically to acid–base balance.60 Comparative phenotypic tests, however, have shown no or very limited variations in serum ammonia, colonic ammonia absorption, clearance of renal ammonia, and liver ammonia accumulation in WT mice versus Aqp8 null mice.57 AQP8 is highly efficient in handling water and ammonia. The substitution of the purified protein enhanced the permeability of modular membranes that mimic epithelial cell apical membranes 3-to-6-fold. AQP8 prohibits ions from passage, as do most members of the AQPs class, allowing for an exclusive transfer of neutral NH3 molecules. AQP8 also promotes the transfer of H2O2 through the plasma membrane and inhibits the downstream H2O2-mediated redox signaling pathway.61 In the inner mitochondrial membrane, the expression of AQP8 has been reported, supporting the idea that AQP8 promotes the release of H2O2 from mitochondria and that its deficiency induces ROS-related depolarization and cell death.62
AQP9, a water, glycerol, and urea receptor, has been identified in mice63 and humans.64 The expression of AQP9 mRNA has been observed in a few tissues, such as human liver and leukocytes, as well as in mouse liver and immature sperm.63 AQP9 protein expression has been observed in a variety of tissues, including the rat and human epididymis,65 multiple cell types in rodent and primate brains,66 the mouse and human small intestine,67 the mouse and human inner ear, the human chorioamnion and placenta, the mouse spinal cord, the rat and human prostate, human adipose tissue, human skeletal muscle.65 AQP9 can function in energy metabolism in the brain and is found in astrocytes, catecholaminergic brain stem neurons, subsets of dopaminergic and hypothalamic nerve cells, and the cells covering the brain ventricles, including the ependymal cells and the tanycytes.63 The study of mice with selective deletion of Aqp9 has demonstrated the existence of tetrameric AQP9 and its expression in the brain and neurons. The expression of AQP9 is deregulated in several brain diseases.64 Furthermore, the similarity of the brain distribution patterns of AQP4 and AQP9 in mice and rats indicates that the two proteins have specific roles and can work in combination to promote the flow of water between CSF and brain parenchyma.65
AQP10 is an aquaglyceroporin that has been shown to be pseudogenic in the human, but not the mouse, intestine.68 AQP10 in humans is primarily distributed in the jejunum and duodenum, where it can act as a carrier of water and small solutes.69 The function of AQPs in small intestinal water transport is, however, uncertain.70 Functionally, in the jejunum and ileum, the osmotic water permeability is only one-tenth that in the kidney cortex, which implies that usable water canals are fewer in the small intestine. The expression of AQP10 mRNA is almost exclusive to the duodenum and jejunum. Furthermore, the expression of AQP10 was higher than that of AQP8 and was found in the epithelial absorbent cells, suggesting that AQP10 may be the main channel for water transport through these regions. Water can move via AQP10, and partly via AQP8, through the apical membrane of the absorbing epithelia, and via AQP3 through the basolateral membrane. AQP10 did not promote adenine permeability relative to AQP9, another aquaglyceroporin. Thus, AQP10's pore seems to be narrower and similar to that of AQP3AQP10 can act as a conduit for intracellular vesicles, carrying water to the granular vesicles and unknown small substrates. Ironically, AQP10 mRNA has been documented to be downregulated in cholera patients. Because serotonin is secreted in cholera, AQP10 may play a role in the regulation of its secretion by enterochromaffin cells. Direct stimulation by cholera toxin, however, did not change the expression of AQP10 mRNA in CaCO2 intestinal epithelial cells. Because serotonin is secreted in cholera, AQP10 may play a role in the regulation of its secretion by enterochromaffin cells. Direct stimulation by cholera toxin, however, did not change the expression of AQP10 mRNA in CaCO2 intestinal epithelial cells.71
AQP11 is strongly expressed in the testis and thymus and expressed at lower levels in the liver, lungs, and intestines.72 It is expressed in the kidney in the proximal tubule. Irregular glycosylation of polycystin-1, mutated in human autosomal polycystic kidney disease, can cause developmental tubular defects.73 AQP11 is expressed in the brain, but its location and function there have not been well understood.72 AQP11 expression in neurons of the rat brain has been reported in one study.73 Due to renal failure with progressive renal cyst formation, Aqp11-null mice die as neonates, indicating the importance of AQP11 in mammalian kidney development.74 AQP11 also plays an important role in the production of mammalian sperm and saliva.74 The role of AQP11 in the brain appears to be different from those of the other brain AQPs. AQP4 is confined throughout the brain to astrocytes, while AQP1 is found in the choroid plexus. AQP9 is present in both astrocytes and a catecholaminergic neuron population. The specific intracellular distribution of AQP11 in Purkinje cells is unique.74 Furthermore, AQP11 is primarily intracellular in vivo. The unusual distribution and apparent lack of involvement in water/solute transport are features specific to AQP11.74
AQP12 was identified by BLAST search using AQP11 as a query sequence. AQP12 is part of the subfamily of superaquaporins.30 It has the strongest sequence identity with AQP11 at 32%, but the sequence identity is less than 15% with other AQPs. AQP12 has been shown to be present in rat cells and the rat RIN-m5F cell line.75 However, the possible involvement of AQP12 in cellular insulin release remains to be assessed. Interestingly, owing to the differences in the reaction of Aqp7−/− and Aqp7+/+ cells to hypoosmolarity (rates and degrees of swelling), it is rational to hypothesize that at least one water-facilitated pathway, such as one involving AQP5 or AQP8 or Na–K–2Cl, should be present in addition to AQP7. Nevertheless, more research will be required to determine the roles of AQP5, AQP8, and Na–K–2Cl in the hypoosmolarity response. In the pancreas, specifically in the cancer cells, AQP12 is selectively expressed, which has been confirmed by RT-PCR and in situ hybridization. Spurious expression of AQP12 in these cells is surprising, as PAC cells express AQP1 and AQP8.
A preliminary study of Aqp12 null mice showed only very minor anomalies.76 Other AQPs appear to compensate for the AQP12 defect, as no anomalies in the pancreas function have been found in the null mouse of AQP1 or AQP8. Although most amino acid sequences among species include strongly homologous AQPs.30 The presence of local gene duplications in human AQP7 and AQP12 are not unique.77
4. AQP inhibitors
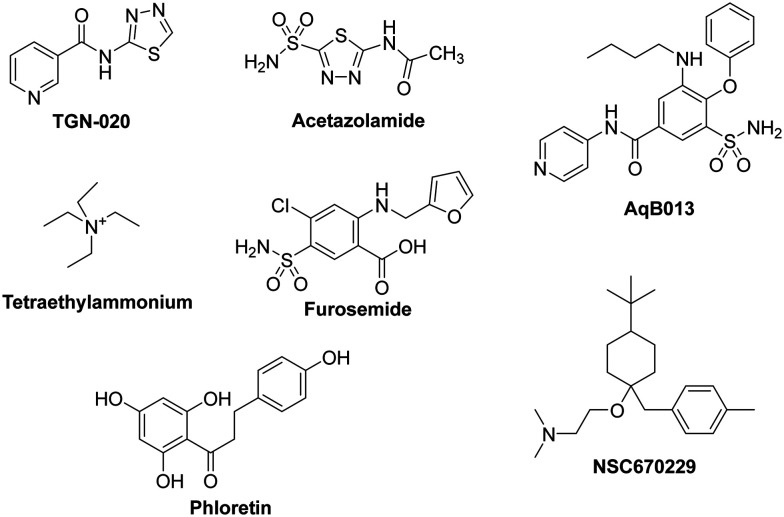
Most of the molecules that are currently under investigation as AQP inhibitors target AQPs 1, 2, 3, or 4. There are many patents, clinical trials, and studies on AQP upregulators, modulators, and inhibitors. We present a partial list of AQP inhibitors in as shown in Fig. 3 and Table 1. Several molecules have been suggested as inhibitors of multiple AQPs. Of these, most are small molecules.78 The similarities between AQPs and ion channels have sparked interest in the idea of ion-channel modulators as AQP inhibitors. Arylsulfonamides, such as acetazolamide, are molecules that have attracted attention as potential AQP inhibitors. Anti-epileptic drugs (AEDs) have also been suggested to have an AQP modulating function; however, they have yet to be proven definitively. A molecule named TGN-020 has shown great promise recently as an inhibitor in mouse models. Mercury and its compounds are known to be toxic due to their non-specificity, which causes many off-target effects.79 Other heavy metal compounds (such as silver and gold compounds) have been investigated as inhibitors, but the challenge is finding a molecule with a side effect profile that is tolerable. The currently known inhibitors are discussed by type in the sections below.
Fig. 3. Molecules that are currently under investigation as AQP inhibitors.
List of AQP inhibitors.
| Drugs | AQP inhibitor | Effect | Ref. |
|---|---|---|---|
| TGN-020 | Inhibits AQP4 water flux | TGN-020 significantly reduced oedema, glial scar, albumin effusion, and apoptosis | 80 |
| Acetazolamide | Inhibits AQP1 and AQP4 water flux | Colon cancer and lung carcinoma | 81 |
| AqB013 | Inhibits AQP1 and AQP4 water flux | Endothelial tube formation and colon cancer | 82 |
| Tetraethylammonium | Inhibits AQP1, AQP2, and AQP4 water flux | It inhibits a wide range of voltage-dependent potassium channels, including those from the Kv1, Kv2, and Kv3 families. It can also inhibit Ca-activated K currents | 83 |
| Furosemide | Inhibits AQP1 and APQ4 | Increased potency of block by intracellular delivery of bumetanide and furosemide via injection into AQP4-expressing oocytes | 84 |
| Phloretin | Inhibits AQP3 and AQP9 | The effects of the putative aquaporin (AQP) inhibitor phloretin on water influx | 85 |
| NSC670229 | Inhibits AQP3 and AQP7 | Differentially affected both AQP4 and AQP1 mediated water transport, with EC50 values between 20 and 50 microM | 15 |
4.1. TGN-020
TGN-020 (2-(nicotinamide)-1,3,4-thiadiazole) was identified based on conserved physicochemical features of drugs found to inhibit AQP4-mediated edema following ischemia when administered before injury.86 There was an approximately 10% reduction in brain volume.86 Therapeutic administration must necessarily occur after a stroke, although a prophylactic treatment could be considered if the side effect profile of TGN-020 was acceptable. TGN-020 was found to inhibit water transport through human AQP4-M23 with an IC50 = 3 μM and 73% maximum inhibition. Subsequent studies, however, suggested that this compound would not be appreciatively selective for AQP4 over AQP1, inhibiting AQP1 mediated hypotonic flux by 56 ± 9% at 20 μM.87 The effect of TGN-020 on infarct volume was more pronounced in the cerebral cortex, with little change in the basal ganglia, which was thought to reflect the relative AQP4 expression levels in those tissues.88
4.2. Tetraethylammonium (TEA)
TEA is a small-molecule inhibitor that was first tested due to perceived similarities between AQPs and ion channels.87,89,90 It is thought to act by binding to a tyrosine residue located on the extracellular end of TM helix 5. Using human AQP1 expressed in Xenopus oocytes, a maximum single dose of 10 mM TEA caused a 33% reduction in water permeability.84 Another study using 4 and 100 μM TEA showed an inhibition of 44%, with no differences between the two concentrations; TEA also inhibited AQP2 and AQP4, with maximal inhibitions of 40% and 57%, respectively, at 100 μM. Further testing of TEA in Xenopus oocytes and in native AQP1-expressing erythrocytes has failed to show inhibition.91
4.3. Acetazolamide (AZA)
AZA a pan-inhibitor of carbonic anhydrase, was reported to inhibit AQP1 in a Xenopus oocyte assay.92,93 AZA-mediated inhibition of AQP1 water transport was evident, with an apparent IC50 in the low micromolar range. Similarly, inhibition of rat AQP1 water transport in HEK cells by AZA using a fluorescence intensity assay and a surface Plasmon resonance assay to detect direct binding of AZA to AQP1 have been reported. Inhibition of AQP1 in HEK cells appeared to be similar to that reported for oocytes, with a KD of 174 μM. AZA inhibition of AQP1 water flux was not reproduced in other functional assay systems. Our own laboratory reported the inhibition of human AQP4-M23 by AZA using a Xenopus oocyte assay system. Therein, we observed a dose dependent decrease in AQP4-mediated hypotonic water flux, with a functional IC50 = 0.9 μM and a maximum inhibition of 85%. AQP4 inhibition by AZA was confirmed in one report but not another; although, no positive controls were reported in the second study. AEDs have also been suggested to be AQP inhibitors. Their anti-epileptic action has been hypothesized to occur by modulation of AQPs. Many AEDs, including topiramate, zonisamide, and lamotrigine, are known to have inhibitory effects similar to that of acetazolamide on carbonic anhydrase. Moreover, there is an in silico predicted binding site on AQP4 similar to that hypothesized for acetazolamide. AEDs have an inhibitory effect on AQP4 in Xenopus oocytes; however, this could not be reproduced in rat thyroid epithelial cells.94 AEDs also are thought to have an inhibitory effect on AQP1, AQP4, and AQP5.86 However, due to unconfirmed mechanisms of action and lack of specificity, there is no conclusive evidence showing that AEDs are effective and safe AQP inhibitors.
4.4. Furosemide
Furosemide, a NKCC1 cotransporter inhibitor, was found to inhibit AQP4-mediated water flux in Xenopus oocytes following internal introduction, but not following external application of the ligand.84 Similarly, furosemide inhibited high osmotic gradient-induced flux through AQP1 in oocytes.95 Although the oocyte assay system was modified from that reported earlier, internal administration of furosemide was also essential for inhibition.95–97
4.5. Phloretin
Phloretin is a small molecule that acts as a non-specific aquaglyceroporin inhibitor. It has also been shown to inhibit the kidney urea transporter UT-A1.95 It has been hypothesized that the same mechanism underpinning urea transport inhibition is responsible for inhibition of AQP3 and AQP9.98 Next, 100 μM of phloretin was used to inhibit AQP9 expressed in Xenopus oocytes, resulting in 86% inhibition. AQP3 glycerol permeability in proteoliposomes was reduced by 83% by 500 μM phloretin. It had no effect on control proteoliposomes with a Pgly of ∼2.8 × 10−6 cm s−1.98
4.6. AqB013
AqB013, structurally related to bumetanide, was recently identified as a non-selective AQP1/AQP4 inhibitor.99 However, unlike the other loop diuretics, AqB013 showed a strong effect when introduced externally in the oocyte system, with an IC50 of 20 μM for both AQP1 and AQP4.84 Computational and protein modification studies suggest that AqB013 binds at a cytosolic position. AqB013 appeared to be inactive against the NKCC1 cotransporter, suggesting that ligands selective for AQPs over competitor proteins can be designed.99–102
4.7. NSC670229
Other candidate AQP modulators include compounds identified from the National Cancer Institute's chemical-screening library using a high-throughput, automated screen.103 In particular, NSC670229 appears to be a promising starting point for future drug-discovery efforts.104 It has an IC50 of 27 μM and has no functional groups that would suggest non-equilibrium binding. While no in vivo data have been reported for NSC670229, its relatively simple structure and reasonable inhibitory effect are attractive.103 A recent study identified new inhibitors of AQP3 and AQP7. The compound DFP00173 was able to inhibit the glycerol permeability of human erythrocytes with an IC50 of ∼0.2 μM. Compound Z433927330 reduced glycerol permeability with an IC50 of ∼0.6 μM. In a Chinese hamster ovary cell line, compound DFP00173 inhibited AQP3 with an IC50 of ∼0.1 μM and was selective for AQP3 over AQP7 and AQP9. Compound Z433927330 was able to inhibit AQP7 in the same cell line with an IC50 of 0.2 μM and with selectivity for AQP7 over AQP3 and AQP9; IC50s for this compound for mouse AQP3 and AQP9 were 0.7 and 1.1 μM, respectively.105
4.8. AQP-based tumor therapeutics
Aquaporins (AQPs), given their involvement in tumor cell infiltration, metastasis, proliferation, and possibly cell adhesion, represent promising therapeutic targets in cancer treatment. AQP modulators, depending on the specific roles AQPs play in tumor biology, could serve as effective anti-cancer agents. For processes relying on the water-transporting function of AQPs, such as tumor cell migration, inhibitors targeting AQP-mediated water transport would be required. Conversely, for roles independent of water transport, such as interactions between AQPs and oncogenes leading to increased proliferation, alternative compounds that disrupt these interactions would be necessary. An example illustrating the potential of AQP-targeted therapies is glioblastoma, the most common and aggressive primary brain tumor. Glioblastoma is characterized by its invasive nature, making it impossible to fully remove surgically, even with aggressive treatments such as radical surgery, radiotherapy, and temozolomide chemotherapy.33,106 Median survival remains about a year post-diagnosis. AQP4, predominantly expressed in glioblastoma cells, facilitates extensive tumor cell infiltration. Inhibitors targeting AQP4 water transport could potentially reduce this infiltration, converting a tumor with diffuse margins into one with well-defined boundaries, making surgical resection more feasible. Additionally, AQP1 is expressed in the vascular endothelial cells within glioblastomas, and AQP1 inhibitors may reduce angiogenesis, indirectly inhibiting tumor growth.
Despite these promising avenues, efforts to develop AQP inhibitors have largely been unsuccessful.15 Inhibitors such as heavy metals (e.g., mercury) effectively block AQP1 but are too toxic for clinical application. Several compounds, including acetazolamide, anti-epileptic drugs, bumetanide, and thiadiazole, have been reported to inhibit AQP4.78 However, follow-up studies using different assay systems have failed to confirm these findings, highlighting the difficulties in discovering reliable and selective AQP inhibitors. For further discussion on these challenges, a recent review by Huber et al.87 provides a comprehensive analysis. An interesting development in AQP-based therapeutics involves the discovery of an autoantibody against AQP4, known as AQP4-IgG or NMO-IgG, found in patients with neuromyelitis optica (NMO), an inflammatory demyelinating disease of the central nervous system.86 AQP4-IgG is pathogenic, binding to AQP4 on astrocytes and triggering complement-dependent astrocyte damage, followed by leukocyte infiltration. Monoclonal AQP4-IgG can now be produced synthetically, and this antibody linked to a toxin could potentially destroy AQP4-expressing glioblastoma cells, which overexpress AQP4.34 However, it remains unclear whether targeting the AQP4-expressing subpopulation of tumor cells will sufficiently reduce glioblastoma aggressiveness.
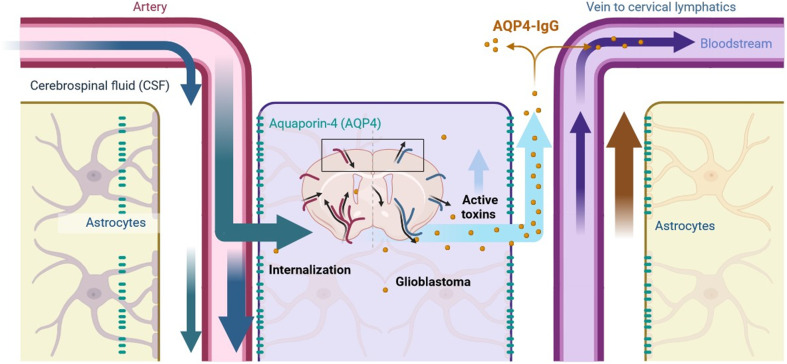
Localized delivery of AQP4-IgG in glioblastoma is feasible, using methods such as wafer placement against the resection cavity wall or convection-enhanced delivery. However, a potential side effect of this treatment could be NMO-like symptoms, caused by AQP4-IgG-mediated damage to normal astrocytes as shown in Fig. 4. A more refined approach would be to link AQP4-IgG to a toxin that activates only upon internalization. Evidence suggests that normal astrocytes, which express AQP4 in perivascular foot processes, do not internalize AQP4-IgG, whereas glioblastoma cells, which express AQP4 throughout the plasma membrane, do internalize it.107 This selective vulnerability of glioblastoma cells to AQP4-IgG internalization could minimize side effects while effectively targeting tumor cells.
Fig. 4. AQP4-IgG conjugated with toxin as a potential glioblastoma therapy: when AQP4-IgG binds to AQP4 located on astrocyte foot processes, the antibody-toxin complex remains on the cell surface without being internalized. In this case, the toxin remains linked to AQP4-IgG, preventing any cytotoxic effects on the astrocyte. Conversely, when AQP4-IgG binds to AQP4 expressed on glioblastoma cells, the complex is internalized into the tumor cell. Upon internalization, the toxin is released from the AQP4-IgG complex, inducing cytotoxicity and leading to the targeted destruction of the glioblastoma cell.
5. Conclusions
AQPs are ubiquitous and have play varied roles in a host of human conditions. The identification of AQPs involved in inflammation will help us understand the complex mechanisms regulating host pathogens. Overall, AQPs are obvious candidates as therapeutic targets for the treatment of edema, cancer, and inflammation. The development in AQP inhibitors has been slow; AQPs continue to be implicated in new diseases, highlighting an urgent need for AQP modulators. Clinical and preclinical studies have shown that AQP expression is increased in a number of cancers. Biological functions and singling pathways of AQPs in cancer are being intensively investigated in mouse AQP knockouts. AQPs play an important role in brain water homeostasis, exocrine gland secretion, urine concentration, skin moisturization, fat metabolism, or neural signal transduction. They are also involved in carcinogenesis, tumor-associated edema, tumor cell proliferation/migration, and tumor angiogenesis. In conclusion, although much remains to be defined for molecular mechanisms in cancer invasion and metastasis, the roles of AQP channel function in cancer progression are likely to inspire new therapeutic targets for improving multiple human diseases.
Future directions
The roles of AQPs in tumor biology are increasingly being elucidated, yet much remains to be understood about the molecular mechanisms underlying AQP-mediated processes such as cell migration and proliferation. It is currently unclear whether the water transport function of AQPs is pivotal to these oncogenic activities or if undiscovered interactions between AQPs and oncogenic pathways exist. Further investigation is needed to identify non-toxic and selective AQP inhibitors, which could serve both as tools for probing AQP function and as potential anticancer therapeutics. AQP inhibitors targeting tumor infiltration, metastasis, and angiogenesis may offer novel treatment strategies when combined with existing therapies aimed at proliferating tumor cells.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Author contributions
DKY and DS: conceptualization, validation, methodology, formal analysis, writing – original draft; DDS and DKY: formal analysis, validation, investigation, project administration, resources, software, supervision, visualization, original draft, writing, editing, and refining of the manuscript. All authors have read and approved the manuscript.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank to the Ministry of Education for funding this research work through the project RS-2020-NR049589. The authors appreciate https://www.Biorender.com's (July 2024) graphics assistance. D. D. S. acknowledge the DST-FIST-AIMT, and DST-PURSE at Amity University, Rajasthan, India.
References
- Agre P. Biosci. Rep. 2004;24:127–163. doi: 10.1007/s10540-005-2577-2. [DOI] [PubMed] [Google Scholar]
- Preston G. M. Carroll T. P. Guggino W. B. Agre P. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Hohmann S. Bill R. M. Kayingo G. Prior B. A. Trends Microbiol. 2000;8:33–38. doi: 10.1016/s0966-842x(99)01645-5. [DOI] [PubMed] [Google Scholar]
- Knepper M. A. Kwon T.-H. Nielsen S. N. Engl. J. Med. 2015;372:1349–1358. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C. Boursiac Y. Luu D.-T. Santoni V. Shahzad Z. Verdoucq L. Physiol. Rev. 2015;95:1321–1358. doi: 10.1152/physrev.00008.2015. [DOI] [PubMed] [Google Scholar]
- Bonhivers M. Carbrey J. M. Gould S. J. Agre P. J. Biol. Chem. 1998;273:27565–27572. doi: 10.1074/jbc.273.42.27565. [DOI] [PubMed] [Google Scholar]
- Day R. E. Kitchen P. Owen D. S. Bland C. Marshall L. Conner A. C. Bill R. M. Conner M. T. Biochim. Biophys. Acta, Gen. Subj. 2014;1840:1492–1506. doi: 10.1016/j.bbagen.2013.09.033. [DOI] [PubMed] [Google Scholar]
- Nagelhus E. A. Ottersen O. P. Physiol. Rev. 2013;93:1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. King L. S. Christensen B. M. Agre P. Am. J. Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M. Kuhlmann E. D. Winkens H. J. Exp. Eye Res. 1979;29:303–313. doi: 10.1016/0014-4835(79)90009-5. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Curr. Biol. 2013;23:R52–R55. doi: 10.1016/j.cub.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn T. P. Møller A. L. B. Zeuthen T. Holm L. M. Klærke D. A. Mohsin B. Kühlbrandt W. Schjoerring J. K. FEBS Lett. 2004;574:31–36. doi: 10.1016/j.febslet.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Moniaga C. S. Nielsen S. Hara-Chikuma M. Biochem. Biophys. Res. Commun. 2016;471:191–197. doi: 10.1016/j.bbrc.2016.01.153. [DOI] [PubMed] [Google Scholar]
- Tricarico P. M. Mentino D. De Marco A. Del Vecchio C. Garra S. Cazzato G. Foti C. Crovella S. Calamita G. Int. J. Mol. Sci. 2022;23:4020. doi: 10.3390/ijms23074020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman A. S. Anderson M. O. Papadopoulos M. C. Nat. Rev. Drug Discovery. 2014;13:259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M. Verkman A. S. J. Am. Soc. Nephrol. 2006;17:39–45. doi: 10.1681/ASN.2005080846. [DOI] [PubMed] [Google Scholar]
- Hara-Chikuma M. Verkman A. S. J. Mol. Med. 2008;86:221–231. doi: 10.1007/s00109-007-0272-4. [DOI] [PubMed] [Google Scholar]
- Saadoun S. Papadopoulos M. C. Watanabe H. Yan D. Manley G. T. Verkman A. S. J. Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Karlsson T. Bolshakova A. Magalhães M. A. O. Loitto V. M. Magnusson K.-E. PLoS One. 2013;8:e59901. doi: 10.1371/journal.pone.0059901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio A. Yool A. J. Cancers. 2023;15:849. [Google Scholar]
- Ozu M. Alvear-Arias J. J. Fernandez M. Caviglia A. Peña-Pichicoi A. Carrillo C. Carmona E. Otero-Gonzalez A. Garate J. A. Amodeo G. Gonzalez C. Int. J. Mol. Sci. 2022;23:12317. doi: 10.3390/ijms232012317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K. Unger L. Salman M. M. Kitchen P. Bill R. M. Yool A. J. Int. J. Mol. Sci. 2022;23:1388. doi: 10.3390/ijms23031388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. F. Egea P. F. Robles-Colmenares Y. Iii J. D. O. Stroud R. M. PLoS Biol. 2003;1:e72. doi: 10.1371/journal.pbio.0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey K. L. Gletten R. B. O'Neale C. V. T. Wang Z. Petrova R. S. Donaldson P. J. Front. Physiol. 2022;13:882550. doi: 10.3389/fphys.2022.882550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. Hockwin O. Crit. Rev. Biochem. 1982;12:1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Shiels A. Differentiation. 2018;102:1–9. doi: 10.1016/j.diff.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. Verkman A. S. Hu J. Verkman A. S. FASEB J. 2006;20:1892–1894. doi: 10.1096/fj.06-5930fje. [DOI] [PubMed] [Google Scholar]
- Singh S. Sadanandam A. Singh R. K. Cancer Metastasis Rev. 2007;26:453–467. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchia G. P. Srinivas M. Li W. Brosnan C. F. Frigeri A. Spray D. C. FASEB J. 2005;19:1674–1676. doi: 10.1096/fj.04-3281fje. [DOI] [PubMed] [Google Scholar]
- Itoh T. Rai T. Kuwahara M. Ko S. B. H. Uchida S. Sasaki S. Ishibashi K. Biochem. Biophys. Res. Commun. 2005;330:832–838. doi: 10.1016/j.bbrc.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Jafari N. V. Rohn J. L. Mucosal Immunol. 2022;15:1127–1142. doi: 10.1038/s41385-022-00565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmeier U. Bink A. Schackert G. Stummer W. Neurooncology. 2008;10:1025–1034. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. L. Lam C. Kalluri S. R. Saikali P. Bautista K. Dupree C. Glogowska M. Case D. Antel J. P. Owens G. P. Gilden D. Nessler S. Stadelmann C. Hemmer B. Ann. Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagströmer C. J. Steffen J. H. Kreida S. Al-Jubair T. Frick A. Gourdon P. Törnroth-Horsefield S. Sci. Rep. 2023;13:14674. doi: 10.1038/s41598-023-41616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri M. Di Mise A. Tamma G. Valenti G. F1000Research. 2019;8:149. doi: 10.12688/f1000research.16654.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. Lu B. Yang J. Li C. Li Y. Chen H. Li N. Duan L. Gu F. Zhang J. Xia W. Front. Endocrinol. 2021;12:665145. doi: 10.3389/fendo.2021.665145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C. Higgins P. J. Zhang W. Cells. 2020;9:2172. doi: 10.3390/cells9102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. Nie X. Lu Q. Bai Y. Jiang Z. Front. Physiol. 2023;14:1264570. doi: 10.3389/fphys.2023.1264570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobasheri A. Wray S. Marples D. J. Mol. Histol. 2005;36:1–14. doi: 10.1007/s10735-004-2633-4. [DOI] [PubMed] [Google Scholar]
- Yadav E. Yadav N. Hus A. Yadav J. S. Respir. Med. 2020;174:106193. doi: 10.1016/j.rmed.2020.106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Chikuma M. Verkman A. S. J. Invest. Dermatol. 2008;128:2145–2151. doi: 10.1038/jid.2008.70. [DOI] [PubMed] [Google Scholar]
- Bhend M. E. Kempuraj D. Sinha N. R. Gupta S. Mohan R. R. Exp. Eye Res. 2023;228:109390. doi: 10.1016/j.exer.2023.109390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri A. Gropper M. A. Umenishi F. Kawashima M. Brown D. Verkman A. S. J. Cell Sci. 1995;108(Pt 9):2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- Jarius S. Aktas O. Ayzenberg I. Bellmann-Strobl J. Berthele A. Giglhuber K. Häußler V. Havla J. Hellwig K. Hümmert M. W. Kleiter I. Klotz L. Krumbholz M. Kümpfel T. Paul F. Ringelstein M. Ruprecht K. Senel M. Stellmann J.-P. Bergh F. T. Tumani H. Wildemann B. Trebst C. Neuromyelitis Optica Study Group (NEMOS) J. Neurol. 2023;270:3341–3368. doi: 10.1007/s00415-023-11634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiezia A. L. Carotenuto A. Iovino A. Moccia M. Gastaldi M. Iodice R. Tedeschi E. Petracca M. Lavorgna L. d'Ambrosio A. Brescia Morra V. Lanzillo R. Int. J. Mol. Sci. 2022;23:14559. doi: 10.3390/ijms232314559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirici I. Balsanu T. A. Bogdan C. Margaritescu C. Divan T. Vitalie V. Mogoanta L. Pirici D. Carare R. O. Muresanu D. F. Int. J. Mol. Sci. 2017;19:46. doi: 10.3390/ijms19010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direito I. Madeira A. Brito M. A. Soveral G. Cell. Mol. Life Sci. 2016;73:1623–1640. doi: 10.1007/s00018-016-2142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues C. Pimpão C. Mósca A. F. Coxixo A. S. Lopes D. Da Silva I. V. Pedersen P. A. Antunes F. Soveral G. Cancers. 2019;11:932. doi: 10.3390/cancers11070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues C. Mósca A. Martins A. Nobre T. Prista C. Antunes F. Gasparovic A. C. Soveral G. Int. J. Mol. Sci. 2016;17:2090. doi: 10.3390/ijms17122090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani S. Saripalli A. Sharma-Walia N. Oncotarget. 2018;9:36392–36405. doi: 10.18632/oncotarget.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J. Lee J. Kim M. S. Jang S. J. Sidransky D. Moon C. Biochem. Biophys. Res. Commun. 2008;367:291–298. doi: 10.1016/j.bbrc.2007.12.073. [DOI] [PubMed] [Google Scholar]
- Woo J. Lee J. Chae Y. K. Kim M. S. Baek J. H. Park J. C. Park M. J. Smith I. M. Trink B. Ratovitski E. Lee T. Park B. Jang S. J. Soria J. C. Califano J. A. Sidransky D. Moon C. Cancer Lett. 2008;264:54–62. doi: 10.1016/j.canlet.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão L. Prista C. Loureiro-Dias M. C. Moura T. F. Soveral G. Biochem. Biophys. Res. Commun. 2014;450:289–294. doi: 10.1016/j.bbrc.2014.05.121. [DOI] [PubMed] [Google Scholar]
- Yasui M. Kwon T.-H. Knepper M. A. Nielsen S. Agre P. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5808–5813. doi: 10.1073/pnas.96.10.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi D. Takeda T. Kakigi A. Okada T. Nishioka R. Kitano H. Acta Oto-Laryngol. 2008;128:832–840. doi: 10.1080/00016480701765691. [DOI] [PubMed] [Google Scholar]
- Moss F. J. Mahinthichaichan P. Lodowski D. T. Kowatz T. Tajkhorshid E. Engel A. Boron W. F. Vahedi-Faridi A. Front. Physiol. 2020;11:728. doi: 10.3389/fphys.2020.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J. C. Bernardino R. L. Gonçalves A. Barros A. Calamita G. Alves M. G. Oliveira P. F. Cells. 2023;12:2003. doi: 10.3390/cells12152003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamita G. Ferri D. Gena P. Liquori G. E. Cavalier A. Thomas D. Svelto M. J. Biol. Chem. 2005;280:17149–17153. doi: 10.1074/jbc.C400595200. [DOI] [PubMed] [Google Scholar]
- Yang B. Zhao D. Verkman A. S. J. Biol. Chem. 2006;281:16202–16206. doi: 10.1074/jbc.M601864200. [DOI] [PubMed] [Google Scholar]
- Sega F. V. D. Zambonin L. Fiorentini D. Rizzo B. Caliceti C. Landi L. Hrelia S. Prata C. Biochim. Biophys. Acta, Mol. Cell Res. 2014;1843:806–814. doi: 10.1016/j.bbamcr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Marchissio M. J. Francés D. E. A. Carnovale C. E. Marinelli R. A. Toxicol. Appl. Pharmacol. 2012;264:246–254. doi: 10.1016/j.taap.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H. Shayakul C. Berger U. V. Mackenzie B. Devidas S. Guggino W. B. Van Hoek A. N. Hediger M. A. J. Biol. Chem. 1998;273:24737–24743. doi: 10.1074/jbc.273.38.24737. [DOI] [PubMed] [Google Scholar]
- Ishibashi K. Kuwahara M. Gu Y. Tanaka Y. Marumo F. Sasaki S. Biochem. Biophys. Res. Commun. 1998;244:268–274. doi: 10.1006/bbrc.1998.8252. [DOI] [PubMed] [Google Scholar]
- Elkjær M.-L. Vajda Z. Nejsum L. N. Kwon T.-H. Jensen U. B. Amiry-Moghaddam M. Frøkiær J. Nielsen S. Biochem. Biophys. Res. Commun. 2000;276:1118–1128. doi: 10.1006/bbrc.2000.3505. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M. Lindland H. Zelenin S. Roberg B. Å. Gundersen B. B. Petersen P. Rinvik E. Torgner I. A. Ottersen O. P. FASEB J. 2005;19:1459–1467. doi: 10.1096/fj.04-3515com. [DOI] [PubMed] [Google Scholar]
- Okada S. Misaka T. Matsumoto I. Watanabe H. Abe K. FEBS Lett. 2003;540:157–162. doi: 10.1016/s0014-5793(03)00256-4. [DOI] [PubMed] [Google Scholar]
- Mobasheri A. Shakibaei M. Marples D. Histochem. Cell Biol. 2024;121:463–471. doi: 10.1007/s00418-004-0657-1. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S. Yoshida Y. Tani T. Koyama Y. Nihei K. Ohshiro K. Kamiie J.-I. Yaoita E. Suda T. Hatakeyama K. Yamamoto T. Biochem. Biophys. Res. Commun. 2001;287:814–819. doi: 10.1006/bbrc.2001.5661. [DOI] [PubMed] [Google Scholar]
- Morinaga T. Nakakoshi M. Hirao A. Imai M. Ishibashi K. Biochem. Biophys. Res. Commun. 2002;294:630–634. doi: 10.1016/S0006-291X(02)00536-3. [DOI] [PubMed] [Google Scholar]
- Flach C.-F. Qadri F. Bhuiyan T. R. Alam N. H. Jennische E. Holmgren J. Lönnroth I. FEBS Lett. 2007;581:3183–3188. doi: 10.1016/j.febslet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Morishita Y. Matsuzaki T. Hara-chikuma M. Andoo A. Shimono M. Matsuki A. Kobayashi K. Ikeda M. Yamamoto T. Verkman A. Kusano E. Ookawara S. Takata K. Sasaki S. Ishibashi K. Mol. Cell. Biol. 2005;25:7770–7779. doi: 10.1128/MCB.25.17.7770-7779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. Sohara E. Kobayashi K. Chiga M. Rai T. Ishibashi K. Horie S. Su X. Zhou J. Sasaki S. Uchida S. J. Am. Soc. Nephrol. 2014;25:2789–2799. doi: 10.1681/ASN.2013060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick D. A. Praetorius J. Tsunenari T. Nielsen S. Agre P. BMC Biochem. 2006;7:14. doi: 10.1186/1471-2091-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Giménez L. Becerril S. Camões S. P. Da Silva I. V. Rodrigues C. Moncada R. Valentí V. Catalán V. Gómez-Ambrosi J. Miranda J. P. Soveral G. Frühbeck G. Rodríguez A. Int. J. Obes. 2017;41:1394–1402. doi: 10.1038/ijo.2017.135. [DOI] [PubMed] [Google Scholar]
- Cho S.-J. Sattar A. K. M. A. Jeong E.-H. Satchi M. Cho J. A. Dash S. Mayes M. S. Stromer M. H. Jena B. P. Proc. Natl. Acad. Sci. U. S. A. 2002;99:4720–4724. doi: 10.1073/pnas.072083499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas L. Kim Y. H. Karimpour-Fard A. Cox M. Hopkins J. Pollack J. R. Sikela J. M. Genome Res. 2007;17:1266–1277. doi: 10.1101/gr.6557307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C. S. Moon D. Kang S. K. Aquaporins in Cancer Biology. Front. Oncol. 2022;12:782829. doi: 10.3389/fonc.2022.782829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S. O'Riordan C. E. Verra C. Aimaretti E. Alves G. F. Dreisch K. Evenäs J. Gena P. Tesse A. Rützler M. Collino M. Calamita G. Thiemermann C. Front. Immunol. 2022;13:900906. doi: 10.3389/fimmu.2022.900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. Lin L. Yin L. Hao X. Tian J. Zhang X. Ren Y. Li C. Yang Y. Front. Immunol. 2022;13:870029. doi: 10.3389/fimmu.2022.870029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin K. Shi-Peng Z. Hepatogastroenterology. 2011;58:1502–1506. doi: 10.5754/hge11154. [DOI] [PubMed] [Google Scholar]
- Dorward H. S. Du A. Bruhn M. A. Wrin J. Pei J. V. Evdokiou A. Price T. J. Yool A. J. Hardingham J. E. J. Exp. Clin. Cancer Res. 2016;35:36. doi: 10.1186/s13046-016-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abir-Awan M. Kitchen P. Salman M. M. Conner M. T. Conner A. C. Bill R. M. Int. J. Mol. Sci. 2019;20:1589. doi: 10.3390/ijms20071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliati E. Meurice N. DuBois P. Fang J. S. Somasekharan S. Beckett E. Flynn G. Yool A. J. Mol. Pharmacol. 2009;76:105–112. doi: 10.1124/mol.108.053744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad S. O'Riordan C. E. Verra C. Aimaretti E. Alves G. F. Dreisch K. Evenäs J. Gena P. Tesse A. Rützler M. Collino M. Calamita G. Thiemermann C. Front. Immunol. 2022;13:900906. doi: 10.3389/fimmu.2022.900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber V. J. Tsujita M. Nakada T. Bioorg. Med. Chem. 2009;17:411–417. doi: 10.1016/j.bmc.2007.12.040. [DOI] [PubMed] [Google Scholar]
- Huber V. J. Tsujita M. Nakada T. Mol. Aspects Med. 2012;33:691–703. doi: 10.1016/j.mam.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Yadav N. Tripathi A. Parveen A. Parveen S. Banerjee M. Pharmaceutics. 2022;14:1326. doi: 10.3390/pharmaceutics14071326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Paik Y. Park S. Int. J. Environ. Res. Public Health. 2022;19:6655. doi: 10.3390/ijerph19116655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. Kwak H. J. Kim B. H. Kim D.-W. Kim H. Y. Kim S. H. Kang K. S. Plants. 2022;11:1658. doi: 10.3390/plants11131658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M. L. Yang L. Jeong K. W. Theranostics. 2022;12:5761–5775. doi: 10.7150/thno.72599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. B. Int. J. Mol. Sci. 2022;23:9624. doi: 10.3390/ijms23179624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulli A. Srinivasu P. N. Sashank M. S. K. Shafi J. Choi J. Ijaz M. F. Sensors. 2022;22:2988. doi: 10.3390/s22082988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B. Zhang H. Verkman A. S. Bioorg. Med. Chem. 2008;16:7489–7493. doi: 10.1016/j.bmc.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozu M. Dorr R. A. Politi M. T. Parisi M. Toriano R. Eur. Biophys. J. 2011;40:737–746. doi: 10.1007/s00249-011-0687-2. [DOI] [PubMed] [Google Scholar]
- Lee S.-Y. Hwang H. J. Ku B. Lee D. W. Anal. Chem. 2022;94:11838–11847. doi: 10.1021/acs.analchem.2c02222. [DOI] [PubMed] [Google Scholar]
- Kang M. Chun Y. S. Park H. K. Cho E. K. Jung J. Kim Y. Ann. Surg. Treat. Res. 2022;102:73. doi: 10.4174/astr.2022.102.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Lucks A. Gena P. Frascaria D. Altamura N. Svelto M. Beitz E. Calamita G. New Biotechnol. 2013;30:545–551. doi: 10.1016/j.nbt.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Tomita Y. Palethorpe H. M. Smith E. Nakhjavani M. Townsend A. R. Price T. J. Yool A. J. Hardingham J. E. Int. J. Mol. Sci. 2019;20:1818. doi: 10.3390/ijms20081818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H. Ahn S. Nam S. Jang Y. Chun Y. Park H. Choi S. Choi H. Kim J. J. Med. Ultrasound. 2022;30:116. doi: 10.4103/JMU.JMU_58_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q. N. Lee S. R. Kim B. Hong J.-H. Jang Y. S. Lee D. E. Pang C. Kang K. S. Kim K. H. Plants. 2022;11:3387. doi: 10.3390/plants11233387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Ahn S. Nam S. Kim Y. Chun Y. Park H. Choi H. J. Med. Ultrasound. 2023;31:147. doi: 10.4103/jmu.jmu_168_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mola M. G. Nicchia G. P. Svelto M. Spray D. C. Frigeri A. Anal. Chem. 2009;81:8219–8229. doi: 10.1021/ac901526k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajracharya R. Song J. G. Patil B. R. Lee S. H. Noh H.-M. Kim D.-H. Kim G.-L. Seo S.-H. Park J.-W. Jeong S. H. Lee C. H. Han H.-K. Drug Delivery. 2022;29:1959–1970. doi: 10.1080/10717544.2022.2089296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag Y. Gena P. Maggio A. Singh T. Artner I. Oklinski M. K. Johanson U. Kjellbom P. Nieland J. D. Nielsen S. Calamita G. Rützler M. J. Biol. Chem. 2019;294:7377–7387. doi: 10.1074/jbc.RA118.006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait M. J. Petrik V. Loosemore A. Bell B. A. Papadopoulos M. C. Br. J. Neurosurg. 2007;21:496–500. doi: 10.1080/02688690701449251. [DOI] [PubMed] [Google Scholar]
- Ratelade J. Bennett J. L. Verkman A. S. J. Biol. Chem. 2011;286:45156–45164. doi: 10.1074/jbc.M111.297275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.