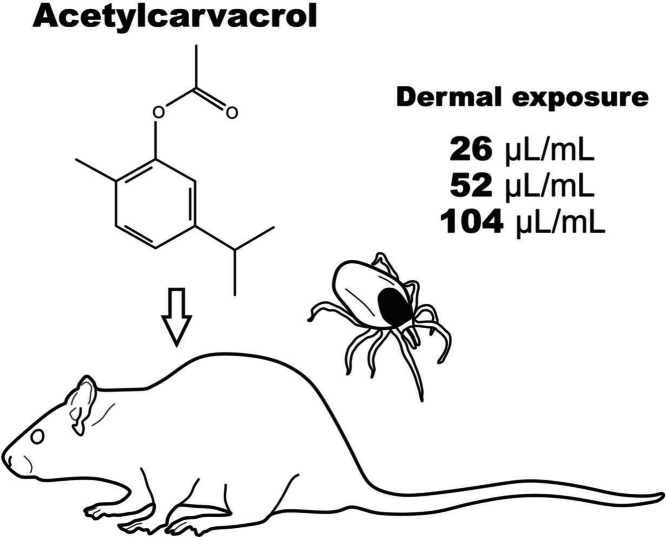
Abstract
Carvacrol, a phenolic monoterpene found in essential oils of plants of the Lamiaceae family, emerges as an alternative acaricide of plant origin. Its acetylation was proposed to obtain a derivative compound with a better pharmacological profile and lower toxicity to non-target organisms. The present study aimed to assess the preclinical safety of acetylcarvacrol after dermal application in Wistar rats, through the examination of hematological and biochemical parameters, as well as histopathological analysis of the skin, liver and kidney. For this, twenty rats were distributed into four groups with five animals each. Three groups received treatment with different concentrations of the substance (26, 52, and 104 µL/mL) based on the lethal concentration for Rhipicephalus sanguineus ticks, and one group (Control) received only the vehicle. Acetylcarvacrol was applied daily to a trichotomized skin area for 21 days. No changes in hematological parameters were observed. Regarding biochemical analysis, a slight increase in urea and alanine transaminase levels was noted. No significant changes were observed in the kidney and liver, although the rats had developed cumulative irritant contact dermatitis at the application site, as corroborated by the histopathological analysis of the skin. In general, the results showed that the dermal application of acetylcarvacrol in the experimental conditions described here is safe. However, it can cause signs of mild systemic toxicity and skin irritation at high concentrations, suggesting that this product should be used in lower therapeutic doses and that the development of less aggressive formulations, including the combination with other acaricides, is desirable.
Keywords: Semi-synthetic, Dermal toxicity, Acaricide, Tick control
Graphical Abstract
Highlights
-
•
Acetylcarvacrol is a potential acaricide for controlling the brown dog tick.
-
•
Its topical application did not result in relevant effects on the liver and kidneys.
-
•
In high concentrations, acetylcarvacrol can cause irritation and changes to the skin.
1. Introduction
The brown-dog-tick Rhipicephalus sanguineus was introduced to the American continent along with the domestic dogs from European colonization [24]. When feeding, these ticks cause spoliation and injury to the hosts and are potential transmitters of pathogens such as protozoa, viruses, and bacteria, causing various diseases [14], [19], [56].
The synthetic chemical groups most used to control ticks in small animals include carbamates, organophosphates, pyrethroids, formamidines, macrocyclic lactones, and phenylpyrazoles, with different modes of action and efficacy [21], [3], [4]. However, the indiscriminate use of these compounds has led to the selection of resistant ticks, environmental contamination, and animal and human poisoning, arousing researchers’ interest in developing new forms of control [4], [69].
Carvacrol (5-isopropyl-2-methylphenol, C10H14O) is a phenolic monoterpene found mainly in essential oils of plants of the Lamiaceae family [54]. This compound, one of the major constituents of Origanum sp. and Thymus sp. essential oils, has also been used in low concentrations as a food flavoring, preservative, and fragrance in cosmetic formulations [59]. Carvacrol has also demonstrated significant acaricidal activity and is considered a promising option for controlling ticks [15], [32], [36], [49]. The effectiveness of carvacrol can be increased by acetylation, conferring greater stability to this compound by converting the phenolic hydroxyl, more susceptible to oxidation, into an ester group [18]. The use of acetylcarvacrol in ticks has already shown high efficacy and several morphological effects in the ovary of R. sanguineus and R. microplus ticks [23], [22], [33], [34], [35], [45].
However, contrary to popular belief, the use of medicinal plants is not without risk. Despite their numerous health benefits for humans and animals, certain plant compounds can exhibit toxic effects, which are influenced by dosage and specific conditions [68]. Information regarding the toxic effects of carvacrol is limited, but this compound is generally considered safe for use [59]. Even so, after acetylation, lower acute toxicity was observed in mice, with a medium lethal dose (LD50) approximately 70 % higher than carvacrol [6]. The difficulty in finding data in the literature to evaluate and discuss the toxicity of acetylcarvacrol reinforces the need for this type of study, ensuring the safe use of this compound in humans and animals [46].
Although some studies have demonstrated the toxic effects of acetylcarvacrol on vertebrates, none of the experiments used concentrations of the product recognizably effective against ticks. Here, we investigated the impact of acetylcarvacrol on Wistar rats (Rattus norvegicus) using concentrations based on the previously defined in vitro lethal concentration values for R. sanguineus ticks [34]. The objective of this study was to assess the safety profile of acetylcarvacrol in Wistar rats through dermal testing with repeated doses, employing clinical, hematological, biochemical, and histopathological analyses.
2. Materials and methods
2.1. Carvacrol acetylation
Carvacrol (5-isopropyl-2-methylphenol, 99 % purity) was obtained from Merck (Darmstadt, Germany), and acetylation was done according to Konig et al. [33]. At room temperature, 5 mL of carvacrol was added to a volumetric flask containing 25 mL of 10 % sodium hydroxide solution. Subsequently, 5.5 mL of acetic anhydride was added to the flask under cooling. The reaction mixture was left under stirring for 15 minutes. The oil was separated from the solution and characterized according to its melting point and infrared (IR) spectroscopy [42], [57].
2.2. Animals
The Ethics Committee on the Use of Animals (CEUA) of the Federal University of Lavras (UFLA) (Protocol 002/2021 of April 26th, 2021) approved all experimental procedures performed in this study.
Twenty adult female Wistar albino rats (Ratus norvegicus), at approximately nine weeks of age and weighing between 160 and 230 g, were obtained from the Central Animal Facility of UFLA. The animals were placed in polypropylene boxes (40x34x17cm) on a ventilated shelf (22 ± 3 ºC, 50–60 % RH), with a photoperiod of 12/12 h (light/dark), and water and food ad libitum. The rats remained in cages for 5 days for acclimatization to laboratory conditions ([44], 2008).
2.3. Experimental design
The experimental design of this work was based on the OECD Guideline 410 of the Organization for Economic Cooperation and Development [44]. The rats underwent trichotomy of 10–20 % of the back using an electric clipper one day before beginning the treatments. The animals were weighed and randomly divided into four groups (n = 5 animals per group). Acetylcarvacrol was diluted in 5 % DMSO (vehicle) at three different concentrations, as suggested by Guideline 410: one with low toxicity to the vertebrate host, one with intermediate toxicity and one with presumable high toxicity [44]. The values used in the different concentrations were based on the work developed by Konig et al. [34]. Based on topical in vitro studies, Konig et al. [34] defined that the concentration of 26 µl/mL is sufficient to generate mortality of approximately 90 % of ticks of the species Rhipicephalus sanguineus (CL₉₀), the brown dog tick. Therefore, this value was used as a basis for the development of the present study, in order to simulate product application conditions in a field situation. Thus: treatment group 1 (T1) was exposed to acetylcarvacrol at a concentration of 26 µl/mL (1 x CL₉₀; 25.2 ×103 ppm); group T2 was treated with a solution at 52 µl/mL (2 x CL₉₀; 50.4 ×103 ppm); and group T3 was exposed to 104 µl/mL (4 x CL₉₀; 100.8 ×103 ppm). The control group received only the vehicle (DMSO at 5 %/day). Daily, 2.5 mL of solution was applied to the trichotomized area using a disposable volumetric pipette for each animal for 21 days. The region was covered with gauze and protected using a crepe bandage fixed with a waterproof plaster. After treatment, the animals were observed for signs of irritation, systemic toxicity, morbidity, and mortality.
At the end of the treatment period, the animals were anesthetized with an intraperitoneal injection of xylazine (10 mg/kg) and ketamine (90 mg/kg) diluted in 0.9 % sodium chloride solution and euthanized by exsanguination by cardiac puncture to collect blood, skin, liver and kidney samples.
2.4. Hematological and biochemical analyses
The hematological analysis was performed using 4 mL of blood taken from each animal and placed in tubes containing EDTA (K3EDTA, Vacuette®). The samples were kept under refrigeration between 2 and 6ºC and processed within a maximum of two hours. The blood was carefully homogenized with the anticoagulant by gently shaking the tube. The following parameters were determined in a Sysmex Poch-100IV.Diff hematological analyzer through impedance detection of cyanide-free hemoglobin and counting of blood cells based on electrical impulses generated when they were immersed in an electrolyte solution: white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, mean corpuscular hemoglobin, and platelet count.
Samples with 4 mL of blood were stored in CAT Serum Sep Clot Activator tubes (Vacuette®) without anticoagulant to determine the values of the following biochemical parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and urea. The blood was centrifuged at 3500 rpm for 15 minutes to separate the serum and clot. Quantifications were carried out using the Beckman Coulter AU480 chemistry analyzer (UV kinetic methodology based on the recommendations of the International Federation of Clinical Chemistry - IFCC - for transaminases, colorimetric kinetic test for the determination of creatinine and UV enzymatic test for urea).
2.5. Morphological and morphometric assessment
Skin, liver and kidney samples were sectioned and immediately immersed in a 4 % formaldehyde fixative solution for 72 h. The samples were then dehydrated in a gradual series of ethyl alcohol (70, 80, 90, and 95 %, for 20 minutes each), diaphanized in xylol, embedded and included in paraffin. The blocks were sectioned in a Lupetec MRP09 microtome, and the histological sections were submitted to the hematoxylin-eosin (H-E) staining technique to observe the general characteristics of the tissue [29]. The skin slides were also subjected to Picrosirius staining to observe the collagen fibers present in the tissue in red [52]. The material was examined and photographed using a Leica DM500 light microscopy equipped with an ICC50W camera.
2.6. Analysis of results
2.6.1. Semi-quantitative analysis
Table 1, Table 2 summarizes the main structural modifications observed in the liver and skin of rats exposed to toxic products based on the information available in the literature. Each alteration received an importance factor (w), defined by the researchers participating in this project, ranging from 1 to 3, according to its relevance in the survival of cells and tissues: (1) minimal importance, when the lesion minimally affects the morphology and physiology of the cell or tissue evaluated; (2) moderate importance, if the function of the cell or tissue is partially lost; and (3) high importance, when the lesion is irreversible, leading to total loss of cell or tissue function. After analyzing the slides, the morphological alterations were classified into scores (a) from 0 to 5, with (0) as no occurrence and values from 1 to 5 indicating different extents of tissue change, expressed as a percentage: (1) 1–20 %; (2) 21–40 %; (3) 41–60 %; (4) 61–80 %; and (5) 81–100 %. The index of each alteration was obtained by multiplying the importance factor by its score (INDEXalt = w x a). The total index of each individual was then calculated by the sum of the indices of each observed alteration, according to the following formula: INDEXind = Σ (INDEXalt) [40].
Table 1.
Main structural modifications observed in the liver of rats exposed to toxic products and their respective importance factors (w) for semiquantitative analysis.
| Morphological changes | Importance factor (w) | References |
|---|---|---|
| Disorganization of the hepatocyte cords | 2 | [17], [59], [7] |
| Increase in interstitial space | 1 | [16], [17] |
| Hepatocytes with irregularly shaped nucleus | 2 | [17], [26], [7] |
| Hepatocyte with pyknotic nucleus | 3 | [7], [17], [26] |
| Chromatin marginalization in hepatocyte nucleus | 3 | [17] |
| Hepatocytes with cytoplasmic vacuolization | 2 | [17], [26], [31], [7] |
| Hepatocytes with cytoplasmic granulation | 1 | [17] |
| Necrosis | 3 | [16], [25], [31], [41], [7] |
| Apoptosis of the hepatocytes | 3 | [17] |
| Hypertrophy of the hepatocytes | 1 | [17], [20], [31] |
| Hyperplasia of the hepatocytes | 1 | [17], [41], [5], [7] |
| Increase in the number of Kupffer cells | 1 | [17], [41], [5], [7] |
| Congestion in blood vessels | 1 | [17], [31], [41], [5] |
| Fibrosis in portal veins | 3 | [16], [41], [7] |
| Cells showing atypical mitosis | 3 | [37] |
| Dilation of the sinusoid lumen | 1 | [16], [31], [41], [7] |
Table 2.
Main structural modifications observed in the skin of rats exposed to toxic products and their respective importance factors (w) for semiquantitative analysis.
| Morphological changes | Importance factor (w) | References |
|---|---|---|
| Epidermal hyperplasia | 2 | [10], [13], [25], [55], [60], [8] |
| Cytoplasmic vacuolization of keratinocytes | 2 | [47], [67] |
| Epidermal ulcers | 3 | [10], [13] |
| Chronic inflammation in the epidermis | 2 | [10], [60] |
| Hyperkeratosis | 2 | [8], [10], [11], [13], [53] |
| Apoptotic keratinocytes | 3 | [25], [67] |
| Necrotic keratinocytes | 3 | [25] |
| Degradation of epidermis | 2 | [25], [47], [55], [60] |
| Hair follicle hyperplasia | 1 | [10], [60] |
| Sebaceous glands hyperplasia | 2 | [10], [13], [60] |
| Dermal fibrosis | 2 | [10], [67] |
| Decreased number of hair follicles | 1 | [11] |
| Increased number of hair follicles | 1 | [10] |
| Inflammatory cell infiltrates in the dermis | 2 | [10], [11], [63], [67] |
| Ulcerative dermatitis | 3 | [25], [63] |
| Munro’s microabscesses | 2 | [30] |
| Parakeratosis | 2 | [1], [13], [25], [30], [63] |
| Spongiosis | 2 | [1] |
| Perifolliculitis | 2 | [1] |
2.6.2. Morphometric analysis
Morphometric analysis was performed using the Image J software (NIH). All individuals in each group were measured.
The area and perimeter of 50 nuclei and 50 hepatocytes in the liver samples were randomly measured. The ratio between the area of the nucleus and the area of the hepatocyte was also obtained (R = area of the nucleus/area of the hepatocyte) for each individual. The numerical density of hepatocytes (Nhep) in the tissue was calculated based on the number of hepatocytes in 20 regions of known area (6000 µ2 each), making it possible to obtain the estimated number of hepatocytes per µm2 by the following formula: Nhep = Σhep/TA, in which Σhep is the number of hepatocytes identified in the focal plane and TA is the total test area. The percentage of altered nuclei (%Nalt) in the tissue was also measured in five random images of each sample (total area 3.54 ×105 µ2). Irregular, hyper-stained, or pycnotic nuclei (Nalt) and the total number of nuclei (Nt) of the evaluated area were counted and used in the following formula: %Nalt = (Naltered/Ntotal) x 100. Furthermore, the percentage of the tissue occupied by sinusoid capillaries was measured in five random images of each sample (total area 3.54 ×105 µ2). For this, a grid of 713 points was established in each image and the number of points that intercept the structure of interest (sinusoids) was counted. The percentage was obtained from the number of points on the sinusoids (Psin) and the total number of points of each image (Ptotal), according to the formula: %sin = (Psin/Ptotal) x 100.
The area occupied by the epidermis in the skin sections was determined in ten random images of each sample and the number of epithelial cells in each image was counted to obtain the number of cells per µm2. The same procedure was adopted for counting connective tissue cells in the dermis. The thickness of the epidermis (distance between the base membrane and the apical surface of the stratum corneum) was measured in ten randomized images of each individual (total area 7.07 ×105 µ2). Three measurements were performed on each image, totaling 30 measurements per individual. Moreover, the percentage of collagen fibers in the tissue was determined on the slides subjected to Picrosirius staining from the Threshold tool of the ImageJ software. Ten images were evaluated (total area 7.07 ×105 µ2), and the areas selected for this measurement corresponded only to the papillary dermis, without the presence of hair follicles.
2.6.3. Statistical analysis
Hematological, biochemical, morphological, and morphometric data were evaluated for normality using the Shapiro-Wilk test and statistically compared by the one-way ANOVA test followed by the post hoc Tukey test (α = 0.05; GraphPad Prism v. 7.00).
3. Results
3.1. Clinical and behavioral assessment
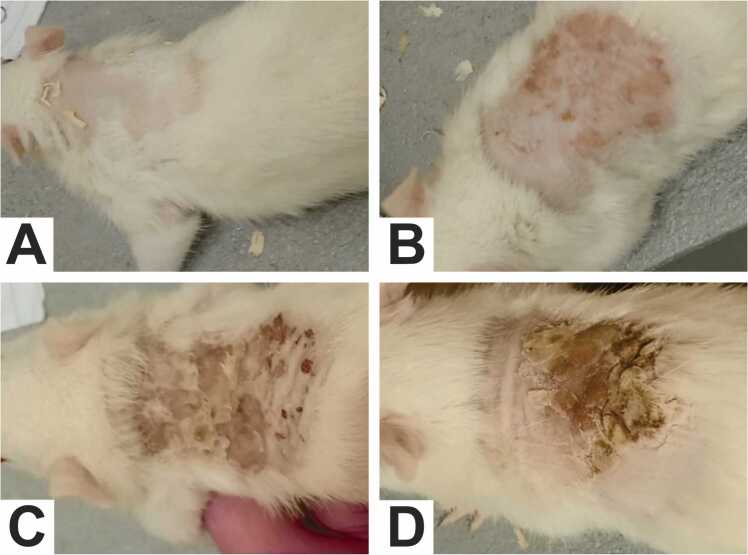
The rats exhibited tolerance to acetylcarvacrol exposure and maintained good overall health throughout the study period. No deaths were observed. However, animals in the treatment groups showed local dermal irritation, pain to the touch, and dermatitis with intense itching. Skin lesions were dose-dependent and more evident in rats treated with the highest concentration from the third day of application (Fig. 1A-D). Abrasions and exudation were associated with skin injury, intensified by self-inflicted trauma due to intense itching, and daily application of the product provoked skin peeling and the appearance of new wounds. Stress and discomfort caused the animals receiving the treatment to vocalize with high-pitched squeaks and become more agitated, restless, and aggressive immediately after applying the substance. Hair growth was inhibited at the site of application.
Fig. 1.
Appearance of the animals' skin at the conclusion of the experiment. It is noted that the occurrence of skin irritation was dose-dependent, compared to the control group. (A) Control Group (DMSO 5 %); (B) T1 Group; (C) T2 Group; (D) T3 Group.
3.2. Hematological and biochemical analysis
No statistical difference was observed in hematological parameters (erythrogram and leukogram) among groups (Table 3, Table 4). In general, the rats did not show significant changes in biochemical parameters (Table 5), Only the animals of the group treated with the highest concentration (T3) showed an slight increase in plasma levels of urea and alanine aminotransferase (ALT) compared with the animals of the control group (p = 0.018). The other indexes analyzed were within normal standards.
Table 3.
Erythrogram of the albino Wistar rats after 21 days of topic exposure to acetylcarvacrol in different concentrations.
| Erythrogram | Groups |
p-value | |||
|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | ||
| Red cells (millions/mm³) | 7.29 ± 0.94a | 7.86 ± 0.21a | 7.16 ± 0.34a | 7.82 ± 0.22a | 0.249 |
| Hemoglobin (g/dL) | 13.82 ± 1.82a | 15.60 ± 0.40a | 14.44 ± 0.37a | 15.43 ± 0.51a | 0.123 |
| Hematocrit (%) | 38.70 ± 4.50a | 42.53 ± 0.85a | 40.02 ± 0.97a | 42.96 ± 1.24a | 0.132 |
| MCV (fL) | 53.17 ± 0.78a | 54.10 ± 0.40a | 55.90 ± 1.86a | 51.43 ± 6.09a | 0.236 |
| MCH (pg) | 18.97 ± 0.09a | 19.83 ± 0.05a | 20.26 ± 0.72a | 19.73 ± 0.11a | 0.197 |
| MCHC (%) | 35.70 ± 0.62a | 36.70 ± 0.20a | 36.20 ± 0.18a | 35.90 ± 0.36a | 0.132 |
| RDW (%) | 12.10 ± 1.54a | 12.90 ± 0.70a | 12.60 ± 0.85a | 11.90 ± 0.52a | 0.599 |
| Erythroblasts (%) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05). (MCV) Mean corpuscular volume; (MCH) Mean corpuscular hemoglobin; (MCHC) Mean corpuscular hemoglobin concentration; (RDW) Red blood cell distribution width; (g/dL) Gram per deciliter; (fL) Femtoliter; (pg) Picogram.
Table 4.
Leukogram of the albino Wistar rats after 21 days of topic exposure to acetylcarvacrol in different concentrations.
| Leukogram | Groups |
p-value | |||
|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | ||
| Total leukocytes (x103/mm³) | 3.52 ± 0.50a | 4.23 ± 0.15a | 3.66 ± 0.90a | 4.63 ± 1.75a | 0.431 |
| Banded neutrophils (%) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - |
| Segmented neutrophils (%) | 15.50 ± 8.90a | 19.00 ± 4.35a | 16.80 ± 7.15a | 24.00 ± 13.74a | 0.627 |
| Lymphocytes (%) | 82.25 ± 9.70a | 79.33 ± 3.21a | 81.60 ± 7.63a | 72.33 ± 14.84a | 0.532 |
| Atypical Lymphocytes (%) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - |
| Monocytes (%) | 1.75 ± 1.50a | 1.66 ± 1.15a | 1.20 ± 0.44a | 2.33 ± 1.15a | 0.572 |
| Eosinophils (%) | 0.50 ± 1.00a | 0.00 ± 0.00a | 0.40 ± 0.50a | 1.33 ± 1.15a | 0.261 |
| Basophils (%) | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | - |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05).
Table 5.
Platelets (x103/mm3), urea (mg/dL), creatinine (mg/dL), AST (U/L) and ALT (U/L) in albino Wistar rats after 21 days of experiment.
| Biochemical exams |
Groups |
p-value |
|||
|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | ||
| Platelets (x103/mm³) | 923.50 ± 93.20a | 1049.00 ± 121.20a | 977.20 ± 177.90a | 972.30± 46.70a | 0.669 |
| Urea (mg/dL) | 49.40 ± 5.32a | 55.80 ± 408ab | 58.40 ± 7.20ab | 59.80 ± 4.20b | 0.033 |
| Creatinine (mg/dL) | 0.30 ± 0.07a | 0.30 ± 0.00a | 0.30 ± 0.00a | 0.28 ± 0.04a | 0.835 |
| AST (U/L) | 150.40 ± 22.30a | 167.60 ± 32.00a | 168.40 ± 5.10a | 168.40 ± 5.10a | 0.205 |
| ALT (U/L) | 57.80 ± 10.30a | 56.40 ± 4.50a | 62.60 ± 16.20ab | 83.20 ± 17.20b | 0.018 |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05). (AST) Aspartate aminotransferase; (ALT) Alanine aminotransferase; (mg/dL) milligrams per deciliter; (U/L) units per liter.
3.3. Histopathology
3.3.1. Skin
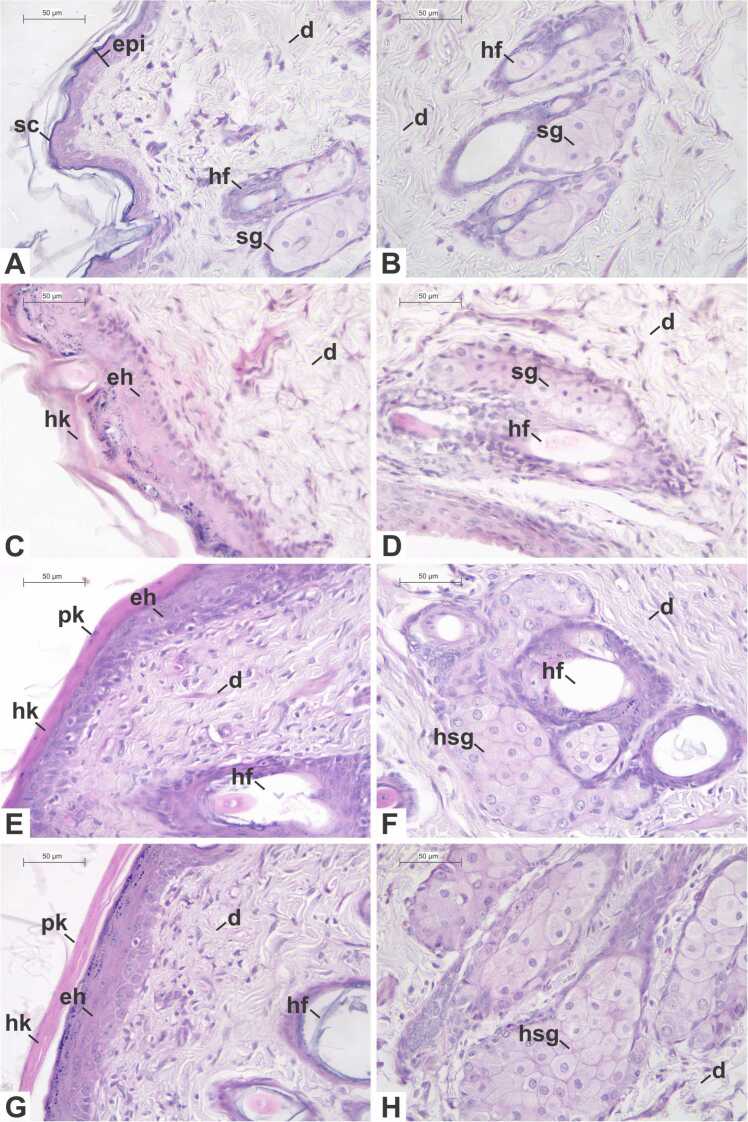
The animals of the control group (Fig. 2A-B) presented normal skin morphology. The epidermis showed mainly keratinocytes, with different morphological characteristics according to the layer in which they were located, and melanocytes, with unstained cytoplasm and a small and rounded nucleus. The underlying dermis presented blood and lymphatic vessels, fibroblasts, hair follicles with sebaceous glands, and sweat glands.
Fig. 2.
Photomicrographs of the skin of Wistar rats subjected to a repeated dose dermal toxicity test with acetylcarvacrol exposure over a 21-day period, stained with hematoxylin-eosin. (A-B) Control Group; (C-D) Treatment Group 1–26 µL/mL; (E-F) Treatment Group 2–52 µL/mL; (G-H) Treatment Group 3–104 µL/mL. Legends: (d) dermis; (eh) epidermal hyperplasia; (epi) epidermis; (hf) hair follicle; (hk) hyperkeratosis; (hsg) hyperplasic sebaceous gland; (pk) parakeratosis; (sc) stratum corneum; (sg) sebaceous gland. Scale bars: (A-H) 50 µm.
In the histopathological analysis, although we observed a small reduction in the number of hair follicles, as well as a discrete parakeratosis and a slight occurrence of dose-dependent nonspecific inflammatory infiltrates, no statistically significant differences in these parameters occurred between the groups (p > 0.05). However, animals belonging to all treatment groups (Fig. 2C-H) presented sebaceous gland hyperplasia (p = 0.0003), when compared with the controls. In the T2 and T3 groups, epidermal hyperplasia (p = 0.0007) was also present, leading to the thickening of epidermis. In addition, vacuolization of keratinocytes within the stratum basale (p = 0.0220) and hyperkeratosis (p = 0.0011) were statistically different between the T3 group and the control group. Due to these differences, the semi-quantitative analysis (Table 8) indicated a significant difference between the control group and the group T3 regarding the INDEXind (p = 0.0006).
Table 8.
Mean ± standard deviation of the indexes of each alteration (INDEXalt) and individual indexes (INDEXind) observed in the skin of rats submitted to the dermal toxicity test with repeated dose.
| Morphological change | (w) | Groups |
p-value | |||
|---|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | |||
| Epidermal hyperplasia | 1 | 0.00 ± 0.00a | 6.00 ± 0.00ab | 7.20 ± 1.10b | 9.20 ± 1.10b | 0.0007 |
| Cytoplasmic vacuolization of keratinocytes | 2 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.80 ± 1.79ab | 1,60 ± 0.89b | 0.0220 |
| Hyperkeratosis | 2 | 0.00 ± 0.00a | 1.60 ± 2.19a | 4.40 ± 0.89ab | 6.80 ± 1.10b | 0.0011 |
| Sebaceous glands hyperplasia | 2 | 0.00 ± 0.00a | 6.00 ± 0.00b | 6.00 ± 0.00b | 6.00 ± 0.00b | 0.0003 |
| Decreased number of hair follicles | 1 | 0.00 ± 0.00a | 0.60 ± 0.89a | 0.00 ± 0.00a | 0.60 ± 1.34a | 0.2839 |
| Inflammatory cell infiltrates in the dermis | 2 | 0.00 ± 0.00a | 1.60 ± 1.67a | 3.20 ± 2.28a | 3.20 ± 1.79a | 0.0523 |
| Parakeratosis | 2 | 0.00 ± 0.00a | 0.00 ± 0.00a | 1.20 ± 2.68 | 3.20 ± 3.03a | 0.0775 |
| INDEX(ind) | 0.00 ± 0.00a | 15.80 ± 3.03ab | 22.80 ± 4.82ab | 30.60 ± 2.61b | 0.0006 | |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05). (w) Importance factor.
Morphometric analysis (Table 9) revealed no significant differences between groups regarding the percentage of collagen fibers in the dermis (p = 0.7680). However, there was an increase in the thickness of the epidermis in the T2 and T3 groups compared with the animals in the control group (p = 0.0002). A significant increase was observed in the number of the epidermal cells (p = 0.0088) and papillary dermis cells (p = 0.0017) of animals belonging to the T2 and T3 groups when compared with the control.
Table 9.
Epidermal and dermal cells numerical density (x 10−3/µm2), thickness of the epidermis (µm) and percentage of collagen in the dermis (%) in rats submitted to the dermal toxicity test with repeated doses of acetylcarvacrol.
| Parameter |
Groups |
p-value | ||||
|---|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | |||
| Numerical density of the epidermal cells (x 10−3/µm2) | 6.35 ± 0.61a | 7.10 ± 1.08ab | 7.78 ± 0.61b | 8.20 ± 0.69b | 0.0088 | |
| Numerical density of the dermal cells (x 10−3/µm2) | 2.36 ± 0.26a | 3.38 ± 0.59ab | 3.95 ± 0.63b | 3.92 ± 0.75b | 0.0017 | |
| Thickness of the epidermis (µm) | 41.09 ± 10.41a | 69.99 ± 11.33ab | 81.20 ± 11.27bc | 111.18 ± 30.57c | 0.0002 | |
| Collagen (%) | 79.10 ± 1.73a | 78.00 ± 1.31a | 77.60 ± 3.93a | 77.94 ± 1.44a | 0.7680 | |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05).
3.3.2. Liver
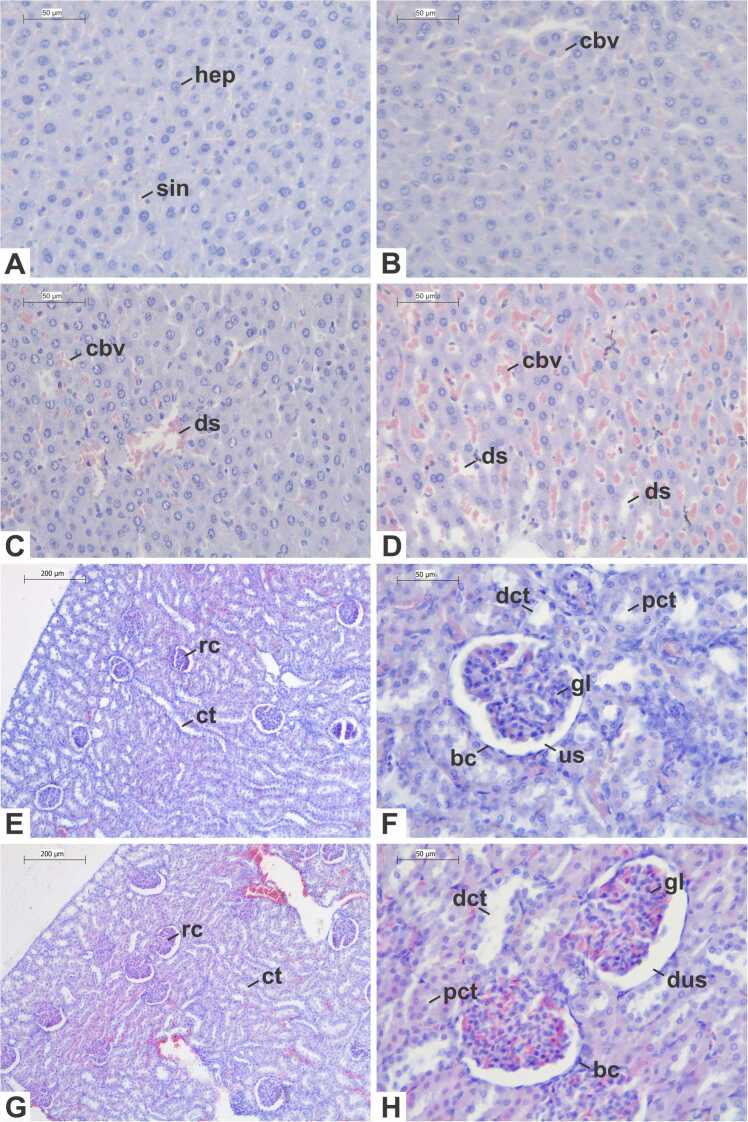
The histological characteristics of the liver remained preserved in the control group (Fig. 3A), with hepatocytes distributed in regular cords and with intact morphology, i.e., polygonal shape and cytoplasm stained pink by eosin, containing one or two rounded nuclei with dispersed chromatin and nucleoli, both stained purple by hematoxylin. Kupffer cells were visualized between the hepatic cords, with an elliptical nucleus, and sinusoidal spaces, veins and arteries with normal appearance were observed.
Fig. 3.
Photomicrographs of the liver (A-D) and kidney (E-H) of Wistar rats subjected to a repeated dose dermal toxicity test with acetylcarvacrol exposure over a 21-day period, stained with hematoxylin-eosin. (A, E, F) Control Group; (B) Treatment Group 1–26 µL/mL; (C) Treatment Group 2–52 µL/mL; (D, G, H) Treatment Group 3–104 µL/mL. Legends: (bc) Bowman capsule; (cbv) congested blood vessel; (ct) convoluted tubules; (dct) distal convoluted tubule; (ds) dilated sinusoid; (dus) dilated urinary space; (gl) glomerulus; (hep) hepatocyte; (pct) proximal convoluted tubule; (rc) renal corpuscle; (sin) sinusoid; (us) urinary space. Scale bars: (A-D, F, H) 50 µm; (E, G) 200 µm.
In general, in rats exposed to acetylcarvacrol (Fig. 3B-D), the liver parenchyma demonstrated a preserved structure, with minimal changes, especially in the groups T1 and T2. Furthermore, no signs of steatosis, necrosis or inflammatory infiltrates were observed in any of the treated groups. Slight sinusoidal lumen dilation and congestion in blood vessels were observed the T1 and T2 groups (FIG). In the T2 group, there was also a slight increase in Kupffer Cells. These same alterations were observed to a greater extent in the T3 group. However, no statistical difference regarding the indices of each alteration (INDEXalt) was observed between the groups in the semiquantitative analysis (Table 6). On the other hand, the individual indices (INDEXind) differed between the control and T3 group (p = 0.037).
Table 6.
Mean ± standard deviation of the indexes of each alteration (INDEXalt) and individual indexes (INDEXind) observed in the liver of rats submitted to the dermal toxicity test with repeated dose.
| Morphological change | (w) |
Groups |
p-value | |||
|---|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | |||
| Increase in the number of Kupffer cells | 1 | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.20 ± 0.40a | 0.80 ± 0.75a | 0.063 |
| Congestion in blood vessels | 1 | 1.40 ± 0.45a | 2.40 ± 0.49a | 2.00 ± 0.63a | 1.80 ± 0.75a | 0.163 |
| Dilation of the sinusoid lumen | 1 | 0.00 ± 0.00a | 0.40 ± 0.49a | 0.80 ± 0.40a | 1.20 ± 0.98a | 0.039 |
| INDEXind | 1.40 ± 0.55a | 2.80 ± 0.84ab | 3.00 ± 1.22ab | 3.80 ± 2.39b | 0.028 | |
Means followed by different lowercase letters in lines differ statistically (Kruskal-Wallis followed by Dunn post-hoc test; p < 0,05). (w) Importance factor.
The morphometric analysis (Table 7) also showed no statistically significant difference between the treatment groups and the control group regarding the area of the and the hepatocyte, nucleus area/hepatocyte area ratio, the percentage of tissue occupied by hepatic sinusoids and the numerical density of hepatocytes in the tissue.
Table 7.
Measurements of the nuclear (µm2) and hepatocyte area (µm2), ratio between the areas of the nucleus and the hepatocyte, percentage of altered hepatocyte nuclei (%), percentage of sinusoids present in the tissue (%) and numerical density of hepatocytes (x 10−3/µm2) in rats submitted to the dermal toxicity test with repeated doses of acetylcarvacrol.
| Parameter |
Groups |
p-value | |||
|---|---|---|---|---|---|
| Control | T1 (26 µL/mL) | T2 (52 µL/mL) | T3 (104 µL/mL) | ||
| Nuclear area (µm2) | 42.76 ± 5.77a | 39.14 ± 12.75a | 44.91 ± 3.00a | 43.54 ± 5.37a | 0.378 |
| Hepatocytes area (µm2) | 179.04 ± 24.23a | 159.94 ± 35.33a | 186.68 ± 13.82a | 181.97 ± 21.91a | 0.675 |
| Nucleus/hepatocyte ratio | 0.24 ± 0.02a | 0.24 ± 0.03a | 0.24 ± 0.02a | 0.24 ± 0.02a | 0.999 |
| Sinusoids (%) | 13.16 ± 1.96a | 13.26 ± 2.78a | 13.24 ± 1.93a | 14.44 ± 2.58a | 0.796 |
| Numerical density (x10−3/µm2) | 33.67 ± 3.61a | 33.33 ± 2.04a | 33.00 ± 1.39a | 32.33 ± 1.90a | 0.836 |
Means followed by different lowercase letters in lines differ statistically (one-way ANOVA followed by Tukey post-hoc test; p < 0,05).
3.3.3. Kidney
In the control group (Fig. 3E-F), individuals exhibited typical morphological characteristics in the kidney. Renal corpuscles contained glomeruli surrounded by the squamous cells of Bowman's capsule, while proximal tubules featured cubic epithelial cells with acidophilic cytoplasm and a distinct striated border, albeit with a sparse lumen. Distal tubules, in turn, showed a wider lumen lined by weakly stained cubic cells with less evident striated border. Collecting ducts were lined by a cubic to columnar epithelium, characterized by pale stained cells. Within the medullary region, the thin components of the loop of Henle were covered by a simple squamous epithelium.
Overall, rats subjected to lower concentration treatments (T1 and T2) showed no discernible morphological alterations. The cortical region displayed unaltered tubules with normal morphology and diameter, alongside renal corpuscles exhibiting characteristics similar to those of the control group. Furthermore, no alterations were noted in the collecting ducts or segments of the loop of Henle. Conversely, in the T3 group, some individuals exhibited urinary space dilation and congested capillaries, while other parameters remained within established normal ranges (Fig. 3G-H).
4. Discussion
The results revealed that dermal application with a repeated dose of acetylcarvacrol in rats for 21 days caused no mortality. The rodents presented a good general condition throughout the study period and clear signs of systemic toxicity were not observed. In fact, hematological and biochemical parameters indicated preserved liver and kidney function in the treated rats, with some exceptions in the T3 group, in which a slight increase in the enzyme alanine aminotransferase (ALT) levels was detected. Andre et al. [5] showed that the subchronic exposure of rats to acetylcarvacrol by gavage at a concentration of 250 mg/kg, for 28 days, caused no significant differences in biochemical and hematological parameters compared to the control group, corroborating our results. Additionally, Oliveira et al. [46] observed no changes in serum levels of hepatic transaminases, urea, and creatinine after administrating acetylcarvacrol orally (2000 mg/Kg) and intraperitoneally (1000 mg/Kg) in mice. Our observations may indicate differences in the metabolism of this substance after exposure to acetylcarvacrol through different routes of administration.
The histopathological examination of the liver and kidneys in the current study further supports the safety profile of the drug, particularly evident in the two lowest concentrations tested and consistent with the laboratory findings. However, the INDEXind of animals subjected to treatment with a higher concentration of acetylcarvacrol (T3 group), indicative of cumulative hepatic damage, may imply mild hepatic toxicity in these subjects. Nonetheless, because the semi-quantitative analysis revealed no statistical disparities among the hepatic alterations identified in this study, caution should be exercised in interpreting these results. In this group, the increased ALT levels may also suggest mild hepatocellular damage. This enzyme, found in the cytosol of hepatocytes, is considered a sensitive marker of cellular injury [39], [43] as it overflows from the cytoplasm due to damage to the plasma membrane [27], [61]. Andre et al. [5] also observed liver changes after acetylcarvacrol administration in rats, such as hepatocyte swelling, Kupffer cell hyperplasia, and congestion of the portal and centrilobular veins. Additionally, treating Mus musculus mice with thymol, a carvacrol isomer, through spray baths caused hepatocyte vacuolation, irregularities in the nucleus, and necrosis [17].
No changes in renal parameters were detected in the groups treated with the two lowest concentrations of acetylcarvacrol; however, a slight increase in plasma urea levels was noted in rats from the T3 group. Nevertheless, this marker lacks specificity, as increased serum urea levels can result from conditions other than acute renal failure, including high protein intake, infection, congestive heart failure, hemorrhage leading to blood absorption in the gut, sodium depletion, trauma, and dehydration [2], [58], which were not assessed here. Although elevated urea levels observed in the T3 group could be attributed to the expansion of urinary space observed in renal corpuscles, no other significant changes in renal parenchyma were present. Furthermore, creatinine levels, an important marker of renal filtration [48], [58], remained unchanged across all groups, including T3, in our study. When collectively analyzed, the data obtained here do not suggest alterations in renal function with clinical significance in the treated animals, nor do they indicate nephrotoxic effects of acetylcarvacrol.
In the treatment groups, the animals showed dose-dependent clinical signs of cumulative irritative contact dermatitis, i.e., pruritus, local dermal irritation, pain to the touch, erythema, and edema. Dermatitis caused by contact with cosmetics containing plant extracts or substances isolated from a plant source in their formulation is very common [64]. Recent studies have shown that topical application of carvacrol induced itching, hyperplasia, and inflammation in the skin, resembling the clinical manifestations of atopic dermatitis [51], [65], [66].
The stratum corneum corresponds to the primary physical barrier of the skin. Therefore, diffusion through this layer is a limiting step for a substance to permeate the skin [50]. Dimethyl sulfoxide (DMSO), used here as a vehicle, is commonly employed as an enhancer of cutaneous penetration of molecules by altering the lipid organization of the stratum corneum [12], [28], [38]. It is also suggested that adding an acetyl radical to the chemical structure of carvacrol increases its hydrophobic properties, improving its cellular permeability [42]. The use of DMSO as a solvent, which favors skin permeation, added to the chemical properties of acetylcarvacrol may have contributed to greater absorption of this substance by the animals.
Our results showed that the effects of acetylcarvacrol on the skin were dose-dependent, as demonstrated by the statistically different INDEXind in the T3 group compared to the control group. Qu et al. [51] applied 3 % carvacrol diluted in a saline solution containing 50 % ethanol for five consecutive days directly to the ear of mice twice daily for five consecutive days, causing inflammatory skin lesions. In turn, topical application of 1 % carvacrol solution diluted in 10 % ethanol solution to the back of mice for 10 minutes, twice daily, for four consecutive days induced epidermal hyperplasia and keratinocyte proliferation [65]. In addition, the application of a 2 % carvacrol solution dissolved in 30 % ethanol for five consecutive days on the trichotomized skin of mice resulted in clinical signs similar to atopic dermatitis, such as severe itching, skin lesions, and epidermal hyperplasia [66]. In our investigation, the application of acetylcarvacrol for 21 days was necessary to trigger skin alterations similar to those observed with carvacrol. It is evident that in shorter and regulated applications of acetylcarvacrol for tick control, such events are likely mitigated. Based on our findings, we recommend a thorough examination of treatment duration in controlling R. sanguineus, particularly at the lower concentrations assessed in this study, in further studies.
Here, especially in groups treated with the highest concentrations of acetylcarvacrol, alterations such as cytoplasmic vacuolization of keratinocytes, acanthosis, parakeratosis and hyperkeratosis were observed in the epidermis. The inflammatory response in the skin is associated with an increased epidermal thickness [63]. Furthermore, epidermal hyperplasia and hyperkeratosis indicate a hyperproliferative reaction consistent with irritative contact dermatitis [9]. In addition, parakeratosis, i.e., the persistence of nuclei in the stratum corneum and the absence of the stratum granulosum occurs because of the accelerated maturation of epidermal cells [30], [62].
5. Conclusion
Despite the good tolerance of rats to acetylcarvacrol, direct damage to the skin was evident. Thus, the use of acetylcarvacrol as an acaricide should be evaluated with caution. Considering its potential in the control of ticks, we suggest that the application of reduced concentrations of acetylcarvacrol for shorter periods of time and/or in association with other chemicals that can act synergistically may enhance its results and reduce its adverse effects mainly in the skin of the hosts.
Author agreement statement
We the undersigned declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We understand that the Corresponding Author is the sole contact for the Editorial process. He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.
Financial support
CNPq (Grant number: 430327/2018-8)
CRediT authorship contribution statement
Graziela Hermínia Andrade Mendonça: Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. José Henrique Silva Rodrigues: Methodology, Investigation, Formal analysis. Camila Souza de Oliveira Guimarães: Writing – review & editing, Validation, Formal analysis. Vitor Luis Tenório Mati: Writing – review & editing, Validation, Formal analysis. Rafael Neodini Remedio: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition, Formal analysis, Conceptualization. Aline Chaves Reis: Methodology, Investigation, Formal analysis. Isaac Filipe Moreira Konig: Methodology, Investigation, Formal analysis. Gabriela Pereira Brito: Methodology, Investigation, Formal analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank for the financial support: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil).
Handling Editor: Prof. L.H. Lash
Data availability
Data will be made available on request.
References
- 1.Adachi K., Yamada N., Yoshida Y., Yamamoto O. Subchronic exposure of titanium dioxide nanoparticles to hairless rat skin. Exp. Dermatol. 2013;22(4):278–283. doi: 10.1111/exd.12121. [DOI] [PubMed] [Google Scholar]
- 2.Addis T., Barret E., Poo L.J., Yuen D.W. The relation between the serum urea concentration and the protein consumption of normal individuals. J. Clin. Invest. 1947;26(5):869–874. doi: 10.1172/JCI101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adenubi O.T., Ahmed A.S., Fasina F.O., McGaw L.J., Eloff J.N., Naidoo V. Pesticidal plants as a possible alternative to synthetic acaricides in tick control: a systematic review and meta-analysis. Ind. Crop. Prod. 2018;123:779–806. doi: 10.1016/j.indcrop.2018.06.075. [DOI] [Google Scholar]
- 4.Agwunobi D.O., Yu Z., Liu J. A retrospective review on ixodid tick resistance against synthetic acaricides: imiplications and perspectives for future research prevention and mitigation. Pestic. Biochem. Phys. 2021;173 doi: 10.1016/j.pestbp.2021.104776. [DOI] [PubMed] [Google Scholar]
- 5.Andre W.P.P., Paiva Junior J.R., Cavalcante G.S., Ribeiro W.L.C., Araújo Filho J.V., Santos J.M.L., Alves A.P.N.N., Monteiro J.P., Morais S.M., Silva I.N.G., Oliveira L.M.B., Abreu F.O.M., Bevilaqua C.M.L. Anthelmintic activity of nanoencapsulated carvacryl acetate against gastrointestinal nematodes of sheep and its toxicity in rodents. Braz. J. Vet. Parasitol.. 2020;29(1):1–16. doi: 10.1590/S1984-29612019098. [DOI] [PubMed] [Google Scholar]
- 6.Andre W.P.P., Ribeiro W.L.C., Cavalcante G.S., Santos J.M.L., Macedo I.T.F., Paula H.C.V., Freitas R.M., Morais S.M., Melo J.V., Bevilaqua C.M.L. Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 2016;218:52–58. doi: 10.1016/j.vetpar.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Bakir M., Geyikoglu F., Colak S., Turkez H., Bakir T.O., Hosseinigouzdagani M. The carvacrol ameliorates acute pancreatitis-induced liver injury via antioxidant response. Cytotechnology. 2015;68(4):1131–1146. doi: 10.1007/s10616-015-9871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behl M., Kadiiska M.B., Hetmancik M.R., Vasconcelos D., Chhabra R. Subacute oral and dermal toxicity of tert-butyl hydroperoxide in Fischer F344/N rats and B6C3F1 mice. Cutan. Ocul. Toxicol. 2012;31(3):204–213. doi: 10.3109/15569527.2011.641194. [DOI] [PubMed] [Google Scholar]
- 9.Bruner J.H. In: Dermal and Ocular Toxicology: Fundamentals and Methods. Robson D.W., editor. CRC Press; Boston: 1991. Pathological processes of skin damage related to toxicant exposure; pp. 80–85. [Google Scholar]
- 10.Catlin N.R., Herbert R., Janardhar K., Hejtmancik M.R., Fomby L.M., Vallant M., Kissling G.E., DeVito M.J. Dose-response assessment of the dermal toxicity of Virginia cedarwood oil in F344/N rats and B6C3F1/N mice. Food Chem. Toxicol. 2016;98:159–168. doi: 10.1016/j.fct.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandra S.A., Peterson R.A., Melich D., Merrill C.M., Bailey D., Mellon-Kusibab K., Adler R. Dermal irritation of petrolatum in rabbits but not in mice, rats or minipigs. J. Appl. Toxicol. 2014;34:857–861. doi: 10.1002/jat.2895. [DOI] [PubMed] [Google Scholar]
- 12.Chen B., Liu D., Pan W., Yang X., Shou J., Wu J., Mao Q., Wang J. Use of lipolanthionine peptide, a toll-like receptor 2 inhibitor, enhances transdermal delivery efficiency. Mol. Med. Rep. 2014;10:593–598. doi: 10.3892/mmr.2014.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chhabra R.S., Elwell M.R., Peters A. Toxicity of 4-Vinyl-l-cyclohexene diepoxide after 13 weeks of dermal or oral exposure in rats and mice. Fund. Appl. Toxicol. 1990;14:745–751. doi: 10.1016/0272-0590(90)90299-y. [DOI] [PubMed] [Google Scholar]
- 14.Contreras M., Alberdi P., Mera I.G.F., Krull C., Nijhof A., Villar M., De la Fuente J. Vaccinomics approach to the identification of candidate protective antigens for the control of tick vector infestations and Anaplasma phagocytophilum infection. Front Cell Infect. Microbiol. 2017;7:1–15. doi: 10.3389/fcimb.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coskun S., Girisgin O., Kürkcüoglu M., Malyer H., Girisgin A.O., Kirimer N., Baser K.H. Acaricidal efficacy of Origanum onites L. essential oil against Rhipicephalus turanicus (Ixodidae) Parasitol. Res. 2008;103(2):259–261. doi: 10.1007/s00436-008-0956-x. [DOI] [PubMed] [Google Scholar]
- 16.Cunha L.C., Azeredo F.S., Mendonça A.C.V., Vieira M.S., Pucci L.L., Valadares M.C., Freitas H.O.G., Sena A.A.S., Lino Junior R.S. Avaliação da toxicidade aguda e subaguda, em ratos, do extrato etanólico das folhas e do látex de Synadenium umbellatum Pax. Ver. Bras. Farm. 2009;19(2a):403–411. doi: 10.1590/S0102-695X2009000300012. [DOI] [Google Scholar]
- 17.Cunha E.L.R., Matos R.S., Pereira N.R.C., Oliveira P.R., Daemon E., Camargo-Mathias M.I. Histopathological changes in the liver and thyroid of mice (Mus musculus) caused by the acaricides: fipronil and thymol. J. Histol. Histopathol. 2017;4(9):1–8. doi: 10.7243/2055-091X-4-9. [DOI] [Google Scholar]
- 18.Damasceno S.R.B., Oliveira F.R.A.M., Carvalho N.S., Brito C.F.C., Silva I.S., Sousa F.B.M., Silva R.O., Sousa D.P., Barbosa A.L.R., Freitas R.M., Medeiros J.R. Carvacryl acetate, a derivative of carvacrol, reduces nociceptive and inflammatory response in mice. Life. Sci. 2014;94(1):58–66. doi: 10.1016/j.lfs.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Dantas-Torres F., Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet. Parasitol. 2015;208(1-2):9–13. doi: 10.1016/j.vetpar.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Engelman M.F.B., Guidugli Neto J., Andrade C.H.V., Hernandez R., Goulart L.B.N.T. Estudo morfométrico do fígado de ratos submetidos a doses supra-fisiológicas de tiroxina. Arq. Bras. Endocrinol. Metab. 2001;45(2):173–179. doi: 10.1590/S0004-27302001000200009. [DOI] [Google Scholar]
- 21.Fernandes J.I., Correia T.R., Ribeiro F.A., Cid Y.P., Tavares P.V., Scott F.B. Eficácia in vitro do nim (Azadirachta indica) no controle de Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) Braz. J. Vet. Med. 2010;32(Supl. 1):64–68. [Google Scholar]
- 22.Gonçalves R.R.P., Peconick A.P., Konig I.F.M., Lunguinho A.S., Ribeiro J.C.S., Gomes S.L., Silva L., Thomasi S.S., Remedio R.N. Acetylation of carvacrol raises its efficacy against engorged cattle ticks Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) Nat. Prod. Res. 2021;35:5475–5479. doi: 10.1080/14786419.2020.1784169. [DOI] [PubMed] [Google Scholar]
- 23.Gonçalves R.R.P., Peconick A.P., Thomasi S.S., Konig I.F.M., Gomes S.L., Remedio R.N. Acaricidal activity and effects of acetylcarvacrol on Rhipicephalus (Boophilus) microplus (Canestrini, 1888) engorged female ticks (Acari: Ixodidae) Int. J. Acarol. 2019;45:404–408. doi: 10.1080/01647954.2019.1665100. [DOI] [Google Scholar]
- 24.Guglielmone A.A., Robbins R.G., Apanaskevich D.A., Petney T.N., Estrada-Peña A., Horak I.G. Springer; Dordrecht: 2014. Hard ticks (Acari: Ixodida: Ixodidae) of the world. [Google Scholar]
- 25.Han J.S., Jang S., Son H., Kim Y., Kim Y., Noh J., Kim M., Lee B. Subacute dermal toxicity of perfluoroalkyl carboxylic acids: comparison with different carbon‑chain lengths in human skin equivalents and systemic effects of perfluoroheptanoic acid in Sprague Dawley rats. Arch. Toxicol. 2019;94(2):523–539. doi: 10.1007/s00204-019-02634-z. [DOI] [PubMed] [Google Scholar]
- 26.Hassan M.E., Hassan R.R., Diab K.A., El-Nekeety A.A., Hassan N.S., Abdel-Wahhab M.A. Nanoencapsulation of thyme essential oil: a new avenue to enhance its protective role against oxidative stress and cytotoxicity of zinc oxide nanoparticles in rats. Environ. Sci. Pollut. Res. Int. 2021;28(37):52046–52063. doi: 10.1007/s11356-021-14427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry, J.B., 2008. Diagnósticos Clínicos e Tratamento por Métodos Laboratoriais. 20th ed. São Paulo: Manole.
- 28.Isik D., Joshi A.A., Guo X., Rancan F., Klossek A., Vogt A., Rühl E., Hedtrich S., Klinger D. Sulfoxide-functionalized nanogels inspired by the skin penetration properties of DMSO. Biomater. Sci. 2021;9:712–725. doi: 10.1039/D0BM01717E. [DOI] [PubMed] [Google Scholar]
- 29.Junqueira L.C.U. Basic Techniques of Cytology and Histology. Bookstore; 1983. Santos. [Google Scholar]
- 30.Karamani C., Antoniadou I.T., Dimou A., Andreou E., Kostakis G., Sideri A., Vitsos A., Gkavanozi A., Sfiniadakis I., Skaltsa H., Papaioannou G.T., Rallis M.C., Maibach H. Optimization of psoriasis mouse models. J. Pharmacol. Tox. Met. 2021;108 doi: 10.1016/j.vascn.2021.107054. [DOI] [PubMed] [Google Scholar]
- 31.Kartheek R.M., David M. Assessment of fipronil toxicity on wistar rats: a hepatotoxic perspective. Toxicol. Rep. 2018;5:448–456. doi: 10.1016/j.toxrep.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koc S., Oz E., Cinbilgel I., Aydin L., Cetin H. Acaricidal activity of Origanum bilgeri P.H. Davis (Lamiaceae) essential oil and its major component, carvacrol against adults Rhipicephalus turanicus (Acari: Ixodidae) Vet. Parasitol. 2013;193:316–319. doi: 10.1016/j.vetpar.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Konig I.F.M., Gonçalves R.R.P., Oliveira M.V.S., Silva C.M., Thomasi S.S., Peconick A.P., Remedio R.N. Sublethal concentrations of acetylcarvacrol strongly impact oocyte development of engorged female cattle ticks Rhipicephalus microplus (Canestrini, 1888) (Acari: Ixodidae) Ticks Tick. -Borne Dis. 2019;10:766–774. doi: 10.1016/j.ttbdis.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Konig I.F.M., Oliveira M.V.S., Gonçalves R.R.P., Peconick A.P., Thomasi S.S., Anholeto L.A., Lima-de-Souza J.R., Camargo-Mathias M.I., Remedio R.N. Low concentrations of acetylcarvacrol induce drastic morphological damages in ovaries of surviving Rhipicephalus sanguineus sensu lato ticks (Acari:Ixodidae) Micron. 2020;129 doi: 10.1016/j.micron.2019.102780. [DOI] [PubMed] [Google Scholar]
- 35.Konig I.F.M., Reis A.C., Gonçalves R.R.P., Oliveira M.V.S., Silva C.M., Melo D.M., Peconick A.P., Thomasi S.S., Remedio R.N. Repellent activity of acetylcarvacrol and its effects on salivary gland morphology in unfed Rhipicephalus sanguineus sensu lato ticks (Acari: Ixodidae) Ticks Tick. Borne Dis. 2021;12(4) doi: 10.1016/j.ttbdis.2021.101760. [DOI] [PubMed] [Google Scholar]
- 36.Lage T.C.A., Monatanari R.M., Fernandes S.A., Monteiro C.M.O., Senra T.O.S., Zerinigota V., Calmon F., Matos R.S., Daemon E. Activity of essential oil of Lippia triplinervis Gardner (Verbenaceae) on Rhipicephalus microplus (Acari: Ixodidae) Parasitol. Res. 2013;112:863–869. doi: 10.1007/s00436-012-3209-y. [DOI] [PubMed] [Google Scholar]
- 37.Llana-Ruiz-Cabello M., Maisanaba S., Puerto M., Prieto A.I., Pichardo S., Moyano R., González-Pérez J.A., Cameán A.M. Genotoxicity evaluation of carvacrol in rats using a combined micronucleus and comet assay. Food Chem. Toxicol. 2016;98:240–250. doi: 10.1016/j.fct.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Lundborg M., Wennberg C.L., Narangifar A., Lindahl E., Norlén L. Predicting drug permeability through skin using molecular dynamics simulation. J. Control Release. 2018;283:269–279. doi: 10.1016/j.jconrel.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Mabeku L.B.K., Beng V.P., Kouam J., Essame O., Etoa F.X. Toxicological evaluation of ethyl acetate extract of Cylicodiscus gabunensis stem bark (Mimosaceae) J. Ethnopharmacol. 2007;111(3):598–606. doi: 10.1016/j.jep.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Marinho J.F.U., Correia J.E., Marcato A.C.C., Pedro-Escher J., Fontanetti C.S. Sugar cane vinasse in water bodies: impact assessed by liver histopathology in tilapia. Ecotoxicol. Environ. Saf. 2014;110:239–245. doi: 10.1016/j.ecoenv.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Mohseni R., Karimi J., Tavilani H., Khodadadi I., Hashemnia M. Carvacrol ameliorates the progression of liver fibrosis through targeting of Hippo and TGF-signaling pathways in carbon tetrachloride (CCl4)- induced liver fibrosis in rats. Immunopharmacol. Immunotoxicol. 2019;41(1):163–171. doi: 10.1080/08923973.2019.1566926. [DOI] [PubMed] [Google Scholar]
- 42.Moraes J., Carvalho A.A.L., Nakano E., Almeida A.A.C., Marques T.H.C., Andrade L.N., Freitas R.M., Sousa D.P. Anthelmintic activity of carvacryl acetate against Schistosoma mansoni. Parasitol. Res. 2013;112(2):603–610. doi: 10.1007/s00436-012-3172-7. [DOI] [PubMed] [Google Scholar]
- 43.Mossa A.T.H., Mohafrash S.M., Chandrasekaran N. Safety of natural insecticides: toxic effects on experimental animals. Biomed. Res. Int. 2018;2018(1):1–17. doi: 10.1155/2018/4308054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.OECD, 1981. Test No. 410: Repeated Dose Dermal Toxicity: 21/28-day Study, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris.
- 45.Oliveira M.V.S., Konig I.F.M., Reis A.C., Silva L., Peconick A.P., Thomasi S.S., Lima-de-Souza J.R., Camargo-Mathias M.I., Remedio R.N. Sublethal concentrations of acetylcarvacrol affect reproduction and integument morphology in the brown dog tick Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) Exp. Appl. Acarol. 2020;82(2):265–279. doi: 10.1007/s10493-020-00538-7. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira G.L.S., Oliveira F.R.A.M., Sousa A.A.C., Moura A.K.S., Lima S.G., Freire J.A.P., Citó A.M.G.L. Carvacryl Acetate: synthesis and toxicological and pharmacological activities. Rev. Virtual Química. 2020;12(3):554–568. doi: 10.21577/1984-6835.20200044. [DOI] [Google Scholar]
- 47.Pappinen S., Pasonen-Seppänen S., Suhonen M., Tammi R., Urtti A. Rat epidermal keratinocyte organotypic culture (ROC) as a model for chemically induced skin irritation testing. Toxicol. Appl. Pharmacol. 2005;208(3):233–241. doi: 10.1016/j.taap.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Pathan S.B., Jawade P., Lalla P. Correlation of serum urea and serum creatinine in diabetics patients and normal individuals. Int. J. Clin. Biochem. Res. 2020;7(1):45–48. doi: 10.18231/j.ijcbr.2020.009. [DOI] [Google Scholar]
- 49.Pereira Júnior A.M., Camargo-Mathias M.I., Daemon E., Peconick A.P., Lima-Souza J.R., Oliveira P.R., Braga A.S., Lara L.J., Remedio R.N. Efficacy of carvacrol on Rhipicephalus (Boophilus) microplus engorged female ticks (Canestrini, 1887) (Acari: Ixodidae): effects on mortality and reproduction. Nat. Prod. Res. 2019;34(23):3428–3431. doi: 10.1080/14786419.2019.1569657. [DOI] [PubMed] [Google Scholar]
- 50.Prow T.W., Grice J.E., Lin L.L., Faye R., Butler M., Becker W., Wurm E.M.T., Yoong C., Robertson T.A., Soyer H.P., Roberts M.S. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011;63:470–491. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Qu Y., Wang G., Sun X., Wang K. Inhibition of the warm temperature–activated Ca2+-permeable transient receptor potential vanilloid TRPV3 channel attenuates atopic dermatitis. Mol. Pharmacol. 2019;96(3):393–400. doi: 10.1124/mol.119.116962. [DOI] [PubMed] [Google Scholar]
- 52.Rittié L. Method for picrosirius red-polarization detection of collagen fibers in tissue sections. Methods Mol. Niol. 2017:395–407. doi: 10.1007/978-1-4939-7113-8_26. [DOI] [PubMed] [Google Scholar]
- 53.Ryu H.J., Seo M.Y., Jung S.K., Maeng E.H., Lee S.Y., Jang D., Lee T., Kim Y., Choh K., Kim M., Lee B.J., Son S.W. Zinc oxide nanoparticles: a 90-day repeated-dose dermal toxicity study in rats. Int. J. Nanomed. 2014;9(2):137–144. doi: 10.2147/IJN.S57930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santoro G.F., Cardoso M.G., Guimarães L.G.L., Salgado A.P.S.P., Menna-Barreto R.F.S., Soares M.J. Effect of oregano (Origanum vulgare L.) and thyme (Thymus vulgaris L.) essential oils on Trypanosoma cruzi (Protozoa: Kinetoplastida) growth and ultrastructure. Parasitol. Res. 2007;100(4):783–790. doi: 10.1007/s00436-006-0326-5. [DOI] [PubMed] [Google Scholar]
- 55.Sharma R., Locke B.R. Jet fuel toxicity: skin damage measured by 900-MHz MRI skinmicroscopy and visualization by 3D MR image processing. Magn. Reson. Imaging. 2010;28(7):1030–1048. doi: 10.1016/j.mri.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 56.Silva A.B., Duarte M.M., Cavalcante R.C., Oliveira S.V., Vizzoni V.F., Duré A.I.L., Iani F.C.M., Machado-Ferreira E., Gazêta G.S. Rickettsia rickettsii infecting Rhipicephalus sanguineus sensu lato (Latreille 1806), in high altitude atlantic forest fragments, Ceara State, Brazil. Acta Trop. 2017;173:30–33. doi: 10.1016/j.actatropica.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 57.Solomons T.W.G., Fryhle C.B., Snyder S.A. Organic Chemistry. 12th ed. John Wiley; Hoboken NJ: 2016. [Google Scholar]
- 58.Stevens L.A., Levey A.S. Measurement of kidney function. Med. Clin. North Am. 2005;89(3):457–473. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 59.Suntres Z.E., Coccimiglio J., Alipour M. The bioactivity and toxicological actions of carvacrol, critical reviews. Crit. Rev. Food Sci. Nutr. 2015;55(3):304–318. doi: 10.1080/10408398.2011.653458. [DOI] [PubMed] [Google Scholar]
- 60.Surh I., Behl M., Elmore S.A., Chhabra R.S. Comparative dermal toxicity of dicyclohexylcarbodiimide and diisopropylcarbodiimide in rodents. Cutan. Ocul. Toxicol. 2012;31(3):177–187. doi: 10.3109/15569527.2011.629384. [DOI] [PubMed] [Google Scholar]
- 61.Tang J., Hu P., Li Y., Win-Shwe T., Li C. Ion imbalance is involved in the mechanisms of liver oxidative damage in rats exposed to glyphosate. Front. Physiol. 2017;8:1083. doi: 10.3389/fphys.2017.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tetzlaff M.T., Nagarajan P., Chon S., Huen A., Diab A., Omar P., Aung P.P., Torres-Cabala C.A., Mays S.R., Prieto V.G., Curry J.L. Lichenoid Dermatologic toxicity from immune checkpoint blockade therapy: a detailed examination of the clinicopathologic features. Am. J. Dermatopathol. 2017;39(2) doi: 10.1097/DAD.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 63.Tewari-Singh N., Jaina K., Orlick D.J., White C.W., Agarwal R. Cutaneous injury-ielated structural changes and their progression following topical Nitrogen Mustard exposure in hairless and haired mice. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veiga Junior V.F., Pinto A.C., Maciel M.A.M. Medicinal plants: safe cure? Quim. Nova. 2005;28:519–528. [Google Scholar]
- 65.Wang Y., Li H., Xue C., Chen H., Xue Y., Zhao F., Zhu M.X., Cao Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 2021;37:313–330. doi: 10.1007/s10565-020-09536-2. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y., Tan L., Kiao K., Xue C., Tang Q., Jiang S., Ren Y., Chen H., El-Aziz T.M.A., Abdelazeem K.N.M., Yu Y., Zhao F., Zhu M.X., Cao Z. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022;179:4792–4808. doi: 10.1111/bph.15913. [DOI] [PubMed] [Google Scholar]
- 67.Winnicka K., Wroblewka M., Sosnowska K., Car H., Kasacka I. Evaluation of cationic polyamidoamine dendrimers’ dermal toxicity in the rat skin model. Drug Des. Develop. Ther. 2015;9:1367–1377. doi: 10.2147/DDDT.S78336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wojtunik-Kulesza K.A. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules. 2022;27:1716. doi: 10.3390/molecules27051716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaim M., Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18(4):161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.