Abstract
Despite years of development in cancer therapy, achieving successful cancer treatment remains a major research topic. Primary means of cancer treatment include chemotherapy, radiotherapy, and surgery. However, these modalities are associated with limitations and adverse effects on normal tissues. Therefore, there is a search for novel therapeutic approaches that will increase the efficacy of the available treatment while minimizing side effects. Naturally occurring bioactive chemicals such as flavonoids have long been used in traditional medicine to treat various illnesses. Baicalein, an active ingredient in Scutellaria baicalensis Georgi, is utilised in traditional medicine to treat conditions such as hypertension, cardiovascular disease, inflammation, and infections. This review focuses on summarizing the data available on cancer prevention and treatment usage of baicalein. Baicalein is thought to prevent cancer progression by inducing apoptosis, autophagy, and genome instability, and its ability to promote chemo-potentiation, anti-metastatic effects, and regulate specific signalling molecules and transcription factors. Baicalein can be a promising option for cancer treatment, either alone or in combination with established anticancer drugs.
Keywords: Baicalein, Cancer prevention, Chemopotentiation, Anticancer properties, Anti-metastatic, Tumor microenvironment, Normal tissue toxicity, Anti-angiogenic properties
1. Introduction
Cancer, the leading cause of death worldwide, is a global concern. Although cancer-related deaths have declined, effective treatment remains a challenge. While surgery, chemotherapy, and radiotherapy are still considered the gold standards for cancer treatment, their adverse effects and lack of specificity have prompted researchers to look for alternative treatment strategies. Chemotherapy prevents the division of continually proliferating cancer cells and can also affect normal cells, such as hair follicles, bone marrow, and gastrointestinal tract cells, resulting in side effects [1]. In addition, cancer cell resistance to chemotherapeutic drugs and agents is another obstacle to chemotherapy. Drug resistance can be inherent drug resistance, where resistance determinants are already present in most tumour cells, whereas acquired drug resistance develops during treatment [2]. One of the explanations for cancer cell resistance to chemotherapy and radiotherapy is the epithelial-mesenchymal transition (EMT), which enhances the migratory potential of cancer cells and results in treatment resistance and invasion [3]. Drug resistance can also occur for various reasons, such as drug efflux, decreased uptake, alterations in membrane lipids, and drug target mutations [4]. Therefore, learning more about the mechanisms needed to develop strategies to address this issue is critical.
There is a high demand for natural compounds and their derivatives that can effectively treat cancer. Considering the nature of innovative treatment solutions with appropriate pharmacotoxicological properties may be advantageous [5]. Flavonoids are polyphenolic secondary metabolites found in abundance in fruits, vegetables, and vascular plants and have long been well-known for their antioxidant properties [6]. The study of flavonoids for establishing anticancer drugs has recently attracted much attention [7,8]. Studies have suggested that dietary intake of flavonoids is correlated with a decreased risk of cancer incidence [9,10]. Baicalein (5,6,7-trihydroxy flavone), the aglycone of the flavonoid baicalin, is obtained from the dried roots of the East Asian skullcap plant Scutellaria baicalensis Georgi, as well as from Scutellaria lateriflora. The whole Scutellaria extract usually contains a combination of four major flavonoids: baicalein, baicalin, wogonoside, and wogonin [11].
Baicalein, one of the four major flavonoids known as Huangqin in traditional Chinese medicine, is used to treat inflammation, cardiovascular diseases, hypertension, atherosclerosis, the common cold, dysentery, and bacterial and viral infections [12,13]. Baicalein has already been proven to be a radical scavenger that acts as an antioxidant [14,15]; it can also reduce inflammation [16] and act as an E2 prostaglandin inhibitor [17]. Baicalein has been shown to have multiple mechanisms of action that make it a promising candidate for cancer prevention. Baicalein properties prevent cell proliferation, induce apoptosis, autophagy, cell cycle arrest, cancer cell migration and invasion, and decrease angiogenesis [18,19]. Moreover, baicalein is a nonmutagenic and nongenotoxic compound with good safety profiles and human pharmacokinetic properties [20]. Furthermore, some studies have suggested that baicalein has a lower toxicity on normal cells than cancer cells, indicating some selectivity for cancer cells. Cancer cells often exhibit altered signalling pathways and genetic mutations, increasing susceptibility to baicalein-induced apoptosis. On the other hand, normal cells may have better mechanisms for repairing DNA damage and preventing apoptosis. However, some normal cells may also have mutations that increase their vulnerability to baicalein-induced apoptosis [21]. In addition to cancer prevention and treatment, the current review emphasises baicaleins' synergistic potential with other chemotherapeutic agents, pharmacokinetics and bioavailability, safety profile, and role in modifying the tumour microenvironment, including its anti-metastatic and anti-angiogenic properties, thereby offering a comprehensive perspective that is currently lacking in the existing literature.
2. Baicalein in cancer prevention
In healthy individuals at risk of cancer, chemoprevention is carried out using natural, biological, or chemical agents to prevent cancer or prevent the progression of carcinogenesis [22]. Because carcinogenesis involves multiple steps, chemopreventive agents must also be administered for an extended period. Therefore, these chemicals must be nontoxic, relatively cheap, and amenable to oral administration [23]. Plant-derived bioactive substances, such as chemopreventive agents, are gaining popularity due to their practical advantages [24]. As a result, the market demand for new, potent, and less toxic phytochemicals has significantly increased [9]. Numerous studies have demonstrated an association between the consumption of phytochemicals and decreased cancer risk.
In cancer cells, baicalein has been shown to induce apoptosis, inhibit cancer cell proliferation, block cell cycle progression and suppress cancer stem cells [[25], [26], [27]]. A 2002 study indicated that baicalein is a better chemopreventive drug for ER-positive breast cancer than genistein because it is an antiestrogen and has a more significant apoptosis-inducing impact [28]. Dose-dependent decreases in the incidences of chemically induced azoxymethane (AOM) and dextran sulfate sodium (DSS) tumours were observed in mice treated with baicalein, indicating that baicalein could be a potential choice for several targets for cancer chemoprevention [29]. Baicalein helps reduce the risk of breast cancer caused by 17-estradiol (E2) by stopping the growth, movement, and spread of cancer cells. It does this by blocking two key E2-driven pathways, binding to both estrogen receptor-α (ERα) and G-protein coupled estrogen receptor 30 (GPR30) [30].
In cancer, the ornithine decarboxylase (ODC) enzyme levels become unbalanced, enabling cancer cells to proliferate uncontrollably. A study confirmed that baicalein could suppress the activity of the ODC enzyme and could, therefore, serve as a cancer chemopreventive agent [31]. In addition, baicalein pretreatment significantly inhibited, decreased and lengthened the latency period of 7,12-dimethylbenz[a]anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mouse skin tumorigenesis by restricting cell proliferation and enhancing apoptosis during the tumour promotion stage [32]. The same study indicated that the inflammatory activity of baicalein correlated with its anticancer properties. Notably, in breast cancer cells, DNA adduct formation due to DMBA is significantly reduced after treatment with baicalein due to the inhibition of CYP1A1 and CYP1B1 enzyme activities and gene expression [33]. Chen et al. verified the chemopreventive properties of baicalein against ovarian cancer by demonstrating that it can limit tumour cell viability by downregulating the expression of cancer-promoting genes such as HIF-1, cMyc, NFkB, and VEGF [34]. This can occur through the reduction of oxidative stress, a process that can cause DNA damage and lead to the development of cancer cells [29]. Baicalein has also been found to inhibit the activity of certain enzymes involved in the initiation and promotion of cancer cells [35].
Baicalein has been shown to activate p53, a tumour suppressor protein that regulates cell growth and division [26]. A study revealed that baicalein regulates the Nrf2/Keap1 pathway via Keap1-dependent and Keap1-independent mechanisms to exert cytoprotective effects and promote cancer chemoprevention [36]. In mice with benzo(a)pyrene (BaP)-induced pulmonary carcinogenesis, baicalein treatment returned the lysosomal enzyme, phase I, and phase II biotransformation enzyme levels to normal. Baicalein treatment also reduces oxidative damage and increases CYP1A1 expression induced by BaP [34]. Therefore, baicalein was determined to regulate lysosomal and microsomal malfunctions to perform chemopreventive actions. Overall, baicalein holds great potential as a natural and effective agent for cancer prevention. However, further studies on the potential of baicalein for preventing cancer development in high-risk populations should be considered. Its multimodal mechanisms of action, including the induction of apoptosis, autophagy, and genomic instability, as well as its chemopreventive properties, make it a promising option for reducing cancer risk.
3. Anticancer properties of baicalein
Developing a substance that eliminates tumour cells while leaving healthy tissues unaffected as much as possible is the greatest obstacle in cancer research. Most already established anticancer medications have a constrained therapeutic window and a high dose-response relationship. Moreover, even the most common types of cancer are still very challenging to treat effectively because targeted anticancer therapies frequently result in adverse side effects or drug resistance. Thus, innovative anticancer medicines with increased efficacy and decreased toxicity are urgently needed. Several reports indicate that baicalein is a potential anticancer agent. In cancer cells, baicalein induces apoptosis and causes genomic instability, leading to death. The anticancer properties of baicalein are mediated through various molecular mechanisms, including inhibition of MMP-2; regulation of cell cycle progression; scavenging of oxidative radicals; attenuation of MAPK, Akt or mammalian target of the rapamycin (mTOR) activities; induction of apoptosis and autophagy; inhibition of cancer stem cells; and activation of p53 [26].
3.1. Induction of apoptosis
Numerous pathological illnesses, including different malignancies, are caused by a slight malfunction of apoptosis in humans. The development of cancer therapy strategies relies primarily on the induction of cancer cell apoptosis. As a result, apoptosis has emerged as one of the most important molecular targets for new drugs, particularly for treating conditions such as cancer [37]. Antiapoptotic genes are highly expressed in cancer cells, so suppressing antiapoptotic genes could be useful for treating various malignancies, particularly aggressive ones [38].
In a study, after bladder cancer cells were treated with baicalein, the expression of antiapoptotic genes (Bcl2, Bcl-xL, XIAP, and survivin) was reduced, and cell viability was decreased [38]. Baicalein caused an increase in the BAX/BCL-2 ratio, leading to apoptosis in breast cancer cells [39] and thyroid cancer cells [8]. However, in follicular undifferentiated thyroid cancer (FRO) cells, baicalein induced apoptosis by upregulating the expression of caspase-3 and caspase-8 and decreased the BCL-2/BAX ratio [16]. In FRO anaplastic thyroid cancer (ATC) cells, baicalein increased the expression of Bcl-2 while reducing the expression of Bax, poly (ADP-ribose) polymerase (PARP), cytochrome c, cleaved caspase-3, and Cox-2 [40]. Furthermore, in lung cancer cells, apoptosis was induced through the downregulation of the Akt/mTOR signalling pathway [25]. In a different investigation, it was observed that baicalein treatment promoted apoptosis in mice with U87 gliomas by downregulating PCNA expression, enhancing the expression of caspase-3 and caspase-9 and improving the Bax/Bcl-2 ratio [41]. Another study revealed that baicalein initiated dose-dependent apoptosis in pancreatic cancer cells by downregulating the expression of caspase-3 and BCL-2 while upregulating cleaved caspase-3 and Bax expression, leading to apoptosis [42]. Baicalein was also found to significantly stimulate the apoptosis of HCT116, A549, and Panc-1 cells by upregulating DEPP/Gadd45a and activating MAPKs [43]. Therefore, baicalein promoted apoptosis, resulting in its antitumour effect. In yet another study, baicalein treatment of lung cancer cells caused a collapse of the mitochondrial membrane potential (MMP), an increase in ROS generation, and enhanced PARP, caspase 3, and caspase 9 cleavage, triggering apoptosis by initiating the mitochondrial apoptotic pathway [44]. In combination with other natural compounds, baicalein inhibited the JAK2/STAT3 pathway, thereby inducing apoptosis in gastric cancer cells [45]. Baicalein also induced cell apoptosis of cervical cancer cells through upregulating miR-183 via inactivation of the JAK2/STAT3 signalling [46]. Moreover, baicalein induced apoptosis in lung cancer cells by suppressing the glutamine-mTOR metabolic pathway. Interacting with glutamine transporters and glutaminase reduced the activation of mTOR, a crucial regulator of cell growth and survival, which in turn drove the cancer cells to undergo apoptosis. Baicalein also modified the amounts of amino acid metabolites, specifically those associated with glutamine, which confirmed its function in disrupting metabolic pathways essential for tumor growth and proliferation [47].
Interestingly, baicalein treatment also suppressed the PI3K/Akt signalling pathway alone [39] or combined with LY294002, a PI3K inhibitor [48]. Baicalein treatment modified the miRNA profile; it upregulated miR-139-3p, targeting NOB1, resulting in increased and downregulated miRNA-targeted ING5 and preventing pancreatic cancer cells from undergoing programmed cell death, preventing the spread of pancreatic cancer [49]. These studies provide insights into the molecular mechanisms involved in baicalein-mediated apoptosis regulation to destroy cancer cells and provide evidence for baicalein as a potential anticancer drug (Table 1).
Table 1.
Molecular mechanisms involved in baicalein-mediated apoptosis.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Pathway involved | Reference |
|---|---|---|---|---|---|
| Bladder cancer | In vitro | T24, 253J and J82 cell lines | 0 and 5 mg/mL | – | [38] |
| Thyroid cancer | In vitro | MDA-T68 cells | 0, 1.25, 2.5, 5, 10, 20, 40, 80, 160 and 320 μM |
NF-kB signalling | [9] |
| Thyroid cancer | In vitro | FRO cells | 10 μM, 20 μM, 40 μM, 80 μM | ERK/PI3K/Akt | [17] |
| Thyroid cancer | In vitro | FRO anaplastic thyroid cancer (ATC) cells | 0, 10, 20, 50, and 100 μM | ERK/p38 MAPK and Akt | [40] |
| Pancreatic cancer | In vitro | CAPAN-2 cell line | 0, 10, 20 and 40 μM | – | [42] |
| Colon cancer | In vitro | e HCT116, A549 and Panc-1 cell lines | 0, 10, 20 and 40 μM | JNK/ERK/p38 MAPK | [43] |
| Gastric cancer | In vitro | AGS and HGC-27 cells | 5–35 mg/ml for AGS cells and 2–12 mg/ml for HGC-27 | JAK/STAT | [45] |
| Liver cancer | In vitro | SMMC-7221 cell line | 0, 1, 2, 5, 10, 20, 50, 100, 200, and 300 μM | PI3K/Akt signalling | [48] |
| Pancreatic cancer | In vitro | Panc-1 cells | 0, 50, and 100 μM | – | [49] |
| Breast cancer | In vitro and in vivo | MCF-7 and MDA-MB-231 cell lines; and Female BALB/c nude mice (3–6 weeks old) | 0, 10, 20, and 40 μM; and 100 mg/kg | PI3K/AKT | [39] |
| Cervical cancer | In vitro | End1/E6E7 cells and Hela cell line | 0, 10 and 100 μM | JAK/STAT | [46] |
| Lung Cancer | In vitro and in vivo | H1299 and A549 NSCLC cells, and Lewis lung cancer cells; C57BL/6 mice (aged between 6 and 8 weeks) | 0, 10, 20, 30, 50, 100, 200 and 500 μM | Glutamine-mTOR metabolic pathway | [47] |
| Lung cancer | In vitro and in vivo | H1650 and H1299 cell lines; and Female BALB/c-nu mice | 0, 50, and 100 μg/ml; and 20 mg/kg | Akt/mTOR Signalling | [26] |
| Lung cancer | In vitro and in vivo | A549, NCI-H1299, and LLC - cell lines; and Male C57BL/6 mice (5–6 weeks old) | 0, 80, 120 and 160 μM; and 50 and 100 mg/kg | AMPK signalling | [44] |
| Intracerebral cancer | In vivo | Male athymic mice (10-week-old) and Fisher 344 (8-week-old) rats | 0, 20 or 40 mg/kg | HIF-1a/VEGF/VEGFR2 | [41] |
3.2. Induction of autophagy
Autophagy is a cellular process that maintains homeostasis, thereby controlling cell death, and can be a potential target in cancer treatment [50]. Autophagy tends to have defensive functions in the early phases of tumour development. However, with cancer progression, autophagy develops into a system of survival adaptation, allowing cancer cells to endure stressful, hypoxic, or starving environments easily [51].
Treatment with baicalein caused an increase in the expression of autophagy-related proteins, such as light chain 3 (LC3), leading to baicalein-induced autophagy in breast cancer cells [39]. In thyroid cancer cells, baicalein-induced autophagy is mediated by the NF-κB (nuclear factor-κB) apoptotic signal, where a noticeable increase in the NF-κB autophagy factor occurred with increasing dose [8]. Baicalein treatment enhanced LC3-II protein expression in ovarian cancer cells and induced the accumulation of acidic vesicular organelles. After administering baicalein and the autophagy inhibitor chloroquine, the cells were protected from baicalein-induced autophagy by reducing cell viability and increasing PARP cleavage. Baicalein-induced autophagy by regulating Beclin-1 and the extracellular signal-regulated kinase (ERK) pathway [52,53]. Another study demonstrated that baicalein induced autophagy in hepatocellular carcinoma HepG2 cells by suppressing the protein kinase B (AKT)/mammalian target of the rapamycin (mTOR) pathway [54]. In FRO cells, baicalein induced autophagy by upregulating the expression of autophagy-related proteins, such as Beclin-1, p62, Atg5, and Atg1. In addition, it decreased the p-ERK/ERK and p-Akt/Akt ratios, thereby inhibiting the ERK and PI3K/Akt signalling pathways [55]. The downstream components of the PI3K/AKT pathway that help in cancer progression are AKT, NF-κB, and mTOR. Treatment with baicalein downregulated p-AKT, p-mTOR, NF-κB, and p-IκB expression while upregulating IκB expression. Moreover, p-AKT and p-mTOR levels were reduced after breast cancer cells were treated with baicalein [39].
Another experiment revealed endoplasmic reticulum stress as the mechanism by which baicalein promotes autophagy in hepatocellular carcinoma (HCC) cells. Additionally, baicalein-induced protective autophagy decreased only when the genes encoding the autophagy regulators Atg5 and Beclin-1 were silenced using siRNA. These findings conclude that baicalein combined with autophagy inhibitors may be a potential intervention for HCC [55]. By increasing the LC3-II/LC3-I ratio and P62 protein expression, baicalein inhibited the PI3K/AKT signalling pathway and promoted autophagy in gastric cancer cells [56]. In a different study, baicalein activated the AMPK/ULK1 pathway and prevented the expression of mTOR/Raptor complex 1, causing autophagic cell death in human cancer cells. It enhanced autophagic flux and stimulated the production of autophagosomes, and this process was influenced by Beclin-1, Vps34, Atg5, Atg7, and ULK1 [57].
In a dose-dependent manner, baicalein alone reduced the viability of multiple CRC (colorectal cancer) cell lines. However, viability was significantly decreased in two cell lines when baicalein was coupled with the autophagy inhibitor chloroquine, suggesting that baicalein treatment accelerated the apoptotic cell death caused by autophagy suppression [58]. Overall, these studies suggested that baicalein induced autophagy in various cancer cells, leading to cell death (Table 2). The induction of autophagy by baicalein is mediated through different molecular mechanisms, including inhibiting the PI3K/AKT pathway and amplifying baicalein-induced apoptosis.
Table 2.
Molecular mechanisms involved in baicalein-mediated autophagy.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Pathway involved | Reference |
|---|---|---|---|---|---|
| Thyroid cancer | In vitro | MDA-T68 thyroid cancer cells | 0, 1.25, 2.5, 5, 10, 20, 40, 80, 160 and 320 μM |
NF-kB signalling | [9] |
| Thyroid cancer | In vitro | FRO cells | 0, 10 μM, 20 μM, 40 μM, 80 μM | ERK/PI3K/Akt | [17] |
| Ovarian cancer | In vitro | Ovarian cancer HEY cells | 0, 12.5, 25, and 50 μM | ERK/PI3K/Akt | [52] |
| Hepatocellular carcinoma | In vitro | SMMC-7721 and Bel-7402 cell lines | 0, 25, 50, 100 and 200 μM | UPR and JNK signalling | [55] |
| Gastric cancer | In vitro | MGC-803 cells | 0, 5, 15, 25 and 50 μmol/L | PI3K/AKT signalling | [56] |
| Adenocarcinoma, breast and prostate cancer | In vitro | PC-3, MDA-MB-231 and DU145 cell lines | 0–10 μg/mL | AMPK/ULK1 pathway | [57] |
| Colorectal Cancer | In vitro | Cell lines HT-29, COLO, HCT-116, LoVo, SW480, and SW620 | 0, 10, 20, 40, 80, and 160 μM | Caspase 3 pathway | [58] |
| Breast cancer | In vitro and in vivo | MCF-7 and MDA-MB-231 cell lines; and Female BALB/c nude mice (3–6 weeks old) | 0, 10, 20, and 40 μM; and 100 mg/kg | PI3K/AKT pathway | [39] |
| Colorectal cancer | In vitro and in vivo | CRC HT29 and DLD1 cell lines; and Balb/c athymic nude mice (4- to 6-week-old) | 0, 10, 20, 30, 40, 50, 60, 80, 100 and 120 μM; and 20 mg/kg/day | ERK signalling | [53] |
3.3. Induction of genome instability
While the induction of genome instability may seem counterintuitive to cancer treatment, inducing genome instability can be an effective strategy for killing cancer cells. DNA is a complex structure that often contains base-pair mismatches and insertions/deletions. The MMR (DNA mismatch repair) pathway identifies and repairs these alterations, preserving genome stability. In MMR2252-proficient cells, baicalein selectively bound to mismatched DNA and MMR protein MutSɑ, leading to the segregation of MutSɑ from CHK2 (checkpoint kinase 2) and the activation of CHK2 by ATM, which allowed the elimination of DNA damage, promoting cell survival. On the other hand, when MMR-deficient cancer cells were treated with baicalein, XPF caused double-strand breaks in mismatched DNA, initiating cell death [59]. Securin is a protein that plays a role in sister chromatid segregation, spindle fibre formation during the cell cycle, and maintenance of genomic stability during mitosis. In addition, securin inhibits the abnormal segregation of chromosomes when spindle fibres are damaged. In bladder cancer cells, baicalein was determined to influence cell survival and genomic stability through its effect on securin and by inhibiting the AKT pathway [60]. Notably, baicalin hydrate inhibited cancer progression in nasopharyngeal carcinoma by affecting genome instability by activating IKKα [61]. Overall, the search results suggested that baicalein-induced genome instability is not a well-studied topic, and additional research is needed to determine the effects of baicalein on genome stability. Table 3 summarises the molecular mechanisms involved in baicalein-induced genome instability.
Table 3.
Molecular mechanisms involved in baicalein-induced genome instability.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Pathway involved | Reference |
|---|---|---|---|---|---|
| Bladder carcinoma, lung adenocarcinoma, colon carcinoma and cervical cancer | In vitro | BFTC9050, A549, RKO, HeLa cells | 0, 10–80 μM | AKT and γ-H2AX pathways | [60] |
| General | In vitro and in vivo | HEC59, HEC59-2, LoVo and HeLa cells; and nude mice | 0–500 μmol/L | DNA mismatch repair (MMR) pathway | [59] |
| Nasopharyngeal carcinoma | In vitro and in vivo | C666-1 cells and HK1 cells; and Female nude mice (5-weeks-old) | 19.38 μM and 31.22 μM; and 10 mg/kg | – | [61] |
4. Other anticancer mechanisms
In addition to the mechanisms discussed above, baicalein has been shown to inhibit cancer through many other pathways. For instance, in a 2019 study by Wang et al., baicalein inhibited gut inflammation and CRC progression in an ApcMin/+ mouse model. Baicalein, produced when the intestinal microbiota of mice biotransforms baicalin, could reduce the number of tumours in the regions of the intestine and colon in mice [62]. In a different investigation, baicalein suppressed melanoma cells in vitro and in vivo by inhibiting tumour glucose metabolism regardless of N-RAS or B-RAF mutations [63]. In another study, breast tumour cells were infected with the measles virus, and treatment with baicalein significantly increased cancer cell inhibition by accelerating apoptosis [64]. These aspects could be exploited as therapeutic techniques to target breast cancer in the future. Additionally, by inducing DNA damage and chromosomal abnormalities, baicalein can trigger cell death pathways that are selective for cancer cells. Studies have indicated that baicalein has multitarget antitumour effects on various cancer types, including hepatocellular carcinoma and triple-negative breast cancer (TNBC). It has been shown to promote antitumour immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells, thereby inhibiting tumour growth [65]. Another study has demonstrated that baicalein enhanced the expression and stability of lysine-specific demethylase 4E (KDM4E), a protein that is reduced in TNBC cells, thereby inhibiting TNBC progression. KDM4E activated the expression of KDM4E activated protein bicaudal D homolog 1 (BICD1), subsequently promoting the endocytosis of protease-activated receptor-1 (PAR1) and blocking its signalling. These findings suggested that targeting the KDM4E-BICD1-PAR1 pathway could provide a novel therapeutic strategy for managing TNBC [66]. Moreover, baicalein has proven to significantly enhance radiosensitivity in CRC by inhibiting the JAK2/STAT3 signalling pathway. In both in vitro and in vivo experiments, baicalein treatment led to increased apoptosis in tumor tissues and improved the effectiveness of ionizing radiation (IR) on radioresistant CRC cells (CT26-R). Additionally, the expression levels of key proteins associated with the JAK2/STAT3 pathway, such as p-STAT3 and JAK2, were downregulated. In contrast, SOCS3 expression was upregulated, indicating that the observed radiosensitizing effects are mediated through this pathway [67]. In a recent study, baicalein was also found to cause ferroptosis in CRC cells by obstructing the JAK2/STAT3/GPX4 signalling pathway. Baicalein was shown to have a dose-dependent effect on the viability of CRC lines HCT116 and DLD1, and the anti-cancer activity of baicalein was confirmed by the fact that this effect decreased when an inhibitor of ferroptosis was co-treated. Furthermore, baicalein's direct interaction with JAK2 lowered GPX4 expression, a critical regulator of ferroptosis, as demonstrated in a CRC xenograft mice model in which baicalein treatment slowed tumour development despite producing considerable ferroptosis in tumour tissues [68]. However, further research is needed to explore the full potential of baicalein and its possible use in combination with established anticancer drugs.
5. Anti-metastatic properties
Metastasis is a multistep process in which cancer cells escape from the primary tumour site, penetrate surrounding tissues, reach new organs via circulatory vessels, and eventually form a secondary tumour. The aggressiveness of metastatic tumours is the leading cause of cancer-related death. The trans-differentiation of stationary epithelial cells into motile mesenchymal cells occurs through the acquisition of invasive ability and the depletion of tight junctions; EMT is a crucial step in cancer metastasis [69]. In CRC cells, baicalein treatment decreased the expression of EMT-promoting factors such as vimentin, Twist1, and Snail while increasing the expression of E-cadherin [69]. Several other investigations support the idea that baicalein inhibits EMT through its antimetastatic effects. Similarly, baicalein could hinder fibronectin-induced EMT and prevent metastasis by inhibiting calpain-2, an EMT-promoting protease, in breast cancer cells and cancer mouse models [70]. Moreover, in non-small cell lung cancer (NSCLC) cells, baicalein limited EMT by silencing the Notch signalling pathway, an EMT-inducing signalling pathway [71].
Ezrin is a membrane-cytoskeleton linker protein that promotes tumour invasion and metastasis. Like that of ezrin, even the S-nitrosylation (SNO) process controls tumour activities that lead to metastasis. Inducible nitric oxide synthase (iNOS) is a primary endogenous NO source that leads to SNO in the tumour microenvironment. It increases ezrin SNO levels and causes an increase in ezrin tension, which eventually induces tumour invasion and metastasis. However, baicalein treatment reduced tumour aggressiveness by decreasing ezrin tension post-treatment [72]. Moreover, baicalein was shown to inhibit metastasis in prostate cancer cells (DU145 and PC-3) by inhibiting the caveolin-1/Akt/mTOR pathway [73]. These results indicated that baicalein could cause apoptosis and limit metastasis by suppressing the caveolin-1/AKT/mTOR pathway. Additionally, baicalein treatment was found to effectively suppress the migration and invasion of breast cancer cells (MDA-MB-231) by downregulating SATB1, as well as the Wnt/β-catenin and MAPK signalling pathways [74,75]. In the case of gastric cancer cells (AGS), baicalein inhibited migration and invasion by suppressing the TGF-β/Smad4 signalling pathway [76]. Baicalein inhibited the generation of interleukin IL-6 and suppressed in vivo and in vitro potential of breast cancer cells to metastasise via inhibition of Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway [77]. Further, GCXXD, a combination of six Chinese herbs, including baicalein, inhibited the JAK2/STAT3 pathway, thereby inhibiting proliferation, invasion and migration [45].
It is known that metastasis results from the breakdown of the extracellular matrix (ECM) promoted by matrix metalloproteinases (MMPs), specifically MMP-2 and MMP-9. The ERK signalling pathway synthesizes these MMPs, and this upregulation results in metastasis. Baicalein inhibited metastasis by downregulating MMP-2/-9 expression [78]. However, the antimetastatic effects were synergistically enhanced when CRC cells were treated with baicalein and an ERK inhibitor [53]. Similarly, baicalein treatment suppressed invasion by downregulating MMP-2/9 expression through the blockade of the AKT signalling pathway [79,80]. Several studies have demonstrated that baicalein can suppress the expression of proteins involved in the promotion of metastasis, such as PI3K/AKT signalling pathway proteins [39] and VEGF, which promote the growth of new blood vessels and are essential for cancer cells to establish new tumours in other parts of the body [81]. Through these experiments, the pathways through which baicalein targets prevent/inhibits cancer progression are known. These studies thereby confirmed the antimetastatic properties of baicalein. Baicalein inhibited the metastatic phenotypes of cancer cells by modulating the expression of the focal adhesion pathway molecule integrin β8 [82]. These studies suggested that baicalein possesses antimetastatic properties and can be a potential therapeutic agent for preventing and treating cancer metastasis. The antimetastatic properties of baicalein are mediated through different molecular mechanisms, including inhibiting the expression of MMP-2 and focal adhesion pathways (Table 4).
Table 4.
Antimetastatic properties of baicalein mediated through different molecular mechanisms.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Pathway involved | Reference |
|---|---|---|---|---|---|
| Non-small cell lung cancer cell | In vitro | A549 and H1299 cell lines | 0, 20, 40, 60, 80, and 100 μM/L | Notch signalling | [71] |
| Prostate cancer (PCa) | In vitro | PCa cell lines | 20 and 40 μM | Caveolin-1/AKT/mTOR | [73] |
| Breast cancer | In vitro | MDA-MB-231 cells | 0, 2, 10 and 50 μM | MAPK signalling | [75] |
| Gastric cancer | In vitro | AGS cell line | 0, 25 or 50 μM | TGF-β signalling | [76] |
| Gastric cancer | In vitro | AGS and HGC-27 cells | 5–35 mg/ml for AGS cells and 2–12 mg/ml for HGC-27 | JAK/STAT | [45] |
| Colorectal cancer | In vitro | HT29 and DLD1 cell lines | 0, 10, 20, 30, 40, 50, 60, 80, 100 and 120 μM | ERK signalling | [53] |
| Colorectal cancer | In vitro | DLD-1 colorectal carcinoma cell line | 0–120 μM | KT signalling | [79] |
| General | In vitro | Human umbilical vein endothelial cells (HUVECs) | 0, 0.5, 1, 2, 5, 10, 20, 50, 100, 200, 400 or 800 μmol/L | p53/Rb signalling | [81] |
| Nasopharyngeal Carcinoma | In vitro | HK-1, SUNE 5–8F, SUNE 6–10B, and TW01 cells | 0, 10, 20, 30, 40, 50, 60, 70 and 80 μM | PI3K/Akt | [82] |
| Breast cancer | In vitro and in vivo | MCF-10A cell line; and Female MMTV-PyMT transgenic mice (about three weeks old) | 0, 2.5, 5, and 10 μM; and 30 mg/kg | – | [70] |
| Breast cancer | In vitro and in vivo | MCF-7 and MDA-MB-231 cell lines | 0, 10, 20, and 40 μM; and 100 mg/kg | PI3K/AKT signalling | [39] |
| Breast cancer | In vitro and in vivo | MCF-7/ADR cells; and Kunming mice (4–6 weeks old) | Baicalein/Doxycycline in a 2/1 ratio | – | [78] |
| Breast cancer | In vitro and in vivo | Immortalized mammary epithelial cells (MCF-10A) cells; MCF7, SKBR3, and MDA-MB-231 cell lines; and nude mice | 0, 10, 20, 40, 60, 80, 100, and 120 μmol/L; and 50 or 100 mg/kg | SATB1 and Wnt/β-catenin | [74] |
| Colorectal cancer | In vitro and in vivo | HT29 and DLD1 cell lines; and male Sprague-Dawley rats (age, 8 weeks) | 0, 20, 30, 40, 60, 80, 100 and 120 μmol/l; and 4.5 g/kg | – | [69] |
| Bladder cancer | In vitro and in vivo | Human bladder papillary transitional cell carcinoma 5637 cells and mouse bladder carcinoma MB49 cells; and female C57BL/6 mice (5–6 weeks) | 0, 25, 50, 75 and 100 μM; and 0.8 mg/mouse | GSK3β pathway | [80] |
| Breast cancer | In vitro and in vivo | Murine cell line 4T1 and human cell line MDA-MB-231; and BALB/c mice (6-week old) | 0, 10 and 100 μM; and 10 μM | JAK/STAT pathway | [77] |
6. Chemopotentiation properties
High doses of chemotherapeutics during cancer treatment give rise to many unwanted side effects. This has drawn the attention of researchers toward combination therapy with drugs. The combined use of anticancer drugs and natural compounds has gained popularity for subduing drug resistance and lowering treatment side effects [83,84]. The use of baicalein as an adjuvant for cancer chemotherapy and targeted therapy has been actively investigated and reported in several studies.
Baicalein has been found to enhance the cytotoxicity and bioavailability of certain cancer therapy drugs when combined [85]. When docetaxel was used in combination with baicalein, the combination of docetaxel and sorafenib synergistically enhanced the expression of Bax and caspase-3 while downregulating BCL-2 and reduced the expression of the metastatic proteins VEGF, TGF-β, E-cadherin, and N-cadherin in ATC cells [83]. Lipid-based nanoparticle systems for co-delivering docetaxel and baicalein in combination with lung cancer chemotherapy have shown promising results [21].
The combination of baicalein with silymarin differentially decreased the viability of HepG2 cells, enhanced the proportion of cells in the G0/G1 phase, upregulated tumour suppressors such as Rb and p53 and CDK inhibitors, and downregulated cyclin D1, cyclin E, CDK4, and phospho-Rb [86]. A study has also shown that using nanoparticles to carry a combination of baicalein and paclitaxel prodrugs could enhance the anticancer effects of paclitaxel while overcoming its multidrug resistance [87]. The codelivery of baicalein and doxorubicin using hyaluronic acid (HA)-decorated nanostructured lipid carriers (NLCs) induced the highest cytotoxicity and synergistic effect on breast cancer cells [78]. Furthermore, baicalein enhanced the antileukemic effects of vincristine. This is significant, as vincristine can be neurotoxic at large dosages [88].
Combined treatment with camptothecin and baicalein-induced apoptosis via intrinsic pathways employed p21 to apprehend the cell cycle in the G1 and G2 phases and caused a fivefold increase in p53 [89]. The combination of baicalein with gemcitabine significantly enhanced the ability of gemcitabine to inhibit pancreatic cancer cell proliferation and decrease pancreatic cancer cell migration [90]. To overcome multidrug resistance (MDR) in breast cancer, paclitaxel and baicalein were encapsulated in nanoemulsions that could accumulate in cancer cells, increase ROS, decrease glutathione, and boost caspase-3 activity [91]. Overall, these studies indicated that combination therapy of baicalein with existing cytotoxic/chemotherapy drugs could be a possible way to enhance tumours by synergistic effects, overcome multidrug resistance, and reduce the drug dose, resulting in less normal tissue toxicity.
By inhibiting P-glycoprotein (P-gp), baicalein can increase the accumulation of chemotherapeutic drugs within cancer cells [21]. Baicalein can also enhance the sensitivity of cancer cells to drugs by modulating various signalling pathways involved in cell growth, proliferation, and survival. For example, baicalein has been found to activate the extracellular signal-regulated kinase (ERK) pathway, which can sensitize cancer cells to the effects of chemotherapy [92]. According to another 2024 study by Jin et al., baicalein improved cisplatin sensitivity in cervical cancer cells by increasing cuproptosis via the Akt signalling pathway. The study showed that treatment with baicalein raised intracellular copper levels, which triggered cuproptosis, while also blocking the Akt pathway, which is implicated in drug resistance in cancer cells. Because of its dual effects, baicalein may prove to be a useful adjuvant medication to enhance the efficacy of cisplatin in the management of cervical cancer [93]. When combined with these drugs, baicalein can enhance anticancer effects, potentially by reducing the required doses and minimizing adverse side effects. The synergistic effect of baicalein with other anti-cancer drugs has been summarized in Table 5.
Table 5.
Synergistic effects of baicalein with other anti-cancer drugs.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Synergistic compound | Drug delivery mechanism | Pathway involved | Reference |
|---|---|---|---|---|---|---|---|
| Anaplastic thyroid cancer | In vitro | Human ATC cell line 8505c cells bearing the p53 gene mutation | 0, 10, 20, 50, and 100 μM | Docetaxel | – | ERK and Akt/mTOR pathway | [83] |
| Hepatocellular carcinoma | In vitro | HepG2 and Chang liver cells | 0, 25, 50, and 100 μM | Silymarin | – | – | [86] |
| Childhood acute lymphoblastic leukemia | In vitro | CCRF-CEM cell line | 0, 25, 50, and 100 μM | Vincristine | – | Caspase-9 and caspase-3 pathway | [88] |
| Breast cancer | In vitro | Human breast cancer MCF-7 and MDA-MB-231 cells | 0, 25, 50, 75, 100, 150 200, and 400 μmol/L | Baicalin | – | ERK/p38 MAPK pathway | [92] |
| Pancreatic cancer | In vitro | Human pancreatic cell line PANC-1, MIA PaCa-2 and HPAF-II | 0 and 10 μM | Gemcitabine | – | caspase 3/PARP signalling pathway | [90] |
| Non-small cell lung cancer | In vitro and in vivo | A549 cell lines; and BALB/c nude mice | 0, 0.01–10 μM | Docetaxel | pH-sensitive transferrin-PEG-Hz-lipid conjugate | – | [21] |
| Lung cancer | In vitro and in vivo | A549 cells and drug-resistant lung cancer A549/PTX cells and Kunming mice (4–6 weeks old) | 0.1g; and 50 mg/kg | Paclitaxel | Self-assembled nanoparticles | – | [87] |
| Breast cancer | In vitro and in vivo | MCF-7/ADR cells; and Kunming mice (4–6 weeks old) | Baicalein/Doxorubicin = 2/1, (w/w) | Doxorubicin | Hyaluronic acid decorated nanostructured lipid carriers | – | [78] |
| Cervical and breast cancer | In vitro and in vivo | BGC823, MCF7 and SMMC7721 cells; and Adult female 4–5 week old athymic nude mice | 0 and 15.625 μM; and 5 mg/kg | 10-hydroxy camptothecin | – | – | [89] |
| Breast cancer | In vitro and in vivo | Human breast cancer cell line MCF-7 and its Taxol-resistant cell line MCF-7/Tax; and Female Balb/C nude mice (6–8 weeks old) | Paclitaxel/Baicalein = 10/1, 5/1, 2/1, 1/1, 1/2, 1/5, and 1/10 ratio | Paclitaxel | Nanoemulsions (through endocytosis pathway) | – | [91] |
| Cervical cancer | In vitro and in vivo | SiHa and C33A cells; BALB/c female nude mice (4 and 6 weeks old) | 2, 4, 8, 16, 32, 64 μM | Cisplatin | – | Akt pathway | [93] |
7. Preventing adverse reactions induced by cancer treatment
Anticancer drugs and radiation effectively inhibit cell proliferation and induce cytotoxicity in cancer cells. However, because of a lack of specificity, this treatment often results in toxicity to normal tissues. Therefore, there is a search for compounds that can attenuate damage to normal tissues and provide radio/chemoprotection [[94], [95], [96]]. Inflammation and oxidative stress are major contributors to the adverse reactions induced by cancer treatment. Baicalein has been shown to have anti-inflammatory, antioxidant, and antitumour effects and may help prevent or reduce some of these adverse reactions. In mice, baicalein reversed the inflammatory response induced by X-ray irradiation via downregulation of the NF-κB pathway and inactivation of its associated proinflammatory genes; it also increased the enzymatic activity of catalase and SOD, which are crucial for preventing the acceleration of the inflammatory response after IR [97]. Similarly, in an IR-induced mouse enteritis model, baicalein treatment attenuated intestinal injury and facilitated the recovery of the intestinal barrier, crypt regeneration, and regulation of inflammation [98].
Chemical chemotherapeutic drug administration for treating cancer has limitations because of the damage it causes to normal tissues [99]. For instance, cisplatin, an anticancer drug, is known to cause acute renal failure as a significant side effect despite having antineoplastic benefits [15]. A study revealed that baicalein treatment reduced kidney damage and dysfunction caused by cisplatin-induced oxidative stress, apoptosis, and inflammation. In brief, this process is accomplished by downregulating the MAPK and NF-κB signalling pathways and strengthening antioxidant defence mechanisms [15]. Similarly, the clinical use of doxorubicin is limited despite its favourable pharmacological characteristics because it induces cardiotoxicity, which may eventually lead to congestive heart failure and degenerative cardiomyopathy [96]. In an in vivo mouse model, baicalein treatment of doxorubicin-induced injured cardiac tissues reduced cardiac damage by restoring myocardial enzymes and nonenzymatic antioxidants, decreasing the expression of NF-κB signalling and intrinsic pathway [15].
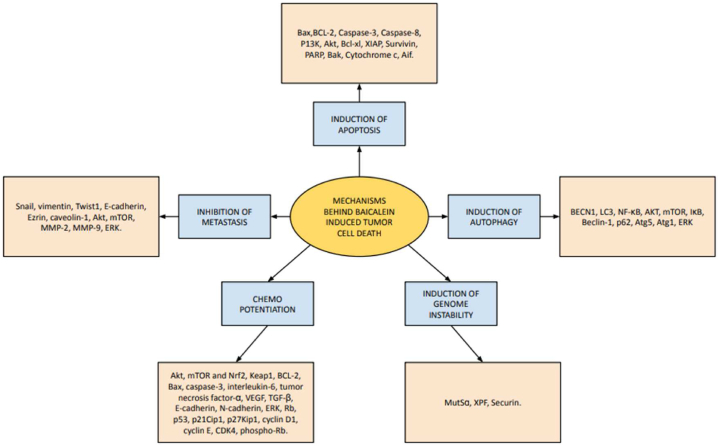
A recent study reported that baicalein could induce ferroptosis cell death in bladder cancer cells via ROS and accumulation of the intracellular chelator iron, and this phenomenon was associated with the downregulation of ferritin heavy chain 1 (FTH1) [100]. Studies have shown that baicalein enhanced the immune response and possibly helped to reduce the risk of infections and other adverse reactions induced by cancer treatment. During chemotherapy, baicalein could protect the liver by reducing inflammation and oxidative stress and enhancing liver function [101]. The overall mechanisms by which baicalein inhibits tumour progression are summarized in Fig. 1.
Fig. 1.
Mechanisms of baicalein-induced tumour cell death.
8. Anti-angiogenic properties
Blood vessels near malignant cells provide them with the nourishment and oxygen they need to proliferate. During angiogenesis, which occurs when pro-angiogenic molecules exceed VEGF-A is crucial in regulating aberrant blood vessel creation [102]. While vascular sprouting is controlled by Notch signalling, platelet-derived growth factor (PDGF) aids in vascular maturation. Additionally, matrix metalloproteinases facilitate angiogenesis by breaking down the basement membrane. By using transmembrane receptors to activate downstream signalling pathways, these compounds promote gene expression and endothelial cell survival, proliferation, and angiogenesis [103]. Plant-based medicines have long been utilised in traditional medicine due to their proven anti-inflammatory, anti-allergic, and anti-infective qualities also modulate angiogenesis [104].
In a study, baicalein significantly suppressed tube formation and cell migration in HUVECs, reducing proliferation and migration and inducing tumour cell death via caspase-3 activation in B16F10 and LLC cells. Additionally, baicalein significantly decreased the expression of the CD31 endothelial cell marker and the mural cell marker α-SMA in the tumours of the baicalein-treated groups, suggesting that baicalein inhibits tumour angiogenesis by hindering the development of the tumour vasculature. These results were demonstrated in mouse models, which also showed that baicalein reduced the tumour volume and significantly reduced the tumour growth rate in the early stages of tumour progression [105]. In lung cancer, baicalein significantly downregulated the expression of the angiogenesis-related protein VEGF-A [106].
An investigation into a novel derivative of baicalein in transgenic zebrafish models revealed that the anti-angiogenic properties came from preventing the formation of new blood vessels rather than from damaging pre-existing vasculature. Furthermore, while exhibiting extremely low toxicity, this compound inhibited HUVEC migration, proliferation, and tube formation in a dose-dependent manner in vitro [107]. The FAK knockdown reduced the microtubule-forming capacity of HUVEC cells, and baicalein was noted to inhibit this tube-forming capacity in both FAK knockdown and overexpressed cells. Moreover, VEGF, MMP-2 and MMP-9 expression was suppressed following baicalein treatment, indicating that baicalein inhibited gastric cancer angiogenesis by regulating FAK [108]. The mechanisms by which baicalein regulates tumour angiogenesis are summarized in Table 6.
Table 6.
Role of baicalein in mediating tumor angiogenesis.
| Type of cancer | Type of Study | Model | Dosage of baicalein | Pathway involved | Reference |
|---|---|---|---|---|---|
| General | In vitro and in vivo | B16F10 cells, Lewis lung carcinoma (LLC) cells, and HUVECs; and pathogen-free C57BL/6 mice | 0, 1, 10, 100 and 200 μM; and 1.5 mg/kg | – | [105] |
| Non-Small Cell Lung Cancer | In vivo | Balb/c male thymic nude mice (6-week-old) | 40 mg/kg | Src/Id1 signalling | [106] |
| Lung cancer | In vitro and in vivo | A549 and HUVECs cell lines; transgenic zebrafish model | 0, 1, 4, 5, 8, 10, 25, 50 and 100 μM; and 1–2.5 μM | – | [107] |
| Gastric cancer | In vitro and in vivo | Cell lines HGC-27, SGC-7901, MGC-803 and BGC-823. 4–6-week-old BALB/c nude female mice |
0, 5, 15, 25 and 50 μmol/l; and 50 mg/kg | miR-7/FAK/AKT signalling | [108] |
9. Bioavailability, pharmacokinetics and toxicity
The main reason hindering the pharmaceutical applications of baicalein is its low extractable quantity from the herbal extract, low aqueous solubility, and low bioavailability. Additionally, the acid hydrolysis method that is used for the biotransformation of baicalin to baicalein is expensive and can cause environmental contamination, which is why there is a need for techniques that can improve the bioavailability of baicalein [46]. Additionally, due to the lesser aqueous solubility of baicalein than its parent drug baicalin, developing relevant delivery systems can enhance their bioavailability and efficacy is needed [109]. Studies have used several methods to enhance the bioavailability of baicalein. For instance, one approach particularly explored the development and application of selenium–baicalein nanoparticles as a targeted therapeutic strategy for NSCLC. This strategy significantly improves the bioavailability of baicalein through several mechanisms. First, the nanoparticles enhance solubility and stability, allowing for better absorption in biological systems compared to free baicalein, which has low water solubility. Second, incorporating selenium facilitated cellular uptake via endocytosis, enabling more effective delivery to target cancer cells. Finally, the nanoparticles' small size and surface modifications promote interaction with cellular membranes, further enhancing bioavailability and therapeutic efficacy against NSCLC [110].
Further, baicalein could easily transfer from the apical to the basolateral side of the intestinal epithelia because of its increased lipophilicity, low molecular weight, and absence of transporters. After entering the bloodstream, baicalein undergoes further metabolism to produce the glucuronidated, sulfated, methylated, and dehydroxylated forms. Hepatic veins allow baicalin and other metabolites from the liver to enter the bloodstream. Baicalein also experienced significant first-pass metabolism in the liver and gut, primarily as sulfation and glucuronidation [111]. Conjugated metabolism is the main metabolic pathway for baicalein [112,113]. Multidrug resistance-associated protein 2 (MRP2) excretes some baicalin and other conjugates into the liver's bile duct, where they are either eliminated in faeces in their various conjugated forms or enter the enterohepatic circulation. Urine excretes a small portion of the blood's baicalin conjugates [114]. A study investigating the pharmacokinetics of a Mannich base derivative of baicalein in monkeys showed that the compound was relatively extensively dispersed in body fluids and did not exhibit any selectivity to tissues. Additionally, it showed outstanding druggability for injectable or oral administration and was able to cross the blood-brain barrier [115]. Overall, baicalein exhibits potential as cancer therapeutic despite these obstacles, and advancements in its pharmacokinetics may make it easier to go from laboratory studies to clinical application [116].
Furthermore, some studies have suggested that baicalein has a lower toxicity on normal cells than cancer cells, indicating some selectivity for cancer cells [22]. For instance, while doxorubicin and hyaluronic acid-baicalein loaded nanostructured lipid carriers demonstrated good co-delivery of the two drugs, suggesting their anti-tumor actions in vivo and in vitro, this approach showed no signs of systemic toxicity [78]. Additionally, baicalein's increase in antioxidant enzymes, inhibition of lipid peroxidation, reduction of inflammatory cytokines, and regulation of the apoptosis pathway contributed to its protective actions against chemical toxicants [117].
10. Conclusion and future Directions
Baicalein primarily exerts its anticancer effects by triggering apoptosis, autophagy, or genomic instability. Additionally, these agents can be used in combination therapy with conventional anticancer medications to synergistically enhance their anticancer effects while reducing the dosages that would otherwise be necessary. Nanotechnology-mediated codelivery of baicalein and anticancer drug combinations was also beneficial. Baicalein alone also functions as a chemopreventive drug, decreasing the likelihood of acquiring cancer by altering the early stages of tumour progression. Furthermore, baicalein has been shown to regulate angiogenesis and EMT processes, prohibiting metastasis. Baicalein was also used to minimize the damage caused by chemotherapy and radiation in normal tissue. Its anticancer effects via the induction of ferroptotic cancer death were further studied. Moreover, baicalin and baicalein have shown promising safety and tolerance in humans.
Future studies should focus on the several limitations in baicalein research. Its anticancer effects have mostly been examined in cellular and animal models, demanding future research using more realistic systems such as patient-derived tumour organoids and xenografts. Baicalein's tendency to establish intramolecular hydrogen bonds results in limited hydrophilicity and bioavailability, encouraging research into improved drug delivery technologies, such as nanoparticles, to improve absorption. Safety evaluations are particularly essential since, although the majority of research shows negligible harm to normal cells, one study suggested possible hepatotoxicity in vivo. Also, improved extraction methods to isolate a good amount of baicalein are required. Ultimately, clinical research is necessary to assess the usefulness of baicalein in the treatment of cancer. Further, research should focus on determining the optimal baicalein dose to achieve the maximum therapeutic effect while minimizing potential side effects, the molecular mechanism of baicalein action, and combination therapy with radiotherapy and immunotherapy.
CRediT authorship contribution statement
Madhu Hegde: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Archana P R: Writing – review & editing. Kamalesh Dattaram Mumbrekar: Writing – review & editing, Supervision, Resources, Conceptualization.
Data availability statement
No primary data is associated with this review article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Manipal School of Life Sciences and Manipal Academy of Higher Education (MAHE) for their support and infrastructure facilities.
References
- 1.Mun E.J., Babiker H.M., Weinberg U., Kirson E.D., Von Hoff D.D. Tumor-treating fields: a fourth modality in cancer treatment. Clin. Cancer Res. 2018 Jan 15;24(2):266–275. doi: 10.1158/1078-0432.CCR-17-1117. [DOI] [PubMed] [Google Scholar]
- 2.Perez C.A., Wei Y., Guo M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009 Mar;103(3):326–332. doi: 10.1016/j.jinorgbio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013 Oct;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 4.Du B., Shim J.S. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016 Jul 22;21(7):965. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 6.Catanzaro E., Greco G., Potenza L., Calcabrini C., Fimognari C. Natural products to fight cancer: a focus on juglans regia. Toxins. 2018 Nov 14;10(11):469. doi: 10.3390/toxins10110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Carlo G., Mascolo N., Izzo A.A., Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–353. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 8.Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009 Feb;35(1):57–68. doi: 10.1016/j.ctrv.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Yi S., Liu G., Wu Y., Liang Q., Li L. Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic catastrophe, apoptosis and autophagy via NF-kB signalling pathway. J BUON. 2020;25(1):389–394. [PubMed] [Google Scholar]
- 10.Kamaraj S., Vinodhkumar R., Anandakumar P., Jagan S., Ramakrishnan G., Devaki T. The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol. Pharm. Bull. 2007 Dec;30(12):2268–2273. doi: 10.1248/bpb.30.2268. [DOI] [PubMed] [Google Scholar]
- 11.Magne Nde C.B., Zingue S., Winter E., Creczynski-Pasa T.B., Michel T., Fernandez X., et al. Flavonoids, breast cancer chemopreventive and/or chemotherapeutic agents. Curr. Med. Chem. 2015;22(30):3434–3446. [PubMed] [Google Scholar]
- 12.Li H.B., Jiang Y., Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Dec 5;812(1–2):277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bie B., Sun J., Guo Y., Li J., Jiang W., Yang J., et al. Baicalein: a review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed. Pharmacother. 2017 Sep;93:1285–1291. doi: 10.1016/j.biopha.2017.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Cheng C.S., Chen J., Tan H.Y., Wang N., Chen Z., Feng Y. Scutellaria baicalensis and cancer treatment: recent progress and perspectives in biomedical and clinical studies. Am. J. Chin. Med. 2018;46(1):25–54. doi: 10.1142/S0192415X18500027. [DOI] [PubMed] [Google Scholar]
- 15.Tian Y., Li X., Xie H., Wang X., Xie Y., Chen C., et al. Protective mechanism of the antioxidant baicalein toward hydroxyl radical-treated bone marrow-derived mesenchymal stem cells. Molecules. 2018 Jan;23(1):223. doi: 10.3390/molecules23010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahu B.D., Mahesh Kumar J., Sistla R. Baicalein, a bioflavonoid, prevents cisplatin-induced acute kidney injury by up-regulating antioxidant defenses and down-regulating the MAPKs and NF-κB pathways. PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0134139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M., Qiu S., Qin J. Baicalein induced apoptosis and autophagy of undifferentiated thyroid cancer cells by the ERK/PI3K/Akt pathway. Am J Transl Res. 2019;11(6):3341–3352. [PMC free article] [PubMed] [Google Scholar]
- 18.Nakahata N., Kutsuwa M., Kyo R., Kubo M., Hayashi K., Ohizumi Y. Analysis of inhibitory effects of scutellariae radix and baicalein on prostaglandin E2 production in rat C6 glioma cells. Am. J. Chin. Med. 1998;26(3–4):311–323. doi: 10.1142/S0192415X9800035X. [DOI] [PubMed] [Google Scholar]
- 19.Yu M., Qi B., Xiaoxiang W., Xu J., Liu X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017 Jun;90:677–685. doi: 10.1016/j.biopha.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y., Snyder S.A., Smith J.N., Chen Y.C. Anticancer properties of baicalein: a review. Med. Chem. Res. 2016 Aug;25(8):1515–1523. doi: 10.1007/s00044-016-1607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S., Wang L., Li N., Liu Y., Su H. Combination lung cancer chemotherapy: design of a pH-sensitive transferrin-PEG-Hz-lipid conjugate for the co-delivery of docetaxel and baicalin. Biomed. Pharmacother. 2017 Nov;95:548–555. doi: 10.1016/j.biopha.2017.08.090. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S., Buttar H.S., Kaur G., Tuli H.S. Baicalein: promising therapeutic applications with special reference to published patents. Pharm Pat Anal. 2022 Jan;11(1):23–32. doi: 10.4155/ppa-2021-0027. [DOI] [PubMed] [Google Scholar]
- 23.Steward W.P., Brown K. Cancer chemoprevention: a rapidly evolving field. Br. J. Cancer. 2013 Jul 9;109(1):1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucuk O. Cancer chemoprevention. Cancer Metastasis Rev. 2002 Dec 1;21(3):189–197. doi: 10.1023/a:1021298508095. [DOI] [PubMed] [Google Scholar]
- 25.Naveenkumar C., Raghunandakumar S., Asokkumar S., Binuclara J., Rajan B., Premkumar T., et al. Mitigating role of baicalein on lysosomal enzymes and xenobiotic metabolizing enzyme status during lung carcinogenesis of Swiss albino mice induced by benzo(a)pyrene. Fundam. Clin. Pharmacol. 2014 Jun;28(3):310–322. doi: 10.1111/fcp.12036. [DOI] [PubMed] [Google Scholar]
- 26.Sui X., Han X., Chen P., Wu Q., Feng J., Duan T., et al. Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating akt/mTOR signaling pathway. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.602282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morshed A.K.M.H., Paul S., Hossain A., Basak T., Hossain M.S., Hasan M.M., et al. Baicalein as promising anticancer agent: a comprehensive analysis on molecular mechanisms and therapeutic perspectives. Cancers. 2023 Apr 3;15(7):2128. doi: 10.3390/cancers15072128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma E., Kumar A., Devi Daimary U., Parama D., Girisa S., Sethi G., et al. Potential of baicalein in the prevention and treatment of cancer: a scientometric analyses based review. J. Funct.Foods. 2021 Nov 1;86 [Google Scholar]
- 29.Kim D.H., Hossain M.A., Kang Y.J., Jang J.Y., Lee Y.J., Im E., et al. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013 Nov;43(5):1652–1658. doi: 10.3892/ijo.2013.2086. [DOI] [PubMed] [Google Scholar]
- 30.Po L.S., Chen Z yu, Tsang D.S.C., Leung L.K. Baicalein and genistein display differential actions on estrogen receptor (ER) transactivation and apoptosis in MCF-7 cells. Cancer Lett. 2002 Dec 10;187(1–2):33–40. doi: 10.1016/s0304-3835(02)00355-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Wang J., Hong D.Y., Chen L., Zhang Y.Y., Xu Y.N., et al. Baicalein has protective effects on the 17β-estradiol-induced transformation of breast epithelial cells. Oncotarget. 2017 Jan 2;8(6):10470–10484. doi: 10.18632/oncotarget.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y.C., Liu Y.L., Hsieh J.Y., Wang C.H., Lin C.L., Liu G.Y., et al. Baicalein, 7,8-dihydroxyflavone and myricetin as potent inhibitors of human ornithine decarboxylase. Nutrients. 2020 Dec 17;12(12):3867. doi: 10.3390/nu12123867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma G.Z., Liu C.H., Wei B., Qiao J., Lu T., Wei H.C., et al. Baicalein inhibits DMBA/TPA-induced skin tumorigenesis in mice by modulating proliferation, apoptosis, and inflammation. Inflammation. 2013 Apr;36(2):457–467. doi: 10.1007/s10753-012-9566-y. [DOI] [PubMed] [Google Scholar]
- 34.Chan H.Y., Chen Z yu, Tsang D.S.C., Leung L.K. Baicalein inhibits DMBA–DNA adduct formation by modulating CYP1A1 and CYP1B1 activities. Biomed. Pharmacother. 2002 Aug 1;56(6):269–275. doi: 10.1016/s0753-3322(02)00192-0. [DOI] [PubMed] [Google Scholar]
- 35.Chen J., Li Z., Chen A.Y., Ye X., Luo H., Rankin G.O., et al. Inhibitory effect of baicalin and baicalein on ovarian cancer cells. Int. J. Mol. Sci. 2013 Mar 15;14(3):6012–6025. doi: 10.3390/ijms14036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X., Yang Y., Li Y., Cao Y., Tang L., Chen F., et al. Baicalein inhibits cervical cancer progression via downregulating long noncoding RNA BDLNR and its downstream PI3K/Akt pathway. Int. J. Biochem. Cell Biol. 2018 Jan;94:107–118. doi: 10.1016/j.biocel.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Goldar S., Khaniani M.S., Derakhshan S.M., Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16(6):2129–2144. doi: 10.7314/apjcp.2015.16.6.2129. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Liu K., Yang L., Zhang G. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the expression of anti-apoptotic genes. Saudi J. Biol. Sci. 2018 Nov;25(7):1478–1482. doi: 10.1016/j.sjbs.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan W., Ma X., Zhao X., Zhang S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther. 2018;12:3961–3972. doi: 10.2147/DDDT.S181939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han S.E., Park C.H., Nam-Goong I.S., Kim Y.I., Kim E.S. Anticancer effects of baicalein in FRO thyroid cancer cells through the up-regulation of ERK/p38 MAPK and Akt pathway. In Vitro. 2019;33(2):375–382. doi: 10.21873/invivo.11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F.R., Jiang Y.S. Effect of treatment with baicalein on the intracerebral tumor growth and survival of orthotopic glioma models. J. Neuro Oncol. 2015 Aug;124(1):5–11. doi: 10.1007/s11060-015-1804-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Chen Z., Li X., He J., Liu Z., Yang L. Baicalein flavone targets cisplatin resistant human pancreatic cancer cells via inducing S-phase cell cycle arrest, inhibition of cell migration and invasion, caspase activation and mitochondrial-dependent apoptosis. J BUON. 2020;25(4):1947–1953. [PubMed] [Google Scholar]
- 43.Su M.Q., Zhou Y.R., Rao X., Yang H., Zhuang X.H., Ke X.J., et al. Baicalein induces the apoptosis of HCT116 human colon cancer cells via the upregulation of DEPP/Gadd45a and activation of MAPKs. Int. J. Oncol. 2018 Aug;53(2):750–760. doi: 10.3892/ijo.2018.4402. [DOI] [PubMed] [Google Scholar]
- 44.Deng X., Liu J., Liu L., Sun X., Huang J., Dong J. Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int. J. Biol. Sci. 2020;16(8):1403–1416. doi: 10.7150/ijbs.41768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Y., Yuan L., Meng F., Lu D., Che M., Zhou X., et al. Gancao Xiexin Decoction inhibits gastric carcinoma proliferation and migration by regulating the JAK2/STAT3 signalling pathway. J. Ethnopharmacol. 2024 Jan 30;319(Pt 2) doi: 10.1016/j.jep.2023.117241. [DOI] [PubMed] [Google Scholar]
- 46.Gong W.Y., Zhao Z.X., Liu B.J., Lu L.W., Dong J.C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017 Jan 27;126:844–852. doi: 10.1016/j.ejmech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 47.Li J., Zhang D., Wang S., Yu P., Sun J., Zhang Y., et al. Baicalein induces apoptosis by inhibiting the glutamine-mTOR metabolic pathway in lung cancer. J. Adv. Res. 2024 Mar 2 doi: 10.1016/j.jare.2024.02.023. Epub ahead of print. PMID: 38432394. S2090-1232(24)00085-7. [DOI] [PubMed] [Google Scholar]
- 48.He K., Yu X., Wang X., Tang L., Cao Y., Xia J., et al. Baicalein and Ly294002 induces liver cancer cells apoptosis via regulating phosphatidyl inositol 3-kinase/Akt signaling pathway. J Cancer Res Ther. 2018 Jun;14(Supplement):S519–S525. doi: 10.4103/0973-1482.235356. [DOI] [PubMed] [Google Scholar]
- 49.Ma D., Chen S., Wang H., Wei J., Wu H., Gao H., et al. Baicalein induces apoptosis of pancreatic cancer cells by regulating the expression of miR-139-3p and miR-196b-5p. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.653061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X., He S., Ma B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020 Jan 22;19(1):12. doi: 10.1186/s12943-020-1138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White E., Mehnert J.M., Chan C.S. Autophagy, metabolism, and cancer. Clin. Cancer Res. 2015 Nov 15;21(22):5037–5046. doi: 10.1158/1078-0432.CCR-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y.F., Xu Y.L., Tang Z.H., Li T., Zhang L.L., Chen X., et al. Baicalein induces Beclin 1- and extracellular signal-regulated kinase-dependent autophagy in ovarian cancer cells. Am. J. Chin. Med. 2017;45(1):123–136. doi: 10.1142/S0192415X17500094. [DOI] [PubMed] [Google Scholar]
- 53.Chai Y., Xu J., Yan B. The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 2017 Apr;37(4):2317–2323. doi: 10.3892/or.2017.5437. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y.F., Li T., Tang Z.H., Chang L.L., Zhu H., Chen X.P., et al. Baicalein triggers autophagy and inhibits the protein kinase B/mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Phytother Res. 2015 May;29(5):674–679. doi: 10.1002/ptr.5298. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z., Jiang C., Chen W., Zhang G., Luo D., Cao Y., et al. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/732516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao D., Li Y., Xing J., Sun P., Wang Y., Zhang Y., et al. [Baicalein inhibits PI3K/AKT signaling pathway and induces autophagy of MGC-803 cells] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019 Jul;35(7):613–618. [PubMed] [Google Scholar]
- 57.Aryal P., Kim K., Park P.H., Ham S., Cho J., Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014 Oct;281(20):4644–4658. doi: 10.1111/febs.12969. [DOI] [PubMed] [Google Scholar]
- 58.Phan T., Nguyen V.H., A’lincourt Salazar M., Wong P., Diamond D.J., Yim J.H., et al. Inhibition of autophagy amplifies baicalein-induced apoptosis in human colorectal cancer. Mol Ther Oncolytics. 2020 Dec 16;19:1–7. doi: 10.1016/j.omto.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Fox J.T., Park Y.U., Elliott G., Rai G., Cai M., et al. A novel chemotherapeutic agent to treat tumors with DNA mismatch repair deficiencies. Cancer Res. 2016 Jul 15;76(14):4183–4191. doi: 10.1158/0008-5472.CAN-15-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang R.H., Su W.C., Liu H.F., Huang H.S., Chao J.I. Opposite expression of securin and γ-H2AX regulates baicalein-induced cancer cell death. J. Cell. Biochem. 2010 Oct 1;111(2):274–283. doi: 10.1002/jcb.22697. [DOI] [PubMed] [Google Scholar]
- 61.Lai W., Jia J., Yan B., Jiang Y., Shi Y., Chen L., et al. Baicalin hydrate inhibits cancer progression in nasopharyngeal carcinoma by affecting genome instability and splicing. Oncotarget. 2018 Jan 2;9(1):901–914. doi: 10.18632/oncotarget.22868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C.Z., Zhang C.F., Luo Y., Yao H., Yu C., Chen L., et al. Baicalein, an enteric microbial metabolite, suppresses gut inflammation and cancer progression in ApcMin/+ mice. Clin. Transl. Oncol. 2020 Jul;22(7):1013–1022. doi: 10.1007/s12094-019-02225-5. [DOI] [PubMed] [Google Scholar]
- 63.Huang L., Peng B., Nayak Y., Wang C., Si F., Liu X., et al. Baicalein and baicalin promote melanoma apoptosis and senescence via metabolic inhibition. Front. Cell Dev. Biol. 2020 Aug 25;8:836. doi: 10.3389/fcell.2020.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuo Y.T., Liu C.H., Wong S.H., Pan Y.C., Lin L.T. Small molecules baicalein and cinnamaldehyde are potentiators of measles virus-induced breast cancer oncolysis. Phytomedicine. 2021 Aug;89 doi: 10.1016/j.phymed.2021.153611. [DOI] [PubMed] [Google Scholar]
- 65.Ke M., Zhang Z., Xu B., Zhao S., Ding Y., Wu X., et al. Baicalein and baicalin promote antitumor immunity by suppressing PD-L1 expression in hepatocellular carcinoma cells. Int Immunopharmacol. 2019 Oct;75 doi: 10.1016/j.intimp.2019.105824. [DOI] [PubMed] [Google Scholar]
- 66.Dong Y., He G., Chen K., He X., Pan M., Huang X., et al. Baicalein promotes KDM4E to induce BICD1 and inhibit triple-negative breast cancer progression by blocking PAR1 signaling. Mol. Carcinog. 2024;63(7):1288–1302. doi: 10.1002/mc.23724. [DOI] [PubMed] [Google Scholar]
- 67.Yu Q., Tang R., Mo W., Zhao L., Li L. Baicalein enhances radiosensitivity in colorectal cancer via JAK2/STAT3 pathway inhibition. Chem. Biol. Drug Des. 2024;104(2) doi: 10.1111/cbdd.14611. [DOI] [PubMed] [Google Scholar]
- 68.Lai J qin, le Zhao L., Hong C., Zou Q ming, xuan Su J., jia Li S., et al. Baicalein triggers ferroptosis in colorectal cancer cells via blocking the JAK2/STAT3/GPX4 axis. Acta Pharmacol. Sin. 2024 Aug;45(8):1715–1726. doi: 10.1038/s41401-024-01258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng Q., Zhang Y., Zhang W., Guo Q. Baicalein suppresses the proliferation and invasiveness of colorectal cancer cells by inhibiting Snail-induced epithelial-mesenchymal transition. Mol. Med. Rep. 2020 Jun;21(6):2544–2552. doi: 10.3892/mmr.2020.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Y., Chen L., Hong D., Chen Z., Zhang J., Fu L., et al. Baicalein inhibits fibronectin-induced epithelial-mesenchymal transition by decreasing activation and upregulation of calpain-2. Cell Death Dis. 2019 Apr 18;10(5):341. doi: 10.1038/s41419-019-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su G., Chen H., Sun X. Baicalein suppresses non small cell lung cancer cell proliferation, invasion and Notch signaling pathway. Cancer Biomark. 2018;22(1):13–18. doi: 10.3233/CBM-170673. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X., Ruan Q., Zhai Y., Lu D., Li C., Fu Y., et al. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020 Oct;111(10):3802–3812. doi: 10.1111/cas.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo Z., Hu X., Xing Z., Xing R., Lv R., Cheng X., et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol. Cell. Biochem. 2015 Aug;406(1–2):111–119. doi: 10.1007/s11010-015-2429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma X., Yan W., Dai Z., Gao X., Ma Y., Xu Q., et al. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. Drug Des Devel Ther. 2016;10:1419–1441. doi: 10.2147/DDDT.S102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L., Ling Y., Chen Y., Li C.L., Feng F., You Q.D., et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010 Nov 1;297(1):42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 76.Chen F., Zhuang M., Peng J., Wang X., Huang T., Li S., et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol. Med. Rep. 2014 Oct 1;10(4):1999–2003. doi: 10.3892/mmr.2014.2452. [DOI] [PubMed] [Google Scholar]
- 77.Susmitha G.D., Miyazato K., Ogura K., Yokoyama S., Hayakawa Y. Anti-metastatic effects of baicalein by targeting STAT3 activity in breast cancer cells. Biol. Pharm. Bull. 2020;43(12):1899–1905. doi: 10.1248/bpb.b20-00571. [DOI] [PubMed] [Google Scholar]
- 78.Liu Q., Li J., Pu G., Zhang F., Liu H., Zhang Y. Co-delivery of baicalein and doxorubicin by hyaluronic acid decorated nanostructured lipid carriers for breast cancer therapy. Drug Deliv. 2016 May;23(4):1364–1368. doi: 10.3109/10717544.2015.1031295. [DOI] [PubMed] [Google Scholar]
- 79.Rui X., Yan X.I., Zhang K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol. Lett. 2016 Jan;11(1):685–688. doi: 10.3892/ol.2015.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu J.Y., Tsai K.W., Li Y.Z., Chang Y.S., Lai Y.C., Laio Y.H., et al. Anti-bladder-tumor effect of baicalein from Scutellaria baicalensis Georgi and its application in vivo. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/579751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ling Y., Chen Y., Chen P., Hui H., Song X., Lu Z., et al. Baicalein potently suppresses angiogenesis induced by vascular endothelial growth factor through the p53/Rb signaling pathway leading to G1/S cell cycle arrest. Exp Biol Med (Maywood) 2011 Jul;236(7):851–858. doi: 10.1258/ebm.2011.010395. [DOI] [PubMed] [Google Scholar]
- 82.Kiatwuthinon P., Narkthong T., Ngaokrajang U., Kumkate S., Janvilisri T. Baicalein inhibits metastatic phenotypes in nasopharyngeal carcinoma cells via a focal adhesion protein integrin β8. Pharmaceuticals. 2021 Dec 21;15(1):5. doi: 10.3390/ph15010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park C.H., Han S.E., Nam-Goong I.S., Kim Y.I., Kim E.S. Combined effects of baicalein and docetaxel on apoptosis in 8505c anaplastic thyroid cancer cells via downregulation of the ERK and akt/mTOR pathways. Endocrinol Metab (Seoul) 2018 Mar;33(1):121–132. doi: 10.3803/EnM.2018.33.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S.H., Cheng Y.C. Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J. Ethnopharmacol. 2012 Apr 10;140(3):614–623. doi: 10.1016/j.jep.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 85.Otsuyama K.I., Ma Z., Abroun S., Amin J., Shamsasenjan K., Asaoku H., et al. PPARbeta-mediated growth suppression of baicalein and dexamethasone in human myeloma cells. Leukemia. 2007 Jan;21(1):187–190. doi: 10.1038/sj.leu.2404462. [DOI] [PubMed] [Google Scholar]
- 86.Chen C.H., Huang T.S., Wong C.H., Hong C.L., Tsai Y.H., Liang C.C., et al. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem. Toxicol. 2009 Mar;47(3):638–644. doi: 10.1016/j.fct.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Xi M., Duan X., Wang Y., Kong F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. IJN. 2015 May 22;10(1):3737–3750. doi: 10.2147/IJN.S80297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y.J., Wu C.S., Shieh J.J., Wu J.H., Chen H.Y., Chung T.W., et al. Baicalein triggers mitochondria-mediated apoptosis and enhances the antileukemic effect of vincristine in childhood acute lymphoblastic leukemia CCRF-CEM cells. Evid. Based Complement Alternat. Med. 2013;2013 doi: 10.1155/2013/124747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang Q., Ji F., Sun W., Wang J., Guo J., Guo L., et al. Combination of baicalein and 10-hydroxy camptothecin exerts remarkable synergetic anti-cancer effects. Phytomedicine. 2016 Dec 15;23(14):1778–1786. doi: 10.1016/j.phymed.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 90.Liu P., Feng J., Sun M., Yuan W., Xiao R., Xiong J., et al. Synergistic effects of baicalein with gemcitabine or docetaxel on the proliferation, migration and apoptosis of pancreatic cancer cells. Int. J. Oncol. 2017 Dec;51(6):1878–1886. doi: 10.3892/ijo.2017.4153. [DOI] [PubMed] [Google Scholar]
- 91.Meng L., Xia X., Yang Y., Ye J., Dong W., Ma P., et al. Co-encapsulation of paclitaxel and baicalein in nanoemulsions to overcome multidrug resistance via oxidative stress augmentation and P-glycoprotein inhibition. Int J Pharm. 2016 Nov 20;513(1–2):8–16. doi: 10.1016/j.ijpharm.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Q mei, Wang S., Zhang H., Lu Y yu, feng Wang X., Motoo Y., et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol. Sin. 2009 Dec;30(12):1648–1658. doi: 10.1038/aps.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin Y., Wu Q., Pan S., Zhou Q., Liu H., Zhang Q., et al. Baicalein enhances cisplatin sensitivity in cervical cancer cells by promoting cuproptosis through the Akt pathway. Biomed. Pharmacother. 2024 Oct 1;179 doi: 10.1016/j.biopha.2024.117415. [DOI] [PubMed] [Google Scholar]
- 94.Anticancer Drug — Friend or Foe | IntechOpen [Internet]. [cited 2024 May 15]. Available from: https://www.intechopen.com/chapters/46653.
- 95.Robbins M.E., Zhao W., Davis C.S., Toyokuni S., Bonsib S.M. Radiation-induced kidney injury: a role for chronic oxidative stress? Micron. 2002;33(2):133–141. doi: 10.1016/s0968-4328(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 96.Wideł M., Przybyszewski W., Rzeszowska-Wolny J. [Radiation-induced bystander effect: the important part of ionizing radiation response. Potential clinical implications] Postepy Hig. Med. Dosw. 2009 Aug 18;63:377–388. [PubMed] [Google Scholar]
- 97.Lee E.K., Kim J.M., Choi J., Jung K.J., Kim D.H., Chung S.W., et al. Modulation of NF-κB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic. Res. 2011 May;45(5):507–517. doi: 10.3109/10715762.2011.555479. [DOI] [PubMed] [Google Scholar]
- 98.Jang H., Lee J., Park S., Kim J.S., Shim S., Lee S.B., et al. Baicalein mitigates radiation-induced enteritis by improving endothelial dysfunction. Front. Pharmacol. 2019;10:892. doi: 10.3389/fphar.2019.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gandhi N.S., Tekade R.K., Chougule M.B. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 2014 Nov 28;194:238–256. doi: 10.1016/j.jconrel.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kong N., Chen X., Feng J., Duan T., Liu S., Sun X., et al. Baicalin induces ferroptosis in bladder cancer cells by downregulating FTH1. Acta Pharm. Sin. B. 2021 Dec 1;11(12):4045–4054. doi: 10.1016/j.apsb.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niu C., Wan J., Bian Y., Li F., Lan J. Baicalein and its underlying mechanism as a protector against liver injury induced by cisplatin in mice. Biotechnol. Biotechnol. Equip. 2017 Jan 2;31(1):193–199. [Google Scholar]
- 102.Li S., Xu H.X., Wu C.T., Wang W.Q., Jin W., Gao H.L., et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019 Feb;22(1):15–36. doi: 10.1007/s10456-018-9645-2. [DOI] [PubMed] [Google Scholar]
- 103.Liu Z.L., Chen H.H., Zheng L.L., Sun L.P., Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023 May 11;8(1):198. doi: 10.1038/s41392-023-01460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu K., Bhat M., Basu S. Plants and their active compounds: natural molecules to target angiogenesis. Angiogenesis. 2016 Jul;19(3):287–295. doi: 10.1007/s10456-016-9512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park Y.G., Choi J., Jung H.K., Kim B., Kim C., Park S.Y., et al. Baicalein inhibits tumor progression by inhibiting tumor cell growth and tumor angiogenesis. Oncol. Rep. 2017;38(5):3011–3018. doi: 10.3892/or.2017.6007. [DOI] [PubMed] [Google Scholar]
- 106.Zhao Z., Liu B., Sun J., Lu L., Liu L., Qiu J., et al. Baicalein inhibits orthotopic human non-small cell lung cancer xenografts via src/id1 pathway. Evid Based Complement Alternat Med. 2019 Mar 4;2019 doi: 10.1155/2019/9806062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang X., Zhou J., Lin Q., Gong G., Sun H., Liu W., et al. Anti-angiogenic and anticancer effects of baicalein derivatives based on transgenic zebrafish model. Bioorg. Med. Chem. 2024;26(15):4481–4492. doi: 10.1016/j.bmc.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 108.Qiao D., Xing J., Duan Y., Wang S., Yao G., Zhang S., et al. The molecular mechanism of baicalein repressing progression of gastric cancer mediating miR-7/FAK/AKT signaling pathway. Phytomedicine. 2022 Jun;100 doi: 10.1016/j.phymed.2022.154046. [DOI] [PubMed] [Google Scholar]
- 109.Chen H., Gao Y., Wu J., Chen Y., Chen B., Hu J., et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014 Nov 1;354(1):5–11. doi: 10.1016/j.canlet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi H., Wang B., Ma H., Li Y., Du J., Zhang B., et al. Preparation of biomimetic selenium–baicalein nanoparticles and their targeted therapeutic application in nonsmall cell lung cancer. Mol Pharmaceutics. 2024 Sep 2;21(9):4476–4489. doi: 10.1021/acs.molpharmaceut.4c00390. [DOI] [PubMed] [Google Scholar]
- 111.Li C., Lin G., Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm Drug Dispos. 2011;32(8):427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 112.Lai M.Y., Hsiu S.L., Chen C.C., Hou Y.C., Chao P.D.L. Urinary pharmacokinetics of baicalein, wogonin and their glycosides after oral administration of Scutellariae Radix in humans. Biol. Pharm. Bull. 2003 Jan;26(1):79–83. doi: 10.1248/bpb.26.79. [DOI] [PubMed] [Google Scholar]
- 113.Li J rong, Zuo F., Zhang L., Nakamura N., Hattori M. [Study on the metabolism of the precipitation of Xiexin decoction in rats I] Zhongguo Zhongyao Zazhi. 2005 Nov;30(21):1673–1676. [PubMed] [Google Scholar]
- 114.Wang R., Wang C., Lu L., Yuan F., He F. Baicalin and baicalein in modulating tumor microenvironment for cancer treatment: a comprehensive review with future perspectives. Pharmacol. Res. 2024 Jan 1;199 doi: 10.1016/j.phrs.2023.107032. [DOI] [PubMed] [Google Scholar]
- 115.Guo H.M., Sun Y.M., Zhang S.X., Ju X.L., Xie A.Y., Li J., et al. Metabolism and pharmacokinetics of 8-hydroxypiperidinylmethyl-baicalein (BA-j) as a novel selective CDK1 inhibitor in monkey. Fitoterapia. 2015 Dec;107:36–43. doi: 10.1016/j.fitote.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Lei C., Yu Y., Zhu Y., Li Y., Ma C., Ding L., et al. The most recent progress of baicalein in its anti-neoplastic effects and mechanisms. Biomed. Pharmacother. 2024 Jul 1;176 doi: 10.1016/j.biopha.2024.116862. [DOI] [PubMed] [Google Scholar]
- 117.Ahmadi A., Mortazavi Z., Mehri S., Hosseinzadeh H. Scutellaria baicalensis and its constituents baicalin and baicalein as antidotes or protective agents against chemical toxicities: a comprehensive review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022 Nov;395(11):1297–1329. doi: 10.1007/s00210-022-02258-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No primary data is associated with this review article.