Abstract
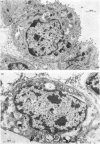
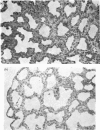
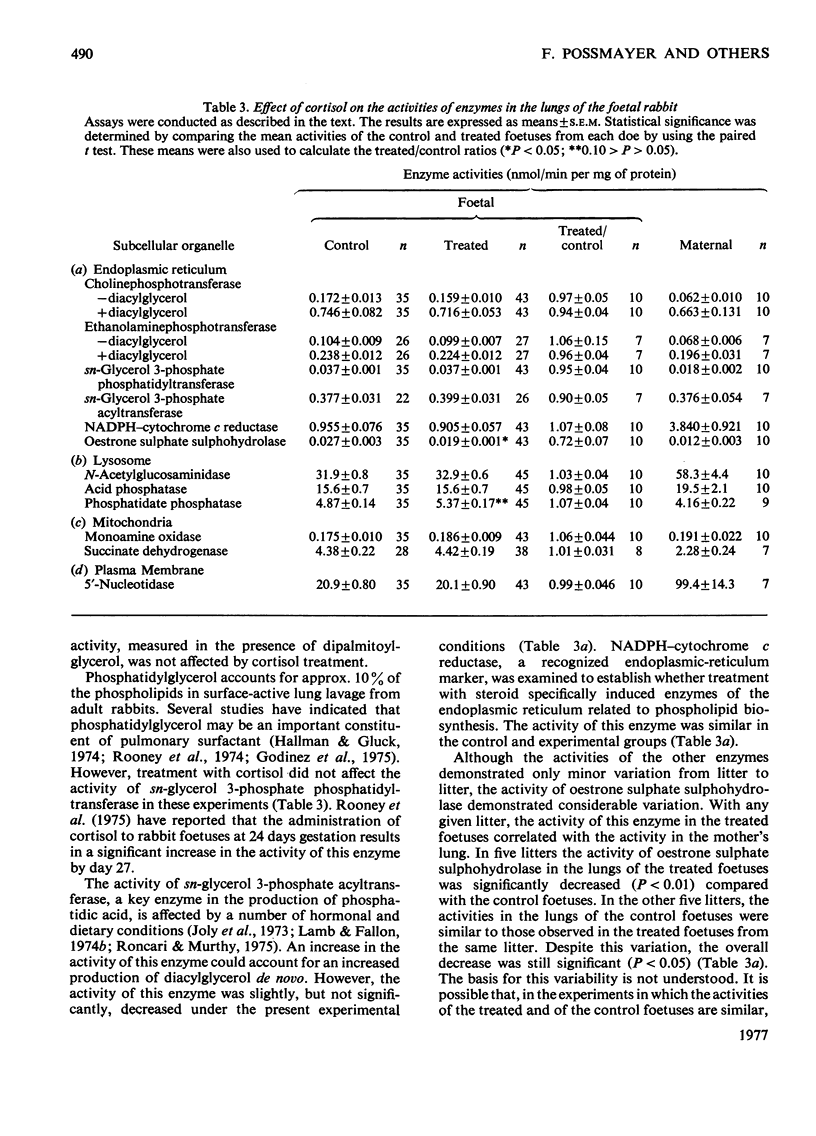
1. Cortisol treatment of rabbit foetuses in utero at 24 days gestation produced a significant decrease in the lung-weight to body-weight ratio compared with littermate controls by day 26. Histological examination revealed that the alveoli of the treated lungs were more open, the walls were thinner and the osmiophilic bodies were more numerous. 2. Cortisol treatment as described above produced significant increases (P<0.05) in the rates of incorporation of [14C]choline into phosphatidylcholine and of [14C]ethanolamine into phosphatidylethanolamine in vitro compared with littermate controls. This indicates that glucocorticoids produce an overall increase in phospholipid metabolism rather than a specific increase in phosphatidylcholine production. 3. The addition of 1,2-diacyl-sn-glycerols from egg phosphatidylcholine produced a 10-fold increase in the activity of choline phosphotransferase and a 3-fold increase in the activity of ethanolamine phosphotransferase in rabbit lung homogenates. The addition of 1,2-dipalmitoyl-sn-glycerol did not affect these activities. These results demonstrate that in the presence of exogenous 1,2-dipalmitoyl-sn-glycerol, the activities of these enzymes are dependent on the presence of endogenous 1,2-diacylglycerols. 4. Cortisol administration had no significant effect on the activity of choline phosphotransferase or ethanolamine phosphotransferase with endogenous or exogenously added diacylglycerols. The activities of other endoplasmic-reticulum enzymes (sn-glycerol 3-phosphate phosphatidyltransferase, sn-glycerol 3-phosphate acyltransferase and NADPH–cytochrome c reductase) were not significantly altered by the hormone administration. Oestrone sulphate sulphohydrolase activity was significantly decreased (P<0.05) by cortisol injection, but this effect varied with the foetuses from different does. 5. Cortisol administration had no effect on the activities of mitochondrial (monoamine oxidase, succinate dehydrogenase), plasma-membrane (5′-nucleotidase) or lysosomal (acid phosphatase, N-acetyl-β-d-glucosaminidase) enzymes. The activity of membrane-associated phosphatidate phosphohydrolase, an enzyme associated with the osmiophilic granules of the type-II alveolar cells, was increased in the lungs of treated foetuses, but the difference was not significant (0.10>P>0.05).
Full text
PDF



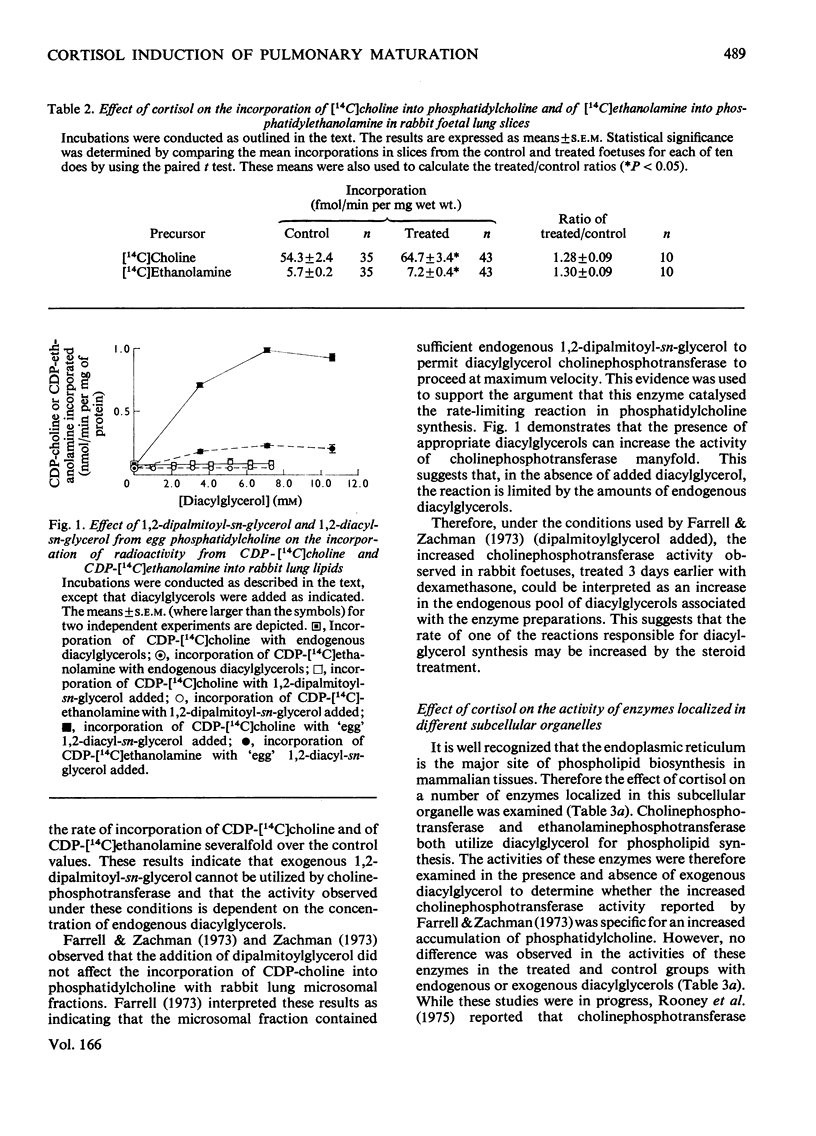




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmann D. W., Bachmann E., Orme-Johnson N., Tan W. C., Green D. E. Membrane systems of mitochondria. VI. Membranes of liver mitochondria. Arch Biochem Biophys. 1968 Jun;125(3):981–1012. doi: 10.1016/0003-9861(68)90537-7. [DOI] [PubMed] [Google Scholar]
- Anfinsen C. B. The formation and stabilization of protein structure. Biochem J. 1972 Jul;128(4):737–749. doi: 10.1042/bj1280737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENDALL D. S., DE DUVE C. Tissue-fractionation studies. 14. The activation of latent dehydrogenases in mitochondria from rat liver. Biochem J. 1960 Mar;74:444–450. doi: 10.1042/bj0740444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. C., Leithauser G., De Loecker W. C., De Wever F. In vitro stimulation of protein synthesis in uterine microsomal supernatant by estrone sulfate. Endocrinology. 1969 Apr;84(4):901–907. doi: 10.1210/endo-84-4-901. [DOI] [PubMed] [Google Scholar]
- Brown B. J., Gabert H. A., Stenchever M. A. Respiratory distress syndrome, surfactant biochemistry, and acceleration of fetal lung maturity: a review. Obstet Gynecol Surv. 1975 Feb;30(2):71–90. doi: 10.1097/00006254-197502000-00001. [DOI] [PubMed] [Google Scholar]
- Carson S. H., Taeusch H. W., Jr, Avery M. E. Inhibition of lung cell division after hydrocortisone injection into fetal rabbits. J Appl Physiol. 1973 May;34(5):660–663. doi: 10.1152/jappl.1973.34.5.660. [DOI] [PubMed] [Google Scholar]
- Coleman R. Phosphatidate phosphohydrolase activity in liver cell surface membranes. Biochim Biophys Acta. 1968 Aug;163(1):111–113. doi: 10.1016/0005-2736(68)90039-4. [DOI] [PubMed] [Google Scholar]
- Cotterrell M., Balázs R., Johnson A. L. Effects of corticosteroids on the biochemical maturation of rat brain: postnatal cell formation. J Neurochem. 1972 Sep;19(9):2151–2167. doi: 10.1111/j.1471-4159.1972.tb05124.x. [DOI] [PubMed] [Google Scholar]
- DeLemos R. A., Shermeta D. W., Knelson J. H., Kotas R., Avery M. E. Acceleration of appearance of pulmonary surfactant in the fetal lamb by administration of corticosteroids. Am Rev Respir Dis. 1970 Sep;102(3):459–461. doi: 10.1164/arrd.1970.102.3.459. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974 Jan 25;249(2):584–593. [PubMed] [Google Scholar]
- Farrell P. M., Avery M. E. Hyaline membrane disease. Am Rev Respir Dis. 1975 May;111(5):657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- Farrell P. M., Zachman R. D. Induction of choline phosphotransferase and lecithin synthesis in the fetal lung by corticosteroids. Science. 1973 Jan 19;179(4070):297–298. doi: 10.1126/science.179.4070.297. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G., Mulay S., Solomon S. Glucocorticoid receptors in lung. II. Specific binding of glucocorticoids to nuclear components of rabbit fetal lung. J Biol Chem. 1973 Jul 25;248(14):5016–5023. [PubMed] [Google Scholar]
- Giannopoulos G. Variations in the levels of cytoplasmic glucocorticoid receptors in lungs of various species at different developmental stages. Endocrinology. 1974 Feb;94(2):450–458. doi: 10.1210/endo-94-2-450. [DOI] [PubMed] [Google Scholar]
- Godinez R. I., Sanders R. L., Longmore W. J. Phosphatidylglycerol in rat lung. I. Identification as a metabolically active phospholipid in isolated perfused rat lung. Biochemistry. 1975 Feb 25;14(4):830–834. doi: 10.1021/bi00675a029. [DOI] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Goldfine H. Use of a filter-paper disk assay in the measurement of lipid biosynthesis. J Lipid Res. 1966 Jan;7(1):146–149. [PubMed] [Google Scholar]
- HAYNES R. C., Jr, MIKHAIL G., ERIKSSON G., WIQVIST N., DICZFALUSY E. OESTRADIOL METABOLISM IN THE PREVIABLE HUMAN FOETUS AND IN THE FOETO-PLACENTAL UNIT. Acta Endocrinol (Copenh) 1964 Feb;45:297–320. doi: 10.1530/acta.0.0450297. [DOI] [PubMed] [Google Scholar]
- Hallman M., Gluck L. Phosphatidyl glycerol in lung surfactant. 1. Synthesis in rat lung microsomes. Biochem Biophys Res Commun. 1974 Sep 9;60(1):1–7. doi: 10.1016/0006-291x(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Hendry A. T., Possmayer F. Pulmonary phospholipid biosynthesis. Properties of a stable microsomal glycerophosphate acyltransferase preparation from rabbit lung. Biochim Biophys Acta. 1974 Nov 18;369(2):156–172. doi: 10.1016/0005-2760(74)90249-5. [DOI] [PubMed] [Google Scholar]
- ISSELBACHER K. J. Enzymatic mechanisms of hormone metabolism. II. Mechanism of hormonal glucuronide formation. Recent Prog Horm Res. 1956;12:134-46; discussion, 146-51. [PubMed] [Google Scholar]
- Jimenez J. M., Schultz F. M., MacDonald P. C., Johnston J. M. Fetal lung maturation. Gynecol Invest. 1974;5(5-6):245–251. doi: 10.1159/000301657. [DOI] [PubMed] [Google Scholar]
- Joly J. G., Feinman L., Ishii H., Lieber C. S. Effect of chronic ethanol feeding on hepatic microsomal glycerophosphate acyltransferase activity. J Lipid Res. 1973 May;14(3):337–343. [PubMed] [Google Scholar]
- KIYASU J. Y., PIERINGER R. A., PAULUS H., KENNEDY E. P. The biosynthesis of phosphatidylglycerol. J Biol Chem. 1963 Jul;238:2293–2298. [PubMed] [Google Scholar]
- Kikkawa Y., Kaibara M., Motoyama E. K., Orzalesi M. M., Cook C. D. Morphologic development of fetal rabbit lung and its acceleration with cortisol. Am J Pathol. 1971 Aug;64(2):423–442. [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Yoneda K. The type II epithelial cell of the lung. I. Method of isolation. Lab Invest. 1974 Jan;30(1):76–84. [PubMed] [Google Scholar]
- Kotas R. V., Avery M. E. Accelerated appearance of pulmonary surfactant in the fetal rabbit. J Appl Physiol. 1971 Mar;30(3):358–361. doi: 10.1152/jappl.1971.30.3.358. [DOI] [PubMed] [Google Scholar]
- Kotas R. V., Fletcher B. D., Torday J., Avery M. E. Evidence for independent regulators of organ maturation in fetal rabbits. Pediatrics. 1971 Jan;47(1):57–64. [PubMed] [Google Scholar]
- Kwun J. K., Emmens C. W. Hormonal requirements for implantation and pregnancy in the ovariectomized rabbit. Aust J Biol Sci. 1974 Jun;27(3):275–283. doi: 10.1071/bi9740275. [DOI] [PubMed] [Google Scholar]
- Köttgen E., van Golde L. M. Selective utilization of endogenous unsaturated phosphatidylcholines and diacylglycerols by cholinephosphotransferase of mouse lung microsomes. Biochim Biophys Acta. 1976 Sep 27;441(3):423–432. doi: 10.1016/0005-2760(76)90239-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. An enzymatic explanation for dietary induced alterations in hepatic glycerolipid metabolism. Biochim Biophys Acta. 1974 Apr 26;348(1):179–188. doi: 10.1016/0005-2760(74)90104-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Glycerolipid formation from sn-glycerol-3-phosphate by rat liver cell fractions. The role of phosphatidate phosphohydrolase. Biochim Biophys Acta. 1974 Apr 26;348(1):166–178. doi: 10.1016/0005-2760(74)90103-9. [DOI] [PubMed] [Google Scholar]
- Liggins G. C., Howie R. N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–525. [PubMed] [Google Scholar]
- Liggins G. C. Premature delivery of foetal lambs infused with glucocorticoids. J Endocrinol. 1969 Dec;45(4):515–523. doi: 10.1677/joe.0.0450515. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meban C. Localization of phosphatidic acid phosphatase activity in granular pneumonocytes. J Cell Biol. 1972 Apr;53(1):249–252. doi: 10.1083/jcb.53.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama E. K., Orzalesi M. M., Kikkawa Y., Kaibara M., Wu B., Zigas C. J., Cook C. D. Effect of cortisol on the maturation of fetal rabbit lungs. Pediatrics. 1971 Oct;48(4):547–555. [PubMed] [Google Scholar]
- Mudd J. B., Van Golde L. M., Van Deenen L. L. Utilization of molecular species of diglycerides in the synthesis of lecithin. Biochim Biophys Acta. 1969 Apr 29;176(3):547–556. doi: 10.1016/0005-2760(69)90221-5. [DOI] [PubMed] [Google Scholar]
- Pack B. A., Brooks S. C. Metabolism of estrogens and their sulfates in rat uterine minces. Endocrinology. 1970 Nov;87(5):924–933. doi: 10.1210/endo-87-5-924. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Balakrishnan G., Strickland K. P. The incorporation of labelled glycerophosphoric acid into the lipids of rat brain preparations. 3. On the biosynthesis of phosphatidyl glycerol. Biochim Biophys Acta. 1968 Sep 2;164(1):79–87. doi: 10.1016/0005-2760(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Duwe G., Hahn M., Buchnea D. Acyl specificity of CDPcholine: 1,2-diacylglycerol cholinephosphotransferase in rat lung. Can J Biochem. 1977 Jun;55(6):609–617. doi: 10.1139/o77-088. [DOI] [PubMed] [Google Scholar]
- Possmayer F., Meiners B., Mudd J. B. Regulation by cytidine nucleotides of the acylation of sn-(14C)glycerol 3-phosphate. Regional and subcellular distribution of the enzymes responsible for phosphatidic acid synthesis de novo in the central nervous system of the rat. Biochem J. 1973 Mar;132(3):381–394. doi: 10.1042/bj1320381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possmayer F., Mudd J. B. The regulation of sn-glycerol-3-phosphate acylation by cytidine nucleotides in rat brain cerebral hemispheres. Biochim Biophys Acta. 1971 Jul 13;239(2):217–233. doi: 10.1016/0005-2760(71)90167-6. [DOI] [PubMed] [Google Scholar]
- Renkonen O. Mono- and dimethyl phosphatidates from different subtypes of choline and ethanolamine glycerophosphatides. Biochim Biophys Acta. 1968 Jan 10;152(1):114–135. doi: 10.1016/0005-2760(68)90014-3. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Murthy V. K. Effects of thyroid hormones on enzymes involved in fatty acid and glycerolipid synthesis. J Biol Chem. 1975 Jun 10;250(11):4134–4138. [PubMed] [Google Scholar]
- Rooney S. A., Canavan P. M., Motoyama E. K. The identification of phosphatidylglycerol in the rat, rabbit, monkey and human lung. Biochim Biophys Acta. 1974 Jul 26;360(1):56–67. doi: 10.1016/0005-2760(74)90179-9. [DOI] [PubMed] [Google Scholar]
- Rooney S. A., Gross I., Gassenheimer L. N., Motoyama E. K. Stimualtion of glycerolphosphate phosphatidyltransferase activity in fetal rabbit lung by cortisol administration. Biochim Biophys Acta. 1975 Sep 19;398(3):433–441. doi: 10.1016/0005-2760(75)90194-0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfaçon R., Possmayer F., Harding P. G. Dexamethasone treatment of the guinea pig fetus: its effects on the incorporation of 3H-thymidine into deoxyribonucleic acid. Am J Obstet Gynecol. 1977 Apr 1;127(7):745–752. doi: 10.1016/0002-9378(77)90250-2. [DOI] [PubMed] [Google Scholar]
- Schultz F. M., Jimenez J. M., MacDonald P. C., Johnston J. M. Fetal lung maturation. I. Phosphatidic acid phosphohydrolase in rabbit lung. Gynecol Invest. 1974;5(4):222–229. [PubMed] [Google Scholar]
- Sedgwick B., Hübscher G. Metabolism of phospholipids. IX. Phosphatidate phosphohydrolase in rat liver. Biochim Biophys Acta. 1965 Jul 7;106(1):63–77. doi: 10.1016/0005-2760(65)90096-2. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Hübscher G. Metabolism of phospholipids. X. Partial purification and properties of a soluble phosphatidate phosphohydrolase from rat liver. Biochim Biophys Acta. 1967 Oct 2;144(2):397–408. [PubMed] [Google Scholar]
- Stoffel W., Schiefer H. G. Biosynthesis and composition of phosphatides in outer and inner mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1017–1026. doi: 10.1515/bchm2.1968.349.2.1017. [DOI] [PubMed] [Google Scholar]
- WILGRAM G. F., KENNEDY E. P. INTRACELLULAR DISTRIBUTION OF SOME ENZYMES CATALYZING REACTIONS IN THE BIOSYNTHESIS OF COMPLEX LIPIDS. J Biol Chem. 1963 Aug;238:2615–2619. [PubMed] [Google Scholar]
- Wang N. S., Kotas R. V., Avery M. E., Thurlbeck W. M. Accelerated appearance of osmiophilic bodies in fetal lungs following steroid injection. J Appl Physiol. 1971 Mar;30(3):362–365. doi: 10.1152/jappl.1971.30.3.362. [DOI] [PubMed] [Google Scholar]
- Weichsel M. E., Jr Glucocorticoid effect upon thymidine kinase in the developing cerebellum. Pediatr Res. 1974 Oct;8(10):843–847. doi: 10.1203/00006450-197410000-00005. [DOI] [PubMed] [Google Scholar]
- Wu B., Kikkawa Y., Orzalesi M. M., Motoyama E. K., Kaibara M., Zigas C. J., Cook C. D. The effect of thyroxine on the maturation of fetal rabbit lungs. Biol Neonate. 1973;22(3):161–168. doi: 10.1159/000240550. [DOI] [PubMed] [Google Scholar]
- Zachman R. D. The enzymes of lecithin biosynthesis in human newborn lungs. 3. Phosphorylcholine glyceride transferase. Pediatr Res. 1973 Jul;7(7):632–637. doi: 10.1203/00006450-197307000-00006. [DOI] [PubMed] [Google Scholar]