Abstract
Aim:
Vaginitis and other vaginal discharge syndromes lead to high healthcare utilization. Molecular tests like syndromic multiplex real-time (RT) polymerase chain reaction (PCR)-based tests are highly sensitive and specific at diagnosing the infectious causes of vaginitis. This study compared the healthcare resource utilization (HCRU) and direct all-cause healthcare costs among patients with vaginitis in the US receiving next-day syndromic multiplex RT-PCR tests with those receiving other PCR tests or no diagnostic test of interest.
Patients & methods:
This retrospective study utilized claims data from IQVIA PharMetrics® Plus database to identify adult patients with a diagnosis for vaginitis (first claim = index) from January 2021 to April 2023, with 6 months of continuous enrollment prior to (baseline) and after index (follow-up). Pairwise comparisons were conducted between RT-PCR and 1:1 propensity matched Other PCR and No Test subcohorts for all-cause HCRU and costs during follow-up.
Results:
Each of the RT-PCR, Other PCR and No Test subcohorts included 1946 matched patients. Mean(SD) follow-up total cost was significantly lower for the RT-PCR than the No Test subcohort ($5607 [$15,122] vs $6680 [$20,751], p = 0.0023). Mean(SD) overall outpatient and other medical service costs were lower for RT-PCR versus Other PCR (outpatient: $2964 [$9666] vs $3174 [$7113], p = 0.0110; other medical: $1961 [$9244] vs $2099 [$6475], p = 0.0002) and No Test subcohorts (outpatient: $2964 [$9666] vs $4067 [$12,341], p < 0.0001; other medical: $1961 [$9244] vs $2973 [$11,685]; p < 0.0001). A lower proportion had any outpatient service HCRU in RT-PCR versus Other PCR subcohort (92.6% vs 94.2%, p = 0.0349). A lower proportion had any other medical service claim in RT-PCR versus Other PCR (78.3% vs 83.2%, p < 0.0001) and No Test subcohorts (78.3% vs 83.0%, p = 0.0001). Physician office, emergency room (ER), prescription use and costs were similar between the subcohorts.
Conclusion:
The use of syndromic multiplex RT-PCR diagnostics with next day test results in patients with vaginitis was associated with lower outpatient costs and total healthcare costs than those in the no test cohort over 6 months. These findings indicate that use of syndromic multiplex RT-PCR diagnostics may contribute to improved patient management compared with clinical diagnosis alone.
Keywords: diagnostics, healthcare costs, healthcare utilization, infectious disease, NAAT, PCR testing, real-world data, vaginitis, women's health
Plain language summary
Healthcare use and costs in women with vaginitis after diagnostic testing
What is this article about?
Vaginitis accounts for over 10 million office visits each year. Time to results from traditional diagnostic testing methods vary from 15 minutes to as long as 7 days.
Molecular tests including multiplex real-time (RT)-PCR tests can offer faster and more accurate results, but their economic impact has not been assessed.
What were the results?
Adult women with vaginitis who received next-day results from multiplex RT-PCR tests had significantly lower mean total and outpatient costs compared with those who received no test and fewer outpatient services compared with those who did not receive any test.
Patients with multiplex RT-PCR also had lower outpatient service costs and lower utilization of outpatient and other medical services than those receiving another PCR test or those who had no diagnostic testing performed.
What do the results mean?
Using multiplex RT-PCR tests for diagnosis of vaginitis leads to lower total costs than not using any tests at all. Also, use of RT-PCR tests is associated with lower utilization and costs for any outpatient service compared with other PCR or no tests during the 6 months after diagnosis.
Background
Vaginal discharge syndromes, attributed to vaginitis and sexually transmitted infections (STIs) are highly prevalent conditions, affecting over 21 million women in the US [1,2]. While attributed to distinct infectious etiologies, symptoms are often similar between vaginitis and STIs, characterized as itching, burning, irritation, vaginal odor, and/or abnormal discharge. Vaginitis is most commonly attributed to three infectious etiologies, bacterial vaginosis (BV) (accounting for 40 to 50% of cases), vulvovaginal candidiasis (VVC) (accounting for 20–25% of cases) and trichomoniasis (TV) (accounting for 15–20% of cases, but incidence can be variable) [1–3]. Although less common, vaginitis symptoms may also be attributed to aerobic vaginitis (AV), desquamative inflammatory vaginosis (DIV) and cytolytic vaginosis (CV) that result from aerobic imbalance of the vaginal microbiota. The incidence of recurrence of BV, VVC and TV is estimated to be high due to insufficient management, inaccurate diagnosis, or continued exposure [3–5]. Together, the high recurrence of these infections contributes to high antibiotic and antifungal use and high outpatient utilization rates, highlighting the need for appropriate and timely diagnosis and prompt initiation of targeted treatment [6–9].
Vaginal discharge syndromes, hereafter referred to as vaginitis, result in substantial healthcare resource utilization (HCRU) and costs, accounting for 10 million office visits and over $1.3 billion spent annually in the US alone [4,10]. A recent study found the average total all-cause follow-up for patients with BV was $8987 per year and as high as $10,968 if the infection was unresolved with initial treatment [11]. Further, the researchers found total costs increased, and the proportion of those costs that were BV-related increased stepwise, as the number of treatment courses increased, underscoring the need for an accurate diagnosis at onset [11]. Traditional methods used by clinicians to diagnose vaginitis include pH testing, potassium hydroxide (KOH) preparation (whiff test), wet mount microscopy, Amsel, criteria and microbiologic culture [6,12]. While wet mount microscopy and Amsel criteria are inexpensive, easy and quick to perform, their sensitivity can vary based on the infectious cause of symptoms [13]. Notably, wet mount microscopy has been found to have high sensitivity for the diagnosis of BV, AV and DIV, but has been shown to vary for the detection of VVC and TV [14–17]. In contrast, culture methods have high sensitivity and specificity, but the time to result is slow, often taking up to 7 days depending on the etiology of the patients symptoms. Before the adoption of polymerase chain reaction (PCR) tests, microscopy-based wet mount was the most commonly used technique for the diagnosis of vaginal infections from TV due to the ease of performing at the point of care; but the requirement for clinics to purchase and maintain microscopes in office led to reduced use in favor of empiric diagnosis [15]. Due to the high sensitivity and specificity of PCR and the convenience of being able to detect multiple microorganism types without the growth restrictions of culture, PCR based diagnostics have become the gold standard for the diagnosis of TV and other sexually transmitted infections, including Neisseria gonorrhoeae, Chlamydia trachomatis and Mycoplasma genitalium among others. These benefits have begun to demonstrate clinical value for the diagnosis of other vaginitis etiologies, like BV and VVC, when point of care microscopy-based methods may not be available [4,6,18]. Further, in select outpatient healthcare settings where symptoms of vaginitis have substantial overlap with STIs, the capability to run a single diagnostic test for all relevant infectious causes may contribute to more streamline and accurate diagnosis [19–21].
There is limited real-world evidence on the clinical and economic impact of these novel multiplex RT-PCR tests for vaginitis. This study aimed to compare all-cause HCRU and costs following the use of multiplex RT-PCR tests with next day results compared with other commercially available PCR tests or when no test was used among adult women with vaginitis in a nationwide claims database of predominantly commercial health plans in the US.
Methods
Study design
We conducted a retrospective propensity-matched cohort study using the IQVIA PharMetrics® Plus claims database from 1 July 2020 to 31 October 2023 (study period). PharMetrics Plus is a health plan claims database comprised of fully-adjudicated medical and pharmacy claims for more than 210 million unique enrollees since 2006. This database is representative of the commercially-insured US national population for patients under 65 years of age. As a retrospective study using secondary data, no interventions were made to patients during this study. In compliance with the Health Insurance Portability and Accountability Act (HIPAA), patient data included in the analyses were de-identified; therefore, this study was not subject to Institutional Review Board (IRB) review. Analysis of existing and anonymized data falls within the exempt criteria 45 CFR 46.101(b)(4) of HHS regulations for the protection of human subjects in research. The PharMetrics Plus database meets ‘safe harbor’ de-identification standards provided in the Privacy Rule of the U.S. HIPAA Privacy Rule section 164.514.
Cohort selection
Adult women (aged ≥18 years) with ≥1 non-ancillary claims with a diagnosis for or a symptom suggestive of vaginitis, vulvovaginitis, or other inflammation of the vagina from 1 January 2021 to 30 April 2023 were identified. An ancillary claim is diagnostic, where the result may not indicate the patient has the disease; therefore, we did not include these claims to ensure confidence in the diagnoses captured. Supplementary Table 1 provides a list of ICD-10-CM codes related to diagnosis or symptoms for vaginitis. The date of first qualifying claim for vaginitis or related symptoms was considered the index date.
To be included for analysis, women were further required to have 6 months of continuous enrollment in health plans prior to and after index date. The 6-month period prior to the index date was termed as the baseline period, and the 6-month period after the index date was termed as the follow-up period. Patients with missing or invalid age or sex were excluded from the analysis.
These patients were categorized depending on the diagnostic test administered, or lack thereof, on the index date, into three mutually-exclusive subcohorts: 1. Patients who received a syndromic multiplex RT-PCR test for vaginitis and sexually transmitted infectious organisms identified by the Current Procedural Terminology (CPT) /Healthcare Common Procedure Coding System (HCPCS) codes listed in Supplemental Table 2 and the National Provider Identifier (NPI) codes for laboratories that provide this test (NPI: 1689639544, 1326743535, 1619346640, 1881352979, 1790470763) (HealthTrackRx, TX, USA); 2. Patients who received any other commercially available diagnostic PCR test independent of laboratory provider or point of care PCR technology, identified using the same CPT code list as cohort 1 (Other PCR subcohort) and absence of NPI codes pertaining to the RT-PCR test cohort (including; 3. Patients who did not receive any diagnostic test of interest for vaginitis (culture, PCR and select point of care tests) on the index date or within 2 days (No Test subcohort). It is important to note that use of wet mount or Amsel criteria for diagnosis were not excluded from the No Test cohort. Full code lists can be found in Supplementary Table 2.
Measures & outcomes
Patient demographic characteristics such as age, sex and geographic region (US Census region) of residence were assessed on the index date. Clinical comorbidities and utilization of relevant treatments for vaginitis were assessed, as well as all-cause baseline total healthcare costs, during the 6-month baseline period. Charlson Comorbidity Index (CCI) score [22] (continuous and categorical) was measured, along with chronic conditions including cancers, congestive heart failure, hepatitis and renal failure. Baseline comorbidities and proportion of patients with previous diagnosis of vaginitis were captured using the presence of any claim with a relevant ICD-10-CM diagnosis code during the baseline period. Baseline use of antibiotics and antifungals were captured using National Drug Codes (NDC). During the 6-month follow-up period, all-cause total and service-specific (overall outpatient and its subcategories including physician office visits, emergency room [ER] visits, other medical services [e.g., radiology, outpatient surgery, ancillary services and other service claims not classified in other outpatient subcategories] and outpatient prescription medications) HCRU, including proportion of patients using the health resource and number of visits per patients, as well as associated healthcare costs, were assessed/evaluated.
Statistical analysis
Categorical measures were presented using frequency (number of patients [n]) and percentage (%) of total study patients observed in each category. Continuous and count variables were presented as the mean, standard deviation (SD) and median. Standardized mean difference (SMD) was assessed for each variable of interest for the two pairs of pre- and post-matched cohorts. SMD was calculated as the difference in means or proportions of a variable divided by the pooled SD. An SMD of ≥0.10 (absolute) between the pairs of subcohorts indicated imbalance.
Propensity Score Matching
Propensity score matching (PSM) was used to adjust for measured confounders and create matched, comparable subcohorts, consisting of patients in the RT-PCR cohort and the other two comparator subcohorts. Based on the observed imbalances in the demographic and clinical characteristics between each pair of subcohorts as well as considering clinical relevance of the variables, the variables for inclusion in the PSM model were selected (Table 1). Post-match balance was assessed based on a SMD cut-off of <0.1. All-cause HCRU and costs were compared pairwise between subcohorts of interest after matching.
Table 1. . Variables for matching between reverse-transcriptase polymerase chain reaction (RT-PCR) subcohort and matched subcohort of patients receiving other tests and no tests among women with vaginitis.
| Baseline characteristics | Pre-match | Post-match | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT-PCR n = 1964 |
Other PCR test n = 244,224 |
SMD | No test n = 238,362 |
SMD | RT-PCR N = 1964 |
Other PCR test n = 1964 |
SMD | No test n = |
SMD | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |||||
| Age group (years) | ||||||||||||||||
| 18–24 | 383 | 19.7% | 65,347 | 26.8% | 0.4711 | 23,806 | 10.0% | 0.3726 | 383 | 19.7% | 383 | 19.7% | 0.0000 | 383 | 19.7% | 0.0000 |
| 25–34 | 404 | 20.8% | 70,246 | 28.8% | 36,211 | 15.2% | 404 | 20.8% | 404 | 20.8% | 404 | 20.8% | ||||
| 35–44 | 362 | 18.6% | 52,181 | 21.4% | 44,406 | 18.6% | 362 | 18.6% | 362 | 18.6% | 362 | 18.6% | ||||
| 45–54 | 332 | 17.1% | 34,200 | 14.0% | 52,952 | 22.2% | 332 | 17.1% | 332 | 17.1% | 332 | 17.1% | ||||
| 55–64 | 303 | 15.6% | 18,970 | 7.8% | 57,821 | 24.3% | 303 | 15.6% | 303 | 15.6% | 303 | 15.6% | ||||
| ≥65 | 162 | 8.3% | 3280 | 1.3% | 23,166 | 9.7% | 162 | 8.3% | 162 | 8.3% | 162 | 8.3% | ||||
| Geographic region | ||||||||||||||||
| Northeast | 22 | 1.1% | 41,756 | 17.1% | 0.6060 | 36,695 | 15.4% | 0.5748 | 22 | 1.1% | 22 | 1.1% | 0.0000 | 22 | 1.1% | 0.0000 |
| Midwest | 630 | 32.4% | 52,523 | 21.5% | 60,206 | 25.3% | 630 | 32.4% | 630 | 32.4% | 630 | 32.4% | ||||
| South | 1086 | 55.8% | 119,079 | 48.8% | 105,556 | 44.3% | 1086 | 55.8% | 1086 | 55.8% | 1086 | 55.8% | ||||
| West | 208 | 10.7% | 30,866 | 12.6% | 30,866 | 12.6% | 208 | 10.7% | 208 | 10.7% | 208 | 10.7% | ||||
| CCI categories | ||||||||||||||||
| 0 | 1624 | 83.5% | 214,435 | 87.8% | -0.1242 | 198,432 | 83.2% | 0.0055 | 1624 | 83.5% | 1624 | 83.5% | 0.0000 | 1624 | 83.5% | 0.0000 |
| ≥1 | 322 | 16.5% | 29,789 | 12.2% | 39,930 | 16.8% | 322 | 16.5% | 322 | 16.5% | 0.0000 | 322 | 16.5% | 0.0000 | ||
| Presence of diabetes | 115 | 5.9% | 6987 | 2.9% | 0.1493 | 13,423 | 5.6% | 0.0119 | 115 | 5.9% | 115 | 5.9% | 0.0000 | 115 | 5.9% | 0.0000 |
| Presence of dyslipidemia | 202 | 10.4% | 13,803 | 5.7% | 0.1748 | 27,850 | 11.7% | -0.0416 | 202 | 10.4% | 202 | 10.4% | 0.0000 | 202 | 10.4% | 0.0000 |
| Use of any antibiotic | 542 | 27.9% | 53,727 | 22.0% | 0.1357 | 51,355 | 21.5% | 0.1466 | 542 | 27.9% | 542 | 27.9% | 0.0000 | 542 | 27.9% | 0.0000 |
| Presence of a previous infection | 73 | 3.8% | 11,393 | 4.7% | -0.0455 | 11,353 | 4.8% | -0.0501 | 73 | 3.8% | 73 | 3.8% | 0.0000 | 73 | 3.8% | 0.0000 |
CCI: Charlson comorbidity index; PCR: Polymerase chain reaction; RT-PCR: Real time-PCR; SMD: Standardized mean difference.
Among the 1,607,248 patients with ≥1 claim with ICD-10 CM diagnosis for vaginitis or relevant symptoms, 1946 women were classified in the pre-match RT-PCR subcohort and 244,224 were classified in the Other PCR subcohort. Of the 1,191,806 women with vaginitis who did not receive any diagnostic tests on the index date or within two days after index, a random 20% sample of 238,362 were classified into the pre-match No Test cohort (Figure 1). The RT-PCR subcohort was matched with the Other PCR subcohort, and separately matched with the No Test subcohort, to create two pairs of matched subcohorts with 1946 patients in each.
Figure 1. . Stepwise attrition to identify patients in each of the three subcohorts of interest.
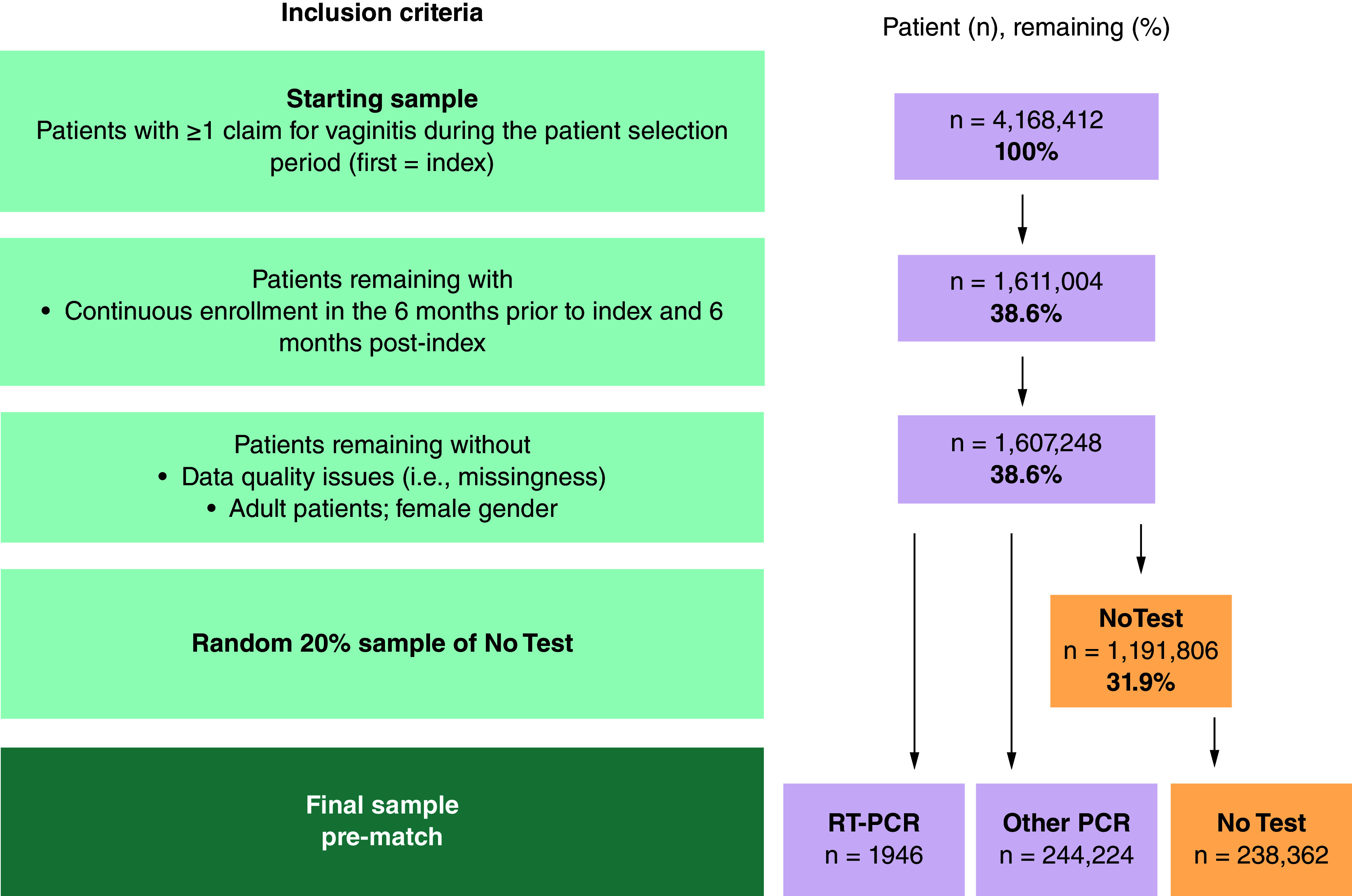
Following PSM, weighted chi-square tests were used to compare categorical and weighted t-tests (mean) or Wilcoxon rank-sum test (median) for continuous outcomes. For comparisons between infrequent independent samples (e.g., among patients with 1 hospitalization), parametric t-test (mean) and non-parametric Wilcoxon rank-sum test (median) were used, as matching no longer applied within subgroups of matched cohorts. A p-value of ≤0.05 was considered statistically significant. All analysis were performed using SAS 9.3 (SAS Institute, NC, USA).
Results
Patient characteristics
Prior to matching, patients in the Other PCR subcohort were, on average, younger (34.9 ± 12.6 years) than the RT-PCR subcohort (41.4 ± 16.4 years). The No Test subcohort was older at 46.6 ± 15.4 years than the RT-PCR subcohort. In all subcohorts, most patients were from the South US region (US Census regions were used to define regions for this study). The mean baseline CCI score in the RT-PCR subcohort was 0.5 ± 1.4 and 0.5 ± 1.5 for the No Test subcohort, and 0.4 ± 1.3 in the Other PCR subcohort. After matching, the average age was approximately 41 years in both sets of matched cohorts. The mean (SD) baseline CCI was 0.5 (1.4), and 3.8% of patients had a previous infection (Table 1).
HCRU & costs in the 6-month follow-up: RT-PCR versus no test
When comparing patients in the RT-PCR subcohort to the patients in the No test subcohort, the RT-PCR subcohort had fewer outpatient services during the 6-month follow-up period (visits per patient: 14.5 vs 16.1, p < 0.0001) (Supplemental Table 3). However, there was no significant difference in the proportion of patients requiring any outpatient service (92.6% vs 93.6%, p = 0.1986) (Figure 2). RT-PCR subcohort had significantly lower mean total outpatient costs ($2964 [$9666] vs $4067 [$12,341], p < 0.0001) (Table 2) than the No Test subcohort. This was driven by a significantly lower proportion of patients in the RT-PCR subcohort with other medical services (78.3% vs 83.0%, p = 0.0001; visits per patient: 8.0 vs 9.2, p < 0.0001) with corresponding lower costs (1961 [$9244] vs $2973 [$11,685], p < 0.0001). A similar proportion of patients in each subcohort had a physician office visit (89.9% vs 90.7%, p = 0.4111), an ER visit (15.1% vs 16.5%, p = 0.2360) and an outpatient pharmacy claim (91.0% vs 89.2%, p = 0.0580).
Figure 2. . Utilization of outpatient medical services in patients with vaginitis during the 6 months following diagnosis for reverse transcriptase polymerase chain reaction (RT-PCR) versus No Test subcohorts.
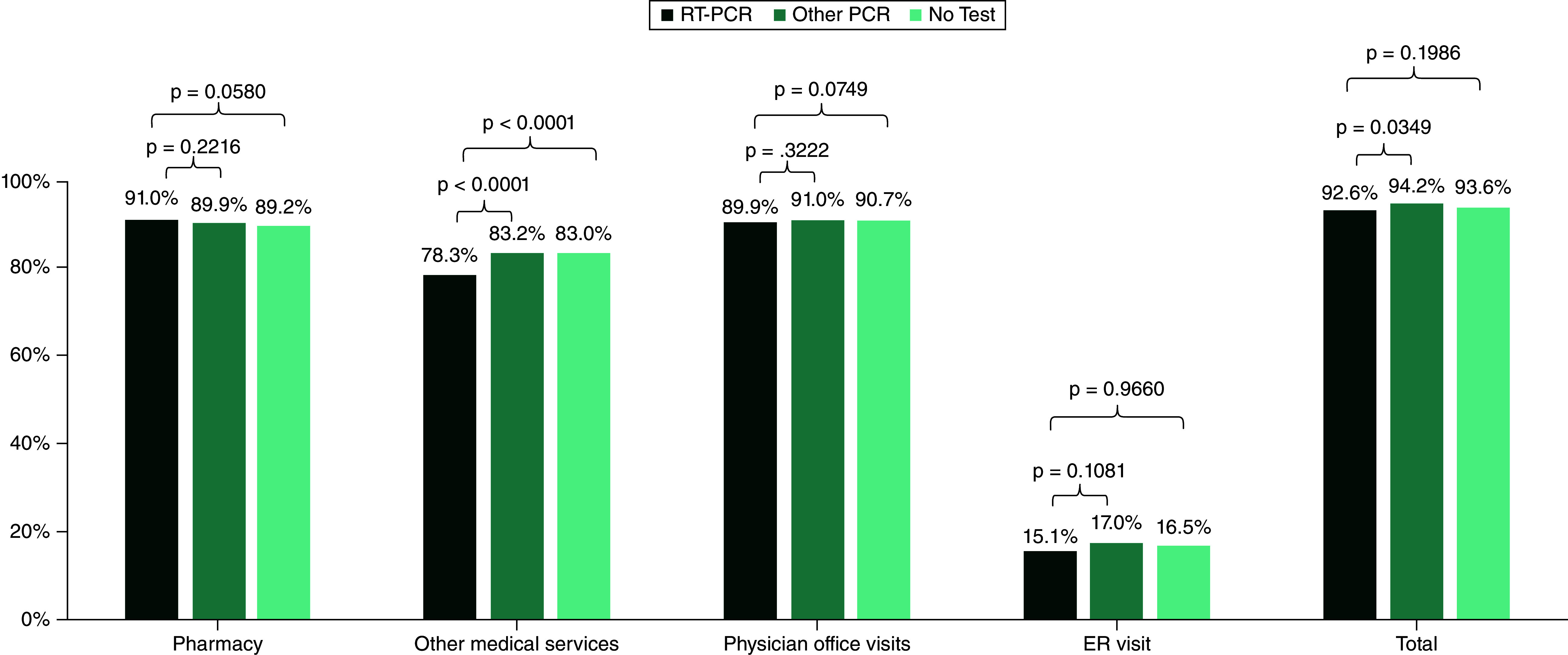
RT-PCR: Real-time polymerase chain reaction.
Table 2. . Healthcare costs in patients with vaginitis during the 6 months following diagnosis among matched RT-PCR versus No Test subcohorts.
| Utilization category | RT-PCR n = 1946 |
No Test n = 1946 |
p-value |
|---|---|---|---|
| Outpatient prescription | |||
| Mean (SD) | $1686 ($6639) | $1319 ($5898) | 0.9805 |
| Median | $163 | $167 | |
| Physician office visits | |||
| Mean (SD) | $725 ($1071) | $779 ($1301) | 0.0749 |
| Median | $397 | $435 | |
| ER visits | |||
| Mean (SD) | $278 ($1044) | $315 ($1290) | 0.9660 |
| Median | $0 | $0 | |
| Other medical services | |||
| Mean (SD) | $1961 ($9244) | $2973 ($11,685) | <0.0001 |
| Median | $219 | $346 | |
| Outpatient | |||
| Mean (SD) | $2964 ($9666) | $4067 ($12,341) | <0.0001 |
| Median | $845 | $1120 | |
| Total Healthcare (medical + pharmacy) | |||
| Mean (SD) | $5607 ($15,122) | $6680 ($20,751) | 0.0023 |
| Median | $1363 | $1675 | |
ER: Emergency room; PCR: Polymerase chain reaction; RT-PCR: Real time-PCR; SD: Standard deviation.
HCRU & costs in the 6-month follow-up: RT-PCR versus other PCR test
When comparing patients in the RT-PCR subcohort to the patients that received Other PCR testing, the RT-PCR subcohort required outpatient services at a lower rate (92.6% versus 94.2%, p = 0.0349 (Figure 2); visits per patient: 14.5 [21.9] versus 15.3 [17.6], p = 0.0011). While this finding shows a small reduction, this difference is statistically significant despite the high degree of variability observed in claims data. Further, the RT-PCR cohort slightly lower outpatient costs compared with the Other PCR cohort ($2964 [$9666] vs $3174 [$7113], p = 0.0110) (Table 3). This was driven by a significantly lower proportion of patients in the RT-PCR subcohort with other medical services (78.3% vs 83.2%, p < 0.0001; visits per patient: 8.0 [17.8] vs 8.7 [12.6], p < 0.0001) and corresponding lower mean costs due to other medical services ($1961 [$9244] vs $2099 [$6,475], p = 0.0002). Both subcohorts had a physician office visit, an ER visit, or outpatient pharmacy use at similar rates (office visit: 89.9% vs 91.0%, p = 0.2470; ER visit: 15.1% vs 17.0%, p = 0.1081; pharmacy 91.0% vs 89.9%, p = 0.2216) (Figure 2). The mean total healthcare costs were similar between subcohorts of RT-PCR and Other PCR tests ($5607 [$15,122]; $5773 [$25,112], p = 0.3057).
Table 3. . Healthcare costs in patients with vaginitis during the 6 months following diagnosis among matched reverse transcriptase polymerase chain reaction (RT-PCR) versus Other PCR subcohorts.
| Utilization category | RT-PCR n = 1946 |
Other PCR test n = 1946 |
p-value |
|---|---|---|---|
| Outpatient prescription | |||
| Mean (SD) | $1686 ($6639) | $1903 ($21,640) | 0.6568 |
| Median | $163 | $180 | |
| Physician office visits | |||
| Mean (SD) | $725 ($1071) | $751 ($1082) | 0.3222 |
| Median | $397 | $429 | |
| ER visits | |||
| Mean (SD) | $278 ($1044) | $325 ($1217) | 0.3048 |
| Median | $0 | $0 | |
| Other medical services | |||
| Mean (SD) | $1961 ($9244) | $2099 ($6475) | 0.0002 |
| Median | $219 | $310 | |
| Outpatient | |||
| Mean (SD) | $2964 ($9666) | $3174 ($7113) | 0.0110 |
| Median | $845 | $1033 | |
| Total Healthcare (medical + pharmacy) | |||
| Mean (SD) | $5607 ($15,122) | $5773 ($25,112) | 0.3057 |
| Median | $1363 | $1560 | |
ER: Emergency room; PCR: Polymerase chain reaction; RT-PCR: Real time-PCR; SD: Standard deviation.
Discussion
Our study aimed to evaluate all-cause HCRU and costs for vaginitis with different diagnostic approaches. Overall, patients receiving syndromic multiplex RT-PCR diagnostic test with next-day results had significantly lower mean total all-cause healthcare (medical + pharmacy) costs than patients who received no test. This finding suggests downstream benefits of more accurate and prompt diagnosis. However, further research leveraging retrospective medical records or prospective comparator testing is needed to determine this.
In our study, patients who received a RT-PCR vaginitis test experienced a small but significant lower utilization of any outpatient services as well as other medical services compared with those who received another PCR test. While this finding does show significance despite the high degree of variability observed in claims analyses, both cohorts receiving any type of PCR testing showed substantial reductions in HCRU and cost compared with patients diagnosed on clinical presentation alone. This finding could indicate a more accurate diagnosis when PCR testing is utilized, subsequently leading to lower HCRU for outpatient and other medical services [23]. It is important to note that the other PCR cohort did not specify the manufacturer or laboratory provider and were identified by CPT/HCPCS code in the analysis. While the proportion of patients using outpatient services were similar in the RT-PCR and No Test subcohorts, the mean number of visits per patient was significantly lower in the RT-PCR subcohort, suggesting a reduced need for follow-up visits when the multiplex RT-PCR test was used. In line with these findings, patients receiving RT-PCR tests with next-day results had significantly lower mean outpatient costs than the Other PCR and No Test subcohorts driven by lower utilization of other medical services claims and associated costs. This is in alignment with recent findings from a retrospective study on vaginitis using administrative claims, which found patients who had a nucleic acid amplification test (including PCR tests) had lower mean total costs in the 12 months following diagnosis compared with clinical evaluation ($7660 vs $8232, p < 0.0001) [10]. Their estimation of mean total cost of $8232 for those patients who were diagnosed with clinical evaluation alone during the 12-months post-diagnosis was higher than our assessment, which was expected as the follow-up period was longer. However, those cost trends generally aligned with our study.
As the scientific landscape continues to evolve, molecular diagnostic tests, including syndromic multiplex RT-PCR tests, are becoming more readily available to diagnose multiple different infectious etiologies of vaginitis with a single test. These and other PCR tests may be able to provide more accurate diagnosis with a faster turnaround time compared with non-molecular based test options. Of those patients receiving a PCR test, less than 1% received the RT-PCR test, which is expected since this test is only administered by select laboratories. To our knowledge, there is no published evidence on the comparative effectiveness of syndromic multiplex RT-PCR diagnostics with next-day results, compared with alternatives, on subsequent HCRU and costs among patients with vaginitis. This study offers a novel evaluation of the cost of multiplex RT-PCR using real-world data, with the potential to positively benefit thousands of patients in the future.
The findings from this study should be interpreted with some limitations. Administrative claims are developed for billing purposes and, hence, some conditions may be undercoded (meaning that some information may be missing from claims analysis). All patients in this analysis had continuous enrollment in a health plan; therefore, these results may not be generalizable to an uninsured population. Another potential limitation is the accuracy of coding detail to reflect the severity of the patient's condition. In other words, symptom severity cannot be directly determined from claims analysis. Finally, identification of specific molecular tests in claims data by CPT/HCPCS code alone can be challenging due to the use of non-specific coding, and as such specific conclusions on the impact of specific commercially available tests within the Other PCR cohort cannot be concluded from this analysis. Further, evidence of prescription claims does not necessarily imply the consumption of medication by the patient, only that the prescription was filled. In addition, any medication obtained outside insurance coverage (i.e., free samples, discount cards, from another country, etc.) and their associated costs were not captured in the analysis. It should be noted that this study utilized PharMetrics Plus adjudicated claims database which is representative of the commercially insured US population aged <65 years. These results cannot be generalized to all US population or to any other countries.
Conclusion
Syndromic multiplex RT-PCR testing with next-day results is a valuable tool for physicians for the diagnosis of vaginitis, allowing for rapid and accurate results and possibly contributing to more targeted treatment after diagnosis. This study demonstrated the use of a novel multiplex RT-PCR test for vaginitis significantly lowers the total cost of follow-up care during the 6 months after the initial visit, compared with patients receiving no diagnostic tests. These findings demonstrate the clinical value this multiplex RT-PCR test offers practitioners, as timely and accurate results allow for selection of the most appropriate treatment resulting in improved patient outcomes.
Summary points
Vaginitis is a common reason for physician office visits and is responsible for over $1.4 billion dollars annually in the US.
Adoption of multiplex polymerase chain reaction (PCR) testing for the diagnosis of vaginal discharge infections is becoming more widespread due to the rapid time to result and the convenience of testing for multiple infectious causes with a single specimen collection. While select traditional diagnostic tests continue to offer reasonable sensitivity and specificity for diagnosis, providers are trending away from frequent use due to the equipment requirements.
This study was undertaken to assess the long-term cost differential between using select multiplex real time (RT)-PCR, other PCR tests and when no diagnostic test was utilized in women with vaginal discharge syndromes.
In this study, we found among women with a vaginitis diagnosis approximately 75% were not tested for vaginitis. When evaluating costs among patients with vaginitis, those that utilized the novel multiplex RT-PCR test had fewer outpatient services and lower outpatient costs compared with those that used a different PCR test or no PCR test.
Molecular techniques for the diagnosis of vaginitis are a valuable tool for physicians, allowing for better patient care with rapid and accurate results and consequently, earlier treatment.
These results may guide decision-makers in developing coverage and reimbursement policies.
Supplementary Material
Acknowledgments
The authors would like to thank K Lovett of IQVIA Inc. for her assistance in manuscript preparation and editorial support.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://bpl-prod.literatumonline.com/doi/10.57264/cer-2024-0173
Author contributions
Concept and design: A Evans, J Reddy, R Doshi and J Yeaw. Analysis and interpretation of data: A Evans, J Reddy, R Doshi, J Yeaw, K Coyle, E Wang, S Goldberg, M Fragala. Drafting of the manuscript: R Doshi, J Yeaw and K Coyle. Critical revision of the paper for important intellectual content: A Evans, J Reddy, R Doshi, J Yeaw, K Coyle, E Wang, S Goldberg and M Fragala. Obtaining funding: A Evans and J Reddy. Administrative, technical and logistic support: R Doshi, A Evans and J Reddy.
Financial disclosure
This study was funded by HealthTrackRx, Inc. A Evans, J Reddy, S Goldberg and M Fragala are employees of HealthTrackRx, Inc. R Doshi, J Yeaw, K Coyle and E Wang are employees of IQVIA, which received consultancy fees in connection with this study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
Assistance in manuscript preparation and editorial support was provided by K Lovett of IQVIA Inc.
Ethical conduct of research
In compliance with the Health Insurance Portability and Accountability Act (HIPAA), patient data included in the analyses were de-identified; therefore, this study was not subject to Institutional Review Board (IRB) review.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.Koumans EH, Sternberg M, Bruce C et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex. Transm. Dis. 34(11), 864–869 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Hildebrand JP, Carlson K, Kansagor AT. Vaginitis. In: StatPearls. StatPearls Publishing, FL, USA: (2024). [PubMed] [Google Scholar]
- 3.Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am. Fam. Physician 97(5), 321–329 (2018). [PubMed] [Google Scholar]
- 4.Brown H, Drexler M. Improving the diagnosis of vulvovaginitis: perspectives to align practice, guidelines, and awareness. Popul. Health Manag. 23(Suppl. 1), S3–S12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benedict K, Singleton AL, Jackson BR, Molinari NAM. Survey of incidence, lifetime prevalence, and treatment of self-reported vulvovaginal candidiasis, United States, 2020. BMC Womens Health. 22(1), 147 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou Chacra L, Fenollar F, Diop K. Bacterial vaginosis: what do we currently know? Front. Cell. Infect. Microbiol. 11, 672429 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell AM, Nyirjesy P. Recurrent vulvovaginitis. Best Pract. Res. Clin. Obstet. Gynaecol. 28(7), 967–976 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Eckert LO. Clinical practice. Acute vulvovaginitis. N. Engl. J. Med. 355(12), 1244–1252 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect. Dis. 18(11), e339–e347 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Kong AM, Jenkins D, Troeger KA, Kim G, London RS. Diagnostic testing of vaginitis: improving the value of care. Popul. Health Manag. 24(4), 515–524 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Watkins E, Chow CM, Lingohr-Smith M et al. Treatment patterns and economic burden of bacterial vaginosis among commercially insured women in the USA. J. Comp. Eff. Res. 13(1), e230079 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Diseases characterized by vulvovaginal itching, burning, irritation, odor or discharge. (2021). https://www.cdc.gov/std/treatment-guidelines/vaginal-discharge.htm
- 13.Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex. Transm. Infect. 89(6), 434–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira-Baptista P, Grincevičienė Š, Oliveira C, Fonseca-Moutinho J, Cherey F, Stockdale CK. The International Society for the Study of Vulvovaginal Disease Vaginal Wet Mount Microscopy Guidelines: how to perform, applications, and interpretation. J. Low. Genit. Tract. Dis. 25(2), 172–180 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74, 14–22 (1983). [DOI] [PubMed] [Google Scholar]
- 16.Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am. J. Obstet. Gynecol. 162, 155–160 (1990). [DOI] [PubMed] [Google Scholar]
- 17.Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J. Clin. Microbiol. 51(11), 3875–3876 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simoes JA, Discacciati MG, Brolazo EM, Portugal PM, Dini DV, Dantas MC. Clinical diagnosis of bacterial vaginosis. Int. J. Gynaecol. Obstet. 94(1), 28–32 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Savicheva AM. Molecular testing for the diagnosis of bacterial vaginosis. Int. J. Mol. Sci. 25(1), 449 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwebke JR, Gaydos CA, Davis T et al. Clinical evaluation of the Cepheid Xpert TV Assay for detection of trichomonas vaginalis with prospectively collected specimens from men and women. J. Clin. Microbiol. 56(2), e01091–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwebke JR, Gaydos CA, Nyirjesy P, Paradis S, Kodsi S, Cooper CK. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J. Clin. Microbiol. 56(6), e00252–18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits. 12(4), 188–197 (2019). [PMC free article] [PubMed] [Google Scholar]
- 23.Teymouri M, Mollazadeh S, Mortazavi H et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol. Res. Pract. 221, 153443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


