Abstract
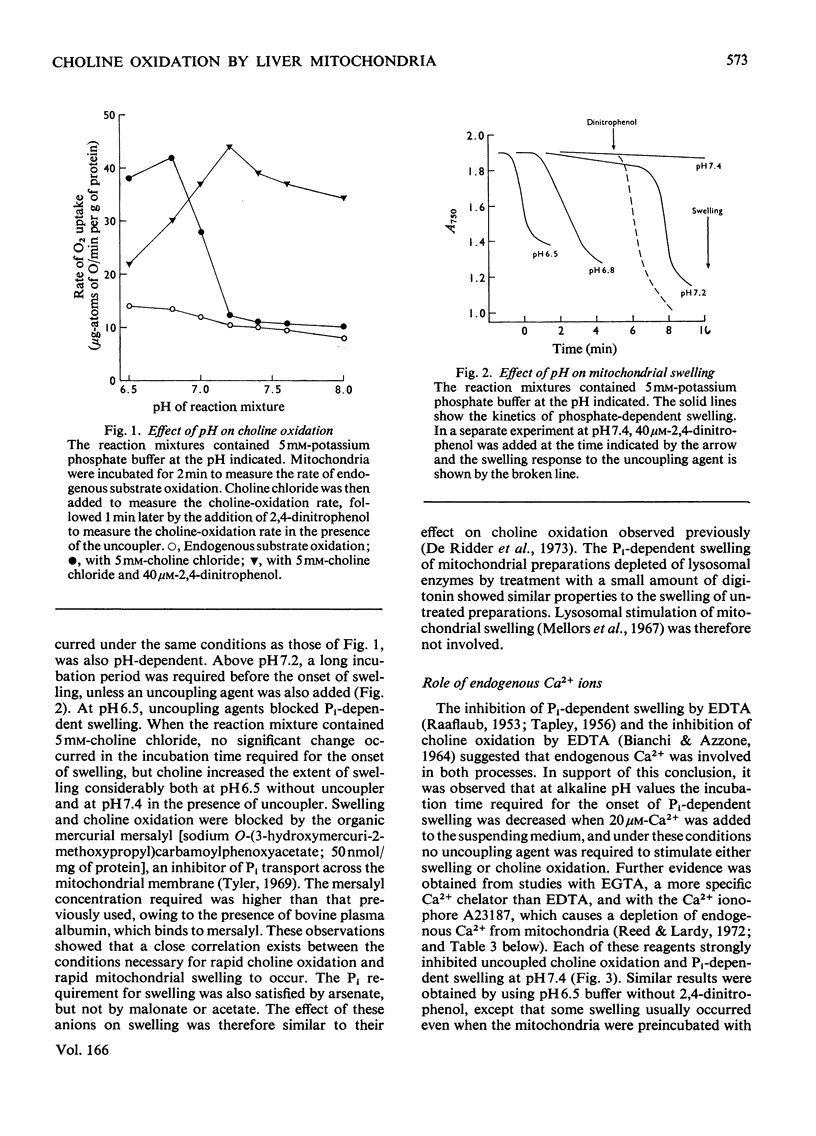
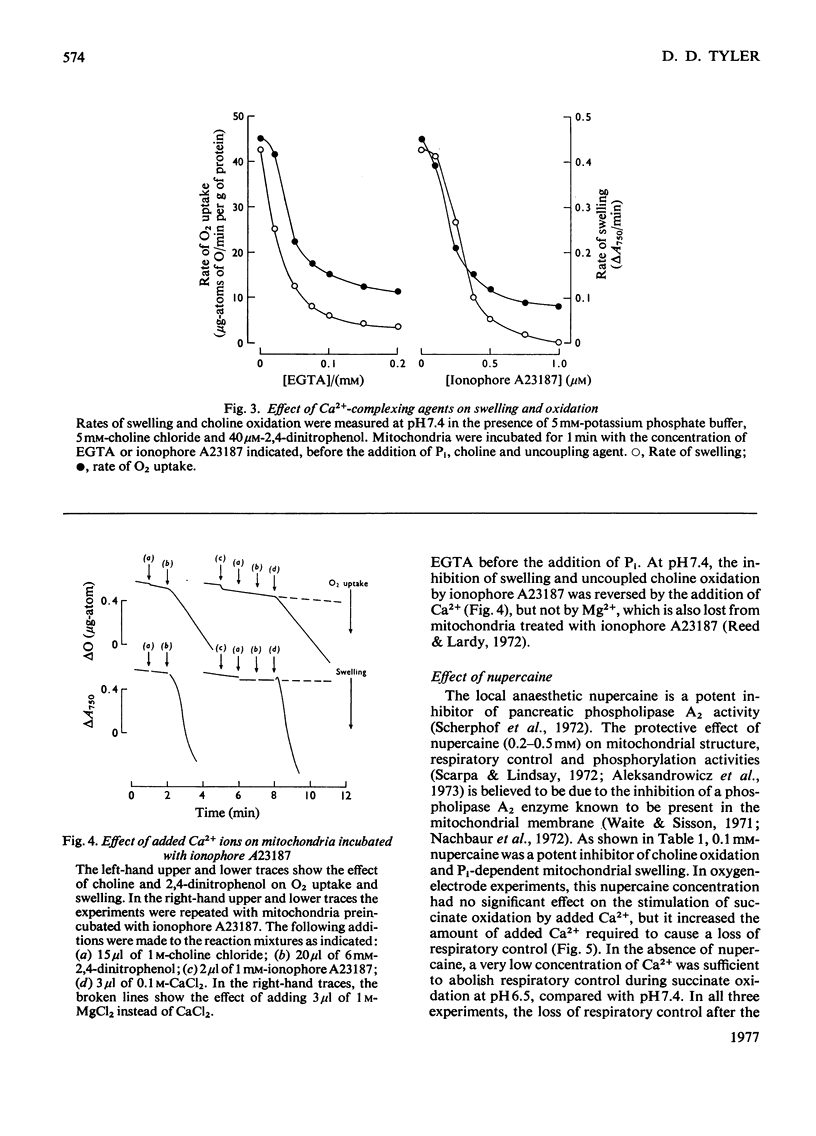
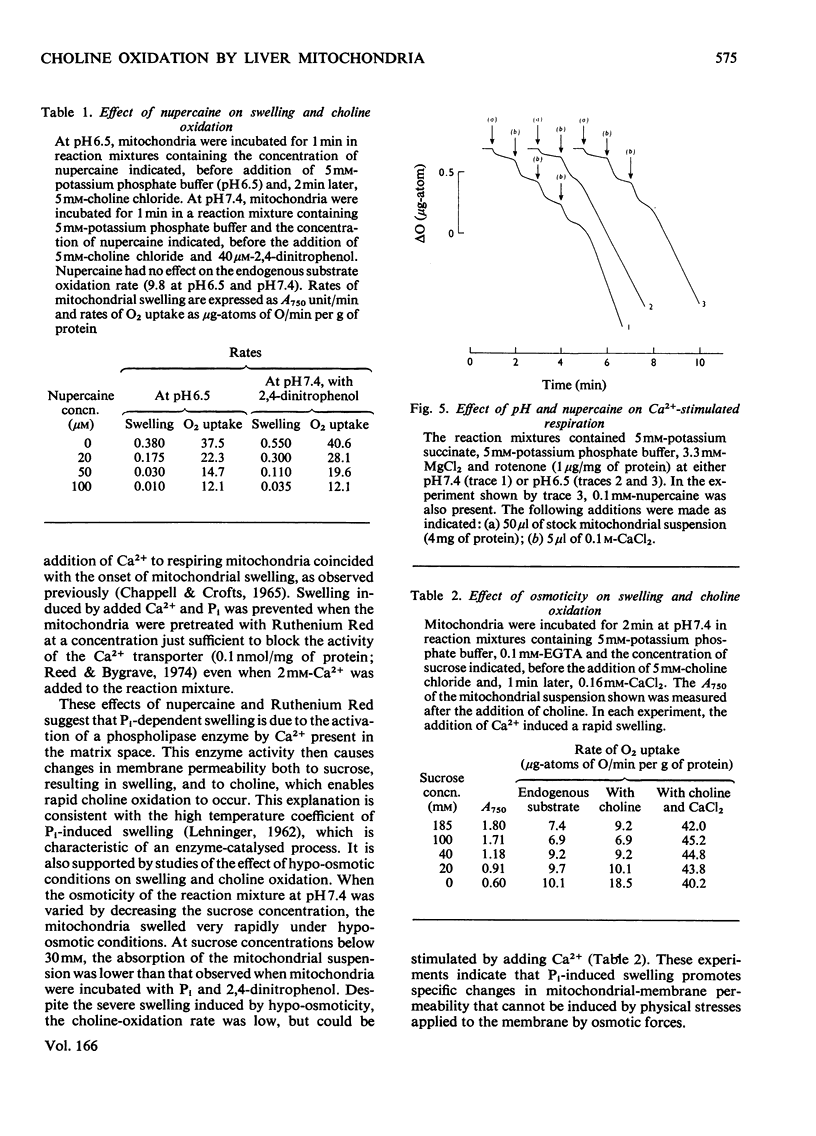
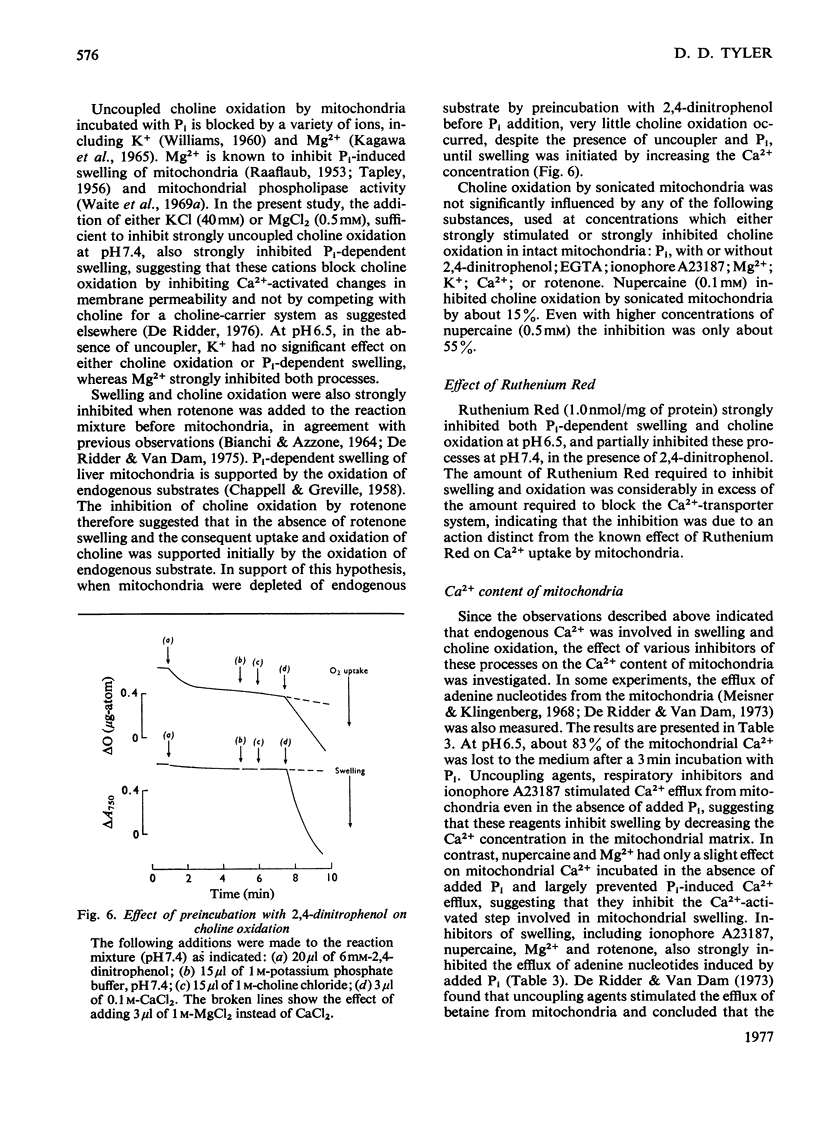
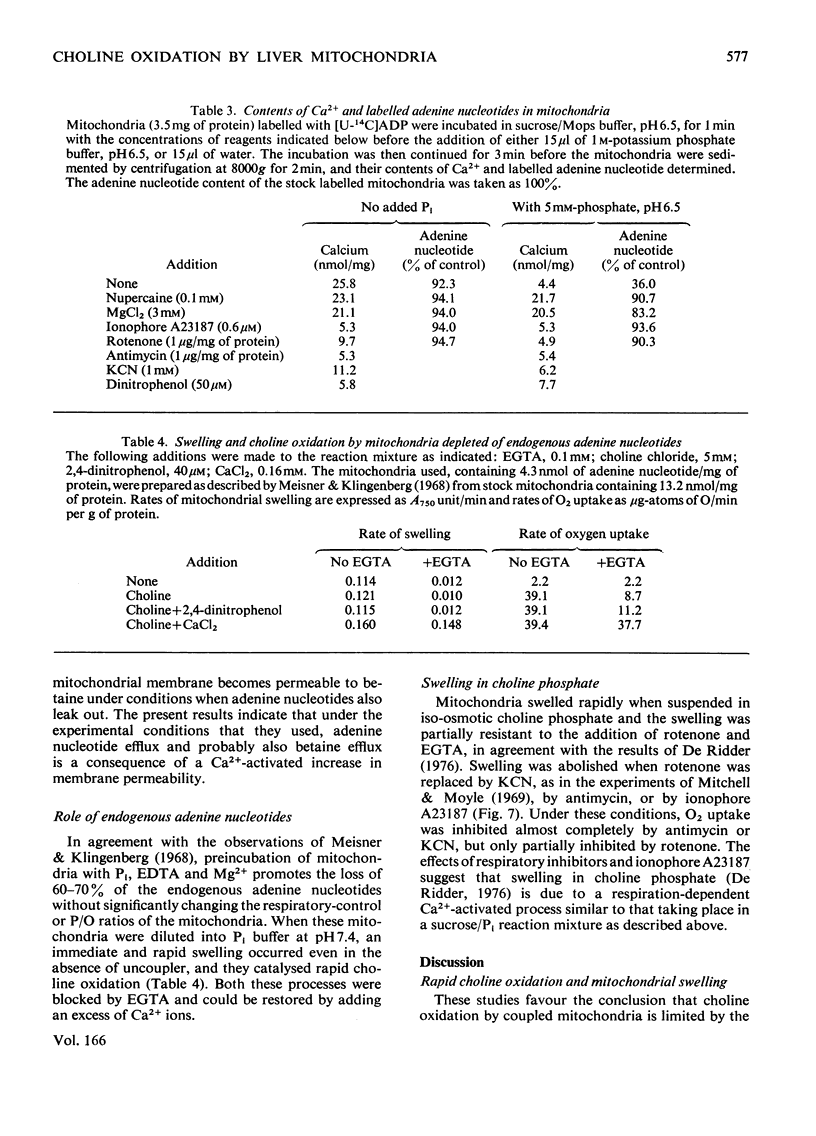
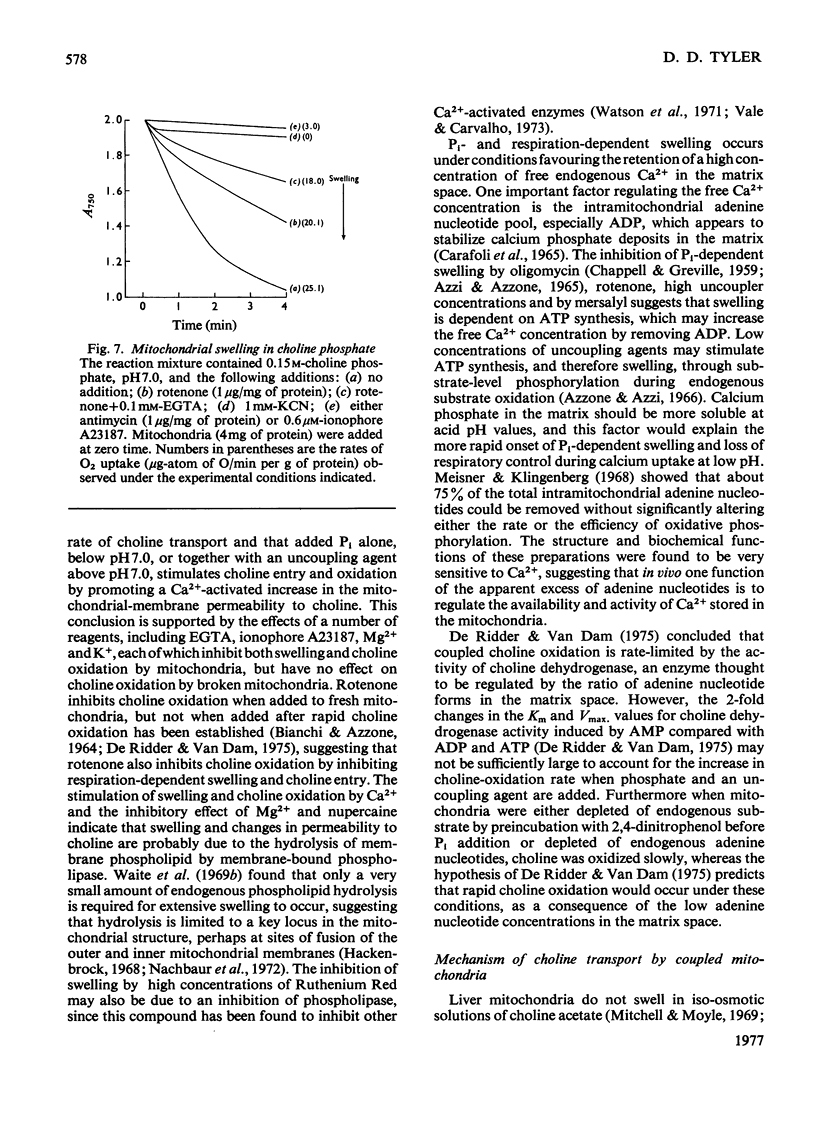
1. Rapid choline oxidation and the onset of Pi-induced swelling by liver mitochondria, incubated in a sucrose medium at or above pH7.0, required the addition of both Pi and an uncoupling agent. Below pH7.0, Pi alone was required for rapid choline oxidation and swelling. 2. Choline oxidation was inhibited by each of several reagents that also inhibited Pi-induced swelling under similar conditions of incubation, including EGTA, mersalyl, Mg2+, the Ca2+-ionophore A23187, rotenone and nupercaine. None of these reagents had any significant effect on the rate of choline oxidation by sonicated mitochondria. There was therefore a close correlation between the conditions required for rapid choline oxidation and for Pi-induced swelling to occur, suggesting that in the absence of mitochondrial swelling the rate of choline oxidation is regulated by the rate of choline transport across the mitochondrial membrane. 3. Respiratory-chain inhibitors, uncoupling agents (at pH6.5) and ionophore A23187 caused a loss of endogenous Ca2+ from mitochondria, whereas nupercaine and Mg2+ had no significant effect on the Ca2+ content. Inhibition of choline oxidation and mitochondrial swelling by ionophore A23187 was reversed by adding Ca2+, but not by Mg2+. It is concluded that added Pi promotes the Ca2+-dependent activation of mitochondrial membrane phospholipase activity in respiring mitochondria, causing an increase in the permeability of the mitochondrial inner membrane to choline and therefore enabling rapid choline oxidation to occur. Nupercaine and Mg2+ appear to block choline oxidation and swelling by inhibiting phospholipase activity. 4. Choline was oxidized slowly by tightly coupled mitochondria largely depleted of their endogenous adenine nucleotides, suggesting that these compounds are not directly concerned in the regulation of choline oxidation. 5. The results are discussed in relation to the possible mechanism of choline transport across the mitochondrial membrane in vivo and the influence of this process on the pathways of choline metabolism in the liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrowicz Z., Swierczyński J., Wrzolkowa T. Protective effect of nupercaine on mitochondrial structure. Biochim Biophys Acta. 1973 Apr 27;305(1):59–66. doi: 10.1016/0005-2728(73)90231-4. [DOI] [PubMed] [Google Scholar]
- Azzi A., Azzone G. F. Swelling and shrinkage phenomena in liver mitochondria. I. Large amplitude swelling induced by inorganic phosphate and by ATP. Biochim Biophys Acta. 1965 Aug 24;105(2):253–264. doi: 10.1016/s0926-6593(65)80150-3. [DOI] [PubMed] [Google Scholar]
- BIANCHI G., AZZONE G. F. OXIDATION OF CHOLINE IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3947–3955. [PubMed] [Google Scholar]
- CARAFOLI E., ROSSI C. S., LEHNINGER A. L. UPTAKE OF ADENINE NUCLEOTIDES BY RESPIRING MITOCHONDRIA DURING ACTIVE ACCUMULATION OF CA++ AND PHOSPHATE. J Biol Chem. 1965 May;240:2254–2261. [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. CALCIUM ION ACCUMULATION AND VOLUME CHANGES OF ISOLATED LIVER MITOCHONDRIA. CALCIUM ION-INDUCED SWELLING. Biochem J. 1965 May;95:378–386. doi: 10.1042/bj0950378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Dependence of mitochondrial swelling on oxidizable substrates. Nature. 1958 Sep 20;182(4638):813–814. doi: 10.1038/182813a0. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effects of 2:4-dinitrophenol and other agents on the swelling of isolated mitochondria. Nature. 1959 Jun 20;183(4677):1737–1738. doi: 10.1038/1831737a0. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The effects of 2,4-dinitrophenol on mitochondrial oxidations. Biochem J. 1964 Feb;90(2):237–248. doi: 10.1042/bj0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder J. J., van Dam K. The efflux of betaine from rat-liver mitochondria, a possible regulating step in choline oxidation. Biochim Biophys Acta. 1973 Jan 26;291(2):557–563. doi: 10.1016/0005-2736(73)90507-5. [DOI] [PubMed] [Google Scholar]
- Gamble J. G., Lehninger A. L. Transport of ornithine and citrulline across the mitochondrial membrane. J Biol Chem. 1973 Jan 25;248(2):610–618. [PubMed] [Google Scholar]
- Gear A. R., Lehninger A. L. Respiration-independent displacement of protons from external anionic groups of mitochondria by alkali metal cations. Biochem Biophys Res Commun. 1967 Sep 7;28(5):840–844. doi: 10.1016/0006-291x(67)90395-6. [DOI] [PubMed] [Google Scholar]
- Gindler E. M., King J. D. Rapid colorimetric determination of calcium in biologic fluids with methylthymol blue. Am J Clin Pathol. 1972 Oct;58(4):376–382. doi: 10.1093/ajcp/58.5.376. [DOI] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, FORD L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J Biol Chem. 1955 Sep;216(1):357–369. [PubMed] [Google Scholar]
- Hackenbrock C. R. Chemical and physical fixation of isolated mitochondria in low-energy and high-energy states. Proc Natl Acad Sci U S A. 1968 Oct;61(2):598–605. doi: 10.1073/pnas.61.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling P. J., Brand M. D., Chappell J. B. Permeability of mitochondria to neutral amino acids. FEBS Lett. 1973 Aug 15;34(2):169–171. doi: 10.1016/0014-5793(73)80785-9. [DOI] [PubMed] [Google Scholar]
- KAGAWA T., WILKEN D. R., LARDY H. A. CONTROL OF CHOLINE OXIDATION IN LIVER MITOCHONDRIA BY ADENINE NUCLEOTIDES. J Biol Chem. 1965 Apr;240:1836–1842. [PubMed] [Google Scholar]
- KLEE W. A., RICHARDS H. H., CANTONI G. L. The synthesis of methionine by enzymic transmethylation. VII. Existence of two separate homocysteine methylpherases on mammalian liver. Biochim Biophys Acta. 1961 Nov 25;54:157–164. doi: 10.1016/0006-3002(61)90948-9. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. The catalytic effect of 2,4-dinitrophenol on adenosinetriphosphate hydrolysis by cell particles and soluble enzymes. J Biol Chem. 1953 Mar;201(1):357–370. [PubMed] [Google Scholar]
- LEHNINGER A. L., REMMERT L. F. An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation. J Biol Chem. 1959 Sep;234:2459–2464. [PubMed] [Google Scholar]
- LEHNINGER A. L. Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation. Physiol Rev. 1962 Jul;42:467–517. doi: 10.1152/physrev.1962.42.3.467. [DOI] [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- McMurray W. C., Magee W. L. Phospholipid metabolism. Annu Rev Biochem. 1972;41(10):129–160. doi: 10.1146/annurev.bi.41.070172.001021. [DOI] [PubMed] [Google Scholar]
- Meisner H., Klingenberg M. Efflux of adenine nucleotides from rat liver mitochondria. J Biol Chem. 1968 Jul 10;243(13):3631–3639. [PubMed] [Google Scholar]
- Mellors A., Tappel A. L., Sawant P. L., Desai I. D. Mitochondrial swelling and uncoupling of oxidative phosphorylation by lysosomes. Biochim Biophys Acta. 1967 Sep 6;143(2):299–309. doi: 10.1016/0005-2728(67)90084-9. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Nachbaur J., Colbeau A., Vignais P. M. Distribution of membrane-confined phospholipases A in the rat hepatocyte. Biochim Biophys Acta. 1972 Aug 9;274(2):426–446. doi: 10.1016/0005-2736(72)90189-7. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Klingenberg M. Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem. 1968 Oct 17;6(1):66–79. doi: 10.1111/j.1432-1033.1968.tb00420.x. [DOI] [PubMed] [Google Scholar]
- RAAFLAUB J. Uber den Wirkungsmechanismus von Adenosintriphosphat als Cofaktor isolierter Mitochondrien. Helv Physiol Pharmacol Acta. 1953;11(2):157–165. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- SCHNEIDER W. C. Biochemical constitution of mammalian mitochondria. J Histochem Cytochem. 1953 Jul;1(4):212–233. doi: 10.1177/1.4.212. [DOI] [PubMed] [Google Scholar]
- SIDRANSKY H., FARBER E. Liver choline oxidase activity in man and in several species of animals. Arch Biochem Biophys. 1960 Mar;87:129–133. doi: 10.1016/0003-9861(60)90133-8. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Lindsay J. G. Maintenance of energy-linked functions in rat-liver mitochondria aged in the presence of nupercaine. Eur J Biochem. 1972 Jun 9;27(3):401–407. doi: 10.1111/j.1432-1033.1972.tb01851.x. [DOI] [PubMed] [Google Scholar]
- Scherphof G. L., Scarpa A., van Toorenenbergen A. The effect of local anesthetics on the hydrolysis of free and membrane-bound phospholipids catalyzed by various phospholipases. Biochim Biophys Acta. 1972 Jun 19;270(2):226–240. doi: 10.1016/0005-2760(72)90234-2. [DOI] [PubMed] [Google Scholar]
- Sundler R., Arvidson G., Akesson B. Pathways for the incorporation of choline into rat liver phosphatidylcholines in vivo. Biochim Biophys Acta. 1972 Dec 8;280(4):559–568. doi: 10.1016/0005-2760(72)90136-1. [DOI] [PubMed] [Google Scholar]
- TAPLEY D. F. The effect of thyroxine and other substances on the swelling of isolated rat liver mitochondria. J Biol Chem. 1956 Sep;222(1):325–339. [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D., Gonze J., Estabrook R. W. Observations on the inhibitor sensitivity of the choline oxidation system. Arch Biochem Biophys. 1966 Aug;115(2):373–384. doi: 10.1016/0003-9861(66)90287-6. [DOI] [PubMed] [Google Scholar]
- Vale M. G., Carvalho A. P. Effects of ruthenium red on Ca2+ uptake and ATPase of sarcoplasmic reticulum of rabbit skeletal muscle. Biochim Biophys Acta. 1973 Oct 19;325(1):29–37. doi: 10.1016/0005-2728(73)90147-3. [DOI] [PubMed] [Google Scholar]
- WILKEN D. R., KAGAWA T., LARDY H. A. THE ROLE OF ADENINE NUCLEOTIDES IN CONTROL OF CHOLINE OXIDATION BY MITOCHONDRIA. J Biol Chem. 1965 Apr;240:1843–1846. [PubMed] [Google Scholar]
- WILLIAMS G. R. The limitation by ionic solutions of the access of substrate to mitochondrial choline oxidase. J Biol Chem. 1960 Apr;235:1192–1195. [PubMed] [Google Scholar]
- WITTENBERG J., KORNBERG A. Choline phosphokinase. J Biol Chem. 1953 May;202(1):431–444. [PubMed] [Google Scholar]
- Waite M., Scherphof G. L., Boshouwers F. M., van Deenen L. L. Differentiation of phospholipases A in mitochondria and lysosomes of rat liver. J Lipid Res. 1969 Jul;10(4):411–420. [PubMed] [Google Scholar]
- Waite M., Sisson P. Partial purification and characterization of the phospholipase A 2 from rat liver mitochondria. Biochemistry. 1971 Jun 8;10(12):2377–2383. doi: 10.1021/bi00788a031. [DOI] [PubMed] [Google Scholar]
- Waite M., Van Deenen L. L., Ruigrok T. J., Elbers P. F. Relation of mitochondrial phospholipase A activity to mitochondrial swelling. J Lipid Res. 1969 Sep;10(5):599–608. [PubMed] [Google Scholar]
- Watson E. L., Vincenzi F. F., Davis P. W. Ca 2+ -activated membrane ATPase: selective inhibition by ruthenium red. Biochim Biophys Acta. 1971 Dec 3;249(2):606–610. doi: 10.1016/0005-2736(71)90140-4. [DOI] [PubMed] [Google Scholar]
- de Ridder J. J., Kleverlaan N. T., Verdouw-Chamalaun C. V., Schippers P. G., van Dam K. Uncoupler-stimulated oxidation of choline by rat-liver mitochondria. Biochim Biophys Acta. 1973 Dec 14;325(3):397–405. doi: 10.1016/0005-2728(73)90200-4. [DOI] [PubMed] [Google Scholar]
- de Ridder J. J. The uptake of choline by rat liver mitochondria. Biochim Biophys Acta. 1976 Nov 9;449(2):236–244. doi: 10.1016/0005-2728(76)90136-5. [DOI] [PubMed] [Google Scholar]
- de Ridder J. J., van Dam K. Control of choline oxidation by rat-liver mitochondria. Biochim Biophys Acta. 1975 Nov 11;408(2):112–122. doi: 10.1016/0005-2728(75)90003-1. [DOI] [PubMed] [Google Scholar]


