Abstract
Background
In recent years, dexmedetomidine (DEX) has been proposed as a useful vasoconstrictor for local anesthesia because it is less effective in circulation than clonidine of antihypertensive drugs. In addition, DEX is expected to act as a vasoconstrictor during local anesthesia. However, histomorphometric studies demonstrating that DEX exerts vasoconstrictive effects are lacking. This study aimed to clarify whether DEX exerts a histomorphologically vasoconstrictive effect on blood vessels in the mandible of rats.
Methods
A total of 12 male Wistar rats were used. General anesthesia was induced and maintained using sevoflurane. Normal saline (0.2 ml) was injected on the left side of the jaw (DEX (−) effect site) and 0.2 ml normal saline containing 12.5 µg/ml DEX was injected on the right side of the jaw (DEX (+) effect site). The puncture point was located on the mesial side of the first molar, 1 mm away from the gingival sulcus. Following decalcification, the specimens were paraffinized and sagittally sliced into 20 µm-thick sections, followed by staining with anti-α smooth muscle actin antibody. The intravascular lumen area was measured in the oral mucosa, periodontal ligament, mandibular bone above the root apex, mandibular bone below the root apex, and dental pulp. The unpaired t-test was used for statistical analysis, and a P value < 0.05 was considered statistically significant.
Results
Compared to the DEX (−) effect site, the intravascular lumen area in the oral mucosa and periodontal ligament of the DEX (+) effect site was significantly decreased. No significant difference was observed in the intravascular lumen area between the DEX (−) and DEX (−) effect sites in the mandibular bone above and below the root apex and dental pulp.
Conclusion
A direct vasoconstrictive effect of DEX was not observed in the intravascular lumen of the mandibular bone above and below the root apex and dental pulp; however, it was observed in the oral mucosa and periodontal ligament.
Keywords: Alpha-Receptor, Dexmedetomidine, Local Anesthesia, Mandible, Rats, Vasoconstriction
INTRODUCTION
Painless dental treatment, which is desired by patients, can be achieved with local anesthesia [1]. Local anesthetics used in clinical dentistry include vasoconstrictors [2,3], especially adrenaline, which may lead to side effects such as increased blood pressure and heart rate [4], endogenous catecholamine release, and hypokalemia [5,6]. Therefore, dentists should be cautious while administering this drug to patients with cardiovascular disease.
Dexmedetomidine (DEX), a selective alpha-2 adrenoreceptor agonist, is clinically used as a sedative for the respiratory management of patients in the intensive care unit (ICU) and for surgeries requiring intravenous sedation with local anesthetics [7,8]. Recently, DEX has been proposed as a useful vasoconstrictor in local anesthesia [9,10,11,12,13,14,15] because it is more selective for alpha-2 adrenoreceptors and less effective in circulation than clonidine, an antihypertensive drug [16]. DEX has also been used in spinal anesthesia [9,10] and nerve block anesthesia [11,12,13,14]. Therefore, DEX is expected to act as a vasoconstrictor of local anesthesia during dental treatment. One study reported that lidocaine combined with DEX enhanced local anesthesia [15]. However, histomorphometric studies have not demonstrated that DEX exerts vasoconstrictive effects. This study aimed to clarify whether DEX exhibits a histomorphometric vasoconstrictive effect on mandibular blood vessels in rats.
METHODS
1. Animals
A total of 12 male Wistar rats (10 weeks old, 362 ± 8 g body weight; Jackson Laboratory Japan, Inc. Kanagawa, Japan) were used in this study. Until the day of experimentation, the rats were housed at a room temperature of 23℃ and a humidity of 60% with free access to food (MF, Oriental Yeast Co., Ltd, Tokyo, Japan) and water.
This study was approved by the Ohu University Animal Research Committee (Animal Experiment permit number 2021-4, 2022-4).
2. General anesthesia in rats
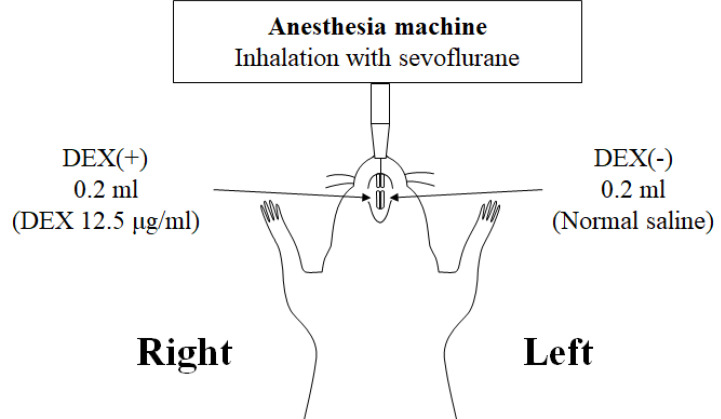
General anesthesia was induced in the rats by inhalation of 5% sevoflurane using an anesthesia machine for small animals (Soft Lander, Shin-Ei Industries, Inc. Tokyo, Japan) and 5 L/min of oxygen via a nasal mask. Sevoflurane (3%) and oxygen (3 L/min) were used to maintain general anesthesia (Fig. 1).
Fig. 1. Preparation for the study of rats. General anesthesia was induced and maintained using sevoflurane. A 0.2 ml of normal saline was injected in the left side (DEX (−) effect site), and 0.2 ml of normal saline containing DEX (12.5 µg/ml) was injected in the right side (DEX (+) effect site). Then, 20 min after DEX administration, perfusion fixation was performed with 4% paraformaldehyde. DEX, dexmedetomidine.
3. DEX injection and tissue removal
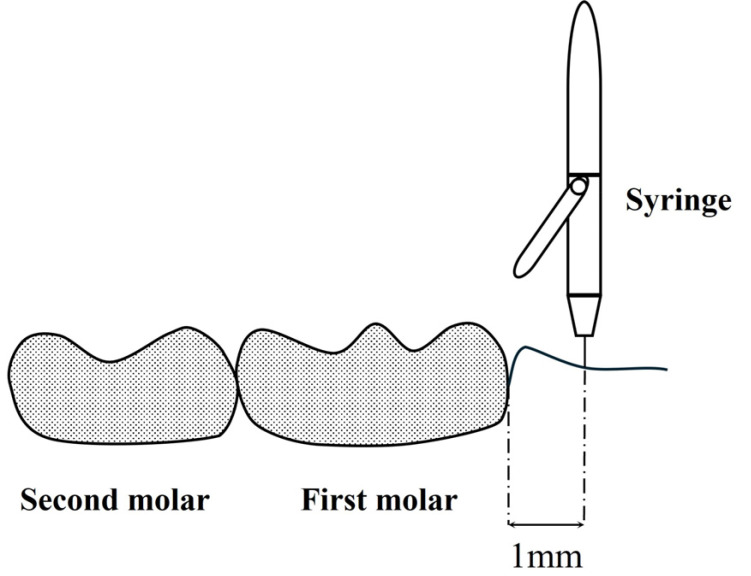
After stable general anesthesia was achieved, a Sopira Citoject Syringe® (Kulzer Japan CO., Ltd., Hanau, Germany) and a 33G needle (0.26 × 12 mm; Niproject Dental Needle®, Nipro Corporation, Tokyo, Japan) were used to control DEX injection by performing one push (delivering 0.05 ml) per second and were conducted four times. The puncture point was located on the mesial side of the first molar, 1 mm away from the gingival sulcus (Fig. 2). Normal saline was administered on the left side of the jaw (DEX (−) effect site) and normal saline containing 12.5 µg/ml on the right side of the jaw (DEX (+) effect site). They were administered to both sides of the rat mandible by the same dentist, without a difference in pressure between the sides.
Fig. 2. Puncture point. The puncture point was located on the mesial side of the first molar, 1 mm away from the gingival sulcus.
Then, 20 min after DEX administration, 4% paraformaldehyde buffer solution (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was injected into the left ventricle, followed by perfusion fixation. For perfusion fixation, a 24G intravenous catheter (BD Insyte IV Catheter, BD, Tokyo, Japan) and a constant-flow pump with a low flow rate (RP-2000P, Tokyo RikaKikai, Co., Ltd., Tokyo, Japan) were used at flow rates of 2–3 L/h. Thereafter, the lower mandible was removed and immersed in a 4% paraformaldehyde buffer solution for 24 h.
4. Histological staining
To clearly visualize vascular smooth muscle [17], we performed histological staining. The specimens were decalcified with a 10% ethylenediaminetetraacetic acid solution (pH 7.0) for 3–6 months. During decalcification, the decalcification depth was checked by trial cutting of the incisors.
The decalcified specimens were embedded in paraffin and then sagittally sliced into 20 µm-thick sections using a Microm HM 360 machine (Micron Technology, Inc., ID, USA). After deparaffinization and hydration, the sections were treated with 0.3% H2O2 in methanol to remove endogenous peroxidase, blocked with goat serum (Vectastain Elite ABC Kit, Vector Lab, Inc., Burlingame, CA, USA) for 1 h, and incubated in a solution of rabbit anti-α smooth muscle actin antibody (α-SMA Polyclonal Antibody; Bioworld Technology, Inc., St Louis Park, MN, USA) as the primary antibody for 10 h. After washing, the sections were treated with biotin-labeled goat anti-rabbit antibody (Vectastain Elite ABC Kit, Vector Lab, Inc., Burlingame, CA, USA) as the secondary antibody for 1 h and incubated in ABC peroxidase (Vectastain Elite ABC Kit, Vector Lab, Burlingame, CA, USA) for 1 h. DAB color development solution (Peroxidase Substrate Kit, Vector Lab, Inc., USA) was used for staining, and nuclei were stained with 5% methyl green (Muto Pure Chemicals Co., Ltd., Tokyo, Japan).
5. Measurement of the intravascular lumen area in each segment of the mandible
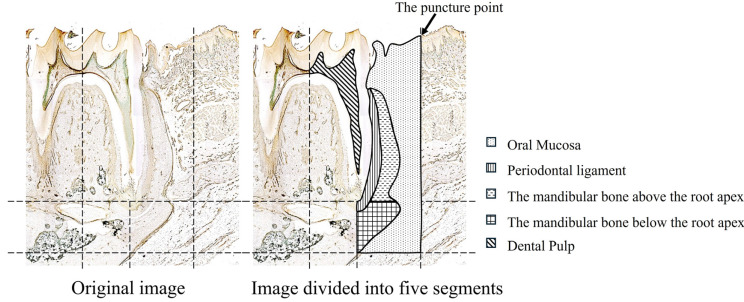
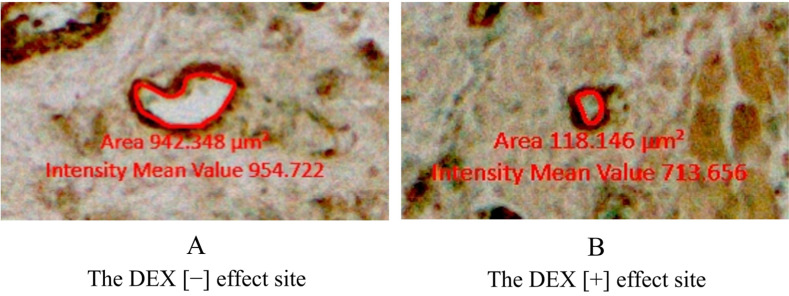
The decalcified specimen of the mandible separated from the rats was sagittally sliced, each 20 µm thick. In total, 240 specimens were created on the left and right sides. The intravascular lumen areas in a three-dimensional range from the mesial gingival sulcus to 1 mm forward were measured at both DEX (−) and DEX (+) effect sites. This part of the jawbone was divided into the following segments: oral mucosa, periodontal ligament, mandibular bone above the root apex, mandibular bone below the root apex, and dental pulp (Fig. 3). In the mandibular bone below the root apex, the vertical range was set from the root apex to the lowest point of the mandibular bone. Using an optical microscope (20 × magnification), the intravascular lumen areas at the DEX (−) and DEX (+) effect sites were measured in the abovementioned five segments using Axio Vision (Carl Zeiss AG, GmbH. software (Oberkochen, Germany). The inner vascular smooth muscle circumference, which was stained dark brown, was drawn as a red line, and the intravascular lumen area was calculated automatically using ZEN (Carl Zeiss AG, GmbH. Oberkochen, Germany) (Fig. 4). Moreover, we measured the intravascular lumen areas and the total number of blood vessels in each of the five segments at the DEX (−) and DEX (+) effect sites. The intravascular lumen area per blood vessel in each segment was calculated and compared between the DEX (−) and DEX (+) effect sites.
Fig. 3. Range of each segment in this study. Part of the lower jawbone was divided into the following segments: oral mucosa, periodontal ligament, mandibular bone above the root apex, mandibular bone below the root apex, and dental pulp.
Fig. 4. Measurement of the intravascular lumen area. (A) DEX (−) effect site and (B) DEX (+) effect site. Dark brown regions around the red line denote vascular smooth muscles. The area inside the red line was calculated using the ZEN software (Carl Zeiss AG, GmbH). Red values indicate intravascular lumen areas. DEX, dexmedetomidine.
In both DEX (−) and DEX (+) effect sites, we measured the total number of blood vessels in each segment and the total area of all intravascular lumens. The mean intravascular luminal area was calculated using the following formula:
Mean intravascular lumen area = total area of all intravascular lumens/total number of blood vessels For the vasoconstriction rate (VR), we used the formula shown below;
VR (%) = {mean intravascular lumen area in DEX (+) effect site/mean intravascular lumen area in DEX (−) effect site} × 100
6. Statistical analysis
The unpaired t-test was used for statistical analysis, and a P value of < 0.05 was considered statistically significant. Statistical analyses were performed using the statistical processing software ystat2018 (Igakutosho Shuppan Co., Ltd., Tokyo, Japan).
RESULTS
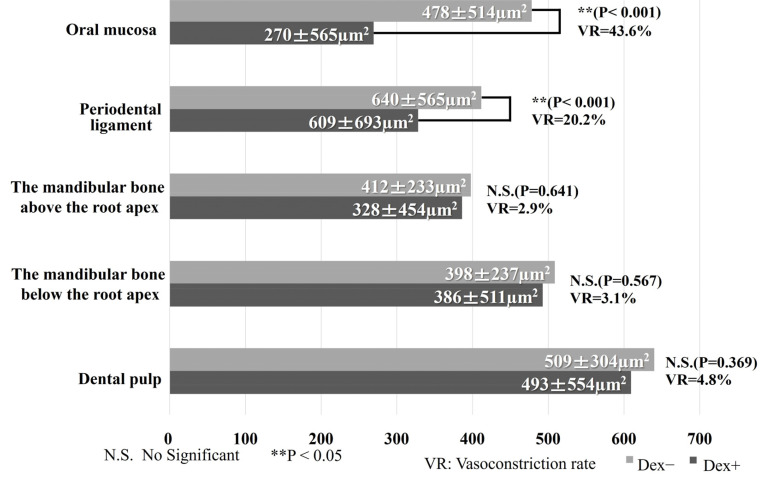
The results are shown in Fig. 5.
Fig. 5. Comparison of the intravascular lumen area in each segment. In the oral mucosa, the intravascular lumen area for the DEX (+) effect site decreased significantly compared to that for the DEX (−) effect site (P < 0.001). Additionally, the intravascular lumen area in the periodontal ligament for the DEX (+) effect site decreased significantly compared to that for the DEX (−) effect site (P < 0.001). However, no significant differences were observed between the DEX (−) and DEX (+) sites in terms of the mandibular bone above and below the root apex and dental pulp. DEX, dexmedetomidine.
1. Oral mucosa
In the oral mucosa, 5827 vessels were measured in the DEX (+) effect site, and 5640 vessels were measured in the DEX (−) effect site. The intravascular lumen area in the oral mucosa for the DEX (+) effect site decreased significantly compared with that for the DEX (−) effect site (270 ± 565 µm2 vs. 478 ± 514 µm2, P < 0.001). The VR of the calculated area is 43.6%.
2. Periodontal ligament
In the periodontal ligament, 679 vessels were measured in the DEX (+) effect site, and 550 vessels were measured in the DEX (−) effect. The intravascular lumen area in the periodontal ligament for the DEX (+) effect site decreased significantly compared with that for the DEX (−) effect site (328 ± 454 µm2 vs. 412 ± 233 µm2, P < 0.001). The VR of the calculated area was 20.2%.
3. Mandibular bone above the root apex
In the mandibular bone above the root apex, 626 vessels were measured at the DEX (+) effect sites, and 493 vessels were measured at the DEX (−) effect sites. The intravascular lumen area in the mandibular bone above the root apex demonstrated no significant differences between the DEX (+) and DEX (−) site effects (386 ± 511 µm2 for and 398 ± 237 µm2, retrospectively; P = 0.641). The VR of the calculated area was 2.9%.
4. Mandibular bone below the root apex
In the mandibular bone below the root apex, 615 vessels were measured at the DEX (+) effect site, and 483 vessels were measured at the DEX (−) effect site. The intravascular lumen area in the mandibular bone below the root apex also exhibited no significant differences between the DEX (+) and DEX (−) site effects (493 ± 554 µm2 and 509 ± 304 µm2 retrospectively; P = 0.567). The VR of the calculated area was 3.1%.
5. Dental pulp
In the dental pulp, 764 vessels were measured at the DEX (+) effect sites, and 655 vessels were measured at the DEX (−) effect sites. Similarly, the intravascular lumen area in the dental pulp did not significantly differ between the DEX (+) and DEX (−) effect sites (609 ± 693 µm2 and 640 ± 565 µm2, retrospectively; P = 0.369). The VR of the calculated area is 4.8%.
DISCUSSION
Alpha-2 agonists are activated through centrally located alpha-2 adrenoceptors and mediate sympatholytic and mild parasympathetic effects, such as decreased heart rate and blood pressure. They also act as direct peripheral vasoconstrictors. The activation of alpha-2 agonists through alpha-2 adrenoceptors in vascular smooth muscle mediates a direct peripheral vasoconstrictive effect [18]. Therefore, the histological staining, was used anti-α smooth muscle actin antibody, was performed to evaluate blood vessel reaction with a focus on the vascular smooth muscles in this study [19].
DEX, an alpha-2 agonist, is frequently used for intravenous sedation for respiratory management in the ICU [20] and implant surgery [21]. One study examined DEX’s direct peripheral vasoconstrictive effect of DEX and found that the duration of nerve block was prolonged when local anesthetics containing DEX were administered to patients with brachial plexus blocks [14,22]. However, the histomorphometric vasoconstrictive effects of DEX have not yet been reported. Thus, we focused on the direct peripheral vasoconstrictive effect of alpha-2 adrenoceptors and histomorphologically investigated the vasoconstrictive effects induced by DEX.
1. DEX concentration
In Japan, 2% lidocaine-containing adrenaline is generally used for local dental anesthesia. Adrenaline is added to local anesthetics as a vasoconstrictor to enhance the anesthetic effect and duration. However, adrenaline included in local dental anesthetics may cause circulation-related side effects such as hypertension and tachycardia. Concerning blood pressure and heart rate, one study using rats has reported that a 12.5 µg/ml concentration of DEX seems to be a suitable alternative to adrenaline in lidocaine formulations. With a view of Akimoto’s study [23], DEX was selected as a vasoconstrictor and 12.5 µg/ml DEX was determined as a DEX concentration in this study.
2. Intravascular lumen area in the oral mucosa and periodontal ligament
The intravascular lumen area in the oral mucosa at the DEX (+) effect site was significantly smaller than that at the DEX (−) effect site. In our study, we injected DEX and normal saline at the DEX (+) effect site and only normal saline at the DEX (−) effect site. Therefore, DEX may exert vasoconstrictive effects on the oral mucosa. Selliseth et al. [24] reported that blood flow to the cervical segment of the periodontal ligament was supplied by the gingiva. In this study, 0.2 ml of DEX was injected into the alveolar crest, suggesting DEX absorption into blood vessels after injection. Consequently, blood containing DEX flows from the oral mucosa around the alveolar crest to the periodontal ligament. Thus, the intravascular lumen area in the periodontal ligament at the DEX (+) site could be significantly decreased compared with that at the DEX (−) effect site.
From a pharmacological point of view, DEX has a comparatively high ratio of alpha-2 to alpha-1 activity (alpha-2:alpha-1 = 1620:1) compared to other drugs such as clonidine (220:1) [25]. Particularly, the alpha-2B adrenoceptor is one of the three alpha-2 adrenoceptor types and exists in peripheral blood vessels [26]. Furthermore, animal research [25] involving continuous DEX infusion indicated that palatal mucosal blood flow was mainly reduced due to vasoconstriction in response to alpha-2B adrenoceptor stimulation by DEX. Hence, the significant decrease in the intravascular lumen area in the oral mucosa and periodontal ligament may be mainly caused by the direct peripheral vasoconstrictive effect of DEX through alpha-2B adrenoceptors.
3. Intravascular lumen area in the mandibular bone above the root apex, mandibular bone below the root apex, and dental pulp
We found no significant differences in the intravascular lumen area of the mandibular bone above and below the root apex between the two groups. Tanaka et al. [27] reported that 2% lidocaine containing adrenaline [12.5 µg/ml) did not significantly decrease the intravascular lumen area in the blood vessels of the Haversian canal/Volkman’s canal or the bone marrow of the mandible, and in my results similar to that report. One possible reason why the intravascular lumen area of the DEX (+) effect site did not significantly decrease is that DEX infiltration may not have reached the alveolar bone around the root apex. In the current study, the mandibular bone was used as the sample, and its cortical bone was thick, making it difficult for DEX to infiltrate the alveolar bone around the root apex. The failure of DEX to infiltrate the alveolar bone around the root apex may also be explained by DEX injection into the top of the gingiva and the long distance between the top and the root apex. Moreover, the possibility that blood vessels in the mandibular bone have a direct vasoconstrictive effect through the alpha-adrenoceptor cannot be excluded.
However, the direct vasoconstrictive effect of DEX was not observed in the mandibular bone above or below the root apex. One study reported that adrenaline had no significant vasoconstrictive effect on bone tissues, such as the mandibular bone, with a contraction rate was 10–20% [27]. In our study, the rate of contraction due to DEX’s direct effect of DEX was only 3% in bone tissue, such as the mandibular bone above and below the root apex. Hence, DEX may be appropriate for surgery to maintain blood flow in the alveolar bone.
Regarding the intravascular lumen areas in the dental pulp, we found no significant difference between the DEX (−) and DEX (+) effect sites. Ibricevic et al. [28] reported that alpha-2 adrenoceptors are present in the blood vessels of the canine dental pulp. The intravascular lumen area for the DEX (+) effect site would decrease more than that for the DEX (+) effect site because of DEX’s direct vasoconstrictive effect of DEX if it reached the dental pulp. Moreover, blood flow in the cervical segment of the periodontal ligament is supplied by the gingiva, whereas blood flow in the dental pulp is mainly supplied through the apical foramen [29,30]. Our results for the dental pulp indicate that DEX did not reach the root apex.
In conclusion, the mandibles of rats were infiltrated with DEX, and histomorphometric analysis was used to analyze DEX’s vasoconstrictive effects of DEX on the blood vessels of the mandible. This direct vasoconstrictive effect was not observed in the intravascular lumen area of the mandibular bone above and below the root apex and dental pulp but was observed in the oral mucosa and periodontal ligament.
Footnotes
- Hikaru Sato: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft.
- Shota Abe: Investigation, Visualization.
- Kimiharu Ambe: Methodology, Project administration, Software, Supervision.
- Shinya Yamazaki: Formal analysis, Software.
- Hiroyoshi Kawaai: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Dionne RA, Yagiela JA, Coté CJ, Donaldson M, Edwards M, Greenblatt DJ, et al. Balancing efficacy and safety in the use of oral sedation in dental outpatients. J Am Dent Assoc. 2006;137:502–513. doi: 10.14219/jada.archive.2006.0223. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS, Dionne R, Sweet J, Gracely R, Brewer HB, Jr, Gregg R, et al. Circulatory, plasma catecholamine, cortisol, lipid, and psychological responses to a real-life stress (third molar extractions): effects of diazepam sedation and of inclusion of epinephrine with the local anesthetic. Psychosom Med. 1982;44:259–272. doi: 10.1097/00006842-198207000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki M, Yokota S, Murata Y, Watanabe H, Nishibori M, Suzuki N, et al. Changes in heart rate and blood pressure during dental procedures with local anesthesia. Anesth Prog. 1989;36:234–235. [PMC free article] [PubMed] [Google Scholar]
- 4.Edmondson HD, Roscoe B, Vickers MD. Biochemical evidence of anxiety in dental patients. Br Med J. 1972;4:7–9. doi: 10.1136/bmj.4.5831.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meechan JG, Rawlins MD. The effect of adrenaline in lignocaine anaesthetic solutions on plasma potassium in healthy volunteers. Eur J Clin Pharmacol. 1987;32:81–83. doi: 10.1007/BF00609962. [DOI] [PubMed] [Google Scholar]
- 6.Kubota Y, Toyoda Y, Kubota H, Asada A. Epinephrine in local anesthetics does indeed produce hypokalemia and ECG changes. Anesth Analg. 1993;77:867–868. doi: 10.1213/00000539-199310000-00045. [DOI] [PubMed] [Google Scholar]
- 7.Mishra N, Birmiwal KG, Pani N, Raut S, Sharma G, Rath KC. Sedation in oral and maxillofacial day care surgery: a comparative study between intravenous dexmedetomidine and midazolam. Natl J Maxillofac Surg. 2016;7:178–185. doi: 10.4103/njms.NJMS_78_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Zhang T, Huang L, Peng W. Comparison between dexmedetomidine and midazolam for sedation in patients with intubation after oral and maxillofacial surgery. Biomed Res Int. 2020;2020:7082597. doi: 10.1155/2020/7082597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–227. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 10.Calasans-Maia JA, Zapata-Sudo G, Sudo RT. Dexmedetomidine prolongs spinal anaesthesia induced by levobupivacaine 0.5% in guinea-pigs. J Pharm Pharmacol. 2005;57:1415–1420. doi: 10.1211/jpp.57.11.0006. [DOI] [PubMed] [Google Scholar]
- 11.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–256. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 13.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–1119. doi: 10.1097/ALN.0b013e3181bbcc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–1551. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 15.Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107:96–101. doi: 10.1213/ane.0b013e318176be73. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 17.Taleb R, Koutlas IG, Argyris PP. Immunohistochemical and histochemical characterization of intraosseous arteriovenous malformations of the jaws: analysis of 16 cases with emphasis on GLUT-1 immunophenotype. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124:165–174. doi: 10.1016/j.oooo.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Talke P, Anderson BJ. Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol. 2018;84:1364–1372. doi: 10.1111/bcp.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gown AM, Vogel AM, Gordon D, Lu PL. A smooth muscle-specific monoclonal antibody recognizes smooth muscle actin isozymes. J Cell Biol. 1985;100:807–813. doi: 10.1083/jcb.100.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weerink MAS, Struys MMRF, Hannivoort LN, Barends CRM, Absalom AR, Colin P. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56:893–913. doi: 10.1007/s40262-017-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaai H, Tomita S, Nakaike Y, Ganzberg S, Yamazaki S. Intravenous sedation for implant surgery: midazolam, butorphanol, and dexmedetomidine versus midazolam, butorphanol, and propofol. J Oral Implantol. 2014;40:94–102. doi: 10.1563/AAID-JOI-D-11-00200. [DOI] [PubMed] [Google Scholar]
- 22.Singh V, Thepra M, Kirti S, Kumar P, Priya K. Dexmedetomidine as an additive to local anesthesia: a step to development in dentistry. J Oral Maxillofac Surg. 2018;76:2091.e1–2091.e7. doi: 10.1016/j.joms.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 23.Akimoto T, Hashimoto S, Sunada K. Dexmedetomidine (12.5 µg/mL) improves tissue distribution, anesthetic action, and hemodynamic effects of lidocaine after palatal infiltration in rats. Odontology. 2016;104:390–396. doi: 10.1007/s10266-015-0221-6. [DOI] [PubMed] [Google Scholar]
- 24.Selliseth NJ, Selvig KA. The vasculature of the periodontal ligament: a scanning electron microscopic study using corrosion casts in the rat. J Periodontol. 1994;65:1079–1087. doi: 10.1902/jop.1994.65.11.1079. [DOI] [PubMed] [Google Scholar]
- 25.Tomita S, Yamazaki S, Togami K, Tada H, Kawaai H. The effect of dexmedetomidine on oral mucosal blood flow and the absorption of lidocaine. Anesth Prog. 2018;65:168–176. doi: 10.2344/anpr-65-03-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talke P, Stapelfeldt C, Lobo E, Brown R, Scheinin M, Snapir A. Alpha-2B adrenoceptor polymorphism and peripheral vasoconstriction. Pharmacogenet Genomics. 2005;15:357–363. doi: 10.1097/01213011-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Kudo K, Ambe K, Kawaai H, Yamazaki S. A histological study of vasoconstriction by local anesthetics in mandible. Anesth Prog. 2018;65:244–248. doi: 10.2344/anpr-65-03-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibricevic H, Heyeraas KJ, Pasic Juhas E, Hamamdzic M, Djordjevic N, Krnic J. Identification of alpha 2 adrenoceptors in the blood vessels of the dental pulp. Int Endod J. 1991;24:279–289. doi: 10.1111/j.1365-2591.1991.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 29.Freezer SR, Sims MR. Morphometry of neural structures in the mouse periodontal ligament mesial to the mandibular first molar. Aust Orthod J. 1989;11:30–37. [PubMed] [Google Scholar]
- 30.Varshavskiĭ AI, Levin VN. Functional anatomy of the blood vessels of the dental pulp in the white rat. Arkh Anat Gistol Embriol. 1983;84:49–56. [PubMed] [Google Scholar]