Abstract
Background:
Urothelial carcinoma is a significant health concern in the United States (US), with high mortality and economic burdens. The CheckMate-901 trial showed promising survival benefits for nivolumab combined with gemcitabine and cisplatin followed by nivolumab maintenance therapy (nivolumab-combination) as first-line treatment of unresectable or metastatic urothelial carcinoma (UC), but its cost-effectiveness is unclear.
Objectives:
This study aimed to evaluate the cost-effectiveness of the nivolumab-combination versus standard chemotherapy (gemcitabine–cisplatin) for advanced UC from the perspective of healthcare payers in the US.
Design:
A model-based pharmacoeconomic evaluation.
Methods:
Based on the CheckMate-901 study, a three-state Markov model (progression-free, progression, and death) was developed to evaluate the cost-effectiveness of nivolumab-combination versus gemcitabine–cisplatin as a first-line treatment for unresectable or metastatic UC. The model’s outputs included quality-adjusted life years (QALYs) and costs and were used to calculate the incremental cost-effectiveness ratio (ICER). Costs included drug prices, adverse event management, and healthcare resource utilization from a US healthcare payer’s perspective. State utilities were derived from published literature. One-way sensitivity analysis and probabilistic sensitivity analysis were used to test model robustness. Scenario analyses for drug costs in the UK and Australian health systems were performed.
Results:
Compared with gemcitabine–cisplatin, the nivolumab-combination resulted in an additional 0.416 QALYs at an incremental cost of $90,523, yielding an ICER of $217,527 per QALY. Sensitivity analyses indicated significant impacts from the cost of nivolumab maintenance therapy.
Conclusion:
Compared with gemcitabine–cisplatin, nivolumab-combination therapy is not cost-effective for unresectable or metastatic UC at a $100,000 per QALY threshold. High drug prices in the US significantly impact cost-effectiveness, highlighting the need for price negotiations and healthcare policy adjustments to balance innovation incentives and patient affordability.
Keywords: cost-effectiveness analysis, nivolumab, urothelial carcinoma
Plain language summary
Evaluating the cost-effectiveness of nivolumab and chemotherapy for advanced bladder cancer treatment
Our study compared a cancer treatment called nivolumab-combination with the standard chemotherapy called gemcitabine-cisplatin for people with a type of cancer called “urothelial carcinoma” that has spread or cannot be removed by surgery. We found that while nivolumab-combination slightly improves quality and length of life, it’s much more expensive. We calculated a number called the “ICER” to help us decide if the extra cost is worth it. The ICER was much higher than what we usually consider a good value, which is $100,000 per extra year of good health. We also discovered that the high drug cost of nivolumab affected the cost-effectiveness most. If all drug prices were like in the UK or Australia, the treatment could be affordable. In short, based on our analysis, nivolumab-combination wasn’t a cost-effective first-line treatment for this cancer from a U.S. healthcare payer’s viewpoint, especially considering the $100,000/QALY threshold. This highlights the need for negotiation on drug pricing and health policy to improve access to affordable treatments.
Introduction
Bladder cancer, accounting for 90% of urothelial carcinoma (UC), 1 is the sixth most common cancer in the United States (US), 2 and is associated with high mortality, morbidity, and substantial treatment costs.3,4 Advances in immuno-oncology have revolutionized the treatment paradigm for unresectable or metastatic UC. Five immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) or programmed death ligand-1 (PD-L1) for metastatic and locally advanced bladder cancer have been approved. However, new immunotherapies often come with steep prices due to high ICI drug development costs.
In the US, high medication costs have raised public concern, burdening patients and the healthcare system, and leading some to abandon treatment. This has prompted the government to seek solutions. The Inflation Reduction Act (IRA) legally authorizes the negotiation of drug prices between Medicare, the Federal Health Insurance Program, and pharmaceutical companies. 5 Attempts to regulate drug prices face resistance from pharmaceutical companies, which argue that drug prices could undermine innovation. 6 Finding solutions that balance incentivizing innovation and protecting patient interests is particularly critical. Increasing constraints on healthcare resources will further integrate cost-effectiveness analysis (CEA) into health policy to maximize outcomes and reduce costs. 7
A phase III CheckMate-901 study reported breakthrough results showing that in the first-line treatment of unresectable or metastatic UC, nivolumab combined with cisplatin-containing chemotherapy achieved significant results in the dual primary endpoints of overall survival (OS) and progression-free survival (PFS) compared to standard cisplatin-containing chemotherapy. 8 The National Comprehensive Cancer Network (NCCN) Bladder Cancer Guidelines 2024 (version 3) upgraded the recommendation for nivolumab, gemcitabine, and cisplatin followed by nivolumab maintenance therapy from category 2A to category 1. 9 However, the cost-effectiveness of this therapy remains unclear. This study assessed the cost-effectiveness of nivolumab combined with gemcitabine plus cisplatin followed by nivolumab maintenance therapy (nivolumab-combination) versus carboplatin plus gemcitabine (gemcitabine–cisplatin) as the first line of therapy (LOT) for patients with unresectable or metastatic UC from a US third-party healthcare payer’s perspective.
Materials (patients) and methods
This study adheres to the Consolidated Health Economic Evaluation Reporting Standards 2022 guidelines 10 (Supplemental Material 2).
Patient population
The model population comprised patients with previously untreated unresectable or metastatic UC eligible for cisplatin-based therapy. The baseline characteristics included a median age of 65 years, 8 and an average body surface area of 1.85 m2. 11
Model structure
A Markov model estimated the health outcomes and costs of nivolumab plus chemotherapy versus chemotherapy alone in the target patient population from a US third-party healthcare payer’s perspective. The Markov model was implemented using Microsoft Excel 2021. Patients transitioned through three health states: PFS, progressive disease (PD), and death. All patients were in a PFS state and remained there until disease progression or death. The PD state included all patients who were alive following progression, whereas death was an absorbing state where patients remained until the model’s end. The model’s outputs included quality-adjusted life years (QALYs) and costs and were used to calculate the incremental cost-effectiveness ratio (ICER). Costs and health outcomes were discounted at an annual rate of 3%. 12 Each model cycle spanned 3 weeks over a 20-year horizon.
Transition probability estimates
Survival data were extracted from the CheckMate-901 survival curves using GetData Graph Digitizer software (version 2.26; http://www.getdata-graph-digitizer.com/download.php). Kaplan–Meier survival analysis was performed using R software (version 4.2.3). A comparison of CheckMate-901 Kaplan–Meier survival curves with corresponding fitted models is shown in Supplemental Figure S1. The Akaike information criterion and the Bayesian information criterion, combined with visual inspection and the clinical plausibility of extrapolated curves, were used to select the best-fit parametric distributions for the base case (Supplemental Figures S1 and S2). The probability of a transition from a progression-free state to a progression-free state was derived from the PFS curves, and the probability of a transition from any state to the death state was derived from the OS curves in the CheckMate-901 trial. 8 Survival curves with log-logistic and log-normal distributions were selected as the base-case curves for the nivolumab and gemcitabine–cisplatin groups, respectively (Supplemental Table S1).
The proportion of patients in each health state at given time points was calculated based on independently estimated parametric survival curves for PFS, OS, and general population mortality calculated from US life tables. 13
Cost estimates
Only direct medical costs were considered, including PD-L1 status detection, routine imaging examinations before treatment, drugs, administrations, hospice care, adverse event (AE) management, and healthcare resource utilization (HRU) (Table 1). The costs of detection, examinations, and administrations were acquired from the 2024 National Physician Fee Schedule Relative Value File. 14 The drug costs were obtained from the Payment Allowance Limits for Medicare Part B Drugs. 15 The costs of hospice care, 11 AE management, 11 and HRU16,17 were derived from references. All costs are presented in US dollars, adjusted to January 2024 values using an annual discount rate of 3%. 12
Table 1.
Baseline values and their variation ranges of all parameters.
| Parameters | Baseline | Variation range | Reference | |
|---|---|---|---|---|
| Costs of drugs per cycle ($) | ||||
| Nivolumab 360 mg | 11,192.04 | 5596.02 | 13,430.49 | HCPCS Code: J929915 |
| Nivolumab 480 mg | 14,922.72 | 7461.36 | 17,907.26 | HCPCS Code: J929915 |
| Pembrolizumab 200 mg | 11146 | 5573 | 13,375.20 | HCPCS Code: J927115 |
| Cisplatin 70 mg/m2 | 28.27 | 22.616 | 33.92 | HCPCS Code: J906015 |
| Carboplatin 400 mg/m2 | 53.27 | 42.616 | 63.92 | HCPCS Code: J904515 |
| Gemcitabine 200 mg/m2 | 67.54 | 54.032 | 81.05 | HCPCS Code: J920115 |
| Docetaxel 100 mg/m2 | 184.08 | 147.264 | 220.90 | HCPCS Code: J917115 |
| Paclitaxel injection 240 mg/m2 | 47.95 | 38.3616 | 57.54 | HCPCS Code: J926715 |
| Paclitaxel protein 260 mg/m2 | 6875.41 | 5500.328 | 8250.49 | HCPCS Code: J926415 |
| Drug administration costs per time ($) | ||||
| Hydration (initial hour) | 31.76 | 25.41 | 38.11 | CPT: 9636014 |
| Hydration (additional hour) | 12.12 | 9.7 | 14.54 | CPT: 9636114 |
| Pretreatment of docetaxel | 15.94 | 12.75 | 19.13 | HCPCS Code: J854014 |
| Pretreatment of paclitaxel | 106.81 | 85.45 | 128.17 | HCPCS Code: J8540, J1200, S002315
CPT: 96372, 9637414 |
| First-hour chemo infusion | 127.05 | 101.64 | 152.46 | CPT: 9641414 |
| Additional hour chemo infusion | 27.18 | 21.74 | 32.62 | CPT: 9641514 |
| Subsequent chemo infusion | 62.54 | 50.03 | 75.05 | CPT: 9641714 |
| Test before treatment | 424.69 | 339.75 | 509.63 | CPT: 88360, 49180, 7701214 |
| Healthcare resource utilization costs per cycle ($) | ||||
| HRU in PFS state (NC group) | 3294.71 | 2635.77 | 3953.65 | 16 |
| HRU in PD state (NC group) | 5596.79 | 4477.43 | 6716.15 | 16 |
| HRU in PFS state (GC group) | 8768.99 | 7015.19 | 10,522.79 | 17 |
| HRU in PD state treated with pembrolizumab (GC group) | 3768.21 | 3014.57 | 4521.85 | 16 |
| HRU in PD state after being treated with pembrolizumab (GC group) | 5694.07 | 4555.26 | 6832.88 | 16 |
| HRU in PD state treated with Taxanes (GC group) | 6690.22 | 5352.18 | 8028.27 | 17 |
| Hospice care | 999.33 | 841.01 | 1157.64 | 11 |
| One-off AE management costs ($) | ||||
| AE management (NC group) | 5838.59 | 5375.37 | 6301.80 | 11 |
| AE management (GC group) | 4566.76 | 4208.64 | 4924.88 | 11 |
| Utilities and disutilities | ||||
| PFS | 0.842 | 0.76 | 0.93 | 22 |
| PD | 0.8 | 0.72 | 0.88 | 22 |
| Disutility of AE (NC group) | 0.068 | 0.06 | 0.07 | 23 |
| Disutility of AE (GC group) | 0.053 | 0.05 | 0.06 | 23 |
| Other parameters | ||||
| Probability of using paclitaxel (paclitaxel or docetaxel) | 0.5 | 0 | 1 | – |
| Probability of using paclitaxel injection (paclitaxel injection or nab-paclitaxel) | 0.5 | 0 | 1 | – |
| Probability of using pembrolizumab (pembrolizumab or taxanes) | 0.5 | 0 | 1 | – |
| Discount rate per cycle (%) | 0.1726 | 0.1553 | 0.1899 | Annual discount rate: 3% 12 |
AE, adverse events; CPT, Current Procedural Terminology, from CMS Physician Fee Schedule; GC, gemcitabine–cisplatin group, carboplatin plus gemcitabine; HCPCS Code, Healthcare Common Procedure Coding System code, Payment Allowance Limits for Medicare Part B Drugs; HRU, healthcare resource utilization; NC, nivolumab-combination group, nivolumab combined with gemcitabine plus cisplatin followed by nivolumab maintenance therapy; PD, progressive disease; PFD, progression-free disease.
The drug costs for the nivolumab-combination group in the PFS state were calculated for patients treated with nivolumab (360 mg) combined with GC (gemcitabine 1000 mg/m² on days 1 and 8, and cisplatin 70 mg/m²) every 3 weeks (E3W) for up to 6 cycles, followed by nivolumab (480 mg) every 4 weeks (E4W) until disease progression or up to a maximum of 2 years. 8 After disease progression, the costs of docetaxel 100 mg/m2 E3W 18 or paclitaxel (paclitaxel injection 80 mg/m2 on days 1, 8, 15, and 22 E4W 19 or nanoparticle albumin-bound paclitaxel (nab-paclitaxel) 260 mg/m² E3W 20 ) for PD patients in the nivolumab combination group were calculated until death. The drug costs for the PFS of patients in the gemcitabine–cisplatin group were calculated for GC (gemcitabine 1000 mg/m2 on days 1 and 8, and cisplatin 70 mg/m2) E3W for up to 6 cycles. After progression, drug costs for the second LOT were calculated for chemotherapy (docetaxel or paclitaxel until death), or pembrolizumab (200 mg E3W) for up to 2 years. 21
All second LOT regimens were based on the NCCN Bladder Cancer Guideline 2024 recommendations and the CheckMate-901 study, 8 with assumed probabilities for each regimen. The second LOT regimen in the gemcitabine–cisplatin group included chemotherapy (docetaxel and paclitaxel) and pembrolizumab. It was assumed that 15% of patients receiving platinum therapy were given carboplatin instead of cisplatin. 8 Treatment-related grade ⩾3 AEs occurring in more than 5% of patients were assigned management costs. One-off average AE costs were totaled for each treatment arm and applied at the start of the model based on each AE probability and corresponding management cost. In the nivolumab-combination group, HRU when treated with PD-1/L1 inhibitor therapies as the first LOT and after discontinuing PD-1/L1 inhibitor therapy (receiving chemotherapy or no treatments) were obtained and defined as HRU in the PFS state and PD state, respectively. In the gemcitabine–cisplatin group, HRU is divided into four parts: receiving platinum-based chemotherapy in the PFS state, receiving PD-1/L1 inhibitor therapy as the second LOT in the PD state, discontinuing PD-1/L1 inhibitor therapy in the PD state, and receiving other chemotherapy therapies as the second LOT in the PD state.
Utility estimates
For each health state, a specific quality-of-life adjustment weight (a utility, where 1 represents full health and 0 represents death) was assigned to calculate the cumulative QALYs over the modeled time horizon. The average utility was 0.842 for progression-free patients and 0.800 for patients with disease progression. 22 The one-off average AE disutilities in both the nivolumab and gemcitabine–cisplatin groups were calculated based on each treatment-related grade ⩾3 AE probability 8 and the corresponding disutility 23 (Table 2) and were applied at the start of the model.
Table 2.
Reported incidence rates and values of each treatment-related grade ⩾3 adverse events.
| Adverse event | Reported incidence rate (%) | Cost and disutility values | ||
|---|---|---|---|---|
| Nivolumab-combination | Gemcitabine–cisplatin | Cost in January 2024 USD (2015 USD) | Disutility | |
| Anemia | 22 | 17.7 | 8796.53 (6944.06) | 0.073 |
| Neutropenia | 22 | 17.7 | 15,512.63 (12,245.81) | 0.2 |
| Decreased neutrophil count | 22 | 17.7 | 15,546.49 (12,272.54) | 0.2 |
| Decreased platelet count | 18.8 | 15.3 | 8277.05 (6533.98) | 0.2 |
| Decreased white-cell count | 18.8 | 15.3 | 12,986.55 (10,251.70) | 0.19 |
| Thrombocytopenia | 18.8 | 15.3 | 12,986.55 (10,251.70) | 0.19 |
Sensitivity analysis
A series of sensitivity analyses were conducted to evaluate the robustness of the model and address the uncertainty in variable estimation. In one-way sensitivity analyses, the value of one parameter at a time was varied over its defined range, and the effect on the ICER was represented using a tornado diagram. Utilities varied within a 10% range. The costs reported in the literature varied according to the range of cost changes they provided. 11 For ICIs, which are typically considered to have more potential for price reductions, the range of price variation was set at 50%–120%. Other costs obtained from the price list varied within 20% of their baseline values (Table 2).
In the probabilistic sensitivity analysis (PSA), the model was run 1000 times, each time all parameters were randomly varied simultaneously according to their sampling distributions.
Scenario analysis
We performed an additional scenario analysis using only drug prices in the US, UK, and Australia for comparison with each country’s gross domestic product (GDP) per capita in 2024. 24 In addition, we substituted the US medicine prices with those of the UK and Australia, assumed that other costs remained unchanged, and evaluated their cost-effectiveness. The cost of medication in the UK was obtained from the British National Formulary. 25 Medication costs in Australia were obtained from the Pharmaceutical Benefits Scheme. 26 All costs are converted into US dollars at the exchange rate as of January 1, 2024.
Results
Base case results
In the base case analysis, patients in the nivolumab-combination group gained an additional 0.416 QALYs at an incremental cost of $90,523, resulting in an ICER of $217,527 per QALY compared with the gemcitabine-cisplatin group.
Sensitivity analysis
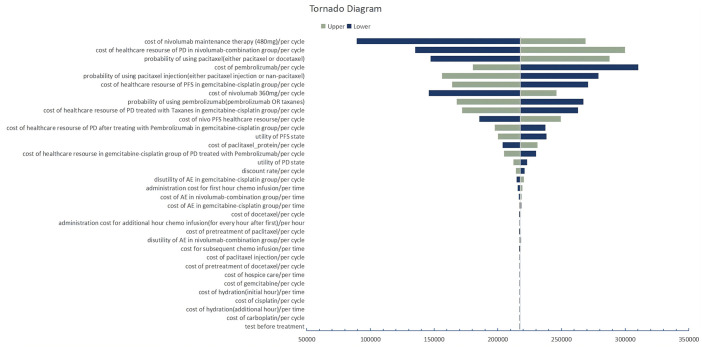
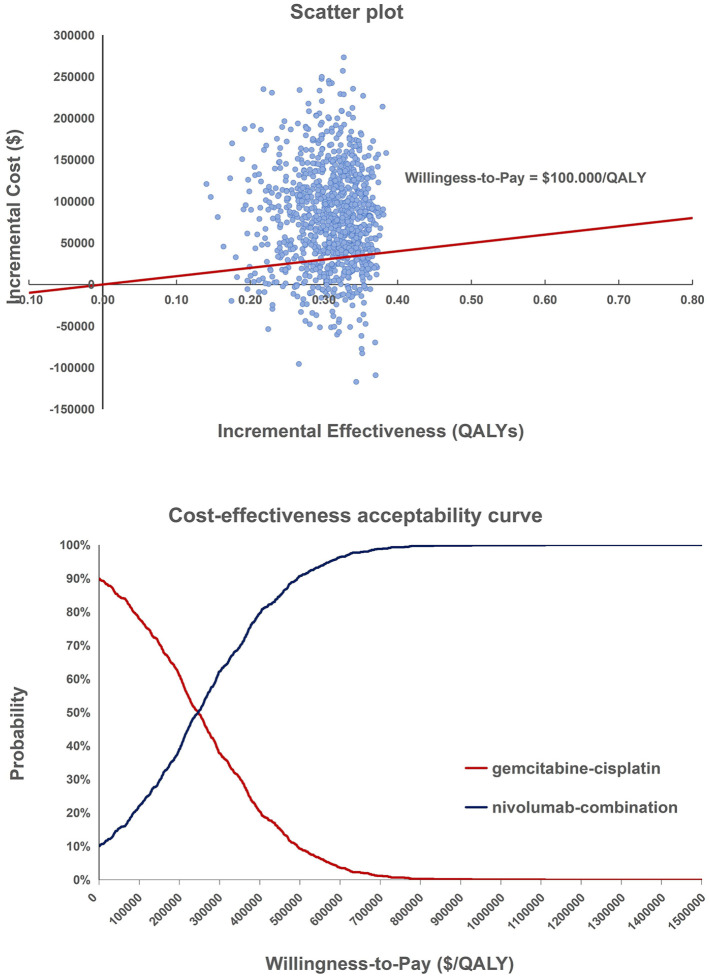
One-way sensitivity analyses revealed that parameters affecting the cost of nivolumab maintenance therapy, the cost of healthcare resources for PD in the nivolumab-combination group, and the probability of using paclitaxel (either paclitaxel or docetaxel) had the greatest impact on the model results (Figure 1). PSA based on 1000 iterations indicated that the nivolumab-combination regimen was more cost-effective than the gemcitabine–cisplatin regimen with a probability of 23.3% at a willingness-to-pay (WTP) threshold of $100,000 (Figure 2).
Figure 1.
Tornado diagram of the one-way sensitivity analysis.
Figure 2.
Scatter plot and cost-effectiveness acceptability curve of the probabilistic sensitivity analysis.
Scenario analysis
The drug costs in the UK and Australian health systems are reported in Supplemental Table S2. The ICERs for the US, the UK, and Australia are 2.996, 2.164, and 1.361 times the GDP per capita of each country, respectively, when only drug prices are considered. When other costs remain unchanged, the ICER was $55,108 after the UK drug price was replaced and $32,894 after the Australian drug price was replaced (Supplemental Table S3).
Discussion
The model results indicate that nivolumab combined with gemcitabine plus cisplatin followed by nivolumab maintenance therapy was not a cost-effective regimen compared to cisplatin plus gemcitabine as the first LOT for patients with unresectable or metastatic UC from a US third-party healthcare payer’s perspective. The ICER for this therapy was well above the WTP threshold of $100,000, making it not a cost-effective option for this population.
According to the sensitivity analysis, the significant impact of choosing paclitaxel or docetaxel on the ICER may be due to the substantial price discrepancy between docetaxel and nab-paclitaxel. The second most significant factor was the cost of HRU for patients who progressed after nivolumab therapy and who received taxanes chemotherapy. The lower Objective Response Rate against PD-L1 in these patients indicates that the disease was more severe and required more expensive HRU. The significant impact of nivolumab maintenance therapy costs could be attributed to two primary factors. First, the cost of the drug exhibited a considerable range of fluctuations, spanning from 50% to 120% of its baseline value. The lower limit of 50% reflected the potential for substantial price reductions due to negotiations and the drug’s margin for cost reduction. The upper limit of 120% accounted for variations within 20% of the baseline values, considering that alternative public and private payers might incur higher expenses. Second, the extended duration of nivolumab use as a maintenance therapy amplifies the impact of these cost variations over time.
For the one-way sensitivity analysis, the parameters were set to fluctuate differently. For those derived from the literature, the reported ranges were the most realistic. For costs, a variation within 20% of their baseline values was considered, accounting for alternative public and private payers who may pay less or more, respectively. For specific ICIs, costs had a lower variation of 50%, as estimated by the Congressional Budget Office, which predicted that net prices would decrease on average due to negotiation. 27
In the currently published research, several studies focus on the CEA of ICIs as the initial first LOT for advanced or metastatic UC.11,28,29 Qin et al. 29 reported that atezolizumab plus platinum-based chemotherapy is not cost-effective as the first LOT for patients with metastatic UC. Hale et al. 11 concluded that pembrolizumab was more cost-effective than carboplatin plus gemcitabine in patients with advanced or metastatic UC who were PD-L1 positive and unsuitable for cisplatin treatment. This discrepancy may be due to the KEYNOTE-052 study focusing on patients with positive PD-L1 expression, who derive greater benefit from immunotherapy. 30 Based on the CheckMate-901 study, Naqvi’s study suggested that, considering only drug costs, nivolumab-combination therapy was likely the most cost-effective option for cisplatin-eligible patients from a U.S. payer perspective, with an ICER of $21,127 per QALY. 28 However, in our research, when considering drug prices alone, the ICER value increased to $255,795.23 per QALY (Supplemental Table S3). The discrepancy may stem from the fact that Naqvi’s study presumed all control group patients received avelumab maintenance therapy as the first LOT, whereas the CheckMate-901 study designated the first LOT as receiving platinum-based chemotherapy alone. In the Supplemental Materials of the CheckMate-901 study, only 10.5% of patients received avelumab treatment. The high cost of avelumab ($12,932 per cycle), coupled with the unlimited treatment duration, significantly increased the cost for the control group, leading to a lower ICER value. Conversely, our study adhered to the design of the CheckMate-901 study. Furthermore, our research considered additional costs associated with drug administration, hospice care, AEs management, and HRU, providing a more authentic representation of the economic burden of treatments. After considering other costs, the ICER value was slightly reduced (from $255,795 to $217,527 per QALY) but still significantly exceeded the WTP threshold of $100,000 per QALY.
According to the scenario analysis, when considering drug prices alone, the ICER in the US was approximately 3 times the GDP per capita, which was 1.5–2 times that of the UK and Australia. When drug prices were replaced, the ICERs decreased significantly from $217,527 to $55,108 (UK) and $32,894 (Australia). This result indicates the high price of drugs in the US.
The issue of high drug prices in the US has a long-standing history. Drug prices in the US were three to five times higher than those in other developed countries 31 and some have risen with an average annual increase of 20%. 32 The heavy burden of fiscal spending has prompted an urgent push for drug pricing reform in the US. In August 2022, the US government formally passed the IRA, which gave Medicare the power to negotiate drug prices for the first time, has commenced substantive price negotiations, 5 and the price caps for selected drugs will be set at 40%–75% of their average price. 33 However, high drug prices have incentivized pharmaceutical companies to innovate and develop while accelerating the rate at which new drugs reach patients. Finding a balance between high drug prices and stimulating innovation while meeting the therapeutic needs and desired benefits remains a complex issue, which enables more US patients to receive the best treatments and increases revenue for pharmaceutical companies through greater drug utilization. A standard, well-validated method is CEA, which considers both cost and efficacy in its specific indication and plays a crucial role in healthcare provider compensation decisions in many European countries. Based on the results of this CEA, the overall ICER may meet the WTP threshold if the drug price is reduced by 50%, which may provide a meaningful reference for US price negotiations.
This study has several limitations and strengths.
This model did not use the specific proportions of subsequent treatments reported by CheckMate-901. The second LOT regimen was based on the NCCN Bladder Cancer Guidelines, 9 excluding the expensive new therapies erdafitinib and enfortumab vedotin-ejfv to avoid significant interference with the results. It was reported that 16 among patients who received subsequent therapy, the most commonly used were single-agent taxanes.
The utility values in this model were based on previous clinical studies, and the same utility values were assigned before and after progression for both groups. 22 However, according to the CheckMate-901 results, the EORTC QLQ-C30 global health status remained stable, with no change of more than 10 points. 8 In addition, by defining the different HRU costs of various treatment options, the model indirectly reflected the potential benefits of these treatments, compensating for the uniform utility values. HRU costs were slightly greater following the discontinuation of PD-1/L1 inhibitor therapy than before discontinuation, largely driven by hospitalizations, outpatient services, and palliative care, which offset reductions in systemic therapy costs. 16 In addition, the parameters indicated that patients receiving chemotherapy alone may incur higher HRU costs than those receiving PD-1/L1 inhibitor treatment.16,17
Conclusion
Compared with gemcitabine-cisplatin, nivolumab-combination therapy is not cost-effective for unresectable or metastatic UC at a $100,000 per QALY threshold. High drug prices in the US significantly impact cost-effectiveness, highlighting the need for price negotiations and healthcare policy adjustments to balance innovation incentives and patient affordability.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241301339 for Cost-effectiveness of nivolumab combined with chemotherapy as a first-line therapy for patients with unresectable or metastatic urothelial carcinoma by Jingwen Lin, Xiaobing Song, Wu Fu, Caicong You, Na Li, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241301339 for Cost-effectiveness of nivolumab combined with chemotherapy as a first-line therapy for patients with unresectable or metastatic urothelial carcinoma by Jingwen Lin, Xiaobing Song, Wu Fu, Caicong You, Na Li, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iDs: Jingwen Lin  https://orcid.org/0009-0001-8441-0298
https://orcid.org/0009-0001-8441-0298
Maobai Liu  https://orcid.org/0000-0003-1519-0778
https://orcid.org/0000-0003-1519-0778
Hongfu Cai  https://orcid.org/0000-0002-4923-4882
https://orcid.org/0000-0002-4923-4882
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jingwen Lin, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian, China; The School of Pharmacy, Fujian Medical University, Fuzhou, Fujian, China.
Xiaobing Song, Department of Quality Management, Ganzhou Fifth People’s Hospital, Ganzhou, Jiangxi, China.
Wu Fu, The School of Pharmacy, Fujian Medical University, Fuzhou, Fujian, China.
Caicong You, The School of Pharmacy, Fujian Medical University, Fuzhou, Fujian, China.
Na Li, Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian, China.
Maobai Liu, Department of Pharmacy, Fujian Medical University Union Hospital, Xinquan Road 29, Fuzhou, Fujian 350001, China.
Hongfu Cai, Department of Pharmacy, Fujian Medical University Union Hospital, Xinquan Road 29, Fuzhou, Fujian 350001, China.
Declarations
Ethics approval and consent to participate: This research did not involve any human or animal subjects, thus ethical approval and consent to participate were not required.
Consent for publication: All authors participated in this study and approved the final version.
Author contributions: Jingwen Lin: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Xiaobing Song: Data curation; Formal analysis.
Wu Fu: Software; Writing – review & editing.
Caicong You: Data curation; Formal analysis; Software.
Na Li: Methodology; Supervision; Visualization.
Maobai Liu: Funding acquisition; Visualization.
Hongfu Cai: Conceptualization.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Data are available for bona fide researchers who request it from the authors.
References
- 1. Antoni S, Ferlay J, Soerjomataram I, et al. Bladder cancer incidence and mortality: a global overview and recent. Eur Urol 2017; 71: 96–108. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute. Cancer stat facts: bladder cancer. SEER, https://seer.cancer.gov/statfacts/html/urinb.html (2024, accessed 23 May 2024). [Google Scholar]
- 3. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet 2016; 388: 2796–2810. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol 2011; 12: 933–980. [DOI] [PubMed] [Google Scholar]
- 5. BBC News. Biden names 10 drugs for Medicare price negotiations, https://www.bbc.com/news/health-57968726 (2023, accessed 23 May 2024).
- 6. Pharmaceutical Research and Manufacturers of America (PhRMA). Litigation asserts price-setting provisions in the inflation reduction act are unconstitutional, https://phrma.org/resource-center/Topics/Access-to-Medicines/Release-NICA-GCCA-PhRMA-Litigation-Asserts-Price-Setting-Provisions-in-the-Inflation-Reduction-Act-are-Unconstitutional (2023, accessed 23 May 2024).
- 7. Walia AS, Sweis RF, Agarwal PK, et al. Cost-effectiveness of immune checkpoint inhibitors in urothelial carcinoma—a review. Cancers (Basel) 2021; 14: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N Engl J Med 2023; 389: 1778–1789. [DOI] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network (NCCN). Recently published guidelines, https://www.nccn.org/guidelines/recently-published-guidelines (2024, accessed 26 April 2024).
- 10. Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022; 25(1): 10–31. [DOI] [PubMed] [Google Scholar]
- 11. Hale O, Patterson K, Lai Y, et al. Cost-effectiveness of pembrolizumab versus carboplatin-based chemotherapy as first-line treatment of PD-L1-positive locally advanced or metastatic urothelial carcinoma ineligible for cisplatin-based therapy in the United States. Clin Genitourin Cancer 2021; 19: e17–e30. [DOI] [PubMed] [Google Scholar]
- 12. Neumann PJ, Sanders GD, Russell LB, et al. (eds). Cost-effectiveness in health and medicine. 2nd ed. New York: Oxford University Press, 2016. [Google Scholar]
- 13. Human Mortality Database. Country: USA, https://mortality.org/Country/Country?cntr=USA (2024, accessed 12 March 2024).
- 14. CMS. Medicare physician fee schedule relative value files, https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a (2024, accessed 26 February 2024).
- 15. United States Department of Health and Human Services. Healthcare cost and utilization project, https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files (2024, accessed 26 February 2024).
- 16. Morgans AK, Hepp Z, Shah SN, et al. Real-world burden of illness and unmet need in locally advanced or metastatic urothelial carcinoma following discontinuation of PD-1/L1 inhibitor therapy: a Medicare claims database analysis. Urol Oncol 2021; 39: 733.e1–733.e10. [DOI] [PubMed] [Google Scholar]
- 17. Aly A, Johnson C, Yang S, et al. Overall survival, costs, and healthcare resource use by line of therapy in Medicare patients with newly diagnosed metastatic urothelial carcinoma. J Med Econ 2019; 22: 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCaffrey JA, Hilton S, Mazumdar M, et al. Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 1997; 15: 1853–1857. [DOI] [PubMed] [Google Scholar]
- 19. Vaughn DJ, Broome CM, Hussain M, et al. Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 2002; 20: 937–940. [DOI] [PubMed] [Google Scholar]
- 20. Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol 2013; 14: 769–776. [DOI] [PubMed] [Google Scholar]
- 21. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patterson K, Prabhu V, Xu R, et al. Cost-effectiveness of pembrolizumab for patients with advanced, unresectable, or metastatic urothelial cancer ineligible for cisplatin-based therapy. Eur Urol Oncol 2019; 2: 565–571. [DOI] [PubMed] [Google Scholar]
- 23. Lu Y, Dai Z, Chang F, et al. Whether and how disutilities of adverse events were used in the economic evaluation of drug therapy for cancer treatment. Pharmacoeconomics 2023; 41: 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Economic Outlook (April 2024)—GDP per capita, current prices (imf.org), https://www.imf.org/external/datamapper/NGDPDPC@WEO/OEMDC/ADVEC/WEOWORLD/GBR (2024, accessed 29 May 2024).
- 25. British National Formulary (BNF). https://bnf.nice.org.uk/ (2024, accessed 26 February 2024).
- 26. Pharmaceutical Benefits Scheme (PBS). A–Z medicine listing, https://www.pbs.gov.au/browse/medicine-listing?initial=n (2024, accessed 11 May 2024).
- 27. American Progress. Medicare drug price negotiation will lower prices by thousands of dollars per month, https://www.americanprogress.org/article/medicare-drug-price-negotiation-will-lower-prices-by-thousandsof-dollars-per-month/ (2024, accessed 23 May 2024).
- 28. Naqvi SAA, Faisal KS, Raina A, et al. Cost-effectiveness analysis of contemporary first-line (1L) agents in locally advanced/metastatic urothelial carcinoma (la/mUC). J Clin Oncol 2024; 42: 4591. [Google Scholar]
- 29. Qin S, Yi L, Li S, et al. Cost-effectiveness of atezolizumab plus chemotherapy as first-line therapy for metastatic urothelial cancer. Adv Ther 2021; 38: 3399–3408. [DOI] [PubMed] [Google Scholar]
- 30. Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 2020; 20: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Government Accountability Office. Drug prices: manufacturer discounts in the 340B program offer benefits, but federal oversight needs improvement, https://www.gao.gov/products/gao-21-282 (2021, accessed 23 May 2024).
- 32. Rome BN, Egilman AC, Kesselheim AS. Trends in prescription drug launch prices, 2008–2021. JAMA 2022; 327: 2145–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Congressional Budget Office. The budget and economic outlook: 2021 to 2031, https://www.cbo.gov/publication/58850 (2023, accessed 23 May 2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241301339 for Cost-effectiveness of nivolumab combined with chemotherapy as a first-line therapy for patients with unresectable or metastatic urothelial carcinoma by Jingwen Lin, Xiaobing Song, Wu Fu, Caicong You, Na Li, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241301339 for Cost-effectiveness of nivolumab combined with chemotherapy as a first-line therapy for patients with unresectable or metastatic urothelial carcinoma by Jingwen Lin, Xiaobing Song, Wu Fu, Caicong You, Na Li, Maobai Liu and Hongfu Cai in Therapeutic Advances in Medical Oncology