Abstract
Minimally manipulated nasal secretions, an accessible form of airway surface fluid, were tested against indigenous and added bacteria by using CFU assays. Antimicrobial activity was found to vary between donors and with different target bacteria and was markedly diminished by dilution of the airway secretions. Donor-to-donor differences in electrophoresis patterns of nasal secretions in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and acid urea-PAGE analyses were readily observed, suggesting that polymorphic genes encode the secreted proteins. Three donors (of twenty-four total), whose nasal fluid yielded similar protein band patterns and did not kill indigenous bacteria, were determined to be heavy nasal carriers of Staphylococcus aureus. Their fluid was deficient in microbicidal activity toward a colonizing strain of S. aureus but the defect was corrected in vitro by a 1:1 addition of nasal fluid from noncarriers. The microbicidal activity of normal fluid was inactivated by heating it for 10 min to 100°C and could not be restored solely by the addition of two major nasal antimicrobial proteins, lysozyme and lactoferrin. Several other known antimicrobial proteins and peptides, including statherin, secretory phospholipase A2, and defensins, were identified in nasal secretions and likely contribute to their total antimicrobial properties. Nasal fluid may serve as a useful model for the analysis of lower-airway secretions and their role in host defense against airway colonization and pulmonary infections.
Host defense is a prominent function of nasal secretions. Among the components of the secretions, mediators of adaptive immunity include immunoglobulin A (IgA) and IgG, which are produced by plasma cells juxtaposed to submucosal glands (29). The immunoglobulins are thought to act in the lumen of the airway and on the mucosa to prevent the attachment and invasion of pathogenic organisms. Mediators of innate mucosal host defense are also found in nasal secretions and include substances that selectively disrupt bacterial cell walls and membranes, sequester microbial nutrients, or act as decoys for microbial attachment. Alexander Fleming discovered the intrinsic antimicrobial properties of human nasal secretions in 1922 and attributed them to lysozyme (8). Since then, additional components of nasal secretions have been identified, including lactoferrin, mucus glycoproteins, secretory leukoprotease inhibitor (SLPI), uric acid, peroxidase, aminopeptidase, immunoglobulins, and neutral endopeptidase (reviewed in references 15 and 16). Systematic analysis of the individual and combined antimicrobial effects of these and other components has not been reported. Pathological alterations in antimicrobial properties of airway secretions may contribute to epithelial colonization by bacteria in such disorders as cystic fibrosis, chronic bronchitis, and chronic sinusitis.
The epithelial lining fluid covering the airways derives its proteins from plasma transudate, mucous and serous cells in submucosal glands, goblet cells, Clara cells, epithelial cells, and other cells within the mucosa (plasma cells, mast cells, phagocytes, and fibroblasts). The epithelial lining fluid is a bilaminar mucous layer consisting of an outer viscous gel (mucus) and a periciliary serous layer (42). Nasal epithelial cilia continually transport mucus with ensnared particulate matter and microbes toward the oropharynx, where they are swallowed. Consequently, in the normal individual, the nasal mucus blanket turns over every 10 to 20 min and must be replenished. However, fluid is relatively stagnant in the anterior portion of the nasal mucosa (15), and there the elimination of impacted microbes must be largely dependent on its intrinsic antimicrobial properties. This stagnant anterior microenvironment might serve as a useful model of torpid viscous secretions in the airways of patients with cystic fibrosis and other forms of bronchiectasis.
The limited available evidence suggests that nasal and bronchial secretions have many similarities in their composition (23). However, unlike bronchial secretions, nasal secretions can be easily collected and could provide a useful foundation for the study of other airway secretions. We reexamined the antimicrobial properties of human nasal secretions by using a CFU microassay, wherein bacteria are directly incubated with minimally manipulated nasal secretions. In contrast to recent studies that have focused on the antibacterial action of individual components of lower airway fluids (12, 27, 35, 44), the analysis of minimally manipulated fluid affords the opportunity to assess the natural antimicrobial activity within the airways.
MATERIALS AND METHODS
Collection of nasal secretions.
Nasal secretions were collected from 24 healthy volunteer donors according to a protocol approved by the University of California at Los Angeles (UCLA) Institutional Review Board. From most donors, nasal secretions were collected by vacuum-aided suction, without chemical stimulation, to avoid introducing foreign substances into the nasal fluids. Gentle manipulation of a narrow rubber-tipped vacuum device inside the nasal passageways mildly stimulated nasal secretions. The range of secretion volumes collected varied between 150 μl and 14 ml, depending on the donor. For selected collections, the secretion of nasal fluids was induced by 25 to 50 mg of methacholine chloride (Spectrum Pharmaceuticals, Irvine, Calif.) administered into one nostril by an atomizing dosimeter. The range of secretion volume varied between 0.5 and 15 ml per donor. Secretions were stored at 4°C for prompt usage or at −20°C for longer-term storage. The microbicidal activity and protein profile by acid urea-polyacrylamide gel electrophoresis (AU-PAGE) of secretions stored for 2 weeks at 4°C or >1 month at −20°C was unchanged compared to fresh samples.
Minimally manipulated fluid.
Vacuum-aspirated nasal fluid collected without methacholine was sonicated for 2 h at 4 to 8°C in a Branson 1200 sonicating water bath (Bransonic Cleaning Equipment Co., Shelton, Conn.) to disrupt the mucoprotein aggregates and to facilitate accurate handling. Unless otherwise stated, fluid samples with a high number (>105/ml) of indigenous bacteria were gamma-irradiated at 4 to 8 krads. Sonication and gamma-irradiation did not produce discernible changes in microbicidal activity (data not shown).
Cationic (poly)peptide extraction from acidified nasal fluid.
Fluids collected by methacholine stimulation were solubilized by 1:20 dilution in 5% acetic acid for 2 h at 4°C. Fluid was cleared by centrifugation at 21,000 × g for 15 min, and the resulting supernatant stored at −20°C. The supernatant was diluted threefold with distilled water (dH2O) and stirred overnight with a weak cation-exchange carboxymethyl (CM) resin (Bio-Rad, Hercules, Calif.) to extract cationic peptides and proteins. The resin was washed once with 25 mM ammonium acetate (pH 6.8), poured into a 1-by-6-cm column, and eluted on a 0.1% acetic acid–0.1 M HCl gradient at 100 μl/min. Peak fractions were tested by radial diffusion assay with Escherichia coli ML-35p and Listeria monocytogenes (39). Active fractions were subjected to reversed-phase high-pressure liquid chromatography (HPLC) (60-min gradient of 5 to 60% acetonitrile in 0.1% trifluoroacetic acid [TFA]; 4.6-by-250-mm Vydac C18 column; The Separations Group, Hesperia, Calif.). Peak fractions were tested by a gel overlay assay with E. coli ML-35p and L. monocytogenes (39). Antimicrobial (poly)peptide bands from duplicate gels were transferred to Trans-Blot polyvinylidene difluoride (PVDF) membranes (Bio-Rad) for 1 h at 0.18 A, stained with amido black (Sigma), and destained with dH2O. Proteins of interest were excised and submitted for microsequencing.
Gel electrophoresis.
Nasal secretions were separated on duplicate gels by AU-PAGE (40) and Tricine-sodium dodecyl sulfate (SDS)-PAGE (33). We used two-dimensional PAGE to improve the resolution of certain proteins and peptides for microsequencing. Nasal secretions were separated on an AU-PAGE gel as the first dimension and transferred to a reducing SDS-PAGE gel as the second dimension. Briefly, an entire lane of an AU-PAGE gel, consisting of electrophoresed nasal secretion proteins, was incubated at room temperature in a 1:1 mixture of 1× Laemmli buffer (25 mM Tris base; 0.19 M glycine; 0.1% SDS) and 3× denaturing sample buffer (0.17 M Tris, pH 8.8; 6% SDS; 21% glycerol; 0.15 M dithiothreitol; bromophenol blue to color) for 10 min. The gel slice was subsequently inserted in the preparative well of an SDS-PAGE gel, sealed with 1% agarose in cathode running buffer (0.1 M Tricine; 0.1 M Tris, pH 8.25; 0.1% SDS), and electrophoresed for 5 h at ∼110 V. Tricine-SDS-PAGE gels for protein sequencing were transferred to Trans-Blot PVDF membranes as described above, and proteins of interest were excised and submitted for microsequencing.
(Poly)peptide identification.
Microsequencing was performed by N-terminal Edman degradation of AU-PAGE or reducing SDS-PAGE gels transferred to PVDF membranes (UCLA Peptide Sequencing Facility). In addition, purified (poly)peptides were analyzed in the UCLA Center for Molecular and Medical Mass Spectrometry by electrospray ionization mass spectrometry on the Sciex API III (Perkin-Elmer Corp., Foster City, Calif.) and matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry on the Voyager RP Instrument (PerSeptive Biosystems, Framingham, Mass.).
Western analysis.
After AU-PAGE, gels were immunoblotted to Immobilon P-PVDF membranes (Millipore, Bedford, Mass.) for 20 min (for very cationic peptides) to 2 h (lactoferrin) in 0.7% acetic acid–10% methanol as described previously (40). The membranes were then incubated with either a 1:250 dilution of rabbit polyclonal anti-human lysozyme (Dako Corp., Carpinteria, Calif.), a 1:5,000 dilution of anti-human lactoferrin (Dako), a 1:1,000 dilution of anti-human beta defensin-1 (HBD-1) (40), a 1:1,000 dilution of anti-HBD-2 (24), or a 1:1,000 dilution of goat anti-human SLPI (R&D Systems, Minneapolis, Minn.) overnight at room temperature. After several washings in 1× TTBS (Tris-buffered saline plus 0.05% Tween 20), membranes were incubated with a 1:2,000 dilution of alkaline phosphatase-conjugated polyclonal goat anti-rabbit secondary antibody (lysozyme, lactoferrin, HBD-1, and HBD-2; Pierce, Rockford, Ill.) or rabbit anti-goat secondary antibody (SLPI; Organon Teknika Corp., West Chester, Pa.) for 1 h at room temperature. The membranes were then developed by using nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP) as a chromogenic substrate. Serial dilutions of standard human milk lysozyme, human milk lactoferrin (Sigma), human recombinant SLPI (a gift from Pieter Hiemstra, University of Leiden), and HBD-1 and HBD-2 (24, 40) were used as positive controls and for semiquantitative studies.
Bacterial strains and culture conditions.
The Pseudomonas aeruginosa strain, designated CF, is an isolate (37) from a cystic fibrosis patient; the isolate was generously donated by M. J. Welsh (University of Iowa). P. aeruginosa CF, E. coli ML-35p, L. monocytogenes EGD, Staphylococcus aureus (clinical isolate; UCLA Clinical Microbiology Facility), and wild-type Salmonella typhimurium were cultured for 18 h at 37°C in 50 ml of 3% Trypticase soy broth (TSB; all strains are described in reference 21). Each strain was subcultured for 2.5 h immediately prior to use in antimicrobial assays in 50 ml of 3% TSB to obtain mid-logarithmic-growth phase. Subcultures were then centrifuged at 1,400 × g for 10 min and washed once in a salt buffer (referred to as 1× nasal buffer [NB]) that approximates the electrolyte composition of nasal secretions: Na, 85 mM; Cl, 97 mM; K, 20 mM; Ca, 1 mM; P, 10 mM; Mg, 0.5 mM; and S, 0.5 mM (19, 25, 37). The bacteria were collected at 1,400 × g for 10 min and resuspended in 1 ml of 1× NB. An optical density at 625 nm of 0.16 to 0.18 approximated 2.5 × 107 CFU/ml. A second CF mucoid strain H246, its nonmucoid revertant H247, a lipopolysaccharide (LPS) mutant strain H234, and their wild-type parent (PAO1) strain H103 were all generously donated by Robert E. W. Hancock (University of British Columbia, Vancouver, Canada). These strains (11) were cultured for 18 h at 30°C but otherwise were treated as described above.
CFU microassays.
To study the effect of nasal fluid on indigenous nonfastidious aerobically growing nasal microbes, 20 μl of nasal secretions (not gamma-irradiated) was incubated in closed 1.5-ml Eppendorf tubes at 37°C for 0, 1, 3, and 24 h and then spread onto 3% TSB–1% agar (TSA) plates with or without 5% sheep blood (Microdiagnostics, Lombard, Ill.). Plates were incubated for 16 to 18 h at 37°C overnight to permit visible colony growth. To determine the effect of nasal secretions on exogenous bacteria, 300 to 600 bacterial CFU per 5 μl in 1× NB were incubated with 20 μl of nasal secretions or 1× NB. The entire sample, or dilutions thereof, was spread after 0, 1, 3, and 24 h of incubation at 37°C onto TSA plates and incubated overnight for 16 to 18 h. With the exception of S. aureus, the colonies formed by resident flora were readily distinguishable from the test organism and could be counted separately. The survival of exogenously added S. aureus was determined by the difference in counts from controls with only indigenous bacteria.
Screening of donors for S. aureus nasal carriage.
Twenty-four donors were screened for nasal carriage of S. aureus by using the Bacto-Staph Latex Test for agglutination (Difco, Detroit, Mich.). The terminal 2 cm of the left nares were swabbed with a sterile cotton swab and immediately streaked onto TSA plates with 5% sheep blood. After 18 h of incubation at 37°C, multiple colonies were individually tested for agglutination (positive for S. aureus) according to the manufacturer’s instructions. Donors’ nasal fluid samples, which exhibited numerous strongly positive colonies, were submitted for confirmatory microbiological testing (UCLA Clinical Microbiology Laboratory).
Lysozyme purification.
To obtain large quantities of human lysozyme for reconstitution studies, 75 ml of human milk was acidified to 5% acetic acid, incubated overnight, and centrifuged 30 min at 10,000 × g. The cleared supernatant was then diluted 1:10 with dH2O, the pH was adjusted to 6.5 to 7.0 with ammonium hydroxide, and 1:100 (vol/vol) CM-Prep weak cation-exchange resin (Bio-Rad) was added and allowed to bind overnight at 4°C with gentle shaking. The resin was washed with 25 mM ammonium acetate (pH 6.8), extracted with 10% acetic acid, lyophilized to dryness, resuspended in 1% acetic acid, and fractionated on a P10 size exclusion column (Bio-Rad). The second of two peak fractions shown by absorbance at 220 nm was pooled, lyophilized to dryness, resuspended in 5% acetic acid, and subjected to reversed-phase C8 HPLC (Vydac). Lysozyme peak fractions, eluting at 40% acetonitrile–0.1% TFA, were lyophilized and resuspended in 0.01% acetic acid. Lysozyme purity and concentrations were confirmed by AU-PAGE, Western, and lysoplate analyses. A 75-ml milk sample yielded 4.5 mg of >90% pure lysozyme.
Lysoplate assay.
Briefly, 0.5 mg of lyophilized Micrococcus lysodeiktikus per ml was suspended in 66 mM sodium phosphate buffer (Na2HPO4-NaH2PO4, pH 7.0), combined with 1% agarose in 66 mM sodium phosphate, and poured in a level 9-by-9-cm square petri dish (30). Sixteen evenly spaced 3-mm wells were punched in the solidified agar, and 5 μl of sample was introduced into each well. The lysozyme enzymatic activity was determined by measuring the diameters of the zones of clearance relative to lysozyme standards.
Statistics.
Bacterial colony counts (CFU assay) were performed in at least triplicate in each independent experiment, except when noted that the quantity of nasal secretions was limiting. Statistical analysis was performed on log10 CFU values to enhance the normal distribution. Sets of independent experiments were compared with a paired t test (SigmaStat; SPSS, Inc., Chicago, Ill.). Dilution experiments were analyzed by groupwise comparison with a two-factor repeated measures analysis of variance, followed by multiple pairwise comparisons by the Tukey Test (SigmaStat). Error bars represent the standard error of the mean and, unless otherwise stated, P values are listed as compared to counts at t = 0 min.
RESULTS
Microbicidal effect of nasal secretions on indigenous microbes.
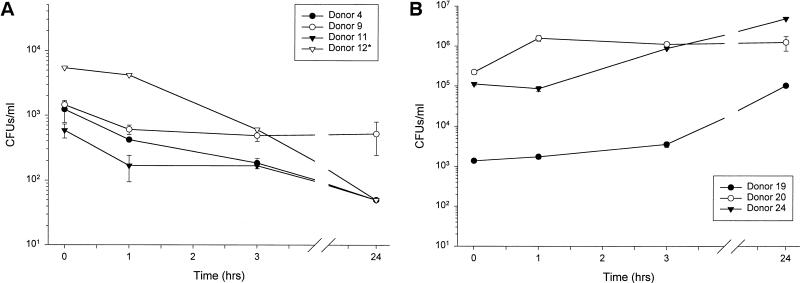
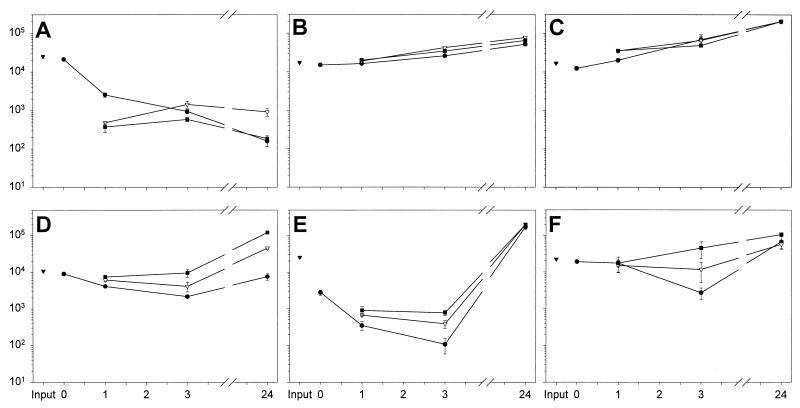
We first assayed the intrinsic antimicrobial properties of nasal secretions against indigenous microbes ex vivo. Minimally manipulated nasal secretions were incubated at 37°C, and microbial counts were measured by a CFU assay at 0, 1, 3 and 24 h (Fig. 1). Although initially all samples contained 103 to 104 CFU/ml, in three samples (donors 4, 11, and 12), the CFU gradually decreased during incubation and none were detected by 24 h (Fig. 1A). In another sample (donor 9) CFU counts diminished with time but were still detectable at 24 h (Fig. 1A). In both cases, CFU counts were significantly decreased by 24 h compared to time zero (P < 0.03). In three other samples (donors 19, 20, and 24) microbes proliferated so that by 24 h a 1 to 2 log increase in CFU (P < 0.03) over input was observed. We next explored whether the dissimilar fates of resident bacteria in nasal secretions were due to differences in the strains of bacteria colonizing the nasal passageway and/or donor variations in antimicrobial or nutritive constituents.
FIG. 1.
The fate of indigenous bacteria in minimally manipulated nasal secretions. (A) The majority of nasal fluid samples were inhibitory to indigenous microbes. (B) Nasal fluid samples from a minority of donors were permissive for indigenous microbes. Asterisk indicates n = 1, due to insufficient amounts of nasal secretions from the indicated donors.
Antimicrobial activity of minimally manipulated nasal secretions against added bacteria.
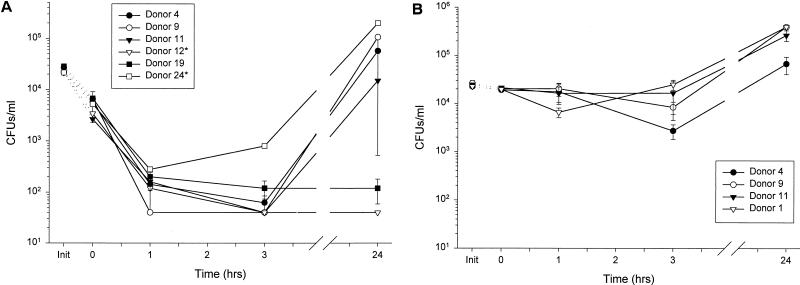
Panels of CFU microassays were employed to ascertain the effect of bacterial species and donor-to-donor variability on the antimicrobial capacity of nasal fluid. The differential effects of a single donor’s secretions (donor 4) against multiple species and strains of bacteria is presented in Fig. 2 and 3, while the various effects of multiple donors’ secretions against an individual bacterial strain is shown in Fig. 4. An inoculum of 2 × 104 to 4 × 104 CFU/ml was used to model the host-defense challenge by moderate numbers of inhaled microbes (Fig. 1A). To avoid selection bias, we included samples from donors from whom only scant amounts of nasal secretions could be collected, but their secretions were tested in a single assay (indicated by an asterisk).
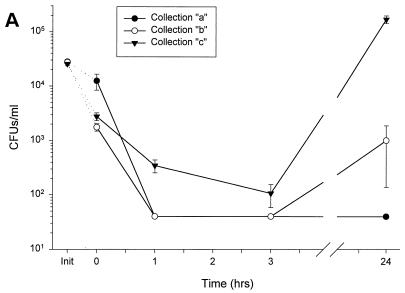
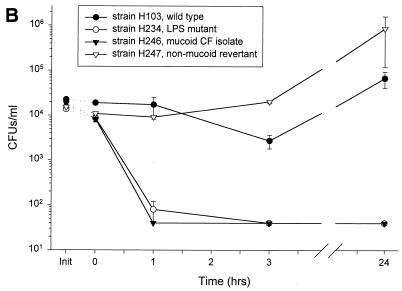
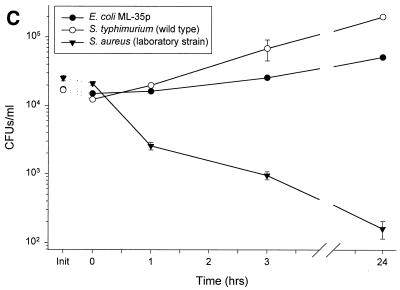
FIG. 2.
Susceptibility of various strains of bacteria to a single donor’s nasal fluid. Nasal secretions from donor 4 were subjected to a modified CFU microassay of nasal fluid targeting four strains of P. aeruginosa, E. coli ML-35p, S. typhimurium wild type, and S. aureus clinical isolate. (A) Secretions tested at three different times showed similar activities against P. aeruginosa CF between 0 and 3 h but varied in the amount of regrowth at 24 h. (B) Fluid incubated with four different strains of P. aeruginosa showed strain-specific antimicrobial activity. (C) Nasal secretions permitted the slow growth of E. coli and S. typhimurium; however, S. aureus was effectively cleared. Init, inoculum (input) at time zero for each sample tested, without the addition of nasal fluid.
FIG. 3.
Effect of fluid dilution on microbicidal activity. Six strains of bacteria were subjected to a CFU microassay of undiluted, 1:1-diluted, and 1:2-diluted minimally manipulated nasal fluid from donor 4. The CFU counts of S. aureus (clinical isolate) (A), E. coli (B), and S. typhimurium (C) were little affected. The antimicrobial effects of nasal secretions on S. aureus (isolate from nasal carrier donor 24) (D), P. aeruginosa CF (E), and P. aeruginosa H103 (F) were decreased with increasing dilution. The effects of undiluted, 1:1-diluted, and 1:2-diluted secretions are represented by closed circles, open triangles, and closed squares, respectively. The input inoculum (closed triangles) is shown for each graph.
FIG. 4.
Susceptibility of P. aeruginosa to multiple donors’ nasal fluids. Activities of multiple donors’ nasal secretions were tested individually against two strains of P. aeruginosa. Different donors’ fluids were tested in a CFU microassay against a cystic fibrosis clinical isolate (A) and the wild-type strain H103 (B). Init, inoculum (input) at time zero for each donor tested, without the addition of nasal fluid. Asterisks indicate n = 1, due to scant fluid from the indicated donors.
Nasal fluid samples obtained on different days from the same donor showed similar antimicrobial activity against P. aeruginosa CF at 3 h of incubation (Fig. 2A). By 24 h, minor regrowth of bacteria was observed in two of the three samples (b and c); however, the differences in CFU counts compared to input were not significant. Thus, variance in the composition of nasal secretions from the same donor may contribute to small fluctuations in bacterial regrowth.
The antimicrobial effect of nasal secretions from a single collection was seen to depend on the properties of the bacterial target (Fig. 2B). An LPS mutant strain, H234, and a mucoid CF strain, H246, were effectively cleared at 1 h with no regrowth at 24 h (P < 0.003). The PAO1 strain, H103, was not cleared but did not significantly grow throughout the observed time period. The nonmucoid revertant strain of H246, H247, slowly grew throughout the entire period of observation with a doubling time of approximately 8 h. Similar differences were observed in the effect of nasal fluid on non-Pseudomonas strains (Fig. 2C). S. aureus (clinical isolate) was effectively eliminated at 24 h compared with time zero (P < 0.003). In contrast, E. coli and S. typhimurium CFU counts slowly increased (P < 0.004 and P < 0.00007, respectively), with a doubling time of 10 to 16 h.
To assess the effect of dilution in a CFU microassay, donor 4’s nasal secretions were diluted 1:1 and 1:2 with 1× NB. Dilution had little effect on antimicrobial activity against S. aureus (clinical isolate), E. coli, and S. typhimurium (Fig. 3A to C), as well as on P. aeruginosa H234, P. aeruginosa H246, and P. aeruginosa H247 (data not shown). However, S. aureus (nasal carrier isolate), P. aeruginosa CF, and P. aeruginosa H103 proliferated in diluted nasal fluid (Fig. 3D to F). In this latter group, the increased dilution resulted in significantly increased bacterial growth either at all of the timepoints tested (Fig. 3D, P < 0.02), at 1 and 3 h (Fig. 3E, P < 0.02), or at 3 h (Fig. 3F, P < 0.003). The observation that thinning of nasal fluids can increase bacterial proliferation indicates that the factors that determine microbial growth in nasal fluid are primarily suppressive and concentration limited.
To detect the variation of the antimicrobial effect between donors, minimally manipulated nasal secretions from seven different donors were tested in a CFU microassay against several strains of P. aeruginosa. Four strains were employed for this study: strains CF (CF isolate), H246 (CF isolate), H234 (LPS mutant), and H103 (wild type) (Fig. 4). Several patterns of antimicrobial effect emerged. All samples tested against strain CF decreased CFU counts by >2 log of the inoculum by 1 h (P < 0.0002) and nearly cleared the nasal secretions of exogenous bacteria by 3 h (Fig. 4A, P < 0.0004). Bacterial regrowth at 24 h appeared to be a donor-specific phenomenon. The wild type, H103, was at least transiently inhibited by nasal secretions from all donors tested, but regrowth at 24 h (P < 0.01) was again observed in secretions from several donors (Fig. 4B). The mucoid (alginate-producing) P. aeruginosa strain, H246, and the LPS-deficient mutant strain, H234, were intermediate in their susceptibilities to nasal secretions (data not shown). Thus, alginate production and LPS structure affect the sensitivity of bacteria to normal nasal fluid.
Identification of antimicrobial (poly)peptides in nasal secretions.
We screened the nasal secretions by Western blotting with specific antibodies against known antimicrobial (poly)peptides (Table 1). Among the proteins so identified, lysozyme, lactoferrin, SLPI, and secretory phospholipase A2 (sPLA2) are antimicrobial components previously noted in nasal secretions. Prominent protein bands that could not have been identified by Western blotting were separated by AU-SDS/2-dimensional PAGE and transferred to PVDF membranes for protein sequencing. Several abundant (poly)peptides were identified as fragments of albumin and the light chain of IgG. Newly described cationic antimicrobial components of nasal secretions included two β-defensins (HBD-1 and HBD-2), an α-defensin (human neutrophil peptide 1 [HNP-1]), statherin, and β-microseminoprotein. The latter three peptides were identified from larger-scale (>5-ml) preparations of acid-extracted nasal secretions and were present at much lower concentrations than lysozyme, lactoferrin, or SLPI.
TABLE 1.
Eleven (poly)peptides identified in nasal secretions by Western analysis, microsequencing, and/or mass spectrometry
| Protein | Molecular size (kDa) | Concn (μg/ml)a | Method of detectionb |
|---|---|---|---|
| Lysozyme | 14.7 | 250–500 | a, b, c |
| Lactoferrin | 78 | 80–200 | a |
| SLPI | 11.7 | 10–80 | a, b, c |
| β-Microseminoprotein | 10.8 | ND | b, c |
| sPLA2 | 14.1 | ND | d |
| Statherin | 5.2 | ND | b, c |
| Light-chain IgG | ∼28 | ND | b |
| Albumin | ∼6 (fragment) | ND | b |
| Albumin | ∼30 (fragment) | ND | b |
| Albumin | ∼31 (fragment) | ND | b |
| HBD-1c | 3.9 | ND | a |
| HBD-2c | 4.3 | 0.3–4 | a |
| HNP-1 | 3.4 | ND | b |
Concentration was determined from five samples by using semi quantitative Western analysis, with each sample containing lysozyme, lactoferrin, and SLPI. Note that three of five donor samples contained measurable amounts (>0.3 μg/ml) of HBD-2. ND, not determined.
a, Western analysis; b, microsequencing by N-terminal Edman degradation; c, mass spectroscopy; d, PLA2 activity determined by using radiolabeled E. coli ML-35p substrate as described previously (31).
Some β-defensin comigrated with higher-molecular-weight proteins.
Variation in antimicrobial (poly)peptide content.
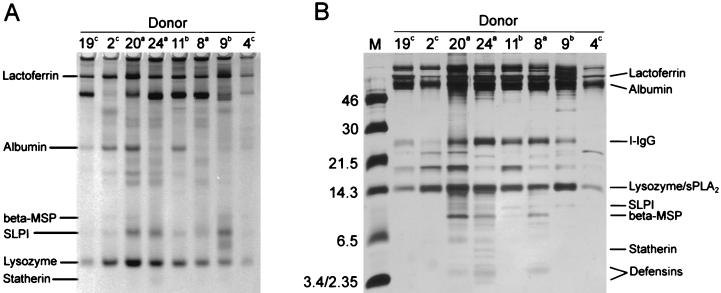
Differences in the antimicrobial activities of nasal secretions could result from donor-to-donor variations in the protein composition of the secretions. Equal volumes of eight donors’ nasal secretions were analyzed by AU-PAGE (Fig. 5A) and stained with Coomassie brilliant blue (Sigma) or analyzed by Tricine-SDS-PAGE and stained with silver stain (Fig. 5B). The variability of the protein patterns among the donors is readily seen. Although a unique protein fingerprint is seen in each donor’s fluid, the electrophoretic patterns can be classified into three groups. Group “a” (donors 8, 20, and 24) had the highest protein concentration, group “b” (donors 9 and 11) had a moderate protein concentration, and group “c” (donors 2, 4, and 19) had the lowest overall protein concentration. Group a also had a number of low-molecular-weight (poly)peptides, several of which are possible antimicrobials. In agreement with previous reports (17), the total protein concentration in our nasal fluid samples (n = 3), as performed by the UCLA Clinical Laboratories by the pyrogallol red molybdate method (Synchron CX Microprotein Calibrator; Beckman Instruments, Inc., Brea, Calif.), was found to be 1 to 3 mg/ml. Concentrations of lysozyme, lactoferrin, and SLPI in crude nasal fluid (n = 5) as tested by semiquantitative Western analysis are given in Table 1. A β-defensin, HBD-2, was also detected by Western blot in three of five donors. Donor-to-donor differences in electrophoretic patterns of nasal secretions in SDS-PAGE and AU-PAGE may indicate that polymorphic genes encode many of the proteins of nasal secretions.
FIG. 5.
Donor-to-donor variability in the protein pattern of nasal secretions. (A) AU-PAGE of eight donor’s nasal secretions shows variations in lactoferrin, albumin, β-microseminoprotein (beta-MSP), SLPI, lysozyme, and statherin concentrations. (B) Reducing Tricine-SDS-PAGE of eight donors’ nasal secretions shows the variability in low-molecular-weight proteins. Superscripts indicate three donor groupings based on protein band similarities. The positions of identified components of nasal secretions are shown at the gel margins.
Nasal S. aureus carriage.
Since the nasal fluid of donors 20 and 24 was permissive for the growth of indigenous bacteria (Fig. 1B) and the two protein band patterns were similar (Fig. 5), the indigenous bacteria were typed to identify microbiological similarities between these two donors. With a Bacto-Staph Latex rapid slide agglutination kit (Difco), both donors were found to have numerous colonies that tested positive for S. aureus. Twenty-two other donors’ secretions were also subjected to the Bacto-Staph Latex agglutination test; only one of these donors had high numbers of colonies positive for S. aureus (donor 8), and two had a few colonies positive for S. aureus (donors 3 and 23). Samples of secretions from donors 8, 20, and 24, suspected of having high S. aureus colonization, were submitted to standard clinical microbiological typing, which confirmed this finding. Heavy S. aureus nasal carriage in 3 of 24 total donors (12.5%) is within the expected frequency based on previous estimates of carriage rates of 8 to 18% (18).
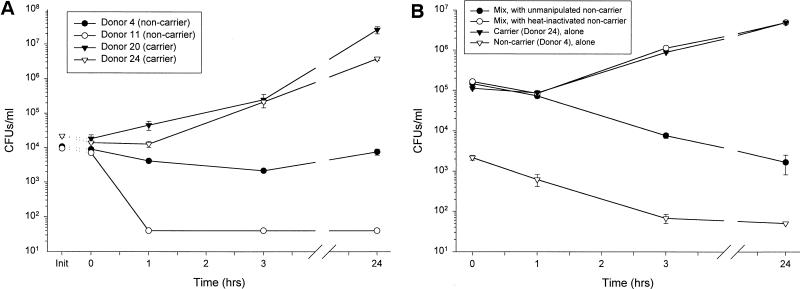
In principle, carriage could result either if the colonizing strains of S. aureus are extraordinarily resistant to secretions or if the carriers’ nasal fluid is favorable to S. aureus proliferation. S. aureus, isolated from a carrier’s nose (donor 24), was tested in CFU microassays of carrier and noncarrier nasal secretions (Fig. 6A). The nasal fluids from four donors, two noncarrier (donors 4 and 11) and two carrier (donors 20 and 24, secretions gamma-irradiated), were tested. While the noncarriers’ secretions were bacteriostatic (donor 4) or bactericidal (donor 11), both carriers’ secretions permitted a 2- to 3-log increase in CFU counts of the S. aureus isolate compared to time zero (P < 0.05). This suggests that the S. aureus strain is not extraordinarily resistant to nasal secretions but that the carriers’ secretions may be deficient in antimicrobial activity.
FIG. 6.
Nasal carriers of S. aureus are deficient in antimicrobial components that are restored by the addition of noncarrier secretions. (A) S. aureus, isolated from donor 24, was used as the target bacterium in a CFU microassay of nasal fluid from two noncarriers (donors 4 and 11) and two carriers (donors 20 and 24, gamma-irradiated fluid). While the noncarriers’ fluids were bacteriostatic or bactericidal, the carriers’ fluids did not inhibit bacterial growth. (B) The 1:1 mix of nasal secretions from an S. aureus carrier (donor 24) with fluid from a noncarrier (donor 4) is bactericidal to indigenous flora, while the mix of nasal secretions from an S. aureus carrier with heat-inactivated (boiling for 10 min) secretions from a noncarrier promoted the growth of indigenous bacteria.
Noncarrier and carrier nasal secretions, with their indigenous flora, were mixed to determine whether the antimicrobial activity of carrier fluid could be restored by the addition of noncarrier fluid. Figure 6B reveals that the indigenous flora of noncarrier secretions (donor 4) was killed throughout incubation (P < 0.01). When carrier fluid (donor 24) was incubated alone, its bacteria proliferated to 1.5 log units above the initial CFU count. Indigenous bacteria in carrier secretions incubated with heat-inactivated (boiling for 10 min) noncarrier fluid showed growth kinetics similar to that of carrier fluid incubated alone. However, a 1:1 (vol/vol) mixture of minimally manipulated carrier and noncarrier fluids was bactericidal for indigenous bacteria in the carrier’s secretions (P < 0.02 compared to carrier fluid with or without the addition of heat-inactivated normal secretions). A similar pattern of activity was seen with the same noncarrier fluid (donor 4) and different carrier fluid (donor 20) (data not shown). A 1:1 (vol/vol) mixture of carrier (donor 20) and noncarrier fluids was bactericidal (P < 0.04 compared to either carrier fluid alone or carrier fluid mixed with heat-inactivated noncarrier fluid). Thus, the noncarrier nasal secretions contain antimicrobial substances that are either deficient or inactive in the carrier’s nasal secretions.
Lysozyme and lactoferrin did not restore the microbicidal activity of heat-inactivated nasal secretions.
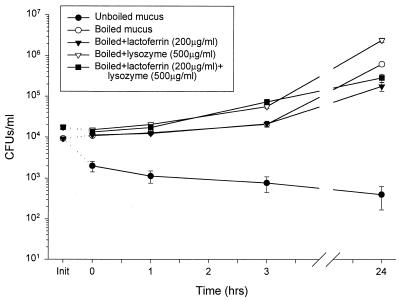
Physiological concentrations of human milk lysozyme and human milk lactoferrin (Sigma), reported as the major antimicrobial agents in nasal secretions (32), were added to heat-inactivated fluid in an attempt to reconstitute the antimicrobial activity of nasal secretions (Fig. 7). While unaltered nasal fluid was bactericidal, P. aeruginosa H246 was insensitive to heat-inactivated secretions. Lysozyme activity in heat-inactivated nasal fluid, as measured by the lysoplate assay, was found to be >25-fold less than in nonboiled control (n = 4; data not shown). The addition of 200 μg of lactoferrin per ml alone, 500 μg of lysozyme per ml alone, or the combination of 200 μg of lactoferrin and 500 μg of lysozyme per ml to heat-inactivated nasal fluid did not restore its antimicrobial activity (P < 0.03). The addition of lysozyme restored the enzymatic activity of nasal fluid towards Micrococcus lysodeikticus in the lysoplate assay (data not shown), but lysozyme added alone increased the growth of P. aeruginosa H246 at 24 h compared to growth in boiled nasal fluid (P < 0.04). The difference in the effect of lactoferrin alone and the lactoferrin-lysozyme combination was not statistically significant at 24 h compared to the boiled nasal fluid alone. Thus, other (poly)peptides besides lysozyme and lactoferrin contribute to the total microbicidal activity of nasal fluid.
FIG. 7.
Physiological concentrations of lysozyme and lactoferrin added to heat-inactivated nasal fluid (donor 4) did not restore microbicidal activity. While nonboiled fluid exhibited the expected bactericidal activity, heat-inactivated (boiling for 10 min) did not inhibit CFU growth. Physiological concentrations of lactoferrin and lysozyme, added separately and concurrently to heat-inactivated secretions, did not restore the antimicrobial activity.
DISCUSSION
Since the pioneering studies of Fleming (8), scant attention has been paid to the antimicrobial activity of nasal secretions and their role in the prevention of microbial colonization. We were motivated to reexamine the antimicrobial activity of nasal fluid because of recent evidence that the bacterial colonization of the airways in cystic fibrosis is due to a local defect in epithelial host defense (26). Our study confirms Fleming’s observation that nasal secretions have intrinsic antimicrobial activity, but it also shows that this activity is precarious. Activity varies depending on the donor and the target bacteria; is often time limited, with regrowth observed by 24 h of incubation; and is ablated by dilution. Under normal conditions in vivo, the contributions of mechanical factors such as mucociliary clearance, phagocytic clearance, or sneezing may shorten the time available to microbes to adapt to the deleterious effects of nasal secretions. It is easy to conceive how the failure of one or more of these mechanisms could permit the colonization of airways by microbes.
Nasal S. aureus carriage has been identified as a risk factor for the pathogenesis of surgical and other hospital-associated infection. Persistent nasal carriers of S. aureus account for approximately 20% of the population, with 8 to 18% heavily colonized (>105 CFU/ml; reviewed in reference 18). Although various epithelial and mucus host factors, such as surface glycoproteins and proteoglycans, have been shown to mediate binding, the precise adhesive molecules on host and bacteria have not been identified. S. aureus appears to attach to cell-associated and cell-free secretions (34) and to involve receptor sites of secretory IgA (3), glycolipids (20), and surfactant protein A (28). Various bacteria, including Staphylococcus epidermidis, are capable of reducing nasal ciliary activity in vitro (6). Enhanced adhesion and diminished mucociliary clearance could explain the retention of S. aureus within the nasal passageway but have no direct bearing on bacterial proliferation. Our observations suggest that the nasal fluid of at least some carriers is deficient in antimicrobial activity. Because of the natural donor-to-donor variation in the composition of nasal fluid, larger studies will be necessary to determine whether this mechanism is the sole or common determinant of nasal colonization by S. aureus and to identify the specific (poly)peptides or other substances that may be deficient in carriers.
Early colonization of cystic fibrosis airways predominantly involves S. aureus, Haemophilus influenzae, or P. aeruginosa. The initially infecting P. aeruginosa organisms are typically nonmucoid; however, they become highly mucoid after continued exposure to the environment of the cystic fibrosis airways. Colonization with P. aeruginosa may induce further airway injury, perhaps due to destructive virulence factors, such as exotoxin A and proteases, and the recruitment of the host’s neutrophils with their proteases, leading to the progressively destructive bronchitis and bronchiolitis. Some studies have attributed Pseudomonas colonization in cystic fibrosis to abnormally glycosylated cell surface receptors with enhanced affinity for pseudomonal adhesive structures (13, 43). Other work suggests that abnormal mucin and the lack of hydration of respiratory secretions entraps bacteria, permitting colonization (22, 26, 36). It has also been proposed that defects in intrinsic antimicrobial activity of airway secretions may be induced by alterations in the salt concentration due to defective chloride transport in cystic fibrosis (37). Our study of nasal secretions suggests that their antimicrobial activity is naturally variable from donor to donor and is often time limited. Factors, such as thick mucus or ciliary dysmotility, that delay the mechanical clearance of particulates from the airway may permit microbes to adapt to the damaging effects of secretions and to recover and resume growth. The ablation of the antimicrobial effects of nasal fluid by dilution ex vivo points to the possibility that reduced concentrations of antimicrobial substances due to abnormalities of fluid movement across epithelia could impair the antimicrobial activity of the airway secretions. The well-recognized phenotypic variability of cystic fibrosis among patients with identical mutations may in part be due to interactions with genes that control the (poly)peptide composition of nasal (and presumably other airway) fluid. Our observations on the variability of the protein patterns in nasal secretions are reminiscent of similar studies with human saliva, whose (poly)peptides are remarkably polymorphic (2). The effects of nasal (poly)peptide polymorphisms on host defense and their possible role as phenotypic modulators in cystic fibrosis should be a productive area for further investigation.
Nasal secretions contain antimicrobial proteins and peptides as a first-line host defense against microbial invaders. Lysozyme and lactoferrin, stored in and secreted from serous cells in nasal submucosal glands, are the most abundant antimicrobial proteins of nasal fluid. They are bactericidal alone for gram-positive bacteria and in combination for some gram-negative bacteria (5, 32). Given their strong combined antibacterial properties and since few other major microbicidal proteins have been previously described in nasal fluid, we expected they would reconstitute the antibacterial activity in heat-inactivated secretions. Our experiments suggest that other factors, or cofactors acting with lysozyme and lactoferrin, contribute to the antimicrobial properties of nasal fluid.
sPLA2 has been previously identified from methacholine-induced nasal lavage fluid and mucosa as a far less abundant (poly)peptide by both immunostain and enzymatic activity (1, 38). Ca2+-dependent antimicrobial activity of sPLA2 has been demonstrated against both gram-positive and gram-negative bacteria (reviewed in reference 10). In the present study we identified sPLA2 in unstimulated nasal fluid as a potential contributor to the total microbicidal activity of nasal fluid.
The current study is the first to identify various small cationic antimicrobial peptides in nasal secretions. HBD-1 has recently been characterized in urogenital tissues (40) and bronchoalveolar lavage (35), while HBD-2 is expressed at sites of inflammation (24, 35). HNP-1 (9) was likely a degranulation product of transudated neutrophils. Statherin, a small antimicrobial phosphoprotein previously identified in saliva (14), was also found to be present in nasal secretions. Another low-molecular-weight protein, β-microseminoprotein, has been localized to several human and rat mucosal tissues (7, 41) and was identified here as a component of nasal fluid (Table 1). Its detection in nasal fluid suggests that β-microseminoprotein is a ubiquitous antimicrobial protein secreted as a component of mucosal defense. We also acknowledge the possibility that anionic antimicrobial (poly)peptides contribute to the antimicrobial activity of nasal fluid, as observed in ovine bronchoalveolar lavage fluid (4); however, our fractionation methodology was not designed to detect such peptides. The abundance of antimicrobial peptides and proteins present in nasal secretions, coupled with the inability of lysozyme and lactoferrin to restore the microbicidal activity of heat-inactivated nasal fluid toward the test strain of P. aeruginosa, suggest that the antimicrobial activity of nasal fluid results from complex effects of its many components.
ACKNOWLEDGMENTS
This work was supported by grants from the Cystic Fibrosis Foundation and the National Institutes of Health (HL46809 and AI40268).
We are grateful to Erika Valore and Christina Park for recombinant HBD-1 and -2 and polyclonal antisera and also for their guidance in protein analysis. We thank Jenny Lee for the purification of human milk lysozyme, Jishu Shi for his assistance in sequencing several peptides, and the UCLA Protein Microsequencing Facility, Mass Spectrometry Facility, and Clinical and Microbiological Laboratories for their invaluable help with this project. We also thank Edith M. Porter for continual discussions on microbiology and the properties of lysozyme.
REFERENCES
- 1.Aho H J, Grenman R, Sipila J, Peuravuori H, Hartikainen J, Nevalainen T J. Group II phospholipase A2 in nasal fluid, mucosa and paranasal sinuses. Acta Otolaryngol. 1997;117:860–863. doi: 10.3109/00016489709114215. [DOI] [PubMed] [Google Scholar]
- 2.Azen E A. Genetics of salivary protein polymorphisms. Crit Rev Oral Biol Med. 1993;4:479–485. doi: 10.1177/10454411930040033201. [DOI] [PubMed] [Google Scholar]
- 3.Biesbrock A R, Reddy M S, Levine M J. Interaction of a salivary mucin-secretory immunoglobulin A complex with mucosal pathogens. Infect Immun. 1991;59:3492–3497. doi: 10.1128/iai.59.10.3492-3497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brogden K A, Ackermann M, Huttner K M. Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium. Infect Immun. 1998;66:5948–5954. doi: 10.1128/iai.66.12.5948-5954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellison R T, III, Giehl T J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson J L, McCaffrey T V, Kern E B, Martin W J. The effects of sinus bacteria on human ciliated nasal epithelium in vitro. Otolaryngol Head Neck Surg. 1988;98:299–304. doi: 10.1177/019459988809800405. [DOI] [PubMed] [Google Scholar]
- 7.Fernlund P, Granberg L B, Larsson I. Cloning of β-microseminoprotein of the rat: a rapidly evolving mucosal surface protein. Arch Biochem Biophys. 1996;334:73–82. doi: 10.1006/abbi.1996.0431. [DOI] [PubMed] [Google Scholar]
- 8.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond (Biol) 1922;93:306–317. [Google Scholar]
- 9.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins: natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 11.Hancock R E, Mutharia L M, Chan L, Darveau R P, Speert D P, Pier G B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harder J, Bartels J, Christophers E, Schroeder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861–862. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 13.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen J L, Xu T, Lamkin M S, Brodin P, Aars H, Berg T, Oppenheim F G. Physiological regulation of the secretion of histatins and statherins in human parotid saliva. J Dent Res. 1994;73:1811–1817. doi: 10.1177/00220345940730120401. [DOI] [PubMed] [Google Scholar]
- 15.Kaliner M A. Human nasal respiratory secretions and host defense. Am Rev Respir Dis. 1991;144:S52–S56. doi: 10.1164/ajrccm/144.3_pt_2.S52. [DOI] [PubMed] [Google Scholar]
- 16.Kaliner M A. Human nasal host defense and sinusitis. J Allergy Clin Immunol. 1992;90:424–430. doi: 10.1016/0091-6749(92)90162-u. [DOI] [PubMed] [Google Scholar]
- 17.Kaulbach H C, White M V, Igarashi Y, Hahn B K, Kaliner M A. Estimation of nasal epithelial lining fluid using urea as a marker. J Allergy Clin Immunol. 1993;92:457–465. doi: 10.1016/0091-6749(93)90125-y. [DOI] [PubMed] [Google Scholar]
- 18.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowles M R, Robinson J M, Wood R E, Pue C A, Mentz W M, Wager G C, Gatzy J T, Boucher R C. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Invest. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee I H, Cho Y, Lehrer R I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect Immun. 1997;65:2898–2903. doi: 10.1128/iai.65.7.2898-2903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J D, Dohrman A F, Gallup M, Miyata S, Gum J R, Kim Y S, Nadel J A, Prince A, Basbaum C B. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci USA. 1997;94:967–972. doi: 10.1073/pnas.94.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindahl M, Stahlbom B, Tagesson C. Two-dimensional gel electrophoresis of nasal and bronchoalveolar lavage fluids after occupational exposure. Electrophoresis. 1995;16:1199–1204. doi: 10.1002/elps.11501601200. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Wang L, Jia H P, Zhao C, Heng H H Q, Schutte B C, McCray P B, Ganz T. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 25.Lorin M I, Gaerlan P F, Mandel I D. Quantitative composition of nasal secretions in normal subjects. J Lab Clin Med. 1972;80:275–281. [PubMed] [Google Scholar]
- 26.Matsui H, Grubb B R, Tarran R, Randell S H, Gatzy J T, Davis C W, Boucher R C. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 27.McCray P B J, Bentley L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–349. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 28.McNeely T B, Coonrod J D. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993;167:91–97. doi: 10.1093/infdis/167.1.91. [DOI] [PubMed] [Google Scholar]
- 29.Meredith S D, Raphael G D, Baraniuk J N, Banks S M, Kaliner M A. The pathophysiology of rhinitis. III. The control of IgG secretion. J Allergy Clin Immunol. 1989;84:920–930. doi: 10.1016/0091-6749(89)90390-4. [DOI] [PubMed] [Google Scholar]
- 30.Osserman E F, Lawlor D P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu X D, Lloyd K C, Walsh J H, Lehrer R I. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect Immun. 1996;64:5161–5165. doi: 10.1128/iai.64.12.5161-5165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphael G D, Jeney E V, Baraniuk J N, Kim I, Meredith S D, Kaliner M A. Pathophysiology of rhinitis. Lactoferrin and lysozyme in nasal secretions. J Clin Invest. 1989;84:1528–1535. doi: 10.1172/JCI114329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 34.Shuter J, Hatcher V B, Lowy F D. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh P K, Jia H P, Wiles K, Hesselberth J, Liu L, Conway B D, Greenberg E P, Valore E V, Welsh M J, Ganz T, Tack B F, McCray P B J. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith A. Pathogenesis of bacterial bronchitis in cystic fibrosis. Pediatr Infect Dis J. 1997;16:91–95. doi: 10.1097/00006454-199701000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 38.Stadel J M, Hoyle K, Naclerio R M, Roshak A, Chilton F H. Characterization of phospholipase A2 from human nasal lavage. Am J Respir Cell Mol Biol. 1994;11:108–113. doi: 10.1165/ajrcmb.11.1.8018333. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg D A, Lehrer R I. Designer assays for antimicrobial peptides. Disputing the “one-size-fits-all” theory. Methods Mol Biol. 1997;78:169–186. doi: 10.1385/0-89603-408-9:169. [DOI] [PubMed] [Google Scholar]
- 40.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Ganz T. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiber H, Lindstrom C, Lilja H, Bjartell A, Fernlund P. Immunohistochemical and in situ hybridization studies of β-microseminoprotein in the human gastric mucosa. Histochem J. 1997;29:839–845. doi: 10.1023/a:1026437706895. [DOI] [PubMed] [Google Scholar]
- 42.Widdicombe J H, Widdicombe J G. Regulation of human airway surface liquid. Respir Physiol. 1995;99:3–12. doi: 10.1016/0034-5687(94)00095-h. [DOI] [PubMed] [Google Scholar]
- 43.Zar H, Saiman L, Quittell L, Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995;126:230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]
- 44.Zhao C Q, Wang I, Lehrer R I. Widespread expression of β-defensin HBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]