Abstract
Aim:
Diabetic Charcot neuro-osteoarthropathy carries a significant worldwide disease burden including diabetic foot infection, ulceration and amputation. The current accepted standard of treatment during the active phase of Charcot neuro-osteoarthropathy is offloading with total contact casting; however, controversy remains regarding weight-bearing status during this period.
Methods:
A systematic review was performed following PRISMA guidelines of Pubmed, EMBASE, MEDLINE and the Cochrane central register of controlled trials for clinical studies from inception until June 2024 investigating weight-bearing and non-weight-bearing total contact casting for active Charcot neuro-osteoarthropathy.
Results:
Four hundred ninety-three studies were identified in the search strategy of which 5 studies met the inclusion criteria comprising 158 patients. These studies found that allowing patients to weight-bear during total contact casting does not have a negative impact on the healing process. There were no comparative studies between weight-bearing and non-weight-bearing total contact casting.
Conclusions:
There is limited evidence to support current practice of non-weight bearing in a total contact casting for active Charcot neuro-osteoarthropathy. Allowing patients to weight bear carries advantages to patient independence and quality of life. Further investigation with randomised control trial should be considered to investigate if weight bearing is associated with negative outcomes.
Keywords: Charcot neuroarthropathy, total-contact-casting, casting, weight-bearing, offloading, deformity, diabetic foot, diabetes, diabetic foot ulceration
Introduction
Charcot neuro-osteoarthropathy (CNO), is a progressive condition leading to the destruction of the bony architecture of the foot and ankle. The leading cause of the condition today is diabetes mellitus, though other causes include alcoholic neuropathy, congenital neuropathy, neurosyphilis and syringomyelia. 1 The global prevalence of diabetes was estimated in 2019 to be 9.3% (463 million people) of the world’s population with this number expected to rise to 10.2% (578 million people) by 2030. 2 The global disease burden of CNO is significant owing to its high morbidity, mortality and growing prevalence. Studies report prevalence of peripheral neuropathy as above 40% of the overall diabetic population. 3 Of these, 0.8%–7.5% were reported have CNO 1 – and 9%–35% of those with a single Charcot joint also have bilateral involvement. 4 The total prevalence reported varies from 0.04% to 0.56% of patients with diabetes. 5
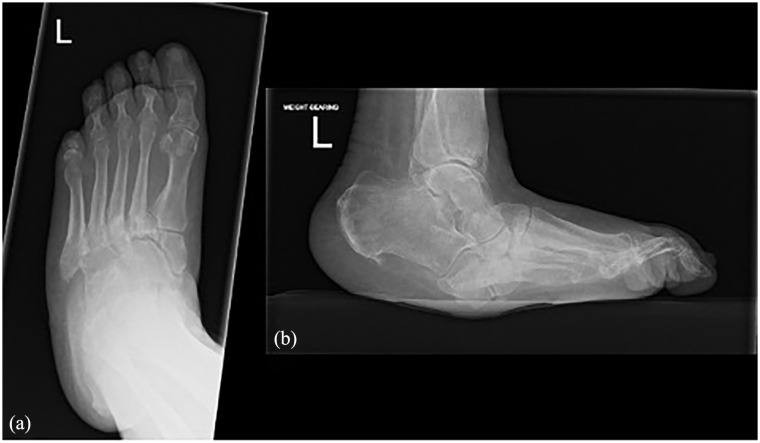
The pathophysiology of the Charcot foot is multifactorial with mechanical, vascular, neurological and biological contributions towards disease progression. The two most widely accepted theories are that of neuro-traumatic and neuro-vascular pathogenesis. 1 The neurotraumatic component is where peripheral neuropathy leads to an insensate foot with progressive microtrauma that goes unnoticed, causing a breakdown in the bony anatomy of the foot and ankle 6 as can be seen in Figure 1.
Figure 1.
(a) AP X-ray of Charcot foot showing destruction along the Tarso-metatarsal joints and (b) lateral weight-bearing X-ray of Charcot foot showing late stage rocker-bottom deformity.
The neurovascular theory relates to the breakdown of autonomic control of localised vasculature resulting in arteriovenous shunting. This is thought to be the cause of the acute presentation of CNO with signs of swelling, erythema and raised local temperature. This increased blood flow allows for increased local monocyte and osteoclast congregation causing osteopenia and osteolysis.7,8
The natural development of CNO was first described by Eichenholtz in 1966 who classified the disease from Stages 0 to 3. 9 Stage 0 is the Initial Phase where the foot loses protective sensation, develops erythema, swelling and mild structural instabilities. In Stage 1 – the Development Phase – erythema and swelling progress, accompanied by warmth and worsening instability and early joint deformation. Stage 2 – The Coalescence Phase – is characterised by radiological signs of absorption of bone fragments, early sclerosis and decreasing erythema, swelling and warmth. The final phase 3 – known as the Consolidation or Remodelling phase – is the consolidation of deformity with joint arthrosis, osteophytes and sclerosis. The inflammatory process begins to withdraw with swelling and erythema subsiding leaving a chronic deformity.
The current management of active CNO in stages 0, 1 and early stage 2 is with total contact casting (TCC), whereby the foot is offloaded in a full fibreglass cast, thereby redistributing plantar pressures, reducing mechanical forces, to try reduce the chance of developing secondary foot deformity whilst the inflammatory response subsides.
The goal of TCC is to achieve a stable, plantigrade and ulcer-free foot, which can be accommodated in bespoke footwear. Casting is usually continued till the affected foot is within 2°C of the contralateral foot and recommended durations are based upon the resolution of oedema and imaging evidence of osseous consolidation.10,11
Currently, non-weight bearing (NWB) TCC is the preferred method of immobilization, although there is little evidence to support this with the existing management based on anecdotal and theoretical principles. 12 Surgeons historically have avoided weight bearing (WB) when concerned about the structural integrity of the foot. This attitude is present not only in the management of CNO but also in wider foot and ankle surgery, with ankle fractures fixations traditionally being kept NWB from a fear of displacing the fixation from excessive load whilst ambulating. WB restrictions however affect patient quality of life, their ability to perform activities of daily living and is associated with increased reliance on social care, and longer hospital stays. NWB TCC has also been shown to cause muscle atrophy, increased thrombo-embolic risk, loss of bone density, 13 reduced overall fitness, weight gain and increased risk of falls. 10 As the general trend in foot and ankle surgery is beginning to change, with recent literature favouring early mobilisation protocols after foot and ankle surgery, 14 we aim to review the evidence surrounding early WB in TCC.
Missed diagnosis and late presentation of CNO reduces the chance of successful non-operative management, and patients at this stage of disease will often require reconstructive surgery in order to obtain an ambulatory plantigrade foot. 15 Though TCC is widely used and accepted, there remains controversy regarding length of treatment, removable versus non-removable casts and WB status. 10 This review aims to evaluate the evidence surrounding the effect of WB on outcomes of patients undergoing TCC for CNO.
Methods
Study design
This systematic review was carried out in accordance with the preferred reporting item for systematic reviews and meta-analyses (PRISMA) guidelines. 16 This study was prospectively registered on PROSPERO – registration number CRD42024539111.
Eligibility criteria
In order for studies to be included in this review, they needed to have an eligible population, intervention, outcome and study design.
Study criteria
The inclusion criteria include studies in full text which report on outcomes related to patients with active Charcot foot in the early stages of the disease (Eichenholtz stages 0, 1 and early stage 2). Studies looking at treatment of neuropathic ulcers with TCC were excluded.
Interventions
The intervention is defined as patients who are treated with either WB or NWB TCC requiring regular cast changes and clinical review. Studies involving removable off-loading orthoses, treatment of ulcers, as well as studies that looked at TCC combined with other interventions were excluded from the review.
Outcomes
Due to the limited number of published studies on the topic, outcomes were kept broad. These include arrested disease progression, prevention of ulceration, deformity and time taken until resolution of disease.
Study design
Eligible studies include randomised controlled trials, non-randomised controlled trials, case–control studies, cohort studies, cross-sectional studies, case series, retrospective studies and systematic reviews. The following types of study were excluded from our review: case reports, biomechanical studies, in vitro studies and studies failing to report clinical or patient reported outcomes.
Search strategy
The search was carried out in May 2024 and the following databases were searched: PubMed, EMBASE, MEDLINE and the Cochrane Central Register of Controlled Trials in the Cochrane Library, as well as the published reference lists of studies included in the review. All eligible studies written in the English language since the inception of these databases were eligible for inclusion. Two independent reviewers (RP and VV) carried out a search using combinations of the following search terms: Charcot, diabetic foot, neuroarthropathy, neuro-osteoarthropathy, arthropathy, TCC, WB and offloading. Any disputes were solved by consensus with a third independent reviewer (BB). An initial scoping search showed us that the number of studies on this subject were extremely limited.
Data extraction
Searches from different databases were arranged in one data set and duplicate papers were removed. Study title and abstracts of all papers were reviewed and those that matched our inclusion criteria were extracted for in depth analysis of the full text. All citations of included studies were also reviewed for any suitable additions.10,17 –20 Relevant data from each study was extracted and presented in Table 1.
Table 1.
Results table summarising studies included in the review.
| Study | Population (n) | Mean age (years) | Study details | WB/NWB/PWB | Intervention | Results | Complications | DOI |
|---|---|---|---|---|---|---|---|---|
| David G. Armstrong (1997) 17 | 55 (27 male, 28 female). | 58.6 (range 50.1–67.1) | Retrospective (1991–1994) | WB | TCC followed by removable cast walkers and prescription therapeutic shoe | Mean casting time 18.5 ± 10.6 weeks | 4 patients (7.3%) developed ulceration during the follow up period | 10.7547/87507315-87-6-272 |
| David R. Sinacore et al. (1998) 18 | 30 (24 male, 6 Female). | 55 (range N/A) | Retrospective (1991–1996) | PWB | PWB in TCC using a heel touch-down method for balance. Casts changed at 1- or 2-week intervals. | Mean casting time 86 days ± 45 days (range: 22–224 days). | 4 patients (13%) went on to have further exacerbations of Charcot neuroarthropathy in the same foot. These required a further 6 week in TCC. | 10.1016/s1056-8727(98) 00006-3 |
| Michael S. Pinzur (2006) 19 | 9 (4 men and 5 women) | 58.2 (range 39–72) | Prospective | WB | TCC with cast changed every 2 weeks, followed by commercial depth-inlay shoes and custom accommodative orthotics. | Mean casting time time of 9.2 weeks (range 8–16). | 1 patient (11%) developed ulceration during follow up | 10.1177/107110070602700503 |
| Leo J. de Souza et al. (2008) 20 | 27 (6 male, 21 female) 7 patients with bilateral involvement resulting in 34 affected feet | N/A | Prospective (1988–2006) | WB | TCC with casts changed at weekly intervals | Mean casting time 14 weeks (range: 4–20). 33 out of the 34 feet reached disease resolution. | 10 patients (37%) developed ulceration during follow up | 10.2106/JBJS.F.01523 |
| Danielle A. Griffiths (2021) 10 | 27 (18 male, 9 female) | 57.9 (range N/A) | Retrospective (2012–2015) | WB | TCC followed by transition into Charcot Restraint Orthotic Walker, knee-high removable offloading boot (e.g. CAM walker or therapeutic footwear. | Median casting time 4.3 months (IQR, 2.7–7.8) | 21 patients (78%) of patients reported minor cast issues including skin irritation and asymmetry pain. 6 patients (22.2%) went on to have soft tissue or bony reconstructive surgery. | 10.1186/s13047-021-00477-5 |
CAM: controlled ankle motion.
Risk-of-bias assessment and study quality
The ROBINS-I tool (risk of bias in non-randomised studies of interventions) was used to assess bias in the methodology of studies included in the analysis. 21 Two authors independently reviewed each study, and any discrepancies were discussed and resolved with the help of a third assessor. The ROBINS-I tool is structured into seven domains. The results are then judged on their overall risk of bias based upon the responses to each domain.
Data analysis
Descriptive statistics were used throughout. Due to the heterogeneity in the included studies, it was not possible to perform a meta-analysis. The synthesis without meta-analysis (SWiM) in systematic reviews guidelines were used to guide reporting in this study. 22
Results
Our search strategy identified a total of 493 papers from the database search. We identified 14 studies for full-text review of which 5 met the inclusion criteria. The total number of patients from all of the studies combined was 148 with 155 affected feet as 7 patients had bilateral involvement included in the study. All of the 155 affected limbs were allowed to either weight-bear or partially weight-bear, and there were no publications looking at resolution times of patients who had been told to strictly non weight-bear.
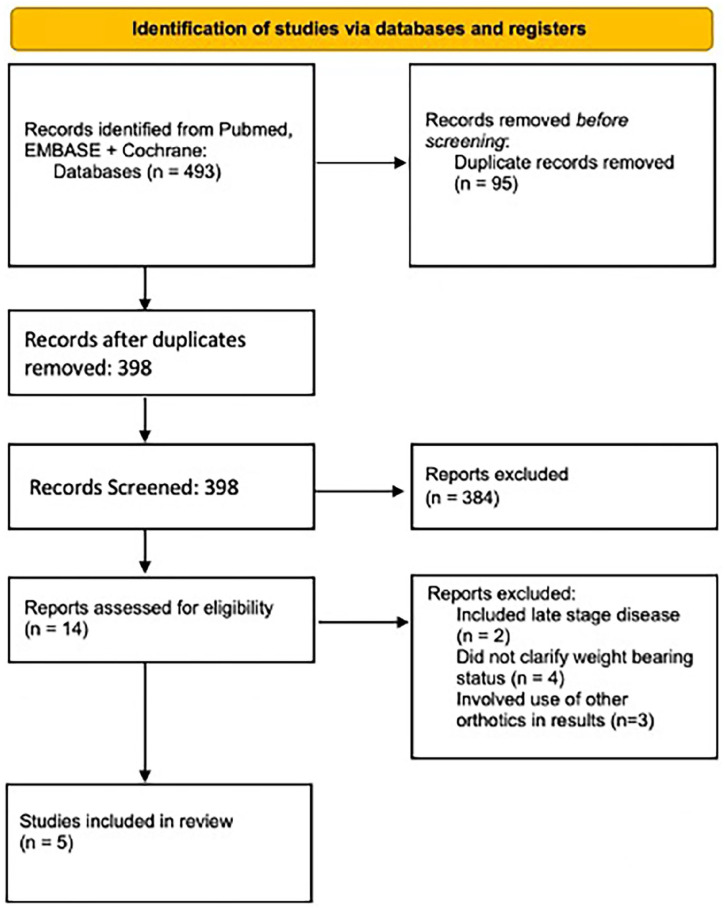
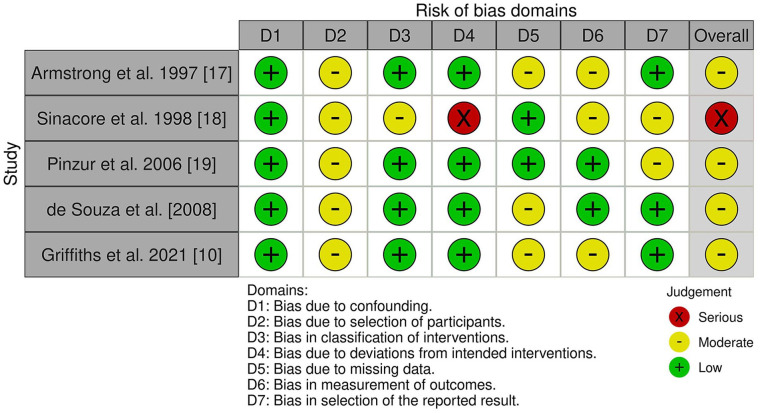
The screening process is demonstrated in Figure 2 below. The study characteristics of included studies can be seen in Table 1. All studies were retrospective, and there were no comparative studies. Details of the intervention and outcome can be seen in Table 1. Risk of bias was assessed for each study and found to be moderate to serious overall as shown in Figure 3.
Figure 2.
PRISMA flow chart showing identification of studies.
Figure 3.
ROBINS-I tool showing levels of bias in the included papers.
The mean time to disease resolution reported by studies in this review ranged from 9.2 to 18.5 weeks with an overall range from 4 to 32 weeks. Mean complication rate was 29.3% ranging from 7.3% to 78%. Age range of patients included is between 39 and 72, with an overall mean age of 57.4. Not all studies provided this data however.
Discussion
All of the studies included in our review evaluated patients who had been on a WB or partial WB protocol throughout their treatment, which highlights the lack of evidence behind the current management of TCC with NWB protocols. These studies showed that WB did not prevent the foot from reaching the consolidation or inactive phase of the disease, whilst remaining free of major deformity and skin breakdown and allowing the patient to maintain a greater level of independence in their daily life. The average time to disease resolution reported by studies in this review ranged from 9.2 to 18.5 weeks with an overall range from 4 to 32 weeks. This large variation in times is likely to be influenced by which stage of the disease patients were in during their initial presentation – which was not reported.
There were no available studies with published data on resolution times in NWB TCC to compare this data with, which implies that existing NWB protocols are based on anecdotal evidence and theory, rather than robust evidence-based clinical practice.
The listed complication rate is variable between the included papers ranging from 7.3% to 78%. This latter number is from a paper which includes minor complications such as skin irritation, when compared to other papers which only include significant complications including ulceration and requirement for corrective surgery. A key question that would require a control group to answer would be the difference in complication rate between NWB and WB TCC.
TCC is widely accepted as the optimal management in treating early CNO. Multiple publications17,23 –26 recommend aggressive and immediate offloading with a non-WB (NWB) cast; however, there are no randomised trials or case series to support this treatment strategy over allowing patients to weight bear. Instead, the current treatment strategy is based on the theoretical premise that offloading the foot by NWB leads to less trauma to the bony architecture when compared with partial or full WB. 19
The two main theories behind the pathophysiology of active Charcot foot are the neuro-traumatic and neuro-vascular theories. Neuro-vascular relates to loss of autonomic control of localised vasculature resulting in arteriovenous shunting, which in turn increases blood flow to the foot and allows congregation of osteoclasts and inflammatory signalling molecules, leading to osteopenia and osteolysis. 27 The neuro-traumatic theory describes how in an insensate foot, irregular plantar pressures lead to progressive unnoticed microtrauma, that leads to a breakdown across the bony and ligamentous architecture of the foot and ankle. 1 The reality is likely a combination of both processes leads to weakening at bone-ligament junctions resulting in fracture dislocations in the foot. As this eventually destroys the longitudinal arch of the foot, the patient can be left with the rocker-bottom deformity synonymous with end-stage Charcot foot. NWB TCC aims to eliminate the neuro-traumatic component by preventing any force from going through the foot, allowing the foot to reach the consolidation phase whilst still in its natural shape. Some of the papers in this review suggest that a well-moulded and well looked after TCC can support the foot sufficiently to withstand the pressures of WB, whilst maintaining the architecture of the foot.
NWB protocols are found widely in all aspects of foot and ankle surgery, with a hesitancy towards early WB post-operatively or post-traumatically, due to fear of early failure or poor outcomes. Recent publications however such as the WAX trial are challenging these principles, with a push towards early mobilisation after ankle fracture fixation, which has been shown to be safe and cost-effective for both patients and health systems. 14
Compliance with a NWB regime can be difficult to enforce and adhere to, 26 with logistical challenges for patients trying to perform activities of daily living. NWB restrictions are associated with immobility-related pathology, dependency on social services and lengthy hospital admissions. Vulnerable patients with co-morbidities including diabetes, suffer from these complications with increased prevalence. 28 Additionally, NWB has been shown to overload the contralateral limb with studies showing an increased prevalence of CNO on the unaffected limb, due to the increased functional burden. 29 Given that this specific cohort of patients suffers from peripheral neuropathy, it is difficult to assess how compliant a patient may be when asked to non-weight bear. Frail patients or those with upper body weakness will struggle more so to limit WB due to existing mobility restrictions.
Limitations
The current literature regarding WB during TCC is limited to case series and uncontrolled cohort studies. The article authored by Sinacore 18 which used a partial weight bearing (PWB) method, does not explain any specific instructions given to patients after casting regarding their WB.
Risk of bias which was assessed and displayed in Figure 3 shows moderate to serious levels of bias in all of the included papers. Four of the included studies were retrospective in nature which introduces bias during the original data collection and data entry, as none of these factors were initially controlled. 30 None of the included studies involved any randomisation or comparison to a control cohort, which leads to selection bias in the study population. The study populations were low ranging from 9 to 55 patients which makes it difficult to extrapolate about the safety or effectiveness of this intervention. 31 Time to resolution was a primary outcome in all the studies; however, this may be affected by how advanced the disease was when the patient presented, which is difficult to control, but has not been quantified. This may be a contributor to the large variation in time to resolution between studies.
The significant levels of bias, as identified by the ROBINS-I tool undermine the validity of the included studies, and therefore make it difficult for us to recommend treatments based on them.
Conclusion
WB in a TCC does not appear to negatively affect healing in patients in stage 0,1 or early stage 2 with active CNO, based on the limited data which have been published on the subject. Given the high levels of bias in the existing literature, as well as the lack of published outcomes in NWB TCC, we are unable to definitively recommend either a WB or NWB protocol for the management of CNO. Further randomised comparative research is urgently needed to identify the optimal WB strategy for patients with active CNO.
Acknowledgments
None.
Footnotes
Author contributions: Rachna Prem: Writing paper; Vikramman Vignaraja: Writing paper; Thomas Lewis : Writing and editing paper; Basil Budair: Final editor. All authors contributed equally towards the publication.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval not required as review article of existing papers.
Informed consent: Informed consent not applicable as this was a systematic review, reviewing already published papers.
ORCID iDs: Vikramman Vignaraja  https://orcid.org/0000-0003-2756-8571
https://orcid.org/0000-0003-2756-8571
Thomas Lewis  https://orcid.org/0000-0002-4167-7427
https://orcid.org/0000-0002-4167-7427
No conflicts in data handling.
Code availability: Not applicable.
References
- 1. Trieb K. The Charcot foot. Bone Jt J 2016; 98-B: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 2. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract; 157. Epub ahead of print 1 November 2019. DOI: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3. Pfannkuche A, Alhajjar A, Ming A, et al. Prevalence and risk factors of diabetic peripheral neuropathy in a diabetics cohort: register initiative ‘diabetes and nerves’. Endocr Metab Sci 2020; 1: 100053. [Google Scholar]
- 4. Sommer TC, Lee TH. Charcot foot: the diagnostic dilemma. Am Fam Physician 2001; 64: 1591–1598. [PubMed] [Google Scholar]
- 5. Wukich DK, Frykberg RG, Kavarthapu V. Charcot neuroarthropathy in persons with diabetes: it’s time for a paradigm shift in our thinking. Diabetes Metab Res Rev 2024; 40: e3754. [DOI] [PubMed] [Google Scholar]
- 6. Chantelau E, Onvlee GJ. Charcot foot in diabetes: farewell to the neurotrophic theory. Horm Metab Res 2006; 38: 361–367. [DOI] [PubMed] [Google Scholar]
- 7. Edmonds ME, Roberts VC, Watkins PJ. Blood flow in the diabetic neuropathic foot. Diabetologia 1982; 22: 9–15. [DOI] [PubMed] [Google Scholar]
- 8. Gilbey SG, Walters H, Edmonds ME, et al. Vascular calcification, autonomic neuropathy, and peripheral blood flow in patients with diabetic nephropathy. Diabet Med 1989; 6: 37–42. [DOI] [PubMed] [Google Scholar]
- 9. Rosenbaum AJ, DiPreta JA. Classifications in brief: Eichenholtz classification of Charcot arthropathy. Clin Orthop 2015; 473: 1168–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Griffiths DA, Kaminski MR. Duration of total contact casting for resolution of acute Charcot foot: a retrospective cohort study. J Foot Ankle Res 2021; 14: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wukich DK, Schaper NC, Gooday C, et al. Guidelines on the diagnosis and treatment of active Charcot neuro-osteoarthropathy in persons with diabetes mellitus (IWGDF 2023). Diabetes Metab Res Rev 2024; 40: e3646. [DOI] [PubMed] [Google Scholar]
- 12. Pinzur MS, Shields N, Trepman E, et al. Current practice patterns in the treatment of Charcot foot. Foot Ankle Int 2000; 21: 916–920. [DOI] [PubMed] [Google Scholar]
- 13. Hastings MK, Sinacore DR, Fielder FA, et al. Bone mineral density during total contact cast immobilization for a patient with neuropathic (Charcot) arthropathy. Phys Ther 2005; 85: 249–256. [PMC free article] [PubMed] [Google Scholar]
- 14. Bretherton CP, Achten J, Jogarah V, et al. Early versus delayed weight-bearing following operatively treated ankle fracture (WAX): a non-inferiority, multicentre, randomised controlled trial. The Lancet. Epub ahead of print 4 June 2024. DOI: 10.1016/S0140-6736(24)00710-4. [DOI] [PubMed] [Google Scholar]
- 15. Chawla R, Madhu SV, Makkar BM, et al. RSSDI-ESI clinical practice recommendations for the management of type 2 diabetes mellitus 2020. Indian J Endocrinol Metab 2020; 24: 1–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armstrong DG, Todd WF, Lavery LA, et al. The natural history of acute Charcot’s arthropathy in a diabetic foot specialty clinic. Diabet Med J Br Diabet Assoc 1997; 14: 357–363. [DOI] [PubMed] [Google Scholar]
- 18. Sinacore DR. Acute Charcot arthropathy in patients with diabetes mellitus: healing times by foot location. J Diabetes Complications 1998; 12: 287–293. [DOI] [PubMed] [Google Scholar]
- 19. Pinzur MS, Lio T, Posner M. Treatment of Eichenholtz stage I Charcot foot arthropathy with a weightbearing total contact cast. Foot Ankle Int 2006; 27: 324–329. [DOI] [PubMed] [Google Scholar]
- 20. de Souza LJ. Charcot arthropathy and immobilization in a weight-bearing total contact cast. J Bone Joint Surg Am 2008; 90: 754–759. [DOI] [PubMed] [Google Scholar]
- 21. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020; 368: l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pinzur MS, Sage R, Stuck R, et al. A treatment algorithm for neuropathic (Charcot) midfoot deformity. Foot Ankle 1993; 14: 189–197. [DOI] [PubMed] [Google Scholar]
- 24. Rogers LC, Frykberg RG, Armstrong DG, et al. The Charcot foot in diabetes. Diabetes Care 2011; 34: 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alpert SW, Koval K, Zuckerman JD. Neuropathic arthropathy: review of current knowledge. J Am Acad Orthop Surg 1996; 4: 100–108. [DOI] [PubMed] [Google Scholar]
- 26. Schade VL, Andersen CA. A literature-based guide to the conservative and surgical management of the acute Charcot foot and ankle. Diabet Foot Ankle 2015; 6: 26627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mascarenhas JV, Jude EB. Pathogenesis and medical management of diabetic charcot neuroarthropathy. Med Clin North Am 2013; 97: 857–872. [DOI] [PubMed] [Google Scholar]
- 28. Aloraibi S, Gladman J, Godfrey D, et al. Optimal care for the management of older people non-weight bearing after lower limb fracture: a consensus study. BMC Geriatr 2021; 21: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Petrova NL, Edmonds ME. Medical management of Charcot arthropathy. Diabetes Obes Metab 2013; 15: 193–197. [DOI] [PubMed] [Google Scholar]
- 30. Norvell DC. Study types and bias—don’t judge a study by the abstract’s conclusion alone. Evid-Based Spine-Care J 2010; 1: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Institute of Medicine (US) Committee on Strategies for Small-Number-Participant Clinical Research Trials. Small clinical trials: Issues and challenges. Washington, DC: National Academies Press; (US). [PubMed] [Google Scholar]