Abstract
Background
Inhibitory neuromuscular transmission in the gastrointestinal tract is mediated by intrinsic nitrergic and purinergic neurons. Purines activate G protein‐coupled receptor P2Y1 receptors, increasing intracellular Ca2+ that activates small conductance calcium‐activated potassium (SKCa) channels. Little is known about the effect of adrenergic receptor activation on intestinal smooth muscle. In vascular tissue, stimulation of α‐adrenoceptors causes smooth muscle contraction, while their effect on intestinal tissue is poorly understood. This study aimed to pharmacologically characterize the effect of α‐adrenoceptor activation in the rat colon, which shares similar inhibitory pathways to the human colon.
Methods
Muscle bath experiments were performed with the rat proximal, mid, and distal colon oriented both circularly and longitudinally.
Results
The α1‐adrenoceptor agonist phenylephrine (PE) (10−8–10−5 M) evoked concentration‐dependent relaxations of the intestinal smooth muscle from all regions and orientations. However, in the mid‐circular colon at low PE concentrations, a contraction sensitive to 10−5 M phentolamine (non‐selective α‐adrenoceptor blocker), the neural blocker tetrodotoxin (TTX; 10−6 M), and atropine (10−6 M) was recorded. PE‐induced relaxations were insensitive to TTX (10−6 M) and the nonselective β‐adrenoceptor blocker propranolol (10−6 M). In contrast, PE‐induced relaxations were blocked by phentolamine (10−5 M), prazosin (10−6 M) (α1‐adrenoceptor blocker), and RS17053 (10−6 M) (α1A‐blocker), but not by yohimbine (10−6 M) (α2‐adrenoceptor blocker). Apamin (10−6 M), a SKCa channel blocker, abolished PE‐induced relaxations.
Conclusions
Contractile responses in the circular muscle of the mid colon could be attributed to α‐adrenoceptors located on enteric cholinergic neurons. Stimulation of α1A‐adrenoreceptors activates SKCa channels to cause smooth muscle relaxation, which constitutes a signaling pathway that shares similarities with P2Y1 receptors.
Keywords: alpha adrenoceptors, apamin, colon, phenylephrine, relaxation
α‐Adrenoceptors cause vasoconstriction, however, its effect in intestinal tissue is poorly characterized. In contrast to what occurs in vascular tissue, activation of α1A‐adrenoceptors cause colonic relaxation attributable to the opening of SKCa channels. The pathway is different from relaxation associated to ß‐adrenoceptor activation.
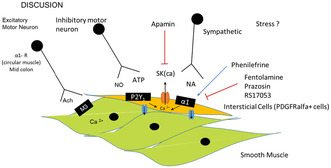
Key Points.
α‐Adrenoceptors cause vasoconstriction, however, its effect in intestinal tissue is poorly characterized.
In contrast to what occurs in vascular tissue, activation of α1A‐adrenoceptors cause colonic relaxation attributable to the opening of SKCa channels.
The pathway is different from relaxation associated to ß‐adrenoceptor activation.
1. INTRODUCTION
Inhibitory neuromuscular transmission in the gastrointestinal (GI) tract is mediated by nitric oxide (NO) and ATP, or a related purine. This mechanism has been described in several species including humans, 1 , 2 rodents, 3 , 4 pigs, 5 and horses. 6 NO binds to cytosolic guanylyl cyclase (Gc), while purines bind on post‐junctional P2Y1 receptors. P2Y1 receptors are G protein‐coupled receptors that upon activation induce an increase in cytosolic Ca2+ in post‐junctional cells. This, in turn, activates apamin‐sensitive SKCa channels (sK3), resulting in smooth muscle hyperpolarization. 7 , 8 Experimental data suggest that NO may play a role in prolonged tonic relaxation, while the activation of P2Y1 receptors likely induces phasic relaxation. 9 Accordingly, the electrophysiological basis of this process consists of a biphasic inhibitory junction potential (IJP), composed of a fast and transient purinergic hyperpolarization (IJPf) followed by a slow, long‐lasting but sustained nitrergic response (IJPs). 10 Moreover, it has been proposed that PDGFRα+ cells, participate in purinergic inhibitory neuromuscular transmission. 11 , 12
Rodents serve as a valuable model for studying neuromuscular transmission in the GI tract due to the presence of a composed IJP. 3 Besides, in mice, distinctions in innervation have been observed not only between the circular and longitudinal layers of the colon 13 but also among the proximal, mid, and distal colon. 4 , 13 In the circular muscle of the colon, an inverse gradient in both inhibitory pathways has been described. 4 , 14 Notably, the proximal and mid colon exhibit predominantly nitrergic inhibitory neuromuscular transmission, whereas in distal areas, the primary inhibitory pathway is purinergic. 4 In contrast, in the longitudinal muscle, neuromuscular innervation was mainly nitrergic. 13
The autonomic nervous system regulates GI motility through the sympathetic, parasympathetic, and enteric nervous systems (ENS). The sympathetic nervous system exerts its effects through epinephrine and norepinephrine released by the adrenal gland (hormonal effect), as well as norepinephrine released by postganglionic sympathetic neurons (neural effect). These catecholamines act on α‐ and β‐adrenoceptors located in various structures, such as neurons of the ENS and intestinal smooth muscle cells, thereby regulating GI motility. Adrenoceptors belong to the superfamily of G protein‐coupled receptors (GPCR) and are activated by catecholamines. α‐adrenoceptors are divided into α1 and α2‐adrenoceptors. 15 The three α1‐adrenoceptor subtypes α1A, α1B, and α1D are activated by the endogenous agonists, adrenaline and noradrenaline. Phenylephrine (PE) is a selective α1–adrenoceptor agonist. α1‐adrenoreceptors are coupled to stimulatory Gq proteins, activate the enzyme phospholipase C, and are mainly found in the smooth muscle cells of blood vessels and the urinary tract, where they induce constriction. β‐adrenoceptor are subdivided into three subtypes β1, β2, and β3. All three β‐adrenoceptor subtypes dilate blood vessels, 16 bronchioles, and relax the muscles of the uterus, bladder, and GI tract (see for review 17 ). In contrast, very little is known about the activation of α‐adrenoreceptors in the GI tract. Two recent papers have reported that α1A‐adrenoceptors activate SKCa channels, leading to smooth muscle hyperpolarization and relaxation. This postulates a non‐nitrergic, non‐purinergic mechanism of relaxation in the human and murine colon. 18 , 19 In another study, region‐specific parasympathetic innervation has been described in the mouse colon affecting both myenteric neurons and post‐junctional cells including PDGFRα+ cells and ICC. 20 Despite these previous studies, the contribution of α‐adrenergic signaling throughout the intestine remains unknown.
The present study aimed to pharmacologically characterize the mechanism of smooth muscle relaxation induced by α‐adrenoceptor activation in the rat colon and to properly assess this mechanism in the proximal, mid, and distal colon from the circular and longitudinal layers.
2. MATERIALS AND METHODS
2.1. Ethical approval
Experimental procedures were approved by the Ethics Committee of the Universitat Autònoma de Barcelona with the approval code MJF‐eut/01 and followed the European Community Council Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
2.1.1. Animals and tissue samples
Thirty‐five Sprague–Dawley rats (20 females and 15 males) of 7‐ to 10‐week old were housed under controlled conditions: temperature (22 ± 2°C) and humidity (55 ± 10%), 12‐h light/dark cycle, and ad libitum access to water and food. Rats were euthanized by decapitation without prior anesthesia.
For functional experiments, the colon was quickly removed and immersed in a cold carbogenated (95% O2 and 5% CO2) Krebs solution. The mesenteric fat was removed, and the colon was carefully opened along the mesenteric border and pinned to a Sylgard base with the mucosa facing upward. Subsequently, the colon was partitioned into three segments: proximal, mid, and distal colon. The mucosal and submucosal layers were removed, and 4 × 10 mm muscle strips were cut in circular and longitudinal directions.
2.1.2. Mechanical studies
Muscle strips were set up in a 10‐mL organ bath filled with Krebs solution and maintained at a temperature of 37 ± 1°C and carbogenated (95% O2 and 5% CO2). A tension of 1 g was applied, and tissues were equilibrated for 1 h. After this period, strips displayed spontaneous phasic contractions. Mechanical activity was measured using an isometric force transducer (UF‐1 Harvard Apparatus) connected to a computer through an amplifier. Data were digitally recorded at a rate of 25 Hz using Data 2001 software (Panlab) coupled with an A/D converter integrated into the computer.
2.1.3. Solutions and drugs
The composition of the Krebs solution was (in mmol/L): glucose 10.10, NaCl 115.48, NaHCO3 21.90, KCl 4.61, NaH2PO4 1.14, CaCl2 2.50, and MgSO4 1.16 bubbled with a mixture of 5% CO2–95% O2 (pH 7.4).
The following drugs were used: L‐phenylephrine (PE; CAS number: 59–42‐7; Merck), isoprenaline hydrochloride (CAS number: 51‐30‐9; Merck), tetrodotoxin (TTX; CAS number: 1078; Tocris), Nω‐Nitro‐L‐arginine (L‐NNA; CAS number: 2149‐70‐4; Merck), apamin (CAS number: 24345‐16‐2; Merck), (N‐[2‐(2‐cyclopropylmethoxyphenoxy)ethyl]‐5‐chloro‐α, α‐dimethyl‐1H‐indole‐3‐ethanamine) hydrochloride (RS17053, CAS number: 0985; Tocris), yohimbine hydrochloride (CAS number: 65‐19‐0; Merck), propranolol hydrochloride (CAS number: 318‐98‐9; Tocris), phentolamine hydrochloride (CAS number: 73‐05‐2; Merck), atropine (CAS number: 51‐55‐8; Merck), Carbachol (CAS number: 51‐83‐2, Merck), and MRS 2500 tetraammonium salt (CAS number: 630103‐23‐0, Tocris).
Stock solutions were prepared by dissolving drugs in distilled water except for RS17053, which was dissolved in DMSO; and L‐NNA, which was dissolved in a physiological saline solution by sonication.
2.1.4. Experimental procedure, data analysis, and statistics
Concentration–response curves were obtained at increasing concentrations of PE (10−8–10−5 M). Each concentration was incubated for 5–10 min until a stable response was obtained. Experiments were also performed with 30 min incubation with different blockers. Isoprenaline was rested at 10−8 M in the presence of different blockers. Each strip was used for one experiment.
The area under the curve (AUC; g × min) of contractions calculated from the baseline was measured to estimate the response to drugs, which was expressed as a percentage of the basal AUC of contractions, being 100 the basal activity before drug addition. Concentration–response curves of phenylephrine under different pharmacological conditions were performed using the following formula Y = 100/ (1 + 10(X‐LogIC50)). Responses to drugs were compared using one‐way and two‐way ANOVA tests. Data are expressed as mean ± SEM. Data were considered significant when p < 0.05. n values represent strips from different animals. Statistical analysis was performed with GraphPad Prism software version 6.01 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
3.1. Effect of PE on the circular and longitudinal layers from the proximal, mid, and distal colon
The α1‐adrenoceptor agonist, PE (10−8–10−5 M) evoked concentration‐dependent relaxations of the colon smooth muscle from all regions and orientations with an IC50 in the range of 10−7 M in all regions and orientations (Table 1). In the longitudinal muscle, no differences were observed between colonic zones (p = 0.9286). In contrast, in the circular muscle, PE elicited differences in the response across colonic zones (p < 0.0001), being more potent in the distal colon with an IC50 of 2.8 × 10−7 M, followed by the proximal colon with an IC50 of 5.7 × 10−7 M and lastly the mid colon with an IC50 of 6.5 × 10−7 M. Moreover, at concentrations from 10−8 to 10−7 M of PE, an increase in the AUC was also observed in the circular muscle from the mid colon (Figure 1).
TABLE 1.
Outcome parameters from PE concentration–response curve evaluation in the proximal, mid, and distal colon from the circular and longitudinal muscle.
| Circular muscle | Longitudinal muscle | |||||
|---|---|---|---|---|---|---|
| Proximal | Mid | Distal | Proximal | Mid | Distal | |
| IC50 (M) | 5.7 × 10−7 | 6.5 × 10−7 | 2.8 × 10−7 | 8.8 × 10−7 | 8.0 × 10−7 | 8.6 × 10−7 |
| Log IC50 ± SE | −6.2 ± 0.03 | −6.2 ± 0.06 | −6.5 ± 0.04 | −6.1 ± 0.03 | −6.1 ± 0.03 | −6.1 ± 0.02 |
FIGURE 1.
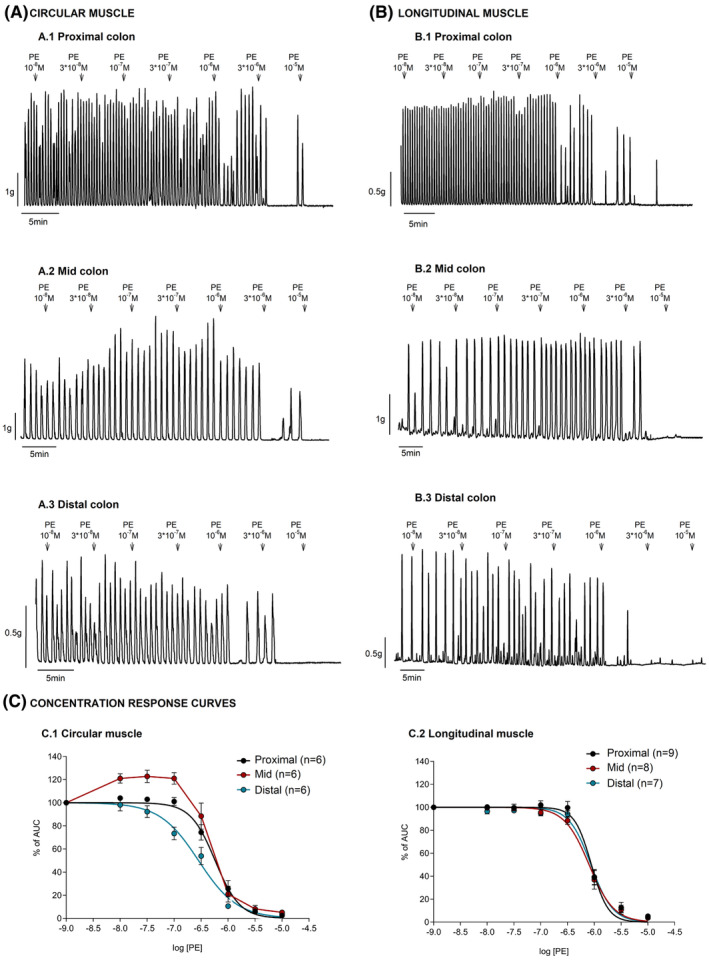
Effect of phenylephrine (PE) on the circular and longitudinal layers from the proximal, mid, and distal colon. Top: Representative mechanical recording showing the effect of PE (10−8–10−5 M) on the circular (A.1–A.3) and longitudinal (B.1–B.3) layers from the proximal, mid, and distal colon. Bottom: Concentration–response curves in each segment and muscle orientation (C.1–C.2). Data were normalized (i.e., 100%) to the basal AUC before drug addition. Data are expressed as mean ± SEM.
3.2. Effect of PE on the circular in control, in the presence of atropine, phentolamine, and propranolol
The response observed with PE in the mid colon, circular muscle was pharmacologically characterized. Tissues were incubated with 10−6 M atropine, 10−5M phentolamine, 10−6 M propranolol, and in control conditions. The increase in the amplitude of the contractile response (measured as increase in AUC) observed in the middle circular colon, at low PE concentrations, was sensitive to 10−5 M phentolamine and 10−6 M atropine. Consistent with the activation of α‐adrenoceptors, PE‐induced relaxation was phentolamine‐sensitive and propranolol‐ and atropine‐insensitive (Figure 2). The contractile response but not the relaxation induced by PE was 10−6 M TTX‐sensitive (Figures 2 and 3). It is important to note that the increase in AUC was mimicked by muscarinic stimulation with low concentration of carbachol (see Figure S1).
FIGURE 2.
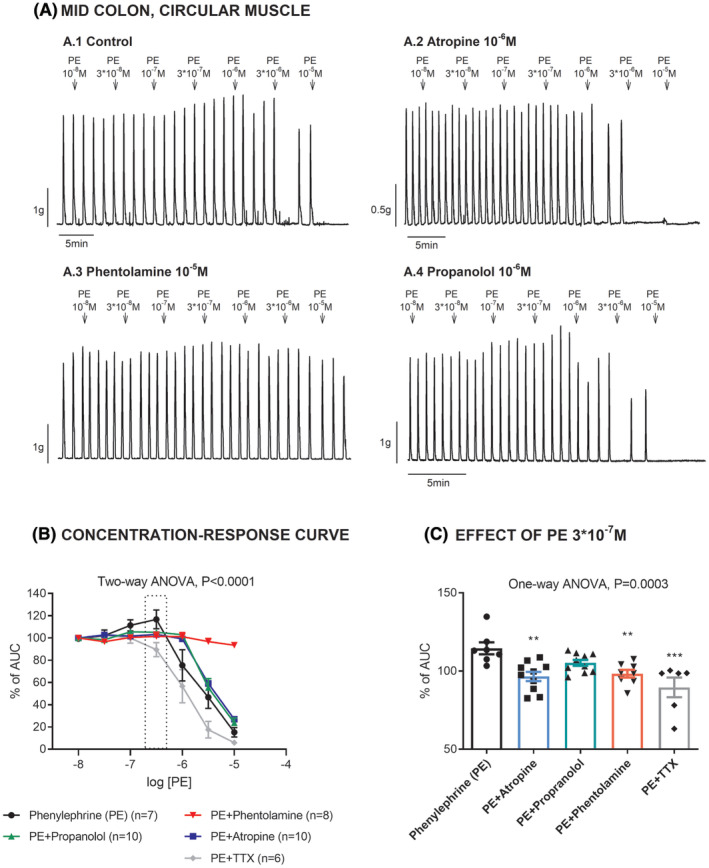
Effect of phenylephrine (PE) on the circular middle colon in control conditions and in the presence of atropine, phentolamine, propranolol, and TTX. (A) Representative mechanical recordings showing the effect of PE in control conditions (A.1), in the presence of atropine (A.2), phentolamine (A.3), and propranolol (A.4). (B) Graph showing the effects of PE in each experimental condition. C. Histogram showing the effect of PE 3 × 10−7 M in each experimental condition. Data were normalized (i.e., 100%) to the basal AUC before drug addition. **p < 0.01, ***p < 0.001, compared with PE alone by one‐way ANOVA with Dunnett's post‐test. Data are expressed as mean ± SEM.
FIGURE 3.
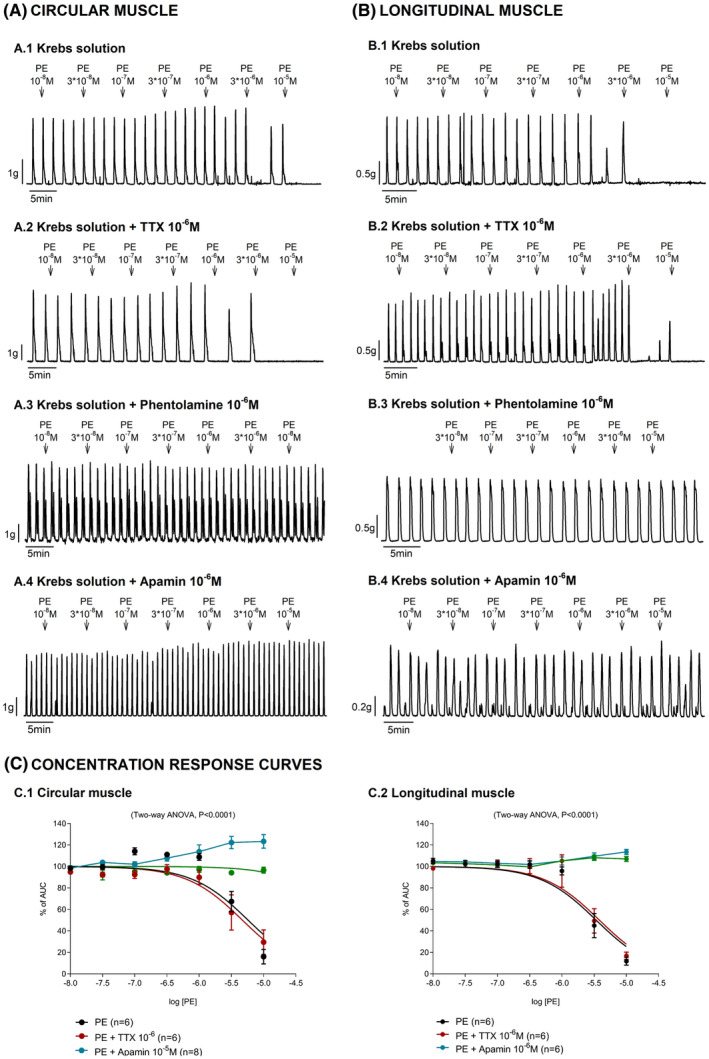
Effect of phenylephrine (PE) on the circular and longitudinal muscle from the mid colon in the presence of TTX, phentolamine, and apamin. Representative mechanical recordings showing the effect of PE in the circular (A: left panel) and longitudinal (B: right panel) muscle in control conditions (A.1, B.1), in the presence of TTX (A.2, B.2), phentolamine (A.3, B.3), and apamin (A.4, B.4). Concentration–response curves assessed in each muscle layer (C). Data were normalized (i.e., 100%) to the basal AUC before drug addition. Data are expressed as mean ± SEM.
3.3. Effect of PE on the circular and longitudinal muscle in control conditions, in the presence of TTX, phentolamine, and apamin
Responses induced by PE were assessed in the presence of 10−6 M TTX, 10−5 M phentolamine, and 10−6 M apamin. PE‐induced relaxations were insensitive to the neural blocker, TTX 10−6 M. In contrast, 10−5 M phentolamine and 10−6 M apamin, an SKCa channel blocker, completely abolished PE‐induced relaxations in both circular and longitudinal muscle strips from the mid colon. To assess whether PE‐induced relaxation was associated with the neural release of inhibitory neurotransmitters, tissue was incubated with a cocktail of blockers including L‐NNA 10−3 M + MRS2500 10−6 M + propranolol 10−6 M and atropine 10−6 M. Under these conditions, PE‐induced relaxations were still observed in both muscle layers. The addition of 10−6 M TTX to the cocktail did not modify PE‐induced relaxations. However, incubation with 10−6 M apamin and 10−5 M phentolamine abolished PE‐induced relaxations (Figure 3). It is important to note that all experiments were performed on different strips. However, in a subset of experiments, the concentration–response curves were performed twice with PE. A washout was performed between the two curves. These experiments correspond to circularly oriented strips and were performed in the presence of TTX (n = 6). Both responses were coincident.
3.4. Effect of PE on the circular and longitudinal muscle in the presence of propranolol, yohimbine, prazosin, and RS17053 in tissue incubated with TTX
Lastly, different α‐adrenoceptor blockers were tested to assess the response induced by PE. Phenylephrine‐induced relaxation in the mid colon was blocked by 10−6 M prazosin (α1‐adrenoceptor blocker) and 10−6 M RS17053 (α1A preferential blocker), but not by 10−6 M yohimbine (α2‐adrenoceptor blocker) in both muscle layers (Figure 4).
FIGURE 4.
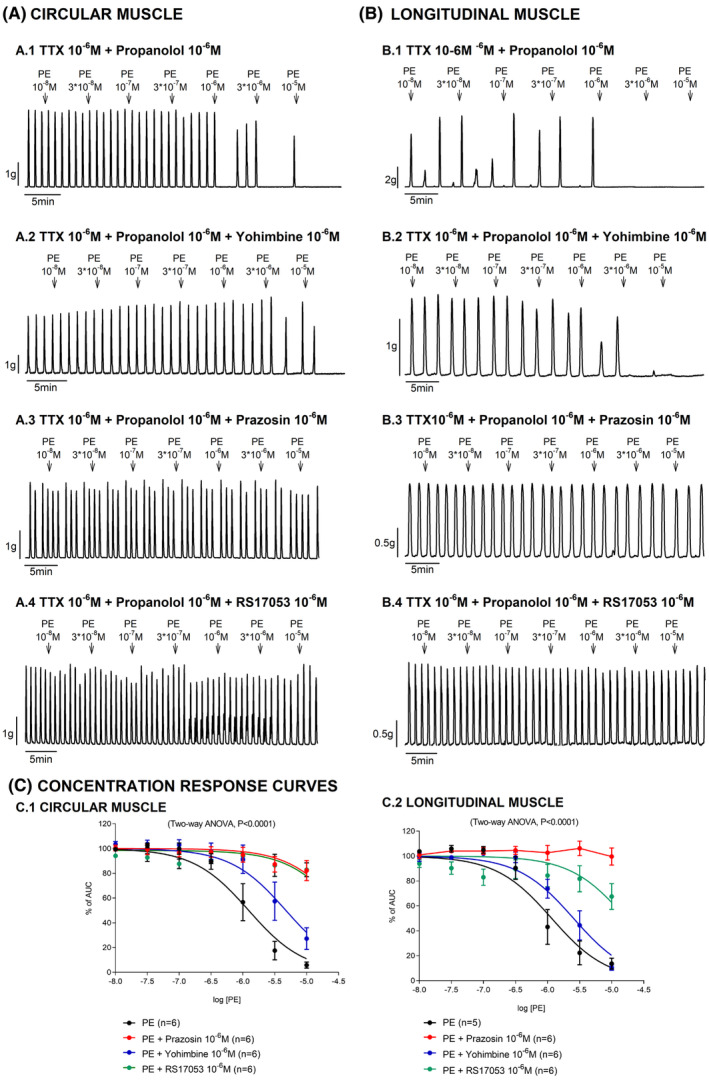
Effect of phenylephrine (PE) on the circular and longitudinal muscle in the presence of propranolol, yohimbine, prazosin, and RS17053 under TTX conditions. Mechanical recordings from the circular (A: left panel) and longitudinal (B: right panel) muscle showing the effect of PE in the presence of propranolol (A.1, B.1), yohimbine (A.2, B.2), prazosin (A.3, B.3), and RS17053 (A.4, B.4). Concentration–response curves assessed in each muscle layer (C). Data were normalized (i.e., 100%) to the basal AUC before drug addition. Data are expressed as mean ± SEM.
3.5. Effect of isoprenaline on the circular muscle
To investigate the involvement of β‐adrenoceptors on colonic inhibitory responses, isoprenaline (β‐agonist) was tested at 10−8 M. Isoprenaline strongly decreased spontaneous phasic contractions. Th effect was reversed by propranolol 10−6 M, and further incubation with Phe at 10−5 M completely abolished spontaneous contractions (n = 6). Incubation with propranolol at 10−6 M prevented the isoprenaline response (n = 7). In contrast, incubation with phentolamine at 10−6 M did not modify the isoprenaline response (n = 7). Tracings and data are shown in Figure 5. These findings are consistent with β‐adrenoceptor activation that is totally different from the previously described response to Phe. Differences are detailed in Table 2.
FIGURE 5.
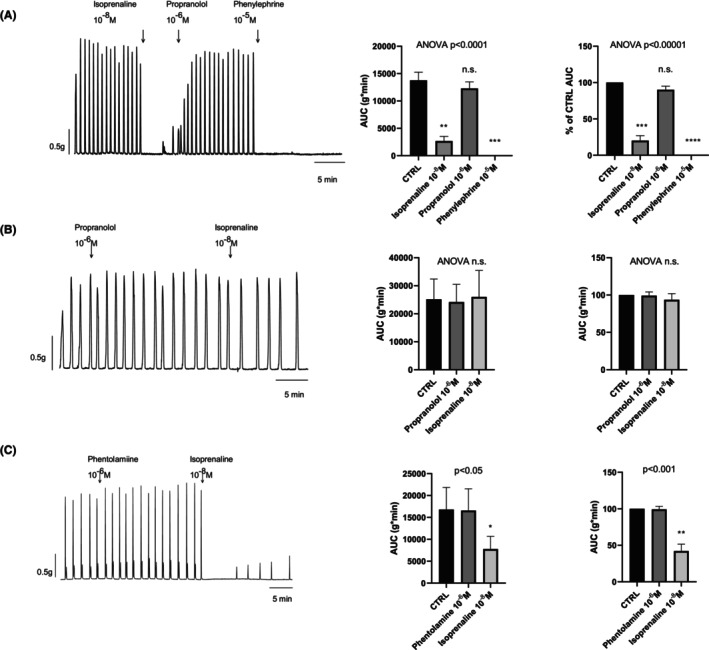
Effect of isoprenaline on the circular muscle from the mid colon. Isoprenaline 10−8 M strongly reduced spontaneous contractions. The effect was reversed by propranolol 10−6 M and further addition of Phe 10−5 M abolished spontaneous contractions (n = 6) (Panel A). Incubation with propranolol 10−6 M prevented isoprenaline 10−8 M responses (n = 8; Panel B). Incubation with phentolamine 10−6 M did not prevent isoprenaline 10−8 M responses (n = 8; Panel C). Data in histograms are shown in AUC and normalized (i.e., control = 100%) for each experimental protocol. Dunnet's post hoc test was performed after paired ANOVA test to check differences from control (CTRL) conditions. To avoid neural‐mediated responses, TTX 10−6 M was added 30 min before the start of the experiments.
TABLE 2.
Pharmacological profile of α‐ and β‐agonists on inhibitory motor responses in the rat colon.
| Agonists | Antagonists that block the inhibitory response | Antagonists that do not block the inhibitory response |
|---|---|---|
|
Phenylephrine (α‐adrenoceptor agonist) |
Phentolamine: α‐adrenoceptor blocker Apamin: sKCa blocker Prazosin: α1‐adrenoceptor blocker RS17053: α1A‐adrenoceptor blocker a | Propranolol: β‐adrenoceptor blocker Yohimbine: α2‐adrenoceptor blocker |
|
Isoprenaline (β‐adrenoceptor agonist) |
Propranolol: β‐adrenoceptor blocker | Phentolamine: α‐adrenoceptor blocker |
Preferential α1A as it might also block α1D‐adrenoceptor.
4. DISCUSSION
In the present study, we found that PE causes a TTX‐insensitive relaxation in both muscle layers and regions of the colon, whereas a TTX‐sensitive contractile response was observed in the circular muscle of the mid colon.
The inhibitory response induced by PE was similar to the one described by Kurahashi et al. in murine distal colon. 19 In this study, contractile responses were evaluated in the circular muscle from the rat distal colon and a reduction in the contractility was observed after PE addition. Our study shows similar results in the different regions of the rat colon analyzed, showing the same mechanism involved in both muscle layers. PE is slightly more potent in rats compared to mice, 19 as the IC50 is between 10−6 and 10−7M. To check a possible involvement of the NO/Purine pathway, we incubated the tissue with the NOS inhibitor L‐NNA and the P2Y1 blocker MRS2500. We also added TTX and atropine to the cocktail to abolish the influence of both neural excitatory and inhibitory pathways. Under these experimental conditions, PE was still able to inhibit spontaneous phasic contractions (SPC), suggesting that inhibitory motor neurons are not involved in PE response (see below).
The receptor involved in the smooth muscle relaxation is probably an α1A‐adrenoceptor. This is consistent with the blockade with prazosin (α1‐adrenoceptor blocker) and RS17053 (α1A preferential blocker), but not with yohimbine (α2‐adrenoceptor blocker). Similar results were obtained with RS100329 (α1A preferential blocker) in the mouse distal colon. 19 Moreover, the reduction in contractility induced by PE was absent in α1A‐receptor KO mice. A similar mechanism has been described in human tissue where at 10−5 M norepinephrine reduced SPC and this mechanism was blocked by prazosin and RS100329. 18 Similar to what we found in our study, the authors observed that the mechanism was present in different regions of the human colon. The pathway described in the present study and in these previous studies 18 , 19 is distinct from the activation of β‐adrenoceptors with isoprenaline, which induces propranolol‐sensitive but phentolamine‐insensitive inhibitory responses. β‐Adrenoceptors couple to Gs‐proteins to activate adenylyl cyclase. Stimulation of adenylyl cyclase leads to the conversion of ATP into cAMP, which subsequently activates protein kinase A, that in turn phosphorylates several substrates. This pathway is responsible for colonic relaxation and differs from α1A‐receptor activation. Understanding these distinct signaling pathways is crucial for elucidating the complex regulation of gastrointestinal motility and may offer insights into potential therapeutic targets for gastrointestinal disorders characterized by abnormal smooth muscle contractility. One important question is where α1A‐adrenoceptors are located in the GI tract. According to cell‐specific transcriptome data, α1A‐adrenoceptors are located in PDGFRα + cells from mouse colon. 19 These cells are interstitial cells that are c‐kit‐negative and constitute another population of interstitial cells different from c‐kit‐positive interstitial cells of Cajal (ICC). 21 Smooth muscle cells are electrically coupled to interstitial cells of Cajal (ICC) and PDGFRα+ cells forming an integrated motor unit, known as SIP syncytium. 21 , 22 In the mouse colon, PDGFRα + cells are located in both muscle layers and are more numerous compared with cKit + cells, which both form a heterologous cellular network. 14 Based on these results it is reasonable to speculate that the inhibitory effect observed in our study is due to activation of α1A‐adrenoceptors in PDGFRα + cells. Consistent with this hypothesis, norepinephrine increased Ca2+ transients in PDGFRα + cells and this response was blocked by the preferential α1A‐adrenoceptors blocker RS100329. 19
Alpha1A‐adrenoceptors belong to the family of G protein‐coupled receptors (GPCR). They activate the Gq/11 and phospholipase Cβ, thus increasing the production of inositol 1,4,5‐triphosphate (IP3) and initiating Ca2+ release from intracellular stores via IP3 receptors. 23 This is probably the basis of contraction in many smooth muscle cells including vascular tissue. However, the opposite result was found in the colon. It is possible that the Ca2+‐dependent increase in PDGFRα + cells activates SKCa channels, and this hyperpolarization is transmitted to smooth muscle cells through the SIP syncytium. Consistent with this hypothesis, PE caused a smooth muscle hyperpolarization (results not shown) that probably reduced open probability of L‐type Ca2+ channels, leading to a decrease in SPC. In this study, we found that the effect of PE was blocked by apamin. Similar results were reported both in mice and human colon. 18 , 19 Therefore, our data support previous evidence in these two species. In addition, previous data from our laboratory showed that the fast component of the IJP is sensitive to apamin and blocked with the P2Y1 blocker MRS2500. 24 , 25 The fast component of the IJP is absent in P2Y1 KO mice, 26 , 27 demonstrating that the IJPfast is due to ATP or a related purine acting on P2Y1 receptors. The IJPf is usually recorded in non‐adrenergic non‐cholinergic conditions and therefore activation of α1A‐adrenoceptors is unlikely to mediate the IJPf and the corresponding relaxation. However, it is possible that P2Y1 receptors in PDGFRalfa+ cells also mediate the purinergic relaxation 26 and consequently α1A‐adrenoceptors share the same inhibitory pathway as P2Y1 receptors.
The physiological significance of our results is associated with an effect of the sympathetic nervous system on colonic motility, which could be overactivated in several pathophysiological conditions such as stress and hypertension. The sympathetic nervous system may exert its effects through epinephrine and norepinephrine released by the adrenal gland (hormonal effect) and norepinephrine released by postganglionic sympathetic neurons (neuronal effect). A recent study in mice showed that postganglionic sympathetic neurons are closely associated with myenteric neurons, as well as with c‐KIT and PDGFRα+ cells. Both electrical and optogenetic stimulation of postganglionic sympathetic neurons inhibit spontaneous contractions. 20 In this study, a decrease in Ca2+ transients were observed in ICC‐MP and this effect was sensitive to prazosin, suggesting the involvement of α‐adrenoceptors. 20 In mice, this effect was most prominent in the proximal colon but according to our results in the rat, the mechanism is present in all regions of the colon and affects both muscle layers.
Sympathetic innervation can also inhibit and activate different populations of enteric neurons. 20 In our study, a contraction was also observed at low concentrations of PE in the circular muscle from the mid colon. This response was found to be sensitive to phentolamine and, therefore, mediated by α‐adrenoreceptors. The contractile response induced by PE was possibly associated with the presence of α‐adrenoceptors located on enteric cholinergic neurons, as the response was TTX and atropine‐sensitive. Consistently, in the human colon, the contractile response observed to norepinephrine was associated to α‐adrenoceptors (possibly α1D adrenoceptors) located in smooth muscle. 18
5. CONCLUSION
We conclude that the contractile responses in the circular muscle of the mid colon could be attributed to α‐adrenoceptors located on enteric cholinergic neurons. Post‐junctional stimulation of α1A‐adrenoreceptors possibly located in PDGFRα+ cells activates SKCa channels to cause smooth muscle hyperpolarization and relaxation. This pathway is coincident with that previously described for P2Y1 receptors in the colon of different species, including humans. Activation of α1A‐adrenoceptors is therefore a new pathway of smooth muscle relaxation through sympathetic regulation of colonic motility.
AUTHOR CONTRIBUTIONS
Sa.T. wrote the paper, prepared the figures, and did some of the experiments. M.G. and So.T. performed part of the experiments. F. J‐A. and P.V. contributed to the design of the study and participated in its funding and M.J. supervised the study.
FUNDING INFORMATION
PID2020‐113634RB‐C22/AEI/10.13039/501100011033 from Ministerio de Ciencia e Innovación and Agencia Estatal de Investigación of Spain.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
Supporting information
Figure S1:
ACKNOWLEDGMENTS
The authors thank Emma Martínez and Antonio Acosta of Universitat Autònoma de Barcelona for their technical assistance.
Traserra S, Grao M, Trujillo S, Jiménez‐Altayó F, Vergara P, Jimenez M. Pharmacological characterization of alpha adrenoceptor‐mediated motor responses in the rat colon. Neurogastroenterology & Motility. 2025;37:e14921. doi: 10.1111/nmo.14921
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gallego D, Gil V, Aleu J, Aulí M, Clavé P, Jiménez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008;295(3):G522‐G533. doi: 10.1152/ajpgi.00510.2007 [DOI] [PubMed] [Google Scholar]
- 2. Gallego D, Gil V, Aleu J, Martinez‐Cutillas M, Clavé P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23(8):792. doi: 10.1111/j.1365-2982.2011.01725.x [DOI] [PubMed] [Google Scholar]
- 3. Grasa L, Gil V, Gallego D, Martín MT, Jiménez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158(6):1641‐1652. doi: 10.1111/j.1476-5381.2009.00454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mañé N, Viais R, Martínez‐Cutillas M, Gallego D, Correia‐de‐Sá P, Jiménez M. Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiol (Oxf). 2016;216(1):120‐131. doi: 10.1111/apha.12599 [DOI] [PubMed] [Google Scholar]
- 5. Gallego D, Vanden Berghe P, Farré R, Tack J, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008;20(2):159‐168. doi: 10.1111/j.1365-2982.2007.01004.x [DOI] [PubMed] [Google Scholar]
- 6. Mas M, Mañé N, Fernández F, Gallego D, Pumarola M, Jiménez M. P2Y(1) receptors mediate purinergic relaxation in the equine pelvic flexure. Vet J. 2016;209:74‐81. doi: 10.1016/j.tvjl.2016.01.002 [DOI] [PubMed] [Google Scholar]
- 7. Gallego D, Hernández P, Clavé P, Jiménez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G584‐G594. doi: 10.1152/ajpgi.00474.2005 [DOI] [PubMed] [Google Scholar]
- 8. Gallego D, Gil V, Martínez‐Cutillas M, Mañé N, Martín MT, Jiménez M. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol. 2012;590(8):1943‐1956. doi: 10.1113/jphysiol.2011.224345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mañé N, Gil V, Martínez‐Cutillas M, Clavé P, Gallego D, Jiménez M. Differential functional role of purinergic and nitrergic inhibitory cotransmitters in human colonic relaxation. Acta Physiol (Oxf). 2014;212(4):293‐305. doi: 10.1111/apha.12408 [DOI] [PubMed] [Google Scholar]
- 10. Jiménez M, Clavé P, Accarino A, Gallego D. Purinergic neuromuscular transmission in the gastrointestinal tract; functional basis for future clinical and pharmacological studies. Br J Pharmacol. 2014;171(19):4360‐4375. doi: 10.1111/bph.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurahashi M, Mutafova‐Yambolieva V, Koh SD, Sanders KM. Platelet‐derived growth factor receptor‐α‐positive cells and not smooth muscle cells mediate purinergic hyperpolarization in murine colonic muscles. Am J Physiol Cell Physiol. 2014;307(6):C561‐C570. doi: 10.1152/ajpcell.00080.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker SA, Hennig GW, Ward SM, Sanders KM. Temporal sequence of activation of cells involved in purinergic neurotransmission in the colon. J Physiol. 2015;593(8):1945‐1963. doi: 10.1113/jphysiol.2014.287599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Traserra S, Villarte S, Traini C, et al. The asymmetric innervation of the circular and longitudinal muscle of the mouse colon differently modulates myogenic slow phasic contractions. Neurogastroenterol Motil. 2020;32(4):e13778. doi: 10.1111/nmo.13778 [DOI] [PubMed] [Google Scholar]
- 14. Lu C, Huang X, Lu HL, et al. Different distributions of interstitial cells of Cajal and platelet‐derived growth factor receptor‐α positive cells in colonic smooth muscle cell/interstitial cell of Cajal/platelet‐derived growth factor receptor‐α positive cell syncytium in mice. World J Gastroenterol. 2018;24(44):4989‐5004. doi: 10.3748/wjg.v24.i44.4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alexander SPH, Christopoulos A, Davenport AP, et al. The concise guide to PHARMACOLOGY 2023/24: G protein‐coupled receptors. Br J Pharmacol. 2023;180(Suppl 2):S23‐S144. doi: 10.1111/bph.16177 [DOI] [PubMed] [Google Scholar]
- 16. Flacco N, Segura V, Perez‐Aso M, et al. Different β‐adrenoceptor subtypes coupling to cAMP or NO/cGMP pathways: implications in the relaxant response of rat conductance and resistance vessels. Br J Pharmacol. 2013;169(2):413‐425. doi: 10.1111/bph.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motiejunaite J, Amar L, Vidal‐Petiot E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann Endocrinol. 2021;82(3–4):193‐197. doi: 10.1016/j.ando.2020.03.012 [DOI] [PubMed] [Google Scholar]
- 18. Kurahashi M, Kito Y, Hara M, Takeyama H, Sanders KM, Hashitani H. Norepinephrine has dual effects on human colonic contractions through distinct subtypes of alpha 1 Adrenoceptors. Cell Mol Gastroenterol Hepatol. 2020;10(3):658‐671.e1. doi: 10.1016/j.jcmgh.2020.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurahashi M, Kito Y, Baker SA, et al. A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB J. 2020;34(4):5563‐5577. doi: 10.1096/fj.201903134R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith‐Edwards KM, Edwards BS, Wright CM, et al. Sympathetic input to multiple cell types in mouse and human colon produces region‐specific responses. Gastroenterology. 2021;160(4):1208‐1223.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94(3):859‐907. doi: 10.1152/physrev.00037.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanders KM, Kito Y, Hwang SJ, Ward SM. Regulation of gastrointestinal smooth muscle function by interstitial cells. Physiology (Bethesda). 2016;31(5):316‐326. doi: 10.1152/physiol.00006.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ciccarelli M, Sorriento D, Coscioni E, Iaccarino G, Santulli G. Chapter 11 adrenergic receptors. Endocrinology of the Heart in Health and Disease. 2017;285‐235. doi: 10.1016/B978-0-12-803111-7.00011-7 [DOI] [Google Scholar]
- 24. Gil V, Gallego D, Maati MO, et al. Relative contribution of SKCa and TREK1 channels in purinergic and nitrergic neuromuscular transmission in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2012;303(3):G412‐G423. doi: 10.1152/ajpgi.00040.2012 [DOI] [PubMed] [Google Scholar]
- 25. Martínez‐Cutillas M, Gil V, Gallego D, et al. α,β‐meATP mimics the effects of the purinergic neurotransmitter in the human and rat colon. Eur J Pharmacol. 2014;740:442‐454. doi: 10.1016/j.ejphar.2014.06.048 [DOI] [PubMed] [Google Scholar]
- 26. Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the'fibroblast‐like cells' in gastrointestinal smooth muscles. J Physiol. 2011;589(Pt 3):697‐710. doi: 10.1113/jphysiol.2010.201129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gil V, Martínez‐Cutillas M, Mañé N, Martín MT, Jiménez M, Gallego D. P2Y(1) knockout mice lack purinergic neuromuscular transmission in the antrum and cecum. Neurogastroenterol Motil. 2013;25(3):e170‐e182. doi: 10.1111/nmo.12060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


