Abstract
Background
Postoperative atrial fibrillation (POAF) is the most frequent cardiac arrhythmia following cardiac operations. It has been associated with an increased risk of postoperative cerebrovascular complications, morbidity and mortality. The aim of this study is to evaluate if the type of venous cannulation to institute the cardiopulmonary bypass (CPB) during major cardiac surgery procedures can influence the rate of POAF and late FA onset.
Methods
We collected data from 2087 consecutive patients who have been operated at our Institution from January 2016 to December 2018. To obtain two homogenous groups we performed a propensity match analyzes: Group 1 for whom the blood drain of the CPB has been granted via peripheral cannulation (PC) through the right common femoral vein and Group 2 with patients who underwent central cannulation (CC) with insertion of a drainage cannula in the right atrium or in the superior and inferior vein cava.
Results
POAF has been observed as statistically similar between the two groups. At 1250‐day follow‐up, While the incidence of POAF was 2.9% and 8.7% in the PC and CC groups, respectively (p = .04).
Conclusions
our data seems to show that the two groups do not differ in terms of POAF, while the CC group may have a significantly higher rate of atrial fibrillation in the follow‐up period.
Keywords: femoral cannulation, atrial fibrillation, risk factors, postoperative atrial fibrillation, late onset of atrial fibrillation, cardiac surgery complications
Abbreviations
- AF
atrial fibrillation
- CC
central cannulation
- CPB
cardiopulmonary bypass
- MISC
minimally invasive cardiac surgery
- PC
peripheral cannulation
- POAF
postoperative atrial fibrillation
1. INTRODUCTION
Postoperative atrial fibrillation (POAF), defined as new onset of AF within 30 days from a cardiac intervention, is the most frequent cardiac complication occurring in almost 30% of patients undergoing cardiac surgery and, when occurs, it has been associated with an increased risk of postoperative cerebrovascular complications, morbidity and mortality including also an eightfold increased risk of subsequent AF at the follow‐up. 1 , 2
Despite considerable efforts by the scientific community aimed to understand the etiology of POAF, an unanimous consensus about the mechanism that generates it and the risk factors that predispose its occurrence has not yet been reached. Nevertheless, there is an almost unanimous consensus that POAF has a multifactorial genesis, such as electrolyte imbalances, sympathetic tone, postoperative inflammation, oxidative stress and type of surgery.
Until a few decades ago, cardiac surgeries always involved central venous cannulation through the right atrium or through the superior and inferior vena cavaThe only conditions that sometimes made surgeons advocate peripheral venous cannulation through the right femoral vein were reinterventions (because of the possibility of injury to a noble structure during isolation of the heart and the consequent need for immediate initiation of cardiopulmonary bypass [CPB]) or extremely fragile atrial tissue.
Since the 1990s, however, with the emergence of minimally invasive cardiac surgery (MICS), the use of femoral venous cannulation (with surgical vessel isolation or percutaneous access) has become increasingly popular among cardiac surgeons 3 and is now the gold standard for the institution of CPB in all minithoracotomy procedures and widely used in ministernotomy procedures.
The idea behind this study stems from the well‐established notion that cardiac scar tissue is a possible irritative focus eventually leading to possible occurrence of late FA insurgence. Considering that central cannulation (CC) (involving one or more surgical accesses to the atrial cavity) at the end of surgery inevitably leaves scar tissue on the wall of the right atrium, we wondered whether these scars due to surgical cannulation could be a possible additional risk factor for POAF therefore increasing the incidence of late FA.
Leveraging data from MICS surgeries performed at our hospital and adding them to those from full sternotomy surgeries during which (for various reasons) the surgeon opted for peripheral venous cannulation, we have investigated whether a different drain strategy for the institution of CPB may change the incidence of POAF (either postoperative and at follow‐up) in elective patients undergoing cardiac surgery.
After careful literature search, we are not aware of any previous studies, in the available literature, that have set out to investigate this eventuality.
2. MATERIALS AND METHODS
From January 2016 to December 2018, 2087 consecutive patients underwent on‐pump major cardiac surgery procedures at our Institution. The method of choice for CPB drenaige was either CC, through the right atrium or the superior and inferior vein cava or peripheral cannulation (PC) inserting a cannula through the right common femoral vein up to the venous tree until the superior vein cava.
To obtain two homogenous groups of patients we performed a propensity match analyzes that resulted in the creation of two homogeneous groups of equal number. A total of 161 patients in whom CPB had been instituted via PC (group 1) and 161 patients underwent CC (group 2). All patients were operated by seven different experienced surgeons (with a track record of at least 1000 successful cardiac surgeries performed as a first operator). We included in the study all patients undergone elective cardiac surgery with peripheral or central venous cannulation via median complete sternotomy or mini‐sternotomy.
In the PC group we had initially also included all patients operated through mini‐thoracotomy, however, we later realized that this particular technique had too many differences to be compared with full sternotomy or ministernotomy (systematic opening of the right pleura, absence of sternal opening, different pericardiotomy, etc.), such that we ran the risk of incurring into study design biases. Therefore, we decided to exclude these patients from the study a priori, preferring a sacrifice of quantitative data in favor of a better quality of the information obtained.
POAF as been assessed by:
-
‐1.
analyzing the 24/7 cardiac telemetry by which each individual patient is monitored during their hospitalization and confirmed by a standard EKG in case of reported arrhythmic event;
-
‐2.
by EKG upon discharge from the rehabilitation center (at which each patient spends 2–3 weeks after discharge from our center);
-
‐3.
studying the discharge letter from the rehabilitation center making sure that no AF episodes occurred during this period.
The new onset of atrial fibrillation (AF) as been evaluated basing on a EKG during the 3‐month follow‐up, analyzing the last follow‐up EKG available and by questioning the patient by telephone to learn about any AF diagnoses during the follow‐up period at other Centers during that period.
EKGs performed during any access to the our emergency department during the analyzed period were considered.
All data, including baseline, intraoperative and perioperative parameters, were retrospectively retrieved and collected from our institutional medical record system and the present study was approved by the local Ethics Committee (CCM1758).
The primary endpoints for the present study were the incidence of POAF, as well the incidence of new‐onset AF at the follow‐up. New onset AF has been defined by the unequivocal, instrumental documentation of AF of any duration, at any point, in the perioperative or follow‐up period by a cardiologist.
3. OPERATIVE TECHNIQUE
The CC was always performed in a standard fashion. This procedure, for the classical atrium cannulation, requires a full‐thickness incision of about 2 cm of the tip of the right atrial appendage (RAA) or somethimes on its side (Figure 1A). The double venous cannulation for the superior and inferior vena cava, on the other hand, involves two similar incisions: one on the superior vein cava right cranially to the superior cavoatrial junction (close to the anatomical position of the sinoatrial node) and the other one on the caudal part of the right atrium (the direct cannulation of the inferior vein cava is usually avoided because of its short length inside the pericardium and its fragility).
Figure 1.
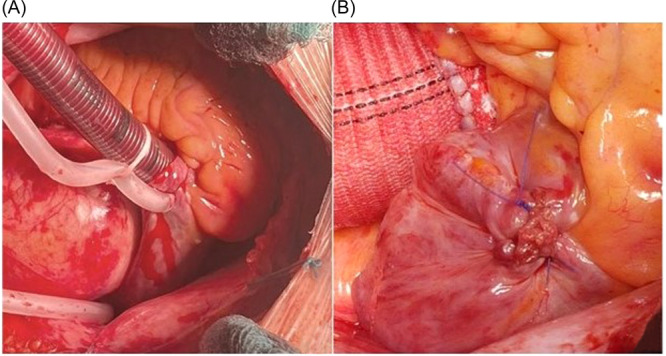
(A) Standard venous cannulation of the right atrium through RAA apex. (B) The atrial incision at the end of surgery is sutured eventually inducing the formation of a scar tissue spot. RAA, right atrial appendage.
This second type of venous cannulation is preferred for all the full sternotomy surgeries involving the opening of at least one of the two atria (operations on the atrioventricular valves, correction of atrial septal defect, etc.) as well as for other types of major cardiac surgery.
All of the previously described incisions, upon removal of the cannulas, must of course be closed by the surgeon with dedicated sutures (in the vast majority of cases with a polypropylene suture) ultimately resulting in scar tissue where the cardiac wall has been incised (Figure 1B).
The peripheral venus cannulation, on the other hand, is achieved by inserting a single or double staged cannula through the right common femoral vein (either by surgically exposing it through a 3–4 cm incision on the groin or by a direct percutaneus insertion). Transesophageal echocardiogram is usually employed to assess correct cannula tip placement (cannula tip right above the superior cavoatrial junction (Figure 2).
Figure 2.
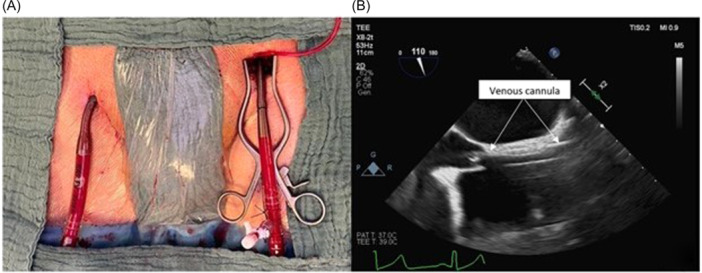
(A) Peripheral percutaneous cannulation of the right common femoral vein. (B) Correct positioning of the cannula through the right atrium assessed by TEE. TEE, transesophageal echocardiogram.
In case of surgical isolation of the vessel, the venous breach, at the end of the procedure, is sutured with a nonabsorbable suture (polypropylene); while for percutaneous access, manual compression lasting few minutes is sufficient to achieve a correct hemostasis.
Of note, this type of cannulation does not require incisions of the heart or major veins wall, resulting in no areas of scar tissue formation on the heart.
4. STATISTICAL ANALYZES
The propensity score was computed, by logistic regression analysis, as the conditional probability of being in the CC group and the PC group, given the following covariates: gender, age, diabetes, OSAS, indexed left atrium volume, hypertension and BMI.
Normally and non‐normally distributed numeric and categorical variables are presented as mean ± SD, as median (interquartile range) or as frequency (%), and they were compared between two groups by independent t‐test, Kruskal–Wallis test or Chi‐square, respectively.
Kaplan–Meier analysis was employed to generate time‐to‐event curves for AF stratified according to groups CC and PC. Log rank test was used to compare strata.
All tests were two‐sided, and a p value of less than .05 was required for statistical significance. All calculations were computed with the aid of the SAS software package (Version 9.4 SAS Institute Inc., Cary, NC).
5. RESULTS
Patient's baseline characteristics of the entire cohort of patients (before matching) is described in Table 1A.
Table 1A.
Preoperative data of the entire cohort of patients before matching.
| Variables | PC group (n = 162 patients) | CC group (n = 1638 patients) | p value |
|---|---|---|---|
| Male sex | 114 (70.4%) | 1093 (66.7%) | 0.36 |
| Age (years) | 64.4 ± 12.3 | 65.1 ± 11.8 | 0.47 |
| BSA | 1.89 ± 0.2 | 1.87 ± 0.2 | 0.12 |
| BMI (kg/m2) | 26.4 ± 4.7 | 25.4 ± 4.3 | 0.75 |
| COPD | 0 (0%) | 1 (0.06%) | 1 |
| OSAS | 2 (1.2) | 7 (0.4) | 0.16 |
| Diabetes | 17 (10.6%) | 299 (18.3%) | 0.02 |
| Hypertention | 106 (65.4) | 1105 (67.5) | 0.60 |
| Sinus rhythm | 147 (90.8%) | 1513 (92.6%) | 0.86 |
| Creatinine (mg/dL) | 0.9 (0.8; 1) | 0.9 (0.8; 1.1) | 0.92 |
| Euroscore II | 1.2 (0.7; 1.8) | 1.3 (0.8; 2.3) | 0.01 |
| Beta‐blockers | 74 (45.68%) | 878 (53.60%) | 0.054 |
| Antiarrhythmic drugs | 14 (8.64%) | 169 (10.32%) | 0.50 |
| PAD | 11 (6.79%) | 156 (9.52%) | 0.25 |
| EF (%) | 61 (56; 65) | 62 (57; 67) | 0.38 |
| Indexed left atrium | 45.2 ± 21.8 | 43.4 ± 19.4 | 0.28 |
Abbreviations: BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; PAD, peripheral artery disease.
Patients' baseline characteristics of the two matched populations are depicted in Table 1B. Of note, no differences in terms of incidence of peripheral vascular disease and preoperative AF were found between the two matched groups. Eventually, 10 patients had paroxysmal AF in the PC group while only one patient had paroxysmal AF in the CC group.
Table 1B.
Matched cohort preoperative data.
| Variables | PC group (n = 161 patients) | CC group (n = 161 patients) | p value |
|---|---|---|---|
| Male sex | 113 (70.2%) | 113 (70.2) | 1 |
| Age (years) | 66 (57; 72) | 67 (59; 73) | 0.61 |
| BSA | 1.87 ± 0.2 | 1.89 ± 0.2 | 0.51 |
| BMI (kg/m2) | 26.3 ± 4.7 | 25.6 ± 4.1 | 0.15 |
| COPD | 0 (0%) | 0 (0%) | 1 |
| OSAS | 1 (0.7) | 1 (0.7) | 1 |
| Diabetes | 17 (10.7%) | 16 (10.1%) | 0.85 |
| Hypertnsion | 109 (67.7) | 105 (65.2) | 0.64 |
| Sinus rhythm | 146 (90.7%) | 150 (93.2%) | 0.78 |
| Creatinine (mg/dL) | 0.9 (0.8; 1) | 0.9 (0.8; 1) | 0.58 |
| Euroscore II | 1.2 (0.8; 2.1) | 1.2 (0.7; 1.8) | 0.42 |
| Beta‐blockers | 73 (45.34%) | 80 (49.69%) | 0.43 |
| Antiarrhythmic drugs | 14 (8.70%) | 18 (11.18%) | 0.46 |
| PAD | 11 (6.83%) | 14 (8.70%) | 0.53 |
| EF (%) | 62 (57; 67) | 61 (56; 65) | 0.32 |
| Indexed left atrium | 45.2 ± 21.8 | 43.5 ± 19.4 | 0.28 |
Abbreviations: BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; PAD, peripheral artery disease.
All patients with a preoperative diagnosis of paroxysmal AF were ad admission under optimal medical therapy in term of rate control and anticoagulation.
Regarding the technical parameters of surgical interventions, cross‐clamp time and CPB time were not significantly longer in PC Group compared to CC Group [118 (93; 154) min to 117 (86; 145) min, p = .15 and 80.5 (61; 121) min to 87 (63; 111) min p = .86].
Intraoperative details as well as type of procedures performed are listed in Table 2.
Table 2.
Intraoperative data.
| Variables | PC group (n = 161 patients) | CC group (n = 161 patients) | p value |
|---|---|---|---|
| Cardiac procedure | |||
| Aortic valve surgery | 101 (62.73%) | 61 (37.89%) | <0.0001 |
| CABG | 42 (26.09%) | 47 (29.19%) | 0.53 |
| Mitral valve surgery | 33 (20.50%) | 65 (40.37%) | 0.0001 |
| Aortic valve surgery + CABG | 13 (8.07%) | −17 (10.56%) | 0.4431 |
| Tricuspid valve surgery | 1 (0.62%) | 7 (4.35%) | 0.067 |
| Aortic valve surgery + ascending aorta surgery and CABG | 0 (0%) | 0 (0%) | 1 |
| Aortic valve and ascending aorta surgery | 0 (0%) | 0 (0%) | – |
| Ascending aortic surgery alone | 0 | 1 (0.62%) | 1 |
| Associated cardiac procedure | |||
| LAA closure | 18 (17.1%) | 11 (11%) | 0.2175 |
| PFO closure | 4 (3.8%) | 3 (2.8%) | 0.7599 |
| PFO closure + LAA | 3 (2.8%) | 1 (1%) | 0.3416 |
| Cardiac tumor excision | 1 (0.9%) | 1 (1%) | 0.9667 |
| Cardioplegia type | <0.0001 | ||
|
122 (75.78%) | 149 (93.13%) | |
|
39 (24.22%) | 11 (6.88%) | |
| CPB and aortic cross clamping data | |||
| Minimum haematocrit in CPB | 30 (26; 393) | 27 (23; 30) | 0.078 |
| Minimum temperature in CPB | 33.05 (32.7; 33.2) | 33.1 (32.8; 33.2) | 0.73 |
| CPB time (min) | 118 (93; 154) | 117 (86; 145) | 0.15 |
| Cross‐clamping time (min) | 80.5 (61; 121) | 87 (63; 111) | 0.86 |
| Priming volume | 1200 (1000; 1200) | 1200 (1100; 1200) | 0.052 |
Abbreviations: CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; LAA, left atrial appendage; PFO, patent foramen ovale.
In‐hospital death occurred in one patient in the PC group (1%; p = 1) while the incidence of major cerebrovascular complications as stroke/TIA occurred in 2% and 1% in the PC and CC, respectively (p 1). Moreover, the incidence of POAF did not differ among the two groups [PC: 45.7% (48/105) vs. CC: 39.4% (39/99); p = .36]. In details, death occurred for multi organ failure secondary to complication of fungal pneumonia, while the patient who reported neurological complication had irreversible paraplegia due to arterial embolism. Both complications occurred in the PC group.
A single patient belonging to the PC group soffered of deep venous thrombosis of the right common vein (surgical cannulation) probably due to too tight a suture of the vein at the end of surgery. Complete recanalization of the vessel was documented 5 days later following anticoagulation therapy with LMWH. A computed tomography scan with contrast medium was performed to exclude pulmonary embolization.
Other postoperative details are listed in Table 3.
Table 3.
Postoperative data.
| Variables | PC group (n = 161 patients) | CC group (n = 161 patients) | p value |
|---|---|---|---|
| Ventricular arrhythmia | 7 (6.7%) | 12 (12.1%) | 0.18 |
| Aortic | 0 (0%) | 0 (0%) | 1 |
| PM implantation | 3 (2.9%) | 3 (2.9%) | 1 |
| Gastrointestinal complicationsa | 1 (1%) | 2 (1.9%) | 1 |
| Pulmonary embolism | 0 (0%) | 0 (0%) | 1 |
| Atrial fibrillation | 48 (45.7%) | 39 (39.4%) | 0.36 |
| AMI | 1 (1%) | 1 (1%) | 1 |
| Sternal wound infection | 0 (0%) | 0 (0%) | 1 |
| Lower limb ischemia | 0 (0%) | 0 (0%) | 1 |
| MOF | 1 (1%) | 1 (1%) | 1 |
| Pneumonia | 2 (1.9%) | 1 (1%) | 1 |
| Inguinal wound reintervention | 4 (3.8%) | 0 (0%) | 0.19 |
| Deep venous thrombosis | 1 (1%) | 0 (0%) | 1 |
| Re‐exploration for bleeding | 10 (29.2%) | 4 (3.7%) | 0.10 |
| Sepsis | 2 (1.9%) | 2 (1.9%) | 1 |
| Stroke | 2 (1.9%) | 1 (1%) | 1 |
| Acute kidney injury | 39 (37, 14%) | 44 (44, 44%) | 0.2856 |
| Exitus | 1 (1%) | 0 (0%) | 1 |
| MACCE | 4 2.4%) | 2 (1.9%) | 0.49 |
| Total bleeding | 532 (377; 762) | 480 (335; 770) | 0.03 |
| Creatinine peak | 1 (0.8; 1.2) | 0.7 (0.8; 1.2) | 0.13 |
| Intubation time (hours) | 4 (3; 6) | 4 (3; 5) | 0.32 |
| ICU stay (days) | 2 (2; 3) | 2 (2; 3) | 0.16 |
| Beta‐blockers | 44 (41.9%) | 40 (50.5%) | 0.82 |
| Antiarrhythmic drugs | 11 (10.5%) | 13 (13.13%) | 0.55 |
| Hospital stay (days) | 13 (9; 17) | 13 (10; 15) | 0.95 |
Abbreviations: AMI, acute myocardial infarction; ICU, intensive care unit; MACCE, major adverse cardiac and cerebrovascular events; MOF, multi organ failure; PM, pace maker.
Gastrointestinal complications include: pancreatitis, liver dysfunction, bowel ischaemia.
Prevalence of major adverse cardiac and cerebrovascular events intended as cardiac death, myocardial infarction, stroke/TIA, did not appear to be different between the two populations (p = .49).
Median mechanical ventilation time [PC: 4 (3; 6) hours vs. CC: 4 (3; 5) hours, p = .32] as well as median hospital stay [PC: 13 (9–17) days vs. CC: 13 (10–15) days, p = .95] were comparable between the two groups.
At 1250‐day follow‐up, the incidence of postoperative new onset of AF was 2.9% and 8.7% in the PC and CC groups, respectively (p = .04) (Figure 3).
Figure 3.
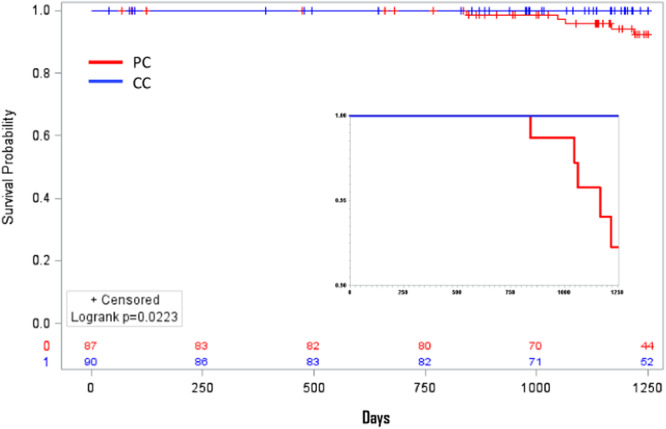
The median follow‐up was 1250 days [1016–1250]. The PC group had a better “free from AF curve” compared with CC group at the end of observation. CC, central cannulation; PC, peripheral cannulation.
6. DISCUSSION
Historically considered an option just in case of reoperation or presence of fragile atrial tissues, the surge of MICS pushed cardiac surgeons to explore and became familiar with alternative peripheral venous cannulation techniques for the institution of the CPB, also for standard operative procedures.
An accurate study of arrhythmogenic complications related to the type of venous cannulation is complex to design because in the vast majority of cases venous cannulation is reserved for minithoracotomy surgery, and this involves too many technical differences from traditional surgery with atrial cannulation eventually leading inevitable statistical bias, thus undermining the possibility of deriving correct data.
Our analyzes planned to exclude all mini‐thoracotomy surgeries from the PC group, considering only patients operated through complete sternotomy or ministernotomy to compare them, after matching, with patients operated with the same surgical access but with central venous cannulation.
We investigated the incidence of AF either perioperatively (POAF) and at follow‐up (late AF) for the two groups. To the best of our knowledge this is the first study designed with this aim.
We found the incidence of POAF not of significance among of the two groups. Interestingly, we found a statistically significant difference in terms of incidence of new onset of AF over a long‐term followup (1250 days).
The incidence of POAF is reported to be around 30% after cardiac surgery and 15% after noncardiac procedures 4 , 5 and when occurs, could prolong the length of hospital stay and increase morbidity and mortality. 6 POAF is also associated with a significant increased risk of late AF, thus confirming the assumption that POAF predicts AF after cardiac surgery. An other well known factor is that late AF at follow‐up in cardiac surgery patients is an independent risk factor for late mortality. 7
The absence of differences in terms of POAF incidence between the two groups and, on the other hand, the statistical significance reported in the long‐term follow‐up, seems to suggest that factors related to the new onset of arrhythmia were not present in the first 30 days after surgery but only came to be present later.
We may assume that our major finding of a significantly reduced incidence of late AF in patients undergoing on‐pump cardiac surgery through PC, could have an impact in the late mortality of this specific subset of patients if considered at the time the surgeon decide the most adequate venous cannulation site for the intervention.
It would be very useful to identify patients at increased risk of developing this type of arrhythmia. As early as 1996, Mathew et al. 8 identified independent predictors of postoperative AF. These included both personal factors such as advanced age, male sex, history of paroxysmal AF or congestive heart failure, and surgery‐related factors such as pulmonary vein venting, bicaval venous cannulation and longer cross‐clamp times. Therefore, several surgical techniques could also represent a trigger or create a favorable substrate for the development of postoperative supraventricular arrhythmias and the scientific community has worked to investigate this topic. (Central Image 1)
Central Image 1.
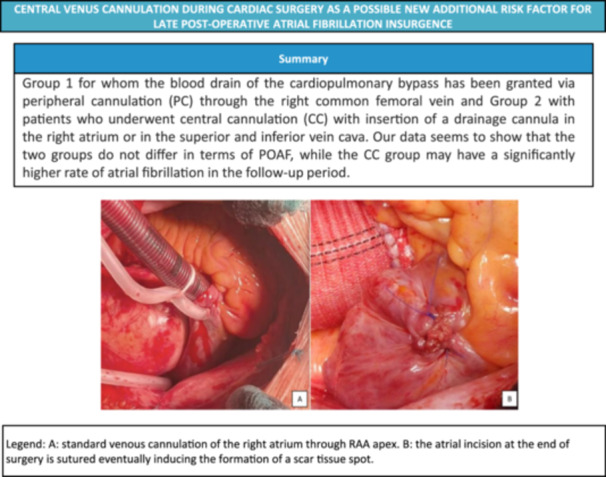
Description of the study population and illustration of the standard venous cannulation of the right atrium through the apex of the RAA. against the atrial incision at the end of the procedure is sutured, possibly inducing the formation of a scar tissue stain.
Despite multifactorial, the interaction between trigger, arrhythmogenic substrate and modulating factors showed to be the most crucial agents intervening in the genesis of POAF. 9 In particular, in the days following cardiac surgery an increased sympathetic tone and a local and systemic inflammatory response, seem to be the most important factors for this secondary arrhythmia. 10 , 11 However, this can only be related with acute POAF having the surgical inflammatory response and the increased sympathetic and vagal tone activation decreasing over the days after the index procedure.
Different factors might be predominant for late recurrence of AF. In a CC context, which implies the use of sutures at the level of the RAA or right atrium, as opposed to a PC, could result in a fibrotic area at the cannulation site thus, generating a potential arrhythmic substrate. A substrate induced by surgery (atriotomy, sutures) together with transient postoperative factors (inflammation and oxidative stress) and perhaps pre‐existing atrial substrate (age, hypertension), may create the preconditions for both increased triggered activity and an arrhythmogenic substrate for re‐entry, essential conditions for the development of AF. 11 Moreover, the presence of a possible underlying chronic cardiovascular disease expressed by the presence of a diastolic disorder, as showed by Melduni et al. 11 could potentially favor the progression of peri‐operative AF towards a late AF in cardiac surgery patients.
To the best of our knowledge, this is the first study specifically investigating relationship between the type of surgical cannulation and the new onset of late AF in cardiac surgery patients. Despite POAF is considered a multifactorial phenomenon, still largely unknown, we showed that PC could potentially reduce the incidence of late AF regardless of the presence or absence of POAF in the weeks following hospitalization.
Further investigation on a large scale is warranted to confirm our preliminary findings and their clinical implications.
7. LIMITATIONS
-
1‐
This is a single‐center study, thus the results may not be generalizable to the clinical practice of different hospital centers. However, our Institution represents a high‐volume center with the highest standard of expertize and competence in cardiac surgery.
-
2‐
The numbers of patients analyzed is limited to the number of variables used to perform the propensity match analyzes to obtain the two most homogenous groups as possible.
-
3‐
We are aware that the way the occurrence of late AF was assessed in this study can rightly be considered a limitation to the quality of the data and that is susceptible to bias, but unless 24/7 continuous monitoring of heart rhythm is possible (e.g., loop recorder), it is impossible to exclude that the occurence of temporary episodes of AF not perceived by the patient may remain undetected during the followup period.
8. CONCLUSIONS
From our data, it seems to emerge that patients undergoing right atrial cannulation for CPB institution may have a higher rate of AF onset in the follow‐up period than those peripherally cannulated, thus possibly adding this parameter to the other known risk factors for late AF onset. Future studies with a larger cohort of patients may validate our hypothesis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
MEMBERS OF VARIATION STUDY GROUP
Giorgio Mastroiacovo, Sergio Pirola, Luigi Sciarra, Fabrizio Rosati, Mattia Petrungaro, Giuseppe Nanci, Daniele Fileccia, Alice Bonomi, Maria Cristina Vinci, Andrea Baggiano, Riccardo Maragna, Gianluigi Bisleri, Claudio Tondo, Gianluca Polvani.
ACKNOWLEDGEMENTS
Open access funding provided by BIBLIOSAN.
Mastroiacovo G, Pirola S, Sciarra L, et al. Central venus cannulation during cardiac surgery as a possible new additional risk factor for late post‐operative atrial fibrillation insurgence. J Cardiovasc Electrophysiol. 2024;35:2296‐2303. 10.1111/jce.16413
Disclosures: None.
Giorgio Mastroiacovo and Sergio Pirola contributed equivalently to the drafting of the manuscript (co‐authorship).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dobromir D, Martin A, Jordi H, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16(7):417‐436. [DOI] [PubMed] [Google Scholar]
- 2. Ahlsson A, Fengsrud E, Bodin L, et al. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37(6):1353‐1359. [DOI] [PubMed] [Google Scholar]
- 3. Chan EY, Lumbao DM, Iribarre A, et al. Evolution of cannulation techniques for minimally invasive cardiac surgery: a 10‐year journey. Innovations. 2012;7(1):9‐14. [DOI] [PubMed] [Google Scholar]
- 4. Filardo G, Damiano Jr, RJ , Ailawadi G, et al. Epidemiology of new‐onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104(12):985‐992. [DOI] [PubMed] [Google Scholar]
- 5. Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989;31(5):367‐378. [DOI] [PubMed] [Google Scholar]
- 6. Mathew JP, Fontes ML, Tudor IC, et al. Investigators of the ischemia research and education foundation; multicenter study of perioperative ischemia research group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291(14):1720‐1729. [DOI] [PubMed] [Google Scholar]
- 7.(a) Melduni RM, Schaff HV, Bailey KR, et al. Implications of new‐onset atrial fibrillation after cardiac surgery on long‐term prognosis: a community‐based study. Am Heart J. 2015;170(4):659‐668. [DOI] [PubMed] [Google Scholar]; (b) Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226(4):501‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. MultiCenter study of perioperative ischemia research group. JAMA. 1996;276:300‐306. [PubMed] [Google Scholar]
- 9. Baeza‐Herrera LA, Rojas‐Velasco G, Márquez‐Murillo MF, et al. Atrial fibrillation in cardiac surgery. Arch Cardiol Mex. 2019;89(4):348‐359. [DOI] [PubMed] [Google Scholar]
- 10. Dobrev D, Aguilar M, Heijman J, Guichard JB, et al. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417‐436. [DOI] [PubMed] [Google Scholar]
- 11. Coumel P, Leenhardt A. Mental cardiac arrhytmias and the autonomic nervous system. J Cardiovasc Electrophysiol. 1993;4(3):338‐355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


