Abstract
Background
CD20-targeted therapies are widely used in the management of B-cell lymphomas. Re-treatment with CD20-directed agents is common; however, previous research has demonstrated loss of CD20 expression at relapse in a subset of patients.
Methods
In this single-center retrospective cohort of 243 patients, CD20 analysis was performed by immunohistochemistry (IHC) and/or flow cytometry at diagnosis and at relapse if a biopsy was performed.
Results
Of 109 patients with relapsed or refractory B-cell lymphoma, 59 patients with CD20-positive lymphoma at diagnosis underwent a biopsy at relapse for a total of 76 biopsies across all relapses. The rate of partial or complete CD20 expression loss was 11.9% (four patients with partial loss, three patients with complete loss). There were four cases of CD20 loss at first relapse (three IHC, one flow cytometry), two at second relapse (one IHC, one IHC and flow cytometry), and one at fifth relapse (IHC and flow cytometry). CD20 antigen escape was observed in marginal zone lymphoma, follicular lymphoma, and diffuse large B-cell lymphoma (DLBCL). All patients with CD20 expression loss previously received rituximab. Among patients with CD20 antigen escape, 85.7% had stage IV disease, and median overall survival after CD20 loss was 4 months. In the group of five patients with indolent lymphoma and CD20 expression loss, three patients (60%) had concurrent transformation to high-grade lymphoma.
Conclusions
This study, which reinforces the importance of repeating a biopsy at relapse before implementing CD20-directed therapy, is particularly relevant given the widespread use of rituximab along with the emerging significance of CD20-targeted bispecific antibodies in the management of B-cell lymphomas.
Keywords: CD20, Lymphoma, Rituximab, Antigen escape
Introduction
Globally, it is estimated that there were 545,000 new cases of non-Hodgkin lymphoma and 260,000 deaths in 2020 [1, 2], and the incidence appears to be increasing [2, 3]. Over the last 25 years, anti-CD20 monoclonal antibodies have revolutionized the management of non-Hodgkin lymphoma. Rituximab has become a foundational element in the management of most B-cell lymphomas [4], and obinutuzumab may have a role as well [5-8]. More recently, novel CD20-targeted bispecific antibodies mosunetuzumab [9, 10], glofitamab [11], epcoritamab [12], and odronextanab [13] have emerged as a promising therapeutic avenue in relapsed or refractory B-cell lymphoma. Furthermore, anti-CD20 chimeric antigen receptor (CAR) T cells are under ongoing investigation [14, 15].
In addition to CD19, CD22, CD79a, and PAX5, CD20 is a marker nearly ubiquitously expressed on mature B cells. Most B-cell non-Hodgkin lymphomas are CD20-positive including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), marginal zone lymphoma (MZL), and mantle cell lymphoma (MCL). CD20-negative B-cell lymphoma is a rare entity (1-2% of all B-cell lymphomas) and can be expected in plasmablastic lymphoma, primary effusion lymphoma, lymphoblastic lymphoma, human herpesvirus 8 (HHV8)-positive large B-cell lymphoma, and anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma [16, 17].
Previous case reports [18-25] and retrospective studies [26-37] have demonstrated loss of CD20 expression at relapse in patients previously treated with rituximab who were CD20-positive at diagnosis. Unfortunately, the sample sizes in most of these studies are relatively small, and the rate of CD20 expression loss is highly variable across studies.
Re-treatment with anti-CD20 therapy in B-cell lymphomas is common; however, loss of CD20 expression at relapse is a clinical concern. The primary aim of this retrospective study was to characterize the rate of CD20 expression loss at the time of relapse or refractory disease in patients with B-cell non-Hodgkin lymphoma.
Materials and Methods
Study design
We performed a retrospective chart review of 288 consecutive adult patients with B-cell non-Hodgkin lymphoma who were seen at Harbor-UCLA Medical Center in Torrance, CA, USA from 2014 to 2023. Harbor-UCLA Medical Center Institutional Review Board (IRB) approval was obtained (IRB number 18CR-32663-01), and the study was performed in accordance with the ethical standards described in the 1964 Declaration of Helsinki and subsequent amendments. Data were collected regarding demographics, lymphoma type, CD20 expression, management, treatment response, and survival.
CD20 expression analysis
Biopsies were obtained from lymph nodes, bone marrow, or most accessible organ site at diagnosis and at time of relapse or refractory disease whenever feasible. Biopsies were obtained per standard practice at the clinician’s discretion and not for the purpose of this study. CD20 protein expression was assessed by immunohistochemistry (IHC) and/or flow cytometry per standard practice at the time of biopsy and not for the intent of the study. In CD20-negative cases, CD79a and/or PAX5 IHC stains and/or CD19 flow cytometry were performed to confirm B-cell lineage. IHC and flow cytometry were performed on-site in the Harbor-UCLA Hematopathology Lab (Torrance, CA, USA).
IHC
The tissues were routinely fixed in 10% neutral buffered formalin and embedded in paraffin. The sections were deparaffinized and rehydrated in graded alcohol. The sections were then put in an automated stainer (Ventana) following the vendor’s protocol. Commercially available ready-to-use monoclonal antibodies to CD20 (clone L26, Roche), CD79a (clone SP18, Roche), and PAX5 (clone SP34, Roche) were used.
Flow cytometry
Flow cytometric analysis was performed with a Beckman Coulter flow cytometer using ready-to-use antibodies (CD20 FITC, clone B9E9; CD19 RD1, clone 89B) according to the vendor’s instructions (Beckman Coulter, Fullerton, CA, USA). We described antigen distribution as “negative” for antigens not expressed, “positive” for antigens expressed, or “partially expressed” for antigens that are expressed in a subset of the population of interest. “Dim” was used to describe antibody fluorescence intensity for a uniformly positive population with lower mean fluorescence intensity than a positive normal cell population.
CD20 antigen escape
Partial CD20 loss was defined by partial or weak CD20 positivity by IHC, dim CD20 expression by flow cytometry, or conflicting IHC and flow cytometry results in a patient with previously identified CD20-positive lymphoma. Complete CD20 loss was defined by complete absence of detectable CD20 expression in a patient who previously had a CD20-positive biopsy.
Results
Patient demographics
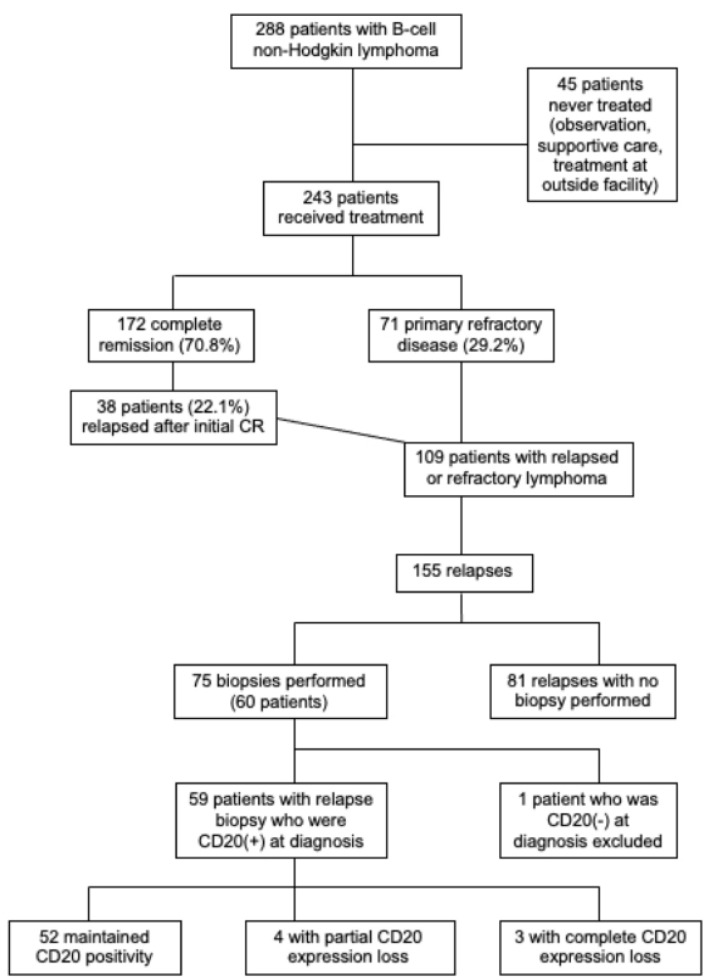
Examination of the electronic health record yielded 288 patients with non-Hodgkin B-cell lymphoma who were seen at Harbor-UCLA Medical Center from 2014 to 2023. Among the 288 patients identified, 243 patients (84.4%) received treatment, and 45 patients (15.6%) were never treated (best supportive care, observation alone, treatment at an outside facility). Therefore, these 45 patients were excluded from the data analysis.
In the cohort of 243 patients with B-cell non-Hodgkin lymphoma who ultimately received treatment, the median age was 56 years old (range 19 - 84 years old). Demographic information for patients included in the study is described in Table 1.
Table 1. Demographics for Patients Who Received Treatment (n = 243).
| Demographics | |
|---|---|
| Gender | |
| Males | 144 (59.3%) |
| Females | 99 (40.7%) |
| Age at diagnosis (years) | |
| Median | 56 |
| Range | 19 - 94 |
| Ethnicity/race | |
| Hispanic | 160 (65.9%) |
| White/European | 34 (14.0%) |
| Black/African-American | 27 (11.1%) |
| Asian | 15 (6.2%) |
| Other | 7 (2.9%) |
| Stage | |
| Stage I | 27 (11.1%) |
| Stage II | 55 (22.6%) |
| Stage III | 28 (11.5%) |
| Stage IV | 111 (45.7%) |
| Primary CNS | 5 (2.1%) |
| Unknown | 17 (7.0%) |
CNS: central nervous system.
Clinical characteristics
The most common types of lymphoma were DLBCL (52.3%), FL (14.0%), MZL (7.4%), high-grade B-cell lymphoma (6.6%), and MCL (4.5%). A complete list of lymphoma subtypes can be found in Table 2. The majority of patients had advanced stage disease (45.7% stage IV and 11.5% stage III versus 22.6% stage II and 11.1% stage I).
Table 2. Cohort of Patients by Histology.
| Number of patients (% cohort) | CRR to first-line treatment | Primary refractory diseasea | Relapse after CRb | Relapsed/refractory | Number of patients with biopsy at relapse (% cohort) | |
|---|---|---|---|---|---|---|
| Overall | 243 | 70.8% | 29.2% | 22.1% | 44.9% | 60 |
| DLBCL | 127 (52.3%) | 80.3% | 19.7% | 16.7% | 33.1% | 20 (33.3%) |
| FL | 34 (14.0%) | 76.5% | 23.5% | 46.2% | 58.9% | 18 (30.0%) |
| MZL | 18 (7.4%) | 55.6% | 44.4% | 40.0% | 66.7% | 10 (16.7%) |
| HGBCLc | 16 (6.6%) | 25.0% | 75.0% | 0% | 75.0% | 3 (5.0%) |
| MCL | 11 (4.5%) | 36.4% | 63.6% | 50.0% | 81.2% | 6 (10.0%) |
| PCNSL | 5 (2.1%) | 40.0% | 60.0% | 50.0% | 80.0% | 1 (1.7%) |
| PMBCL | 4 (1.6%) | 100% | 0% | 25.0% | 25.0% | 1 (1.7%) |
| Burkitt | 4 (1.6%) | 75.0% | 25.0% | 0% | 25.0% | 0 (0%) |
| Plasmablastic | 2 (1.6%) | 50.0% | 50.0% | 100% | 100% | 1 (1.7%) |
| NOS/other | 22 (9.1%) | 69.6% | 30.4% | 0% | 30.4% | 0 (0%) |
aPrimary refractory disease includes patients with partial remission, stable disease, or progressive disease after first-line treatment. bAmong patients with initial CR to first-line treatment. cDouble expressor, triple expressor, or HGBCL-NOS. CR: complete remission; CRR: complete remission rate; DLBCL: diffuse large B-cell lymphoma; FL: follicular lymphoma; HGBCL: high-grade B-cell lymphoma; MCL: mantle cell lymphoma; MZL: marginal zone lymphoma; NOS: not otherwise specified; PCNSL: primary central nervous system lymphoma; PMBCL: primary mediastinal B-cell lymphoma.
First-line treatment
For patients with B-cell lymphoma who received first-line treatment (n = 243), the complete remission rate (CRR) was 70.8% (Table 2). Among 71 patients (29.2%) with primary refractory disease, there were 24 patients (9.9%) with partial remission (PR), 14 patients (5.8%) with stable disease (SD), and 33 patients (13.6%) with progressive disease (PD). In the first-line setting, 218 patients (89.7%) received a rituximab-containing regimen. The group who did not receive rituximab first-line included patients who received radiation alone and patients with CD20-negative lymphoma.
CD20 antigen escape in patients with relapsed or refractory disease
Among 172 patients with complete remission (CR) with first-line therapy, 38 patients (22.1% of those with initial CR) later relapsed. Overall, 109 patients (44.9% treated patients) had relapsed or refractory disease, among whom 60 received a biopsy at relapse (Fig. 1).
Figure 1.
Flow diagram of patient eligibility.
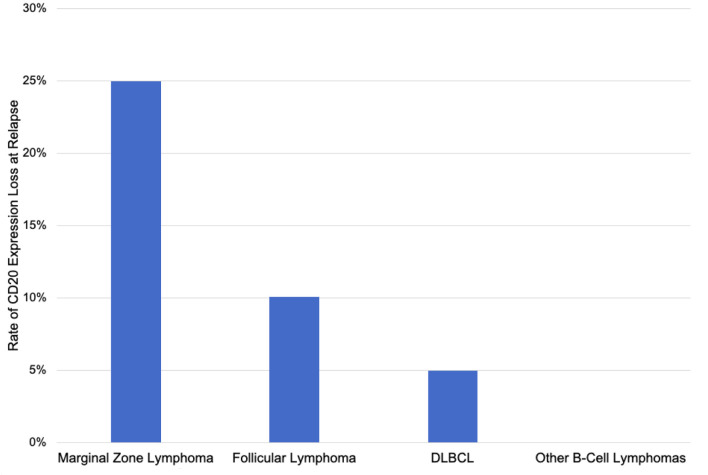
The rate of CD20 antigen escape was 11.9% including four patients (6.8%) with partial CD20 loss and three patients (5.1%) with complete CD20 loss (Table 3). CD20 loss was detected by IHC in four cases, flow cytometry in one case, and both IHC and flow cytometry in two cases. In six out of seven cases with CD20 loss, expression of other B-cell markers (CD19, CD45, CD79b, PAX5) was maintained. In one case of CD20 antigen escape (patient 5 in Table 4), there was also loss of expression of CD79b, PAX5, and CD45 (weakly positive). The rate of CD20 expression loss was 30% for MZL, 11.1% for FL, 10% for DLBCL, and 0% for all other lymphoma types (Fig. 2).
Table 3. CD20 Expression Analysis by Relapse.
| CD20 expression | First relapse (n = 52) | Second relapse (n = 13) | Third relapse (n = 4) | Fourth relapse (n = 3) | Fifth relapse (n = 2) |
|---|---|---|---|---|---|
| Positive | 48 | 11 | 4 | 3 | 1 |
| Partial | 3 | 1 | 0 | 0 | 0 |
| Negative | 1 | 1 | 0 | 0 | 1 |
| Rate of CD20 expression loss | 7.7% | 15.4% | 0% | 0% | 50% |
Table 4. Patients With Complete or Partial Loss of CD20 Expression (n = 7).
| Patient and lymphoma characteristics | Biopsy at diagnosis | First-line treatment | First relapse biopsy and treatment | Second relapse biopsy and treatment | Third relapse and later |
|---|---|---|---|---|---|
| 1) 51M DLBCL ABC type Stage IV Partial CD20 loss at R1 |
Lymph node: IHC CD20(+) | RCHOP => CR | Lymph node: IHC CD20(+), Flow CD20(+) dim RICE => PD |
Death 3 months after R1 | |
| 2) 33M DLBCL ABC type Stage IV Partial CD20 loss at R1 |
Lymph node: IHC CD20(+) | RCHOP => CR | Lymph node: IHC CD20(+) weak RICE => PR Autologous SCT => CR |
Alive 70 months after R1 | |
| 3) 47M Follicular Stage IV CD20 loss at R1 |
Bone marrow: IHC CD20(+), Flow CD20(+) Chest wall mass: IHC CD20(+), Flow CD20(+) |
RCHOP + rituximab maintenance => PD |
Lymph node: IHC CD20(-), transformation to HGBCL RICE => PD |
Death 3 months after R1 | |
| 4) 54F Marginal zone Stage IV Partial CD20 loss at R1 |
Bone marrow: IHC CD20(+), Flow CD20(+) | Rituximab => PD |
Bone marrow: IHC CD20(-), Flow CD20(+) Ibrutinib => PD |
Death 4 months after R1 | |
| 5) 56F Follicular Stage IV CD20 loss at R2 |
Lymph node: IHC CD20(+) Bone marrow: IHC CD20(+) |
RCHOP + rituximab maintenance => CR |
No biopsy, relapse on PET BR => PR |
Lymph node: IHC CD20(-), transformation to DLBCL Peripheral blood: Flow CD20(-), no evidence of transformation ICE => PD |
Death 3 months after R2 |
| 6) 58M Marginal zone Stage III Partial CD20 loss at R2 |
Orbital mass: IHC CD20(+) | FCR => CR | Biopsy results unavailable FCR => CR |
Lymph node: IHC CD20(-), Flow CD20(+) dim, transformation to DLBCL RCHOP => PD |
Death 5 months after R2 |
| 7) 60M Marginal zone Stage IV CD20 loss at R5 |
Peripheral blood: IHC CD20(+), Flow CD20(+) Bone marrow: IHC CD20(+), Flow CD20(+) |
RCHOP => PR | Bone marrow: IHC CD20(+), Flow CD20(+) FR => PR |
Peripheral blood: Flow CD20(+) Spleen: IHC CD20(+), Flow CD20(+) Idelalisib + splenectomy => SD |
R3: Peripheral blood: Flow CD20(+) Ibrutinib => PD R4: Peripheral blood: Flow CD20(+) Bone marrow: IHC CD20(+) Bendamustine/ofatumumab + ofatumumab maintenance => PD R5: Peripheral blood: Flow CD20(-) Abdominal wall mass: IHC CD20(-), Flow CD20(-) Duodenal mass: IHC CD20(-) ICE => PD Death 4 months after R5 |
BR: bendamustine + rituximab; CR: complete remission; DAREPOCH: dose-adjusted rituximab + etoposide + prednisone + vincristine + cyclophosphamide + doxorubicin; DLBCL: diffuse large B-cell lymphoma; F: female; FCR: fludarabine + cyclophosphamide + rituximab; Flow: flow cytometry; FR: fludarabine + rituximab; HGBCL: high-grade B-cell lymphoma; ICE: ifosfamide + carboplatin + etoposide; M: male; PR: partial remission; PET: positron emission tomography; PD: progression of disease; R1: first relapse; R2: second relapse; R3: third relapse; R4: fourth relapse; R5: fifth relapse; RCHOP: rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone; RICE: rituximab + ifosfamide + carboplatin + etoposide; SD: stable disease; SCT: stem cell transplant.
Figure 2.
Rate of CD20 expression loss at relapse by lymphoma type.
All seven patients with loss of CD20 expression previously received rituximab. In the group of seven patients with CD20 loss, six patients (85.7%) had stage IV disease at diagnosis, and median overall survival after CD20 loss was 4 months. Among the five patients with MZL or FL with CD20 antigen escape, three patients (60%) had transformation to high-grade lymphoma at the time of CD20 loss.
At first relapse, three patients had partial CD20 loss (two DLBCL, one MZL), and one patient had complete CD20 loss (FL with transformation to high-grade B-cell lymphoma). At second relapse, two patients had CD20 antigen escape (one FL patient with complete CD20 loss, one MZL patient with partial CD20 loss). Both patients received two prior lines of rituximab-containing therapy and had transformation to DLBCL at the time of CD20 loss (Table 4). No cases of CD20 antigen escape were observed at third or fourth relapse. The MZL patient with CD20 loss at fifth relapse received two rituximab-containing regimens as well as ofatumumab as fourth-line therapy. In all previous biopsies from diagnosis to fourth relapse, lymphoma cells were CD20-positive. At fifth relapse, complete CD20 expression loss was shown via peripheral blood flow cytometry, duodenal mass biopsy IHC, and abdominal wall mass biopsy IHC and flow cytometry (Table 4).
Discussion
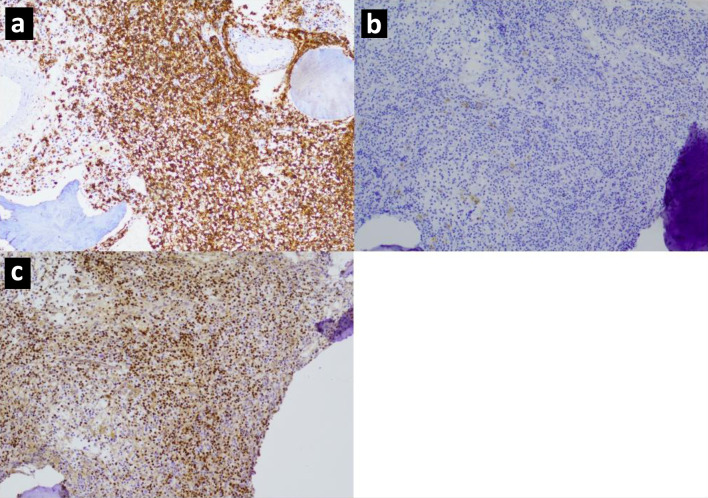
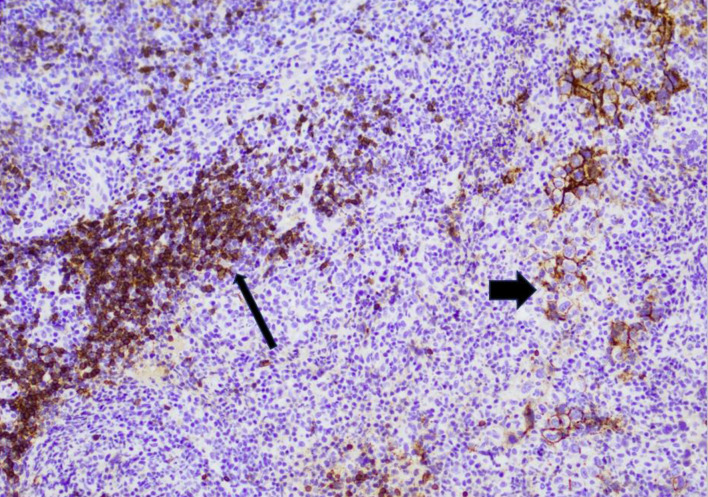
While there is an expanding body of evidence regarding CD20 antigen escape at relapse, the exact rate of CD20 loss and the underlying mechanism are not well defined. In this retrospective cohort of 243 patients with non-Hodgkin B-cell lymphoma, 109 patients had relapsed or refractory disease. Among 59 patients with CD20-positive B-cell lymphoma at diagnosis who had a biopsy at relapse, the rate of CD20 expression loss was 11.9% including 5.1% complete loss (Fig. 3) and 6.8% partial loss (Fig. 4). In comparison, the rate of CD20 loss ranges from 10% to 40% across previous studies [26-31, 33, 34, 36, 37]. The lower rate observed in our study may be related to the fact that the large majority of biopsies were from first relapse (70.3%), and CD20 antigen escape seems to occur more frequently at later relapses [28].
Figure 3.
Immunohistochemistry images for a marginal zone lymphoma with complete CD20 loss showing (a) CD20 expression at diagnosis, (b) CD20 negativity at second relapse, (c) PAX5 positivity, thereby confirming B-cell lineage, at second relapse.
Figure 4.
Immunohistochemistry image for a diffuse large B-cell lymphoma with partial weak CD20 expression at first relapse, representing partial CD20 loss. A subset of large lymphoma cells are weakly positive for CD20 (wide arrow), while the background small B cells are strongly positive for CD20 (thin arrow).
Notably, six of seven patients (85.7%) with CD20 loss had stage IV disease at diagnosis, and three of five indolent lymphoma patients (60%) with loss of CD20 expression had transformation to high-grade lymphoma at the time of CD20 loss. Previous studies have described an association between CD20 loss and transformation to DLBCL [27, 30]. In the present study, median overall survival after CD20 phenotypic conversion was 4 months. Likewise, other studies have suggested that CD20 loss may be associated with aggressive disease and poor prognosis [27, 28, 30, 34].
The cohort of patients in this study represented a balanced mixture of the most common non-Hodgkin B-cell lymphomas, and the relative proportions were compatible with the distribution of lymphomas in clinical practice. The rate of CD20 expression loss was highest in MZL (30%) with phenotypic conversions also observed in FL (11.1%) and DLBCL (10%). Other lymphoma subtypes did not demonstrate any instances of CD20 loss. However, previous research has demonstrated CD20 loss in nearly all types of B-cell non-Hodgkin lymphoma [26, 27, 29-34, 36].
Many potential mechanisms of CD20 expression loss have been explored with particular interest in how the mechanism may help explain resistance to anti-CD20 therapy, transformation to high-grade lymphoma, and aggressive disease as has been noted after CD20 loss. The two primary avenues for CD20 loss after rituximab therapy include selection of a preexisting CD20-negative clone and directly induced loss of CD20 expression in a previously CD20-positive clone. Preferential selection of a CD20-negative clone may be supported by re-emergence of CD20 expression in subsequent relapses after initial CD20 loss in a small subset of patients [26, 31, 32].
Possible mechanisms of direct CD20 loss include CD20 gene mutation [19, 33, 35, 38, 39], decreased gene promoter activity [40], downregulation of CD20 mRNA [39], impaired CD20 transport to cell membrane surface [40], and antibody-mediated internalization of CD20 by B cells [41]. Exploring therapies which evade these mechanisms of CD20 loss and subsequent therapeutic resistance remains a vital ongoing research endeavor.
Limitations of the present study include the lack of precise quantification of CD20 expression in patients with partial CD20 loss (patients 1, 2, 6 in Table 4) and patients with contradictory IHC and flow cytometry results (patients 1, 4, 6 in Table 4). Previous studies on CD20 loss have reported comparable partial expression loss [28], discrepancy between IHC and flow cytometry [27, 36], and variation between biopsy sites [26]. Additional limitations include inconsistency in performing a biopsy at relapse and the retrospective nature of the study. Lastly, this study was essentially limited to patients who received rituximab, with no patients receiving obinutuzumab or a CD20-targeted bispecific antibody and one patient receiving ofatumumab with subsequent CD20 loss.
Partial or complete loss of CD20 expression was observed in 11.9% of relapses, highlighting the importance of repeating a biopsy at relapse before initiating CD20-targeted therapy. Our rate of re-biopsy at relapse was 48.4%, which while suboptimal, may be reflective of real-world clinical practice. In fact, the landmark trials which resulted in the approvals of mosunetuzumab [9, 10] and glofitamab [11] did not require CD20 testing at relapse prior to treatment. The efficacy of CD20-targeted bispecific antibodies in CD20-negative lymphomas is unclear. As these novel agents become more prevalent in clinical practice, CD20 expression should ideally be analyzed before treatment with a bispecific antibody and after treatment at the time of disease progression.
With the continued and expanding use of rituximab and obinutuzumab along with advancements in CD20 bispecific antibodies, further research is needed regarding CD20 loss and management of CD20-negative lymphomas. Most research involving CD20 antigen escape has been reported in the rituximab era; however, there is a paucity of research regarding the effect of treatment with CD20-targeted bispecific antibodies (mosunetuzumab, glofitamab, epcoritamab, odronextamab) on CD20 expression. There are limited clinical data regarding treatment of relapsed/refractory B-cell lymphoma after CD20 antigen escape. Historically, CD20-negative relapsed B-cell lymphoma has been treated with salvage cytotoxic chemotherapy, such as ifosfamide + carboplatin + etoposide (ICE) or dexamethasone + cytarabine + cisplatin (DHAP) [29]. Novel strategies including CAR T-cell therapy have been reported [42], and further investigation is warranted.
Conclusion
In this single-center retrospective cohort of patients with B-cell non-Hodgkin lymphoma, the rate of CD20 expression loss at relapse was 11.9%. CD20 antigen escape was observed in MZL, FL, and DLBCL. In general, repeat biopsy should be performed at relapse prior to re-treatment with CD20-targeted therapy.
Acknowledgments
The authors would like to acknowledge the Harbor-UCLA Hematopathology Lab for assistance with pathologic analysis.
Funding Statement
ST has received funding from Novartis, Merck, Principia Biopharma, Sanofi, Seagen, and Rigel Pharmaceuticals. JM, XQ, and MD have no relevant financial or non-financial interests to disclose.
Conflict of Interest
The authors have no relevant conflicts of interest to disclose.
Informed Consent
Informed consent was waived due to the retrospective nature of the study. Identifiable patient information was removed at the time of data analysis.
Author Contributions
ST and MD conceived and designed the study. ST implemented the software for patient procurement. JM collected, analyzed, and curated data. XQ analyzed biopsy samples and provided relevant pathologic images. JM and XQ prepared the original draft of the manuscript. All authors were involved in manuscript editing and review. ST was responsible for project supervision. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chu Y, Liu Y, Fang X, Jiang Y, Ding M, Ge X, Yuan D. et al. The epidemiological patterns of non-Hodgkin lymphoma: global estimates of disease burden, risk factors, and temporal trends. Front Oncol. 2023;13:1059914. doi: 10.3389/fonc.2023.1059914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.Salles G, Barrett M, Foa R, Maurer J, O'Brien S, Valente N, Wenger M. et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E. et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 6.Bachy E, Houot R, Feugier P, Bouabdallah K, Bouabdallah R, Virelizier EN, Maerevoet M. et al. Obinutuzumab plus lenalidomide in advanced, previously untreated follicular lymphoma in need of systemic therapy: a LYSA study. Blood. 2022;139(15):2338–2346. doi: 10.1182/blood.2021013526. [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH, Chua N, Mayer J, Dueck G, Trneny M, Bouabdallah K, Fowler N. et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081–1093. doi: 10.1016/S1470-2045(16)30097-3. [DOI] [PubMed] [Google Scholar]
- 8.Morschhauser F, Le Gouill S, Feugier P, Bailly S, Nicolas-Virelizier E, Bijou F, Salles GA. et al. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol. 2019;6(8):e429–e437. doi: 10.1016/S2352-3026(19)30089-4. [DOI] [PubMed] [Google Scholar]
- 9.Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J. et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–1065. doi: 10.1016/S1470-2045(22)00335-7. [DOI] [PubMed] [Google Scholar]
- 10.Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, Matasar MJ. et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-cell lymphomas: phase I dose-escalation study. J Clin Oncol. 2022;40(5):481–491. doi: 10.1200/JCO.21.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, Khan C. et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2022;387(24):2220–2231. doi: 10.1056/NEJMoa2206913. [DOI] [PubMed] [Google Scholar]
- 12.Thieblemont C, Phillips T, Ghesquieres H, Cheah CY, Clausen MR, Cunningham D, Do YR. et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: dose expansion in a phase I/II trial. J Clin Oncol. 2023;41(12):2238–2247. doi: 10.1200/JCO.22.01725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA. et al. Odronextamab, a human CD20xCD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–e339. doi: 10.1016/S2352-3026(22)00072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang W, Shi M, Yang J, Cao J, Xu L, Yan D, Yao M. et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9(16):5827–5838. doi: 10.1002/cam4.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NN, Johnson BD, Schneider D, Zhu F, Szabo A, Keever-Taylor CA, Krueger W. et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–1575. doi: 10.1038/s41591-020-1081-3. [DOI] [PubMed] [Google Scholar]
- 16.Chu PG, Loera S, Huang Q, Weiss LM. Lineage determination of CD20- B-Cell neoplasms: an immunohistochemical study. Am J Clin Pathol. 2006;126(4):534–544. doi: 10.1309/3WG32YRAMQ7RB9D4. [DOI] [PubMed] [Google Scholar]
- 17.Katchi T, Liu D. Diagnosis and treatment of CD20 negative B cell lymphomas. Biomark Res. 2017;5:5. doi: 10.1186/s40364-017-0088-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Zhao Y, Dong X, Zhang S, Li Y, Wu G. Loss of CD20 expression in relapsed diffuse large B cell lymphoma after rituximab therapy: a case report and review of the literature. Chin-Ger J Clin Oncol. 2013;12:148–151. [Google Scholar]
- 19.Nakamaki T, Fukuchi K, Nakashima H, Ariizumi H, Maeda T, Saito B, Yanagisawa K. et al. CD20 gene deletion causes a CD20-negative relapse in diffuse large B-cell lymphoma. Eur J Haematol. 2012;89(4):350–355. doi: 10.1111/j.1600-0609.2012.01838.x. [DOI] [PubMed] [Google Scholar]
- 20.Massengale WT, McBurney E, Gurtler J. CD20-negative relapse of cutaneous B-cell lymphoma after anti-CD20 monoclonal antibody therapy. J Am Acad Dermatol. 2002;46(3):441–443. doi: 10.1067/mjd.2002.108490. [DOI] [PubMed] [Google Scholar]
- 21.Duman BB, Sahin B, Ergin M, Guvenc B. Loss of CD20 antigen expression after rituximab therapy of CD20 positive B cell lymphoma (diffuse large B cell extranodal marginal zone lymphoma combination): a case report and review of the literature. Med Oncol. 2012;29(2):1223–1226. doi: 10.1007/s12032-011-9955-3. [DOI] [PubMed] [Google Scholar]
- 22.Alvaro-Naranjo T, Jaen-Martinez J, Guma-Padro J, Bosch-Princep R, Salvado-Usach MT. CD20-negative DLBCL transformation after rituximab treatment in follicular lymphoma: a new case report and review of the literature. Ann Hematol. 2003;82(9):585–588. doi: 10.1007/s00277-003-0694-1. [DOI] [PubMed] [Google Scholar]
- 23.Haidar JH, Shamseddine A, Salem Z, Mrad YA, Nasr MR, Zaatari G, Bazarbachi A. Loss of CD20 expression in relapsed lymphomas after rituximab therapy. Eur J Haematol. 2003;70(5):330–332. doi: 10.1034/j.1600-0609.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 24.Bellesso M, Xavier FD, Costa RO, Pereira J, Siqueira SA, Chamone DA. Disease progression after R-CHOP treatment associated with the loss of CD20 antigen expression. Rev Bras Hematol Hemoter. 2011;33(2):148–150. doi: 10.5581/1516-8484.20110036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreri AJ, Dognini GP, Verona C, Patriarca C, Doglioni C, Ponzoni M. Re-occurrence of the CD20 molecule expression subsequent to CD20-negative relapse in diffuse large B-cell lymphoma. Haematologica. 2007;92(1):e1–2. doi: 10.3324/haematol.10255. [DOI] [PubMed] [Google Scholar]
- 26.Foran JM, Norton AJ, Micallef IN, Taussig DC, Amess JA, Rohatiner AZ, Lister TA. Loss of CD20 expression following treatment with rituximab (chimaeric monoclonal anti-CD20): a retrospective cohort analysis. Br J Haematol. 2001;114(4):881–883. doi: 10.1046/j.1365-2141.2001.03019.x. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy GA, Tey SK, Cobcroft R, Marlton P, Cull G, Grimmett K, Thomson D. et al. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br J Haematol. 2002;119(2):412–416. doi: 10.1046/j.1365-2141.2002.03843.x. [DOI] [PubMed] [Google Scholar]
- 28.Maeshima AM, Taniguchi H, Fujino T, Saito Y, Ito Y, Hatta S, Yuda S. et al. Immunohistochemical CD20-negative change in B-cell non-Hodgkin lymphomas after rituximab-containing therapy. Ann Hematol. 2020;99(9):2141–2148. doi: 10.1007/s00277-019-03853-1. [DOI] [PubMed] [Google Scholar]
- 29.Rasheed AA, Samad A, Raheem A, Hirani SI, Shabbir- Moosajee M. Cd20 Expression and Effects on Outcome of Relapsed/ Refractory Diffuse Large B Cell Lymphoma after Treatment with Rituximab. Asian Pac J Cancer Prev. 2018;19(2):331–335. doi: 10.22034/APJCP.2018.19.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraga J, Tomita A, Sugimoto T, Shimada K, Ito M, Nakamura S, Kiyoi H. et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: its prevalence and clinical significance. Blood. 2009;113(20):4885–4893. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- 31.Maeshima AM, Taniguchi H, Nomoto J, Maruyama D, Kim SW, Watanabe T, Kobayashi Y. et al. Histological and immunophenotypic changes in 59 cases of B-cell non-Hodgkin's lymphoma after rituximab therapy. Cancer Sci. 2009;100(1):54–61. doi: 10.1111/j.1349-7006.2008.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeshima AM, Taniguchi H, Fukuhara S, Morikawa N, Munakata W, Maruyama D, Kim SW. et al. Follow-up data of 10 patients with B-cell non-Hodgkin lymphoma with a CD20-negative phenotypic change after rituximab-containing therapy. Am J Surg Pathol. 2013;37(4):563–570. doi: 10.1097/PAS.0b013e3182759008. [DOI] [PubMed] [Google Scholar]
- 33.Chu PG, Chen YY, Molina A, Arber DA, Weiss LM. Recurrent B-cell neoplasms after Rituximab therapy: an immunophenotypic and genotypic study. Leuk Lymphoma. 2002;43(12):2335–2341. doi: 10.1080/1042819021000040044. [DOI] [PubMed] [Google Scholar]
- 34.Michot JM, Buet-Elfassy A, Annereau M, Lazarovici J, Danu A, Sarkozy C, Chahine C. et al. Clinical significance of the loss of CD20 antigen on tumor cells in patients with relapsed or refractory follicular lymphoma. Cancer Drug Resist. 2021;4(3):710–718. doi: 10.20517/cdr.2020.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rushton CK, Arthur SE, Alcaide M, Cheung M, Jiang A, Coyle KM, Cleary KLS. et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma. Blood Adv. 2020;4(13):2886–2898. doi: 10.1182/bloodadvances.2020001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wada N, Kohara M, Ogawa H, Sugiyama H, Fukuhara S, Tatsumi Y, Kanamaru A. et al. Change of CD20 Expression in Diffuse Large B-Cell Lymphoma Treated with Rituximab, an Anti-CD20 Monoclonal Antibody: A Study of the Osaka Lymphoma Study Group. Case Rep Oncol. 2009;2(3):194–202. doi: 10.1159/000249152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takiar R, Ng WL, Shah E, Boonstra PS, Karimi YH, Carty SA, Wilcox RA. et al. A multi-center study: outcomes among relapsed / refractory diffuse large B cell lymphoma patients with CD20 loss. Blood. 2022;140(Supplement 1):9510–9511. [Google Scholar]
- 38.Terui Y, Mishima Y, Sugimura N, Kojima K, Sakurai T, Mishima Y, Kuniyoshi R. et al. Identification of CD20 C-terminal deletion mutation associated with loss of CD20 expression in non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15(7):2523–2530. doi: 10.1158/1078-0432.CCR-08-1403. [DOI] [PubMed] [Google Scholar]
- 39.Czuczman MS, Olejniczak S, Gowda A, Kotowski A, Binder A, Kaur H, Knight J. et al. Acquirement of rituximab resistance in lymphoma cell lines is associated with both global CD20 gene and protein down-regulation regulated at the pretranscriptional and posttranscriptional levels. Clin Cancer Res. 2008;14(5):1561–1570. doi: 10.1158/1078-0432.CCR-07-1254. [DOI] [PubMed] [Google Scholar]
- 40.Tsai PC, Hernandez-Ilizaliturri FJ, Bangia N, Olejniczak SH, Czuczman MS. Regulation of CD20 in rituximab-resistant cell lines and B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2012;18(4):1039–1050. doi: 10.1158/1078-0432.CCR-11-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beers SA, French RR, Chan HT, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS. et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood. 2010;115(25):5191–5201. doi: 10.1182/blood-2010-01-263533. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Cai D, Gao R, Miao Y, Cui Y, Liu Z, Zhang H. et al. Case Report: CD19 CAR T-cell therapy following autologous stem cell transplantation: a successful treatment for R/R CD20-negative transformed follicular lymphoma with TP53 mutation. Front Immunol. 2023;14:1307242. doi: 10.3389/fimmu.2023.1307242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.