Abstract
Background
Coronary artery bypass grafting (CABG) provides superior long-term outcomes to percutaneous coronary intervention (PCI) for complex multivessel coronary artery disease (CAD). People with chronic kidney disease (CKD) have increased prevalence of multivessel CAD, but also increased surgical risk. We investigated whether CKD predicted real-world use of CABG, versus PCI, in patients revascularized for acute coronary syndrome (ACS).
Methods
Embase, MEDLINE, Scopus and CENTRAL were searched to identify articles referring to ACS and invasive coronary intervention in high-income countries (2012 - 2023). Articles were included if CABG rates were reported in ACS patients with and without CKD receiving revascularization. CKD was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2; proxy definitions were accepted. Random effect meta-analyses were used to determine the average effect of CKD on odds of CABG, stratified by ACS type and dialysis use.
Results
Searches generated 15,138 articles, of which 13 observational studies were included (n = 1,682,207). Amongst revascularized ACS patients, those with CKD were more likely to receive CABG than those without (pooled odds ratio (OR) = 1.50 (95% confidence interval (CI) = 1.30 - 1.72). This association was stronger following ST-elevation myocardial infarction (STEMI) than non-ST-elevation ACS (NSTE-ACS) (OR: 1.54 (95% CI: 1.23 - 1.93)) versus 1.16 (1.10 - 1.23), respectively).
Conclusions
In high-income countries, revascularized ACS patients with CKD receive CABG (versus PCI) more frequently than those without kidney disease. However, accounting for lower use of coronary angiography in the CKD population removed this association following NSTE-ACS. Greater use of invasive angiography in those with NSTE-ACS and CKD might therefore increase access to revascularization, and thereby improve outcomes.
Keywords: Chronic kidney disease, Meta-analysis, Revascularization
Introduction
Acute coronary syndrome (ACS) is a major cause of mortality and morbidity amongst patients with chronic kidney disease (CKD). The incidence of ACS in CKD patients is four-times higher compared to the general population [1], due to cumulative detrimental effects of traditional cardiovascular risk factors and the pro-inflammatory and uremic milieu of kidney disease [2]. ACS and other cardiovascular diseases are the most common causes of death in patients with CKD [3]. Elderly individuals with CKD are more likely to die from cardiovascular disease than to progress to end-stage kidney disease [4].
CKD patients experience worse outcomes after ACS than those without kidney disease [5-7]. Mortality risk increases with CKD severity [8]. CKD is associated with prolonged hospital admission, lower likelihood of return to work and increased risk of recurrent ACS events [9]. Our understanding of the pathophysiology and optimal management strategies for ACS in CKD patients is however limited, as those with moderate to severe CKD (stages 3 - 5) have been systematically excluded from most randomized controlled trials in this area [10].
CKD influences clinical decision-making regarding ACS patients. In high-income countries, people with CKD are significantly less likely to receive invasive coronary angiography (ICA) or revascularization following an ACS event, than those without kidney disease [5-7, 11-14]. The extent of these differences is not in keeping with current guidelines on ACS care [15], which reflects the growing body of observational evidence demonstrating a mortality benefit associated with invasive management, which appears to be independent of existing kidney disease [5, 16-18].
Coronary artery revascularization improves survival and quality of life after ACS [16]. Revascularization improves blood supply to ischemic myocardium leading to relief of angina, reduced risk of recurrent ACS, and cardiovascular death. Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) offer different advantages and disadvantages. Compared with PCI, CABG is associated with increased risk of stroke, acute kidney injury (AKI) and mortality during hospitalization, but with lower rates of recurrent ACS, further revascularization procedures, and death in the longer-term. CKD increases the procedural mortality risk of both PCI and CABG, particularly regarding the former [7, 16, 19].
The SYNTAX scores have been developed to 1) quantify the complexity of coronary artery disease (CAD); and 2) estimate relative mortality differences associated with CABG versus PCI for individual patients [20]. SYNTAX II considers coronary anatomy, patient demographics and comorbidities including kidney function. Reduced kidney function increases the predicted benefit from CABG over PCI [20]. Yet, clinicians may be reluctant to refer for, and/or undertake coronary surgery in people with CKD due to a perceived limited prognosis, greater postoperative risks of AKI, wound infection, and mortality versus those without kidney disease [21, 22]. Furthermore, patients with CKD may prefer to avoid cardiac surgery. It is unknown therefore, how SYNTAX scoring translates into clinical practice for this specific population. To our knowledge, choice of type of revascularization strategy has not previously been systematically compared between the CKD and non-CKD ACS populations.
Our research question, framed within the “Population, Exposure, Comparison and Outcomes (PECO)” framework was as follows: Amongst patients who receive revascularization during their index admission for ACS, does CKD influence the type of revascularization received?
Materials and Methods
On the April 21, 2022 (updated on the September 29, 2023), Embase, MEDLINE, Scopus, CENTRAL and the NIHR’s website of funded studies were searched to identify studies with medical subject headings terms and text words for ACS and invasive coronary management (ICA with or without revascularization) over the past 10 years. The search strategy was developed with assistance from University of Bristol librarians (Supplementary Material 1, cr.elmerpub.com). Studies conducted in low or middle-income countries (LMICs) were excluded as ACS management is likely to be influenced by available resources and healthcare funding.
Study titles and abstracts were screened against pre-determined eligibility criteria (Supplementary Material 2, cr.elmerpub.com) by one of four authors (JS, ML, WHA, HMC) using Rayyan software. Observational and qualitative studies, systematic reviews, and meta-analyses of 10 or more adult humans (> 17 years), published on or after January 1, 2012, were eligible for inclusion if the abstract was written in English. The abstract or title was required to indicate that ICA or revascularization in people with ACS (non-ST-elevation myocardial infarction (NSTEMI), unstable angina (UA) or ST-elevation myocardial infarction (STEMI)) was reported. The same four authors assessed the eligibility of the full papers according to pre-specified criteria (Supplementary Material 3, cr.elmerpub.com). The reference lists of systematic reviews and meta-analyses were hand-searched. To be eligible, articles were required to report crude numbers and/or effect estimates for the receipt of CABG by people with and without CKD, who had received some form of revascularization following ACS. We defined CKD as an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2 within 48 h of ACS. However, we accepted as a proxy for this any definition of CKD other than where the authors concluded CKD reflected acute kidney injury. Data extraction was undertaken by two authors independently. Corresponding authors for 10 studies were emailed to request clarification on definitions or supply additional data/analyses. Replies were received from four study groups.
Two authors (JS and ML) assessed the risk of bias using the Risk of Bias in Non-Randomized Studies of Exposures (ROBINS-E) tool (Supplementary Material 4, cr.elmerpub.com) [23]. The assessments relate to the outcomes examined in this systematic review, and do not reflect the bias of the studies regarding their own specified outcomes. JS and ML used the GRADE approach to independently rate certainty of evidence for each outcome [24, 25] (Supplementary Material 5, cr.elmerpub.com). Differences in opinion were resolved by discussion. As an observational study design was deemed the most appropriate for this review question, all ratings were started as ‘high certainty’. Certainty of the precision and magnitude of effect estimate were judged according to the Cochrane guidance and after seeking consensus amongst study authors (Supplementary Materials 6, 7, cr.elmerpub.com) [26].
Statistical analysis was conducted in Stata/MP 17.0. Due to the anticipated heterogeneity between studies, we used random-effects models employing the restricted maximum likelihood (REML) method to pool effect estimates [27]. Receipt of CABG was reported as odds ratios (ORs) in most studies. Where only a hazard ratio (HR) was available, this was analyzed as an OR [28]. Unadjusted and covariate-adjusted effect estimates were extracted where available. Where unadjusted effect estimates were not available, raw data from the paper were used to calculate crude ORs.
We pre-specified sub-group analyses to determine the impact of ACS type and severity of CKD on effect estimates. Studies could contribute data to both non-ST-elevation ACS (NSTE-ACS) and STEMI subgroups if distinct effect estimates were reported or could be calculated. The proportion of the population with CKD was not calculated in studies that matched CKD cases to controls without CKD [29].
We examined the robustness of our findings by performing sensitivity analyses, in which we excluded studies 1) at serious or critical risk of bias; 2) of small study size (n < 3,000); 3) that examined outcomes in dialysis users versus non-users; 4) where the follow-up period was longer than the duration of the index ACS admission; and 5) any study including UA from the NSTE-ACS subgroup analysis.
We used random-effects meta-regression to attempt to explore potential statistical heterogeneity due to differences in population characteristics (mean age (Supplementary Material 8, cr.elmerpub.com), ACS type, percentage of sample with CKD, percentage of total cohort receiving CABG, prevalence of diabetes within total cohort, date of reported data, small study size, sex, and country of origin) and methodology (risk of bias).
Funnel plots were drawn to qualitatively explore symmetry of effect estimates. We did not use quantitative tests of funnel plot asymmetry due to low number of studies and significant statistical heterogeneity.
The Institutional Review Board approval was not required for this review of published data, and ethical compliance is not applicable.
Registration and protocol
The protocol for this review combined with related work investigating receipt of ICA and revascularization (any) is available here (Supplementary Material 9, cr.elmerpub.com). The following changes have been made since the protocol was first written: 1) the timeframe for data collection has been updated to include the period May 2022 - October 2023; 2) the ROBINS-E rather than the ROBINS-I tool was used for assessment of the risk of bias in included studies as it was later deemed more appropriate; 3) no subgroup analyses pre and during the coronavirus disease 2019 (COVID-19) pandemic were performed due to lack of data specific to the COVID era; 4) the GRADEpro GDT software was not utilized unnecessarily; 5) Egger’s test was not used to quantify funnel plot asymmetry because of the significant amount of between study heterogeneity identified [30].
This report was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidance (Supplementary Material 10, cr.elmerpub.com).
Results
Overview
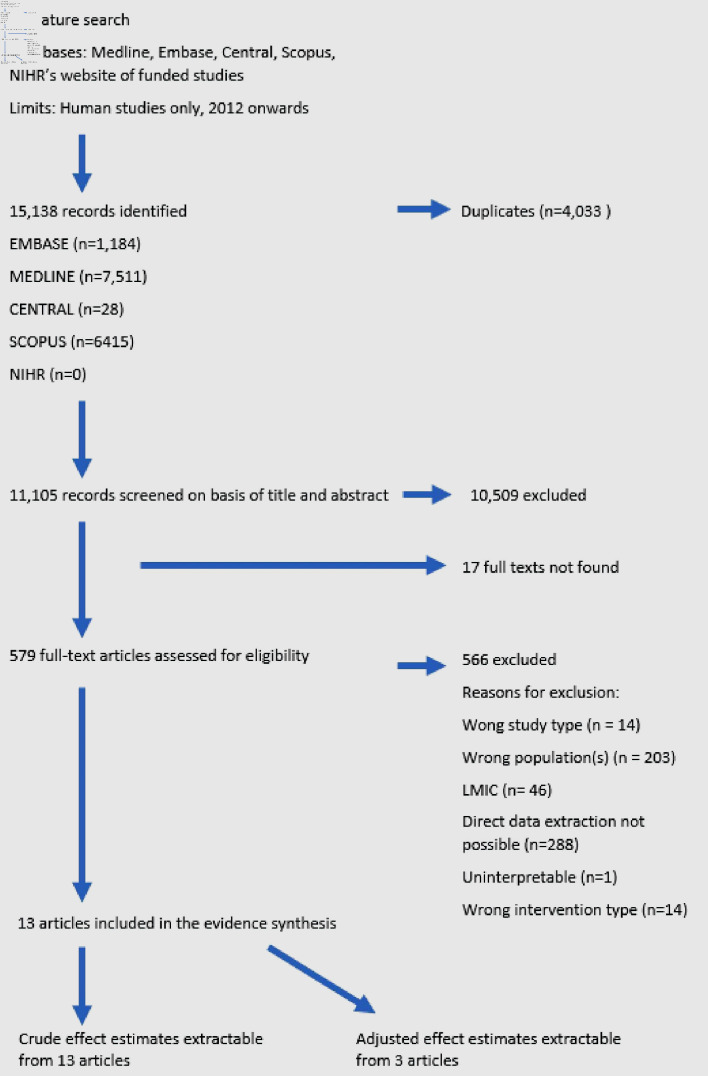
Initial searches generated 15,138 articles, of which 13 observational studies fulfilled predefined criteria and were included in the systematic review (Fig. 1). One study was prospective [29], while the remainder were retrospective. The selected studies included a total of 1,682,207 participants (sample size range: 438 to 478,919, mean age range 61 to 79 years), from four geographical areas (North America: n = 7, Europe and the UK: n = 4, Australasia: n = 1, East Asia: n = 1). Outcomes were reported for people with NSTE-ACS (with or without inclusion of UA), STEMI or any ACS. CKD was defined in five different ways (Supplementary Material 11, cr.elmerpub.com). Of note, two studies excluded any patient receiving more than one form of revascularization during the index admission [13, 31]; the remainder of studies did not mention how they managed these individuals. Characteristics of the included studies are summarized in Table 1 [2, 4-7, 13, 19, 28, 29, 32-38].
Figure 1.
Flowchart of study selection.
Table 1. Characteristics of Included Studies.
| Study design, country | Hospital PCI capability | N | Dates | Definition of impaired CKD function | Definition of control group | ACS type(s) | Age range | Crude figures | Effect estimates, OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Alushi et al, 2021 [29] | Prospective, Germany | All | 438 | 2012 - 2017 | eGFR < 30 or dialysis duration > 30days at the time of MI | eGFR > 90 | NSTEMI, UA | Not specified | NSTE-ACS 25/222 CKD vs. 18/216 non-CKD | Crude OR NSTE-ACS 1.36 (0.75 - 2.46) |
| Adjusted OR NSTE-ACS 3.20 (1.28 - 8.00) | ||||||||||
| Bagai et al, 2018 [7] | Retrospective, USA | All | 615,425 | 2007 - 2015 | eGFR < 60 | eGFR ≥ 60 | NSTEMI, STEMI | Not specified | All ACS 21,191/171,140 CKD vs. 49,707/444,285 non-CKD | Crude OR all ACS 1.12 (1.10 - 1.14) |
| NSTE-ACS 16,344/91,021 CKD vs. 37,087/216,244 non-CKD | Crude OR NSTE-ACS 1.12 (1.10 - 1.14) | |||||||||
| STEMI 4,847/80,119 CKD vs. 37,087/228,041 non-CKD | Crude OR STEMI 1.10 (1.06 - 1.14) | |||||||||
| Blicher et al, 2013 [28] | Retrospective, Denmark | Mixed | 34,722 | 2000 - 2009 | Code for CKD/dialysis [4] | No CKD code [5] | NSTEMI, STEMI [2] | > 25 years | All MI 239/904 CKD vs. 6,337/33,818 non-CKD | Crude OR all ACS 1.56 (1.34 - 1.81) |
| Kawsara et al, 2022 [33] | Retrospective, USA | Mixed | 69,281 | 2016 - 2019 | Dialysis code | No dialysis code | STEMI | ≥ 18 years | STEMI 37/522 CKD vs. 3,438/68,759 non-CKD | Crude OR STEMI 1.45 (1.04 - 2.03) |
| Khan et al, 2020 [34] | Retrospective, USA | Mixed | 149,983 | 2012 - 2017 | ICD-9 CM code for ESRD | Other | STEMI | ≥ 18 years | STEMI 251/1,753 CKD vs. 63,675/12,735/148,230 non-CKD | STEMI OR 1.78 (1.55 - 1.03) |
| Kotwal et al, 2017 [35] | Retrospective, Australia | Mixed | 12,662 | 2004 - 2008 | ICD-10 code for CKD | No CKD code | NSTEMI, STEMI | ≥ 18 years | All ACS 272/811 CKD vs. 1,741/11,851 non-CKD | All ACS OR 2.93 (2.51 - 3.42) |
| Lin et al, 2022 [13] | Retrospective, Taiwan | Mixed | 67,534 | 2001 - 2013 | Code for CKD/dialysis | No code for CKD or dialysis | NSTEMI, STEMI | ≥ 20 years | All ACS 2,433/16,477 CKD vs. 4,894/51,057 non-CKD | All ACS OR 1.62 (1.55 - 1.72) |
| Murray et al, 2018 [36] | Retrospective, USA | Mixed | 236,284 | 2001 - 2012 | ICD-9 code for CKD1-5 or dialysis | No code for CKD or dialysis | NSTEMI | ≥ 18 years | NSTE-ACS 7,227/22,891 CKD vs. 47,517/208,293 non-CKD | NSTE-ACS OR 1.18 (1.14 - 1.21) |
| Panchal et al, 2021 [37] | Retrospective, USA | Mixed | 87,883 | 2012 - 2014 | ICD-9 code for CKD1 - 5, unspecified CKD, or dialysis | No code for CKD or dialysis | STEMI | Not specified | STEMI 838/7,476 CKD vs. 5,734/80,407 non-CKD | STEMI OR 1.67 (1.55 - 1.81) |
| Sakhuja et al, 2016 [38] | Retrospective, USA | Mixed | 141,838 | 2006 - 2010 | ICD-9 code for ESRD or OPCS codes for PD or HD, without a code for CKD | No ESRD/PD/HD code | STEMI | ≥ 20 years | STEMI 292/1,248 CKD vs. 13,746/140,590 non-CKD | STEMI OR 1.67 (1.43 - 1.95) |
| Sanchis et al, 2021 [32] | Retrospective, Spain | Not specified | 7,211 (total study size) | 2002 - 2017 | eGFR < 60 calculated from admission creatinine | Admission eGFR ≥ 60 | NSTEMI, UA | ≥ 70 years | Crude figures not available | Crude OR NSTE-ACS 0.85 (0.70 - 1.03) |
| Adjusted OR NSTE-ACS 1.11 (0.92 - 1.35) | ||||||||||
| Shaw et al, 2014 [6] | Retrospective, England & Wales | Mixed | 9,732 | 2008 - 2010 | eGFR < 60 calculated from admission creatinine | Admission eGFR ≥ 60 | NSTEMI, UA | Not specified | NSTE-ACS 274/2,318 CKD vs. 679/7,414 non-CKD | Crude OR NSTE-ACS 1.32 (1.15 - 1.54) |
| Smilowitz et al, 2017 [19] | Retrospective, USA | Mixed | 388,592 | 2007 - 2012 | ICD9 codes for CKD3 - 5 or HD | No code for CKD3 - 5 or HD | NSTEMI, STEMI | Not specified | All ACS 9,775/43,242 CKD vs. 55,389/345,350 non-CKD | Crude OR all ACS 1.53 (1.49 - 1.57) |
| NSTE-ACS 7,783/30,657 CKD vs. 38,324/175,921 non-CKD | Adjusted OR all ACS 1.06 (1.03 - 1.09) | |||||||||
| STEMI 1,992/12,585 vs. 17,065/169,429 non-CKD | Crude OR NSTE-ACS 1.22 (1.19 - 1.26) | |||||||||
| Adjusted OR NSTE-ACS 1.02 (0.99 - 1.06) | ||||||||||
| Crude OR STEMI 1.69 (1.60 - 1.77) | ||||||||||
| Adjusted OR STEMI 1.21 (1.14 - 1.28) |
PCI: percutaneous coronary intervention; ACS: acute coronary syndrome; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ICD: international classification of diseases; NSTE-ACS: non-ST-elevation acute coronary syndrome; NSTEMI; non-ST-elevation myocardial infarction; OR: odds ratio; CI: confidence interval; STEMI: ST-elevation myocardial infarction; UA: unstable angina; PD: peritoneal dialysis; HD: hemodialysis; ESRD: end-stage renal disease; ICD-9 CM: International Classification of Diseases, Ninth Revision, Clinical Modification.
Main analyses
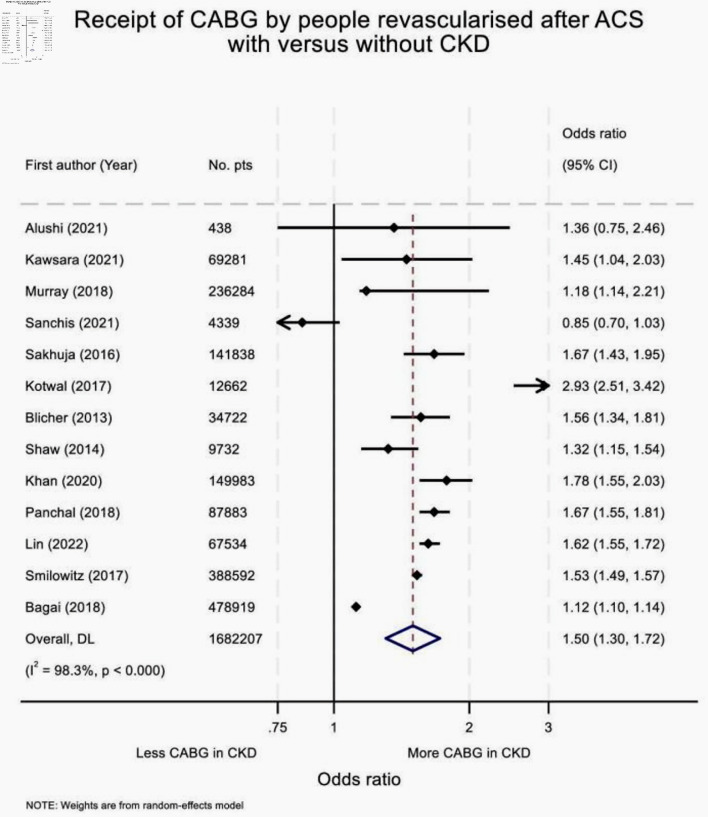
CKD was associated with higher odds of receipt of CABG (versus PCI) following ACS (OR: 1.50 (95% CI: 1.30 - 1.72)) (Fig. 2). There was marked heterogeneity between the effect estimates (I2 = 98.3%, P < 0.001). The risk of bias was estimated as low (n = 3), some concerns (n = 6), high risk (n = 3) and very high (n = 1).
Figure 2.
Forest plot summarizing effect estimates for the receipt of CABG by people revascularized after ACS with versus without CKD. In real-world practice, people with CKD are more likely to receive CABG (versus PCI) if revascularized following ACS, than those without kidney disease. ACS: acute coronary syndrome; CKD: chronic kidney disease; CABG: coronary artery bypass graft; OR: odds ratio; CI: confidence interval.
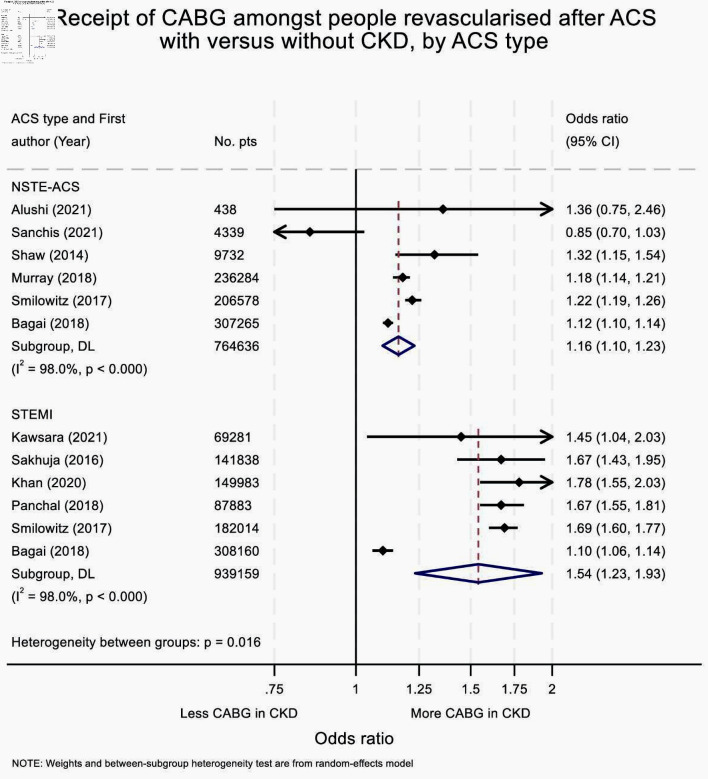
Greater use of CABG vs. PCI was seen in patients with CKD versus those without CKD, independent of either ACS type or use of dialysis. The strength of the association between CKD and CABG treatment was influenced by ACS type; a stronger association was seen in those with STEMI (OR: 1.54 (95% CI: 1.23 - 1.93) than those with NSTE-ACS (1.16 (1.10 - 1.23), interaction P value = 0.016) (Fig. 3). In contrast, receipt of preoperative dialysis did not meaningfully influence the strength of the association (OR: 1.25 (95% CI: 1.12 - 1.40 in dialysis users and 1.44 (1.20 - 1.71) in those not using dialysis, interaction P value = 0.206) (Supplementary Material 12, cr.elmerpub.com). Only one study reported receipt of CABG by eGFR category [16]. In this NSTE-ACS population, compared to people without CKD, increased odds of receipt of CABG (versus PCI) were observed in people with an eGFR of 30 - 45 and 45 - 60 mL/min/1.73 m2 (OR: 1.43 (95% CI: 1.13 - 1.82) and 1.33 (1.11 - 1.60), respectively), but not amongst those with an eGFR < 30 (including those receiving dialysis) (OR: 1.05 (95% CI: 0.68 - 1.61)).
Figure 3.
Forest plot summarizing effect estimates for the receipt of CABG, versus PCI, amongst people revascularized after ACS with versus without CKD, by ACS type. Amongst those revascularized for ACS, people with CKD are more likely to receive CABG (versus PCI) than those without kidney disease, independent of ACS type. However, this association is stronger following STEMI, than NSTE-ACS. ACS: acute coronary syndrome; CABG: coronary artery bypass graft; CKD: chronic kidney disease; NSTE-ACS: non-ST-elevation acute coronary syndrome; OR: odds ratio; STEMI; ST-elevation myocardial infarction.
Summary of studies with adjusted effect estimates
Covariable-adjusted effect estimates for the receipt of CABG (versus PCI) in CKD patients compared to those without CKD were available in three studies [19, 29, 32]. In two (rated as “high” and “very high” risk of bias) studies, adjustment for confounders of the association between CKD and receipt of CABG made no meaningful difference to effect estimates. In contrast, Smilowitz et al [19] found adjustment for baseline demographics, cardiovascular risk factors and features at ACS presentation to weaken the association between CKD and receipt of CABG (OR for STEMI: 1.21 (95% CI: 1.14 - 1.28) from 1.69 (1.60 - 1.77), and NSTE-ACS OR: 1.02 (95% CI: 0.99 - 1.06) from 1.22 (1.19 - 1.26)). There were “some concerns” regarding risk of bias for this study. Meta-analysis of these results was not performed due to low study number.
Sensitivity analyses
In sensitivity analyses, no meaningful changes to the results of the main analyses were found when we excluded studies with high or very high risk of bias or those whose defined CKD as the receipt of dialysis (Supplementary Materials 13, 14, cr.elmerpub.com). Small attenuations in the average exposure effects were seen following both exclusion of small studies [29] and of studies with a follow-up period longer than the duration of the index ACS hospitalization [28] (OR: 1.29 (95% CI: 1.28 - 1.31) for both), and when a fixed effects model was used (OR: 1.29 (1.28 - 1.31)) (Supplementary Materials 15-17, cr.elmerpub.com). Exclusion of studies including people with UA made no meaningful impact on the effect estimate for receipt of CABG following NSTE-ACS (Supplementary Material 18, cr.elmerpub.com).
Exploration of heterogeneity
The ability of pre-defined characteristics of the study populations or methodology to explain the observed inter-study heterogeneity was explored. In univariable random effects meta-regression, STEMI versus NSTE-ACS, lower population mean age group, low percentage of the population identified as having CKD and geographical area (Australasian study population), were all associated with increase receipt of CABG versus PCI by people with CKD. An example bubble plot demonstrating the association between percentage population with CKD and effect estimate is provided (Supplementary Material 19, cr.elmerpub.com). In multivariable regression, lower percentage population identified as having CKD, Australasia as a geographical region, and lower mean population age group, were predictive of an increased effect size, together accounting for 81.9% of the heterogeneity between study results. Although the impact of geographical region was driven by a single study, there was no clinical or methodological reason to suggest that we should remove this effect estimate.
Funnel plots
The funnel plot was symmetrical, suggesting that serious publication bias was unlikely. In this review, we have some unusually large studies whose point estimates lie outside the expected plot area, likely due to clinical and/or methodological heterogeneity [30] (Supplementary Material 20, cr.elmerpub.com).
Summary of findings
A summary of outcomes and the certainty of these conclusions is presented in Table 2.
Table 2. Summary of Findings.
| Finding | No. of studies | No. of participants | Average exposure effect (OR (95% CI)) | Certainty of evidence |
|---|---|---|---|---|
| CKD is associated with increased odds of receipt of CABG vs. PCI amongst people revascularized following ACS. | 13 | 1,682,207 | 1.50 (1.30 - 1.72) | Moderate |
| CKD is associated with increased odds of receipt of CABG vs. PCI amongst people revascularized following NSTE-ACS. | 6 | 764,636 | 1.16 (1.10 - 1.23) | Moderate |
| CKD is associated with increased odds of receipt of CABG vs. PCI amongst people revascularized following STEMI. | 6 | 939,159 | 1.54 (1.23 - 1.93) | Moderate |
| CKD is associated with increased odds of receipt of CABG vs. PCI amongst people revascularized following ACS in people who do not receive dialysis. | 5 | 1,020,773 | 1.44 (1.20 - 1.71) | Low |
| CKD is associated with increased odds of receipt of CABG vs. PCI amongst people revascularized following ACS in people who receive dialysis. | 5 | 838,935 | 1.25 (1.12 - 1.40) | Moderate |
ACS: acute coronary syndrome; CABG: coronary artery bypass graft; CKD: chronic kidney disease; NSTE-ACS: non-ST-elevation acute coronary syndrome; OR: odds ratio; CI: confidence interval; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Discussion
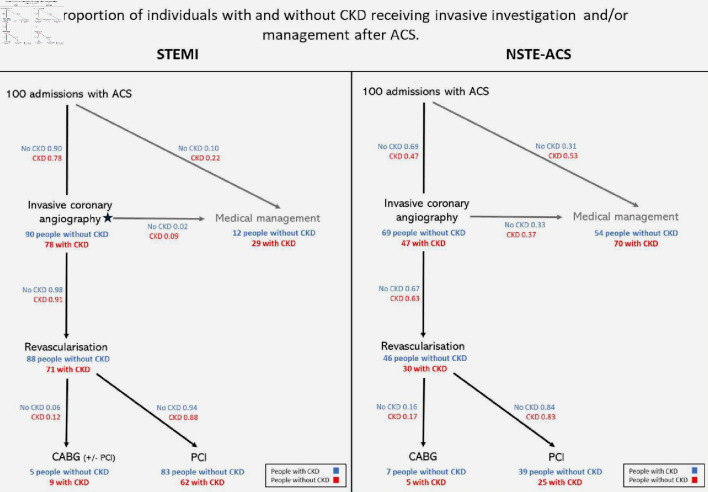
In this systematic review spanning the last 12 years, we found that amongst patients revascularized following ACS, CKD was associated with a relative increase in CABG treatment versus PCI. Compared to those without kidney disease, patients with CKD were almost 1.5 times as likely to receive CABG (OR: 1.47 (95% CI: 1.30 - 1.66)). Taken in isolation, these findings might be misleading, as it is known that patients with CKD are less likely to receive ICA or to be revascularized (CABG or PCI) compared to those without kidney disease [5-7, 19]. However, the findings of this review reflect the management of only those individuals who have been selected to receive revascularization. In contrast, when the entire pathway of invasive management is considered, the absolute rates of CABG were similar between those with or without CKD (Fig. 4).
Figure 4.
Flowchart demonstrating the crude proportions of individuals receiving invasive management by ACS type and CKD status. People with CKD are less likely to receive either invasive coronary angiography or revascularization (of any form) following ACS, than those without kidney disease. In this figure, invasive coronary angiography and revascularization are portrayed as distinct steps, however in clinical practice, angiography is typically associated with immediate revascularization in STEMI. ACS: acute coronary syndrome; CABG: coronary artery bypass graft; CKD: chronic kidney disease; NSTE-ACS: non-ST-elevation acute coronary syndrome; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction.
Greater use of CABG (versus PCI) amongst those with CKD is unsurprising, given the higher prevalence of complex multivessel CAD in this population, compared to those without kidney disease [39, 40]. When compared to multivessel PCI, CABG treatment is associated with reduced rates of recurrent ACS, need for further revascularization and improved long-term survival, independent of CKD status [41-45]. Furthermore, although CKD is associated with reduced survival following any type of revascularization treatment, it has a greater impact on postprocedural mortality in those treated with PCI than those treated with CABG. Hence, current ACS guidelines recommend CABG in favor of PCI for complex multivessel CAD in people with CKD and with an “acceptable” surgical risk profile, other than those for whom expected prognosis is less than 1 year [46].
The association between CKD and CABG treatment versus PCI amongst the revascularized ACS population was stronger following STEMI than following NSTE-ACS. Furthermore, in STEMI patients this association persisted after taking into consideration reduced receipt of both ICA and any type of revascularization in the CKD population (Fig. 3). This was less obvious in NSTE-ACS cases. These differences are likely to reflect variation in the underlying wider risk profile of patients and in management pathways. STEMI results from acute coronary obstruction. In these cases, immediate invasive angiography followed by primary PCI reduces mortality, independent of patient demographics or comorbidity. Treatment pathways have therefore been established whereby ST-elevation triggers almost de-facto acceptance onto an immediate invasive management pathway. Kidney function is often not known prior to intervention being received. Therefore, although people with CKD are less likely to receive invasive management for STEMI than those without kidney disease, the absolute numbers of conservatively treated individuals are low. Once accepted onto an “active treatment pathway”, cognitive biases may drive clinicians to pursue further revascularization including CABG.
In contrast to the STEMI pathway, angiography in NSTE-ACS is frequently a standalone investigation used to delineate coronary anatomy in the subset of individuals perceived suitable for later revascularization. In contrast to the “treatment de facto” STEMI pathway, deliberate decision-making is required to determine which individuals with NSTE-ACS should receive: 1) ICA; and 2) revascularization. The patient’s demographics and comorbidities, including kidney function, are central to this decision-making process.
After NSTE-ACS, reduced use of revascularization in the CKD, versus non-CKD population, is primarily the result of reduced use of ICA. However, less ICA cannot alone explain why the relative receipt of CABG versus PCI is so much lower in the CKD population after NSTE-ACS, versus STEMI. It appears possible therefore that either: 1) amongst people with CKD, ICA is undertaken less frequently amongst those with complex multivessel than those with single-vessel disease, requiring clinicians to be able to predict multivessel disease based on comorbidities or noninvasive imaging results; or 2) people with CKD and evidence of complex multivessel disease on ICA, are more likely to be managed medically, or with PCI (only) after NSTE-ACS than STEMI. Clinicians and/or patients may choose to avoid surgery in those with perceived “lower risk” ACS events.
Most of the heterogeneity between effect estimates in this review was explained by differences in: 1) mean age; and 2) the proportion of individuals identified as having CKD. Studies that examined younger populations and fewer people with CKD demonstrated stronger associations between CKD and receipt of CABG. Older people may be less likely to receive CABG due to greater risks of AKI, infection, bleeding, short-term mortality [47], and lower expectation of long-term survival. As a result, CKD may have a lower relative influence on decision-making between revascularization strategies in this population. The proportion of individuals identified as having CKD was associated with CKD severity. For example, studies in which the CKD cohort comprised less than 5% of the total population relied on codes for renal dialysis to identify CKD. Greater effect size in studies with smaller CKD cohorts may reflect either more extensive CAD in these individuals, and/or absence of risk of precipitating the need for dialysis.
Strengths and limitations
This review has many strengths. We performed a thorough search of the literature from high-income countries from 2012 onwards and included more than 8 million patients. Cochrane and PRISMA guidance were followed throughout. We undertook multiple sensitivity analyses to determine how robust our findings were, and extensively examined reasons for heterogeneity between effect estimates. However, we also recognize some limitations. Regarding methodology, despite our broad search strategy, we may have missed relevant grey literature or missed reports where the abstract was not available in English. However, there is no indication of publication bias from qualitative inspection of our funnel plot. Secondly, due to the array of CKD definitions it was not possible to fully assess the influence of this on heterogeneity between effect estimates. Thirdly, it was unclear from most papers how the authors analyzed people who had received both CABG and PCI. It is likely that many people denoted as having received CABG, also received previous PCI, especially following STEMI. Underestimation of CABG rates is unlikely however, as the cost associated with cardiac surgery will drive recording. Fourth, we were unable to investigate whether differences in sex, ethnicity, granular CKD stage or diabetes mellitus influenced effect estimates due to lack of detail in the included studies. Furthermore, most variables were only available at a population level, and mortality was not described, as data were not collected for this purpose. Lastly, GRADE ratings of the certainty of evidence relating to each outcome were moderate to low.
We are also aware of some statistical limitations. Firstly, ORs may overestimate the effect of an exposure in the presence of commonly occurring outcomes, such as invasive management [48]. Secondly, between study heterogeneity was high. The I2 values may also however, be artificially elevated due to the large sample sizes in the included observational data [49].
Further research
Further research is needed to determine: 1) whether people with CKD receive revascularization, by PCI or CABG, concordant with the preferred strategy, as predicted by the SYNTAX II score; and 2) if not, what are the reasons for the discordance between recommendations and applied rates of CABG treatment? Qualitative methodology would be most appropriate to meet the second aim, with specific focus on investigating: 1) what factors drive decisions regarding revascularization strategy in the presence of CKD; and 2) the involvement and wishes of patients in the decision-making process. It is also essential that we improve our understanding of the balance of risks and benefits, and net outcomes, of CABG versus PCI in people with different degrees of severity of CKD and seek the views of people with CKD disease towards these risk-benefit weightings.
Conclusions
Following ACS, people with CKD are approximately 50% less likely to receive angiography or revascularization compared to those without kidney disease. Amongst those with STEMI who are revascularized however, CKD is associated with a 50% increase in the use of CABG, versus PCI. This appears consistent with the increased prevalence of complex multivessel CAD in the kidney disease population. However, adjustment for differential receipt of angiography and revascularization (any) in people with CKD removes the association between CKD and receipt of CABG (versus PCI) after NSTE-ACS. This raises the question of whether complex multivessel disease is being appropriately managed in those with CKD and NSTE-ACS, and whether greater use of coronary angiography in people with NSTE-ACS could increase access to revascularization and improve outcomes as a result.
Supplementary Material
Search strategies by database.
Criteria for screening titles and abstracts.
Criteria for screening full texts.
Risk of bias assessments.
Grade ratings of certainty of outcome, by domain.
Study group consensus for assessing magnitude of effect estimate within grade rating of certainty.
Study group consensus and Cochrane guidance for assessing precision of effect estimate within grade rating of certainty.
Method for determining mean age of sample population.
Systematic review protocol.
Prisma 2020 checklist.
Definitions of CKD amongst included studies.
Forest plot depicting meta-analysis of studies reporting receipt of revascularization in people with CKD receiving or not receiving dialysis, versus those without CKD.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people with versus without CKD, excluding those with serious or critical risk of bias.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding those that defined CKD as receipt of dialysis.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding small studies.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding those that defined the follow-up period as other than the duration of the index hospitalization.
Forest plot depicting a fixed-effects meta-analysis of the receipt of CABG in people revascularized after ACS with versus without CKD following ACS.
Forest plot depicting meta-analysis of the receipt of CABG in people revascularized after NSTEMI with versus without CKD.
Bubble plot demonstrating meta-regression of the log odds of the effect estimates for receipt of CABG against % of population identified as having CKD.
Funnel plot of the log odd of the effect estimates for CABG in people revascularized after ACS with versus without CKD.
Acknowledgments
We would like to thank Professor Julian Higgins, Professor of Evidence Synthesis and Senior Editor of the Cochrane Handbook for Systematic Reviews of Interventions, for his advice regarding the optimal method of combining multiple effect estimates within individual studies. Also, we would like to thank Sarah Herring, subject librarian for Biochemistry and Social and Community Medicine at the University of Bristol, for her assistance with our search for the medical literature. These data are to be presented in abstract form at the European Society of Cardiology, 2024.
Funding Statement
This research was supported by the National Institute for Health and Care Research (NIHR), UK. JS, Doctoral Research Fellow, NIHR 300906, is funded by the NIHR for this research project. The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS or the UK Department of Health and Social Care. LS is a NIHR Career Development Fellow (CDF-2018-11-ST2-009).
Conflict of Interest
The authors declare that there is no conflict of interest.
Informed Consent
Informed consent was not required for this review of published data.
Author Contributions
JS, PB, LS, FJC, TJ and YBS conceived and designed the study. JS, ML, WHA and HMC analyzed the data. All members of the cardiorenal working group provided additional data and/or additional analyses to support the study. JS drafted the manuscript. JS, PB, LS, FJC, TJ, RA, ML, WHA, HMC and YBS and all members of the cardiorenal group critically appraised the results and edited the manuscript. All authors approved the final version of the manuscript. The lead authors confirm that all authors meet ICJME authorship criteria, and no one who meets ICJME criteria has been excluded.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Author Note
Cardio-CKD working group: Juan Sanchis Fores (Department of Cardiology, University Clinic Hospital of Valencia, University of Valencia, CIBERCV, Spain), Albert Ariza-Sole (Department of Cardiology, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain), Brunilda Alushi (Heart Centre, Alter Hof, Munich, Germany), Fabian Artusa (Department of Hepatology and Gastroeneterology, Charite - Universitatsmedizin Berlin, Campus Virchow Klinikum (CVK) and Campus Charite Mitte (CCM), Berlin, Germany).
Abbreviations
- ACS
acute coronary syndrome
- AKI
acute kidney injury
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CI
confidence interval
- CKD
chronic kidney disease
- ICA
invasive coronary angiography
- LMICs
low- and middle-income countries
- NSTE-ACS
non-ST-elevation acute coronary syndrome
- NSTEMI
non-ST-elevation myocardial infarction
- OR
odds ratio
- PCI
percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction, UA, unstable angina
References
- 1.de Chickera SN, Bota SE, Kuwornu JP, Wijeysundera HC, Molnar AO, Lam NN, Silver SA. et al. Albuminuria, reduced kidney function, and the risk of ST - and non-ST-segment-elevation myocardial infarction. J Am Heart Assoc. 2018;7(20):e009995. doi: 10.1161/JAHA.118.009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Major RW, Cheng MRI, Grant RA, Shantikumar S, Xu G, Oozeerally I, Brunskill NJ. et al. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS One. 2018;13(3):e0192895. doi: 10.1371/journal.pone.0192895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M. et al. Cause of Death in Patients with Reduced Kidney Function. J Am Soc Nephrol. 2015;26(10):2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briasoulis A, Bakris GL. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013;15(3):340. doi: 10.1007/s11886-012-0340-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Arora S, Bhatia SM, Al-Hijji M, Reddy YNV, Patel P, Rihal CS. et al. Non-ST-segment-elevation myocardial infarction among patients with chronic kidney disease: a propensity score-matched comparison of percutaneous coronary intervention versus conservative management. J Am Heart Assoc. 2018;7(6) doi: 10.1161/JAHA.117.007920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw C, Nitsch D, Steenkamp R, Junghans C, Shah S, O'Donoghue D, Fogarty D. et al. Inpatient coronary angiography and revascularisation following non-ST-elevation acute coronary syndrome in patients with renal impairment: a cohort study using the Myocardial Ischaemia National Audit Project. PLoS One. 2014;9(6):e99925. doi: 10.1371/journal.pone.0099925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagai A, Lu D, Lucas J, Goyal A, Herzog CA, Wang TY, Goodman SG. et al. Temporal trends in utilization of cardiac therapies and outcomes for myocardial infarction by degree of chronic kidney disease: a report from the NCDR chest pain-MI registry. J Am Heart Assoc. 2018;7(24):e010394. doi: 10.1161/JAHA.118.010394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santopinto JJ, Fox KA, Goldberg RJ, Budaj A, Pinero G, Avezum A, Gulba D. et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE) Heart. 2003;89(9):1003–1008. doi: 10.1136/heart.89.9.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt JH, Rorth R, Kragholm K, Kristensen SL, Torp-Pedersen C, Gislason GH, Kober L. et al. Return to the workforce following coronary artery bypass grafting: A Danish nationwide cohort study. Int J Cardiol. 2018;251:15–21. doi: 10.1016/j.ijcard.2017.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Konstantinidis I, Nadkarni GN, Yacoub R, Saha A, Simoes P, Parikh CR, Coca SG. Representation of patients with kidney disease in trials of cardiovascular interventions: an updated systematic review. JAMA Intern Med. 2016;176(1):121–124. doi: 10.1001/jamainternmed.2015.6102. [DOI] [PubMed] [Google Scholar]
- 11.Sederholm Lawesson S, Alfredsson J, Szummer K, Fredrikson M, Swahn E. Prevalence and prognostic impact of chronic kidney disease in STEMI from a gender perspective: data from the SWEDEHEART register, a large Swedish prospective cohort. BMJ Open. 2015;5(6):e008188. doi: 10.1136/bmjopen-2015-008188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kinsara AJ, Alsaleh A, Taher ZA, Alshamiri M, Elshaer F Sr. The primary management strategies for ST-elevation myocardial infarction patients in Saudi Arabia: a sub-study of the Saudi acute myocardial infarction registry. Cureus. 2020;12(11):e11783. doi: 10.7759/cureus.11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DS, Lin YS, Lee JK, Kao HL. Sex differences following percutaneous coronary intervention or coronary artery bypass surgery for acute myocardial infarction. Biol Sex Differ. 2022;13(1):18. doi: 10.1186/s13293-022-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadlacki B, Horton D, Hossain S, Hariharaputhiran S, Ngo L, Ali A, Aliprandi-Costa B. et al. Long term survival after acute myocardial infarction in Australia and New Zealand, 2009-2015: a population cohort study. Med J Aust. 2021;214(11):519–525. doi: 10.5694/mja2.51085. [DOI] [PubMed] [Google Scholar]
- 15.Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ. et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. 2024;13(1):55–161. doi: 10.1093/ehjacc/zuad107. [DOI] [PubMed] [Google Scholar]
- 16.Shaw C, Nitsch D, Lee J, Fogarty D, Sharpe CC. Impact of an early invasive strategy versus conservative strategy for unstable angina and non-ST elevation acute coronary syndrome in patients with chronic kidney disease: a systematic review. PLoS One. 2016;11(5):e0153478. doi: 10.1371/journal.pone.0153478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallacher PJ, Miller-Hodges E, Shah ASV, Farrah TE, Halbesma N, Blackmur JP, Chapman AR. et al. High-sensitivity cardiac troponin and the diagnosis of myocardial infarction in patients with kidney impairment. Kidney Int. 2022;102(1):149–159. doi: 10.1016/j.kint.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Fong KY, Low CHX, Chan YH, Ho KW, Keh YS, Chin CT, Chin CY. et al. Role of invasive strategy for non-ST-elevation myocardial infarction in patients with chronic kidney disease: a systematic review and meta-analysis. Am J Cardiol. 2023;205:369–378. doi: 10.1016/j.amjcard.2023.07.178. [DOI] [PubMed] [Google Scholar]
- 19.Smilowitz NR, Gupta N, Guo Y, Mauricio R, Bangalore S. Management and outcomes of acute myocardial infarction in patients with chronic kidney disease. Int J Cardiol. 2017;227:1–7. doi: 10.1016/j.ijcard.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 20. Gameren Mv. Syntax Score. Available from: https://syntaxscore.org/
- 21.Holzmann M, Jernberg T, Szummer K, Sartipy U. Long-term cardiovascular outcomes in patients with chronic kidney disease undergoing coronary artery bypass graft surgery for acute coronary syndromes. J Am Heart Assoc. 2014;3(2):e000707. doi: 10.1161/JAHA.113.000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaipov A, Molnar MZ, Potukuchi PK, Sumida K, Szabo Z, Akbilgic O, Streja E. et al. Acute kidney injury following coronary revascularization procedures in patients with advanced CKD. Nephrol Dial Transplant. 2019;34(11):1894–1901. doi: 10.1093/ndt/gfy178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA, Lemeris C. et al. A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E) Environ Int. 2024;186:108602. doi: 10.1016/j.envint.2024.108602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE. et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25. Ryan R HS. How to GRADE the quality of the evidence. 2016. Version 3.0. Available from: http://cccrg.cochrane.org/author-resources.
- 26.Schunemann HB. Handbook for grading the quality of evidence of the strength of recommendations using the GRADE approach. 2013. Guyatt, Gordon; Oxman, Andrew. GRADE Handbook. [Google Scholar]
- 27.SW R. In: The handbook of research synthesis and meta-analysis. H Cooper LH, Valentine JC, editors. New York: Russell Sage Foundation; 2009. Analyzing effect sizes: random-effects models; pp. 295–315. [Google Scholar]
- 28.Blicher TM, Hommel K, Olesen JB, Torp-Pedersen C, Madsen M, Kamper AL. Less use of standard guideline-based treatment of myocardial infarction in patients with chronic kidney disease: a Danish nation-wide cohort study. Eur Heart J. 2013;34(37):2916–2923. doi: 10.1093/eurheartj/eht220. [DOI] [PubMed] [Google Scholar]
- 29.Alushi B, Jost-Brinkmann F, Kastrati A, Cassese S, Fusaro M, Stangl K, Landmesser U. et al. High-sensitivity cardiac troponin T in patients with severe chronic kidney disease and suspected acute coronary syndrome. J Clin Med. 2021;10(18) doi: 10.3390/jcm10184216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 31.Ogunbayo GO, Bidwell K, Misumida N, Ha LD, Abdel-Latif A, Elayi CS, Smyth S. et al. Sex differences in the contemporary management of HIV patients admitted for acute myocardial infarction. Clin Cardiol. 2018;41(4):488–493. doi: 10.1002/clc.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchis J, Garcia Acuna JM, Raposeiras S, Barrabes JA, Cordero A, Martinez-Selles M, Bardaji A. et al. Comorbidity burden and revascularization benefit in elderly patients with acute coronary syndrome. Rev Esp Cardiol (Engl Ed) 2021;74(9):765–772. doi: 10.1016/j.rec.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Kawsara A, Sulaiman S, Mohamed M, Paul TK, Kashani KB, Boobes K, Rihal CS. et al. Treatment effect of percutaneous coronary intervention in dialysis patients with ST-elevation myocardial infarction. Am J Kidney Dis. 2022;79(6):832–840. doi: 10.1053/j.ajkd.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Khan MZ, Syed M, Osman M, Faisaluddin M, Sulaiman S, Farjo PD, Khan MU. et al. Contemporary trends and outcomes in patients with ST-segment elevation myocardial infarction and end-stage renal disease on dialysis: insight from the national inpatient sample. Cardiovasc Revasc Med. 2020;21(12):1474–1481. doi: 10.1016/j.carrev.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotwal S, Ranasinghe I, Brieger D, Clayton PA, Cass A, Gallagher M. The influence of chronic kidney disease and age on revascularization rates and outcomes in acute myocardial infarction - a cohort study. Eur Heart J Acute Cardiovasc Care. 2017;6(4):291–298. doi: 10.1177/2048872616640995. [DOI] [PubMed] [Google Scholar]
- 36.Murray J, Balmuri A, Saurav A, Smer A, Alla VM. Impact of chronic kidney disease on utilization of coronary angiography and percutaneous coronary intervention, and their outcomes in patients with non-ST elevation myocardial infarction. Am J Cardiol. 2018;122(11):1830–1836. doi: 10.1016/j.amjcard.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Panchal HB, Zheng S, Devani K, White CJ, Leinaar EF, Mukherjee D, Mamas M. et al. Impact of chronic kidney disease on revascularization and outcomes in patients with ST-elevation myocardial infarction. Am J Cardiol. 2021;150:15–23. doi: 10.1016/j.amjcard.2021.03.057. [DOI] [PubMed] [Google Scholar]
- 38.Sakhuja A, Wright RS, Schold JD, McCarthy JT, Williams AW, Amer H, Albright RC. National impact of maintenance dialysis or renal transplantation on outcomes following ST elevation myocardial infarction. Am J Nephrol. 2016;44(5):329–338. doi: 10.1159/000450834. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Yan L, Ma GS, Zhang LP, Gao M, Wang YL, Wang SP. et al. Association of chronic kidney disease and coronary artery disease in 1,010 consecutive patients undergoing coronary angiography. J Nephrol. 2012;25(2):219–224. doi: 10.5301/JN.2011.8478. [DOI] [PubMed] [Google Scholar]
- 40.Engelbertz C, Reinecke H, Breithardt G, Schmieder RE, Fobker M, Fischer D, Schmitz B. et al. Two-year outcome and risk factors for mortality in patients with coronary artery disease and renal failure: The prospective, observational CAD-REF Registry. Int J Cardiol. 2017;243:65–72. doi: 10.1016/j.ijcard.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Hu L, Zheng W. Percutaneous coronary intervention versus coronary artery bypass graft in acute coronary syndrome patients with renal dysfunction. Sci Rep. 2018;8(1):2283. doi: 10.1038/s41598-018-20651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Formica F, Gallingani A, Tuttolomondo D, Hernandez-Vaquero D, Singh G, Pattuzzi C, Maestri F. et al. Long-term outcomes comparison between surgical and percutaneous coronary revascularization in patients with multivessel coronary disease or left main disease: a systematic review and study level meta-analysis of randomized trials. Curr Probl Cardiol. 2023;48(7):101699. doi: 10.1016/j.cpcardiol.2023.101699. [DOI] [PubMed] [Google Scholar]
- 43.Yong J, Tian J, Zhao X, Yang X, Xing H, He Y, Song X. Optimal treatment strategies for coronary artery disease in patients with advanced kidney disease: a meta-analysis. Ther Adv Chronic Dis. 2021;12:20406223211024367. doi: 10.1177/20406223211024367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts JK, Rao SV, Shaw LK, Gallup DS, Marroquin OC, Patel UD. Comparative efficacy of coronary revascularization procedures for multivessel coronary artery disease in patients with chronic kidney disease. Am J Cardiol. 2017;119(9):1344–1351. doi: 10.1016/j.amjcard.2017.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kilic A, Sultan I, Gleason TG, Wang Y, Smith C, Marroquin OC, Thoma F. et al. Surgical versus percutaneous multivessel coronary revascularization in patients with chronic kidney disease. Eur J Cardiothorac Surg. 2020;57(5):994–1000. doi: 10.1093/ejcts/ezz336. [DOI] [PubMed] [Google Scholar]
- 46.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA. et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14(14):1435–1534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]
- 47.Lemaire A, Soto C, Salgueiro L, Ikegami H, Russo MJ, Lee LY. The impact of age on outcomes of coronary artery bypass grafting. J Cardiothorac Surg. 2020;15(1):158. doi: 10.1186/s13019-020-01201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelsey JL. Methods in observational epidemiology. Monographs in Epidemiology. 1996 [Google Scholar]
- 49.Rucker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies by database.
Criteria for screening titles and abstracts.
Criteria for screening full texts.
Risk of bias assessments.
Grade ratings of certainty of outcome, by domain.
Study group consensus for assessing magnitude of effect estimate within grade rating of certainty.
Study group consensus and Cochrane guidance for assessing precision of effect estimate within grade rating of certainty.
Method for determining mean age of sample population.
Systematic review protocol.
Prisma 2020 checklist.
Definitions of CKD amongst included studies.
Forest plot depicting meta-analysis of studies reporting receipt of revascularization in people with CKD receiving or not receiving dialysis, versus those without CKD.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people with versus without CKD, excluding those with serious or critical risk of bias.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding those that defined CKD as receipt of dialysis.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding small studies.
Forest plot depicting meta-analysis of studies reporting receipt of CABG in people revascularized after ACS with versus without CKD, excluding those that defined the follow-up period as other than the duration of the index hospitalization.
Forest plot depicting a fixed-effects meta-analysis of the receipt of CABG in people revascularized after ACS with versus without CKD following ACS.
Forest plot depicting meta-analysis of the receipt of CABG in people revascularized after NSTEMI with versus without CKD.
Bubble plot demonstrating meta-regression of the log odds of the effect estimates for receipt of CABG against % of population identified as having CKD.
Funnel plot of the log odd of the effect estimates for CABG in people revascularized after ACS with versus without CKD.
Data Availability Statement
The authors declare that data supporting the findings of this study are available within the article.