Abstract
Tubulocystic Renal Cell Carcinoma (TC-RCC) and Polycythemia Vera (PV) are both infrequent medical conditions. TC-RCC was recognized as a distinct subtype of kidney cancer by the World Health Organization in 2016, while PV is a rare myeloproliferative neoplasm distinguished by the excessive production of red blood cells. The coexistence of these two conditions is exceptionally uncommon and lacks comprehensive documentation. This study presents a case report of a 35-year-old male patient who has been diagnosed with PV for the past 20 years. The patient underwent a radical nephrectomy to remove the renal tumor, and subsequent histopathological analysis confirmed the presence of TC-RCC. Throughout the 6-month follow-up period, the patient exhibited no signs of abnormalities. The rarity of the coexistence of TC-RCC and PV highlights the intricate nature of managing such instances, necessitating a cautious approach to diagnosis and treatment, particularly in surgical interventions. The present study serves as a valuable resource for diagnosing and treating individuals presenting with concurrent renal neoplasms and PV.
Keywords: case report, polycythemia vera, radical nephrectomy, tubulocystic renal cell carcinoma
Introduction
Tubulocystic renal cell carcinoma (TC-RCC), first described by Amin et al. in 2004, was recognized as a rare subtype of renal cancer by the World Health Organization (WHO) in 2016.1,2 TC-RCC is predominant in individuals with a mean age of 55.7 years, with a male-to-female ratio of 5:1.2–4 Most patients are asymptomatic upon admission, but occasional symptoms include gross hematuria, abdominal pain, or distension. It exhibits a favorable prognosis and has low metastasis potential.
Polycythemia Vera (PV) is a rare myeloproliferative neoplasm with an annual incidence rate of 0.4–2.8/100,000 individuals. 5 It is characterized by excessive red blood cell production, which often leads to increased white blood cells and platelets, thus causing increased blood viscosity and volume. Almost all patients with PV have Janus kinase 2 (JAK2; 9p24) mutations, with 96 and 3% of patients having a mutation in exon 14 (JAK2 V617F) and 12, respectively.6,7 PV can lead to peripheral neuropathy, manifesting symptoms such as headache, dizziness, and ataxia. Other complications, including myelofibrosis, severe anemia, hypoalbuminemia, deep vein thrombosis, and refractory infections, may occur in advanced stages. 8
This study presents a unique case involving both TC-RCC and PV, which, to our knowledge, has not been previously documented. With the emphasis on the rarity of these diseases, accurate diagnosis and effective treatment are necessary.
Case
A 35-year-old man with a 16-day history of recurrent gross hematuria and left flank pain was admitted to the West China Fourth Hospital (Figure 1). The patient did not experience any symptoms, such as nausea, vomiting, fever, or lower urinary tract symptoms. He had a previous diagnosis of PV 20 years ago, which was treated with oral hydroxyurea and transfer factor for 9 years. Physical examination revealed massive erythema and papules in the body and limbs. Hematological analysis revealed red blood cell count of 7.11 × 1012/L (normal range: 4.0–5.8 × 1012/L), hemoglobin (Hb) level of 197 g/L (normal range: 120–175 g/L), and hematocrit (Hct) of 61.6% (normal range: 40–50%), white blood cell and platelet count within the normal range, and increased activated partial thromboplastin time by 5 s, with other coagulation indices within normal limits.
Figure 1.
Case timeline: diagnosis and treatment overview.
Three urinary cytologic examinations detected no tumor cells. Computed tomographic urography (CTU) identified a parenchymal alteration adjacent to the renal pelvis (Figure 2). In light of the patient’s gross hematuria, a flexible ureterorenoscopy was performed to comprehensively examine the entirety of the left upper urinary tract. No exophytic lesions were observed except for extravasated blood from the renal pelvis mucosa and a few small clots within the renal pelvis.
Figure 2.
In CTU, both coronal (A) and transverse sections (B) show a solid mass located in the middle near the upper pole of the left kidney, next to the renal pelvis.
In compliance with tumor size and location, as well as patient preferences, a left radical laparoscopic nephrectomy was performed. Postoperatively, aspirin (100 mg/day) was administered for 1 week, which was discontinued after 1 week and replaced with low molecular weight heparin (5000 IU/daily subcutaneous injection) as a bridging agent to reduce the risk of thrombotic events during the perioperative period.
The dimensions of the surgical specimen and the length of the ureter segment were 13 × 7 × 5.7 cm and 5.5 cm, respectively. A nodular neoplasm of 5 × 4 × 3 cm size in the middle upper pole of the kidney was identified (Figure 3). The tumor cut surface exhibited a spongy texture, distinct boundary, and gray-white color.
Figure 3.
Macroscopic view shows the tumor located at mid-upper pole of kidney, adjacent to the renal collecting system.
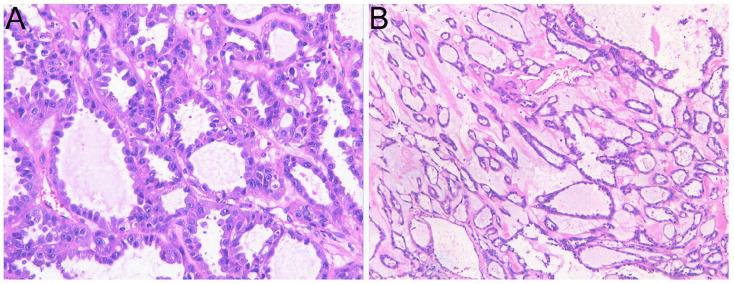
Microscopically, the tumor regions exhibited a comprehensive tubulocystic configuration devoid of any solid, papillary, or cribriform architectural features (Figure 4). The composition encompassed tubules of diverse dimensions, spanning from diminutive to moderate, alongside dilated tubules housing cysts. These tubules were surrounded by a hypocellular and fibrotic stroma. The cellular lining of the surface displayed a varying layer, ranging from flat to cuboidal to hobnailed. Predominantly, abundant pink cytoplasm and large nuclei with prominent nucleoli (indicating a nucleolar grade three by the WHO/ISUP classification) having no perinucleolar halos were observed in cells.
Figure 4.
The tubules and cysts are delineated by a uniform layer of epithelial cells, displaying a variety of morphologies including flat and hobnail to cuboidal and columnar. The majority of cells feature copious eosinophilic cytoplasm. (Haematoxylin and Eosin staining (HE), magnification ×400 (A), magnification ×100 (B).).
Tumor invasion was not observed in the ureter or renal sinus, and there was no evidence of vascular or perineural invasion, lymph node metastasis, sarcomatoid or rhabdoid features, or invasion in the capsule and perinephric fat.
Immunohistochemical staining confirmed the diagnosis and revealed tumor cell positive results for CA-IX (focal+), PAX-8 (+), vimentin (+), P504S (+), AMACR (+), SDHB (+), FH (+), Ki-67 (+, approximately 25%), and CK (AE1/AE3) (focal+), while negative results for CD10 (−), CK (−), CK20 (−), WT1 (−), HMB45 (−), CD57 (−), TFE3 (−), and HCK (−).
The analysis of morphological characteristics and immunohistochemistry confirmed the diagnosis of TC-RCC, with tumor staging classified as pT1bN0M0. Following a successful recovery, the patient was discharged with a recommendation for ongoing monitoring. A subsequent 6-month follow-up revealed no signs of tumor or hematuria recurrence, with normal levels of Hb and Hct.
Discussion
TC-RCC remains a rare disease, with less than 100 cases reported worldwide.3,4 Due to its rarity, there is a lack of research examining the demographic features or risk factors associated with TC-RCC. Imaging examinations, such as CT or magnetic resonance imaging, provide limited diagnostic insights into TC-RCC 9 due to its resemblance with adult cystic nephroma and mixed epithelial and stromal tumors, both exhibiting multilocular cystic manifestations. 10 TC-RCC cases have been reported to impact either the renal cortex or the corticomedullary junction, potentially resulting in gross hematuria in patients. 11 Additionally, the proximity of the neoplasm to the renal pelvis indicates that urothelial malignancy should be considered as a potential differential diagnosis before obtaining pathology results. To determine the surgical extent and obtain a biopsy for definitive diagnosis, invasive diagnostic methods, including the use of a flexible ureteroscope, are considered appropriate.
The clinical symptoms of PV often include fatigue, headaches, pruritus, and nocturnal diaphoresis, with some patients also exhibiting hematuria. 12 PV therapy is aimed at reducing the incidence of thrombotic events, which can be achieved through therapeutic phlebotomy, low-dose aspirin therapy, and various cytoreductive therapies. 8 When the patient cannot tolerate or respond adequately to phlebotomy, which is the primary treatment for managing PV, hydroxyurea can be considered a first-line treatment option. In our case, the patient did not respond adequately to phlebotomy; therefore, long-term oral hydroxyurea chemotherapy was administered to achieve cytoreduction and symptom control. However, patients with PV have a higher risk of developing secondary malignancies, and they often exhibit elevated levels of pro-inflammatory cytokines, including interleukin-6, tumor necrosis factor-α, and C-reactive protein, which play a significant role in tumor development and progression. Rampant inflammation may also lead to poor treatment outcomes, potentially facilitating the progression and poor prognosis of renal carcinoma. 13 The prognosis is even worsen for patients with end-stage PV with concurrent renal malignancies. Several studies on patients receiving chemotherapy, radiotherapy, or targeted therapy have indicated that albumin can serve as a prognostic biomarker in cancer patients, with lower albumin levels associated with a higher risk of mortality or disease progression. 14 Moreover, patients with end-stage PV may present with cachexia and low albumin levels, along with an increased burden on the liver due to myeloproliferation and hepatotoxic effects of hydroxyurea and interferon. 8 It results in reduced albumin synthesis capacity and decreased plasma albumin levels, leading to a poorer prognosis. Beyond treatment, quality of life is an important focus for such patients. 15
TC-RCCs are characterized by an average size of 4 cm and classified as indolent or having low metastatic potential, indicating that surgical interventions could be beneficial for most patients with TC-RCC.2–4 Given the T1b tumor stage of our patient, radical nephrectomy is the preferred treatment option, although nephron-sparing surgery remains a viable alternative for T1b-stage tumors. 16 The immunohistochemical findings following surgery demonstrate a positive expression of FH markers, which suggests a low risk of progression. 17 However, cases involving large masses, multiple lesions, or metastases have also been reported.2,4,11 The current knowledge and treatment strategies are inadequate in effectively managing the TC-RCC recurrence and metastatic potential. We conducted a comprehensive literature review and found that only a few studies have documented the use of adjuvant treatment, which are summarized in Table 1.
Table 1.
Summary of studies on adjuvant treatment for TC-RCC cases.
| Author(s) | Number of cases (n) | Gender (M/F) | Mean age | Size of tumor (mean and range) (cm) | Stage | Adjuvant treatment | Metastasis |
|---|---|---|---|---|---|---|---|
| Amin et al. 2 | 31 | 27/4 | 54 | 4.2 (0.7–17) | pT1 (24 patients); pT2 (4 patients); pT3 (3 patients) | Adjuvant chemotherapy* (3 patients) | Local (1 patient), liver and bone (2 patient) |
| Bhullar et al. 18 | 1 | 1/0 | 33 | 10.1 × 8.0 | NA | Sorafenib | Bone and peritoneum |
| Hora et al. 19 | 1 | 5/0 | 56 | 5.8 (5.1–6.5) | pT1 (4 patients); pT3 (1 patient) | Gemcitabine, cisplatin, sunitinib (1 patient); temsirolimus, sunitinib (1 patient) | Pleura, retroperitoneum and mediastinal lymph nodes (1 patient); sternum, ribs and mediastinal lymph nodes (1 patient) |
| Sangle et al. 20 | 1 | 1/0 | 45 | 4.5 | pT3 | Adjuvant chemotherapy* | Brain, paraspinal tissue, peritoneal sidewall and adrenal gland |
| Banerjee et al. 21 | 1 | 0/1 | 45 | 14.6 × 10.8 × 14.6 | NA | Sunitinib, Everolimus | Peritoneum and omentum |
| Urabe et al. 22 | 1 | 1/0 | 51 | 9.2 × 6 × 5.4 | pT2a | Sunitinib, Axitinib, Temsirolimus | Lung and bone |
| Salvatori et al. 23 | 1 | 1/0 | 70 | NA | pT3 | Pazopanib, Nivolumab | Left hypochondrium, liver and bone |
NA: not available; M: male; F: Female.
Drug specifics not detailed in the article.
The combination of these two rare diseases has prompted some reflections. For PV patients with advanced-stage renal carcinoma, subsequent consideration of chemotherapy, immunotherapy, and targeted therapy is warranted. These treatments can have certain side effects, including peripheral neuropathy. Additionally, using PV treatments, such as hydroxyurea, can significantly increase the risk of developing this condition, potentially leading to difficult-to-manage symptoms such as headaches and neuropathic pruritus. 24 In such cases, simultaneous adjustment of treatment plans for both diseases may be necessary, highlighting the importance of collaboration among oncologists, urologists, and hematologists.
Currently, there are few cases of TC-RCC and a lack of international collaboration, resulting in a paucity of research on molecular mechanisms. More therapeutic targets need exploration, with future research focusing on developing adjuvant therapeutic drugs with fewer side effects.24,25 Additionally, the direct link between JAK2 mutations and renal cancer remains unrecognized, with most studies focusing on the role of JAK2 mutations in hematologic malignancies. A few studies suggest that inhibiting the JAK2/STAT3 pathway may suppress the proliferation, invasion, and migration of renal cancer cells, while also promoting apoptosis within these cells.26,27 Exploring the potential efficacy of JAK2 inhibitors in renal cancer treatment could provide a dual-effective regimen for patients with PV and renal cancer, thereby offering greater benefits.
Conclusion
The rare coexistence of TC-RCC and PV highlights the complex nature of managing such conditions, necessitating a cautious approach to diagnosis and treatment, particularly in surgical interventions. Our case study provides a reference for physicians for managing simultaneous renal neoplasms and PV. However, additional research is necessary to explore the interaction and mechanisms underlying the coexistence of PV and renal cancer, aiming to develop optimal treatment strategies.
Acknowledgments
We thank West China School of Public Health and West China Fourth Hospital, Sichuan University for supporting our work
Footnotes
Abbreviations: TC-RCC, Tubulocystic Renal Cell Carcinoma;
PV, Polycythemia Vera;
WHO, The World Health Organization;
CTU, Computed Tomographic Urography;
Hb, Hemoglobin;
Hct, Haematocrit.
Author contributions: Renjie Wei and Ruitu Ran: Protocol/Project development, data analysis, drafting the manuscript, Figures design.
Fudong Liu and Xu Luo: Protocol/Project development, data collection.
Chunyu Gong: manuscript editing, data analysis.
All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Ruitu Ran  https://orcid.org/0000-0001-7804-9451
https://orcid.org/0000-0001-7804-9451
References
- 1. Moch H, Cubilla AL, Humphrey PA, et al. (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. European Urology 70(1): 93–105. [DOI] [PubMed] [Google Scholar]
- 2. Amin MB, MacLennan GT, Gupta R, et al. (2009) Tubulocystic carcinoma of the kidney: Clinicopathologic analysis of 31 cases of a distinctive rare subtype of renal cell carcinoma. The American Journal of Surgical Pathology 33(3): 384–392. [DOI] [PubMed] [Google Scholar]
- 3. Laddha A, Thomas A, Bindhu MR, et al. (2020) Tubulocystic renal cell carcinoma in young adult. Indian Journal of Surgical Oncology 11(Suppl 2): 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xing S, Liu A, Yang X, et al. (2021) Tubulocystic renal cell carcinoma: Two-case report and literature review. International Journal of Immunopathology and Pharmacology 35: 20587384211002966. DOI: 10.1177/20587384211002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moulard O, Mehta J, Fryzek J, et al. (2014) Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. European Journal of Haematology 92(4): 289–297. [DOI] [PubMed] [Google Scholar]
- 6. Pardanani A, Lasho TL, Finke C, et al. (2007) Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 21(9): 1960–1963. [DOI] [PubMed] [Google Scholar]
- 7. Vannucchi AM, Antonioli E, Guglielmelli P, et al. (2008) Clinical correlates of JAK2V617F presence or allele burden in myeloproliferative neoplasms: A critical reappraisal. Leukemia 22(7): 1299–1307. [DOI] [PubMed] [Google Scholar]
- 8. Tefferi A, Barbui T. (2019) Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. American Journal of Hematology 94(1): 133–143. [DOI] [PubMed] [Google Scholar]
- 9. Cornelis F, Hélénon O, Correas JM, et al. (2016) Tubulocystic renal cell carcinoma: A new radiological entity. European Radiology 26(4): 1108–1115. [DOI] [PubMed] [Google Scholar]
- 10. Honda Y, Nakamura Y, Goto K, et al. (2018) Tubulocystic renal cell carcinoma: A review of literature focused on radiological findings for differential diagnosis. Abdominal Radiology 43(7): 1540–1545. [DOI] [PubMed] [Google Scholar]
- 11. Bhullar JS, Varshney N, Bhullar AK, et al. (2014) A new type of renal cancer–tubulocystic carcinoma of the kidney: A review of the literature. International Journal of Surgical Pathology 22(4): 297–302. [DOI] [PubMed] [Google Scholar]
- 12. Oliveira SCS, Santos LTR, Esmeraldo MA, et al. (2020) Macroscopic hematuria as the initial presentation of polycythemia vera. Cureus 12(10): e10800. DOI: 10.7759/cureus.10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahin TK, Rizzo A, Aksoy S, et al. (2024) Prognostic Significance of the Royal Marsden Hospital (RMH) score in patients with cancer: A systematic review and meta-analysis. Cancers 16(10): 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guven DC, Sahin TK, Erul E, et al. (2022) The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Frontiers in Molecular Biosciences 9: 1039121. DOI: 10.3389/fmolb.2022.1039121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rizzo A, Mollica V, Dall’Olio FG, et al. (2021) Quality of life assessment in renal cell carcinoma phase II and III clinical trials published between 2010 and 2020: A systematic review. Future Oncology 17(20): 2671–2681. [DOI] [PubMed] [Google Scholar]
- 16. Campbell SC, Clark PE, Chang SS, et al. (2021) Renal mass and localized renal cancer: Evaluation, management, and follow-up: AUA guideline: Part I. The Journal of Urology 206(2): 199–208. [DOI] [PubMed] [Google Scholar]
- 17. Choi TS, Lee DG, Won KY, et al. (2021) Tubulocystic renal cell carcinoma is not an indolent tumor: A case report of recurrences in the retroperitoneum and contralateral kidney. Medicina 57(8): 851. DOI: 10.3390/medicina57080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhullar JS, Thamboo T, Esuvaranathan K. (2011) Unique case of tubulocystic carcinoma of the kidney with sarcomatoid features: A new entity. Urology 78(5): 1071–1072. [DOI] [PubMed] [Google Scholar]
- 19. Hora M, Urge T, Eret V, et al. (2011) Tubulocystic renal carcinoma: A clinical perspective. World Journal of Urology 29(3): 349–354. [DOI] [PubMed] [Google Scholar]
- 20. Sangle NA, Mao R, Shetty S, et al. (2013) Novel molecular aberrations and pathologic findings in a tubulocystic variant of renal cell carcinoma. Indian Journal of Pathology & Microbiology 56(4): 428–433. [DOI] [PubMed] [Google Scholar]
- 21. Banerjee I, Yadav SS, Tomar V, et al. (2016) Tubulocystic renal cell carcinoma: A great imitator. Reviews in Urology 18(2): 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Urabe F, Miki J, Yanagisawa T, et al. (2016) A case of metastatic tubulocystic carcinoma of the kidney treated with molecularly targeted therapy. Hinyokika Kiyo. Acta Urologica Japonica 62(11): 569–574. [DOI] [PubMed] [Google Scholar]
- 23. Salvatori F, Macchini M, Misericordia M, et al. (2020) A simple cyst is not always simply a cyst: A case of cystic recurrence after nephrectomy for tubulocystic renal cell carcinoma and literature review. Urologia 87(3): 119–124. [DOI] [PubMed] [Google Scholar]
- 24. Rizzo A, Santoni M, Mollica V, et al. (2021) Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: The MOUSEION-02 study. Expert Opinion on Drug Metabolism & Toxicology 17(12): 1455–1466. [DOI] [PubMed] [Google Scholar]
- 25. Dall’Olio FG, Rizzo A, Mollica V, et al. (2021) Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: A meta-analysis. Immunotherapy 13(3): 257–270. [DOI] [PubMed] [Google Scholar]
- 26. Chae IG, Song NY, Kim DH, et al. (2020) Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food and Chemical Toxicology 139: 111253. DOI: 10.1016/j.fct.2020.111253. [DOI] [PubMed] [Google Scholar]
- 27. Xu Z, Wu D, Fu D, et al. (2020) Nobiletin inhibits viability of human renal carcinoma cells via the JAK2/STAT3 and PI3K/Akt pathway. Cellular and Molecular Biology 66(5): 199–203. [PubMed] [Google Scholar]