Abstract
Background and Aims:
Endoscopic ultrasound–guided pancreatic cyst chemoablation is safe and effective for appropriately selected patients; however, the proper frequency of radiographic surveillance after successful chemoablation is unknown. Here we report the long-term follow-up of 2 randomized prospective Chemotherapy for Ablation and Resolution of Mucinous Pancreatic Cysts (ChARM) clinical trials. In addition, the performance of a postablation-reduced radiographic surveillance protocol was evaluated according to clinical and economic outcomes and patient experience metrics.
Methods:
Patients who successfully completed 1 of the 2 ChARM randomized control trials were evaluated for durability of response and clinical outcomes. Patients were eligible if 2 years or more of follow-up were available and complete. We calculated economic outcomes according to Medicare allowable costs applicable to endoscopic ultrasound, magnetic resonance imaging, and outpatient clinic visits. We modeled costs of a patient followed by the ChARM Post-treatment Reduced Radiographic Surveillance Protocol compared with a similar patient followed under Fukuoka or American College of Gastroenterology (ACG) guidelines over 5 years. In addition, patients under long-term surveillance in our clinic were interviewed via a 4-question Likert-type questionnaire.
Results:
A total of 52 patients were eligible and included in the study. At the most recent follow-up of the 52 patients, 36 (69.2%) achieved complete response, an additional 11 (21.2%) showed partial response, and only 5 (9.6%) showed nonresponse. All patients were successfully reduced to annual or less surveillance without recurrence or the development of cyst-associated malignancy. Compared with Fukukoa or ACG guidelines, a patient treated and followed under the ChARM Post-treatment Reduced Radiographic Surveillance Protocol incurred a Medicare allowable cost of $7200.00 versus $19,437.44 and $12,526.52 if untreated and observed under Fukukoa and ACG guidelines, respectively. The patient experience questionnaire was returned completed by 49 participants.
Conclusions:
The ChARM Post-treatment Reduced Radiographic Surveillance Protocol safely allows a reduction in radiographic surveillance. A reduction in cost associated with cyst management under the ChARM protocol, compared with management following Fukukoa or ACG guidelines, was shown. According to the questionnaire, most patients reported a moderate level of logistical and emotional burden associated with magnetic resonance imaging surveillance, and a majority were in favor of reducing the frequency of radiographic surveillance if it could be done without a marked increase in oncologic risk.
As the fourth leading cause of cancer-related mortality, pancreatic cancer is a lethal malignancy with a dismal 5-year survival rate of 8%, the lowest of any major cancer.1,2 Although the majority of pancreatic cancers are ductal adenocarcinomas, approximately 15% of pancreatic cancer develops from mucinous-type pancreatic cysts, and this percentage may be underreported.1,3 Pancreatic cysts are common, with a prevalence of 2% in American adults and 37% in those older than 80 years of age.4 Although certain types of pancreatic cysts carry little to no malignant potential, the majority are neoplastic and include mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN), which carry a significant potential for malignant transformation over a period of time. The majority of these cysts are low risk; however, the natural history of mucinous pancreatic cysts is variable, with the overall risk of progression to pancreatic cancer generally linked to the number of high risk features.5,6 Identification of a mucinous pancreatic cyst requires the clinician and patient to choose between indefinite radiographic surveillance (magnetic resonance imaging [MRI] or CT) or therapy by surgical resection, both of which can have considerable limitations.7 Surveillance for malignancy carries significant economic and likely psychologic burdens, and surgical resection possesses a substantial risk for serious adverse events (20%-40%) and mortality (1%-5%) and still requires postoperative surveillance.6,8,9
In this respect, endoscopic ultrasound (EUS)–guided pancreatic cyst ablation has emerged as an innovative and promising minimally invasive approach for the treatment of appropriately selected pancreatic cysts (Fig. 1).10-13 Early trials involved the lavage of a target mucinous cyst with dehydrated alcohol, which had relatively low efficacy and a significant risk of serious adverse events. The subsequent innovation of alcohol lavage followed by the infusion of paclitaxel resulted in increased efficacy rates of 50% to 79%, but was still limited by serious adverse event rates of 3% to 10%, primarily pancreatitis, peritonitis, and venous thrombosis thought to be secondary to the potent inflammatory and toxic effects of alcohol.11 In 2017, the randomized, prospective, double-blind ChARM trial (Chemotherapy for Ablation and Resolution of Mucinous Pancreatic Cysts; NCT01475331) was published, in which 46 patients were randomized to either alcohol lavage followed by infusion of 38 mg/mL gemcitabine + 6 mg/mL paclitaxel versus alcohol-free saline lavage followed by the same infusion.14 At 1 year after treatment, 61% of the patients in the alcohol arm and 67% in the alcohol-free arm achieved complete ablation, demonstrating that alcohol is not required for effective pancreatic cyst ablation when a chemotherapy cocktail specifically designed for pancreatic neoplasia is used. More importantly, the rate of adverse events was significantly lower in the alcohol-free arm (P = .01), with all minor (22%) and serious (6%) adverse events occurring in the alcohol arm. This demonstrated that removal of alcohol from the pancreatic cyst ablation process significantly reduces adverse event rates, improving the risk profile of the procedure while preserving its efficacy and offering a new therapeutic option for patients with appropriately selected, mucinous-type, pancreatic cysts. This trial led to the larger National Institutes of Health–funded randomized, prospective, multicenter ChARM II trial (NCT03085004), which is currently underway.
Figure 1.
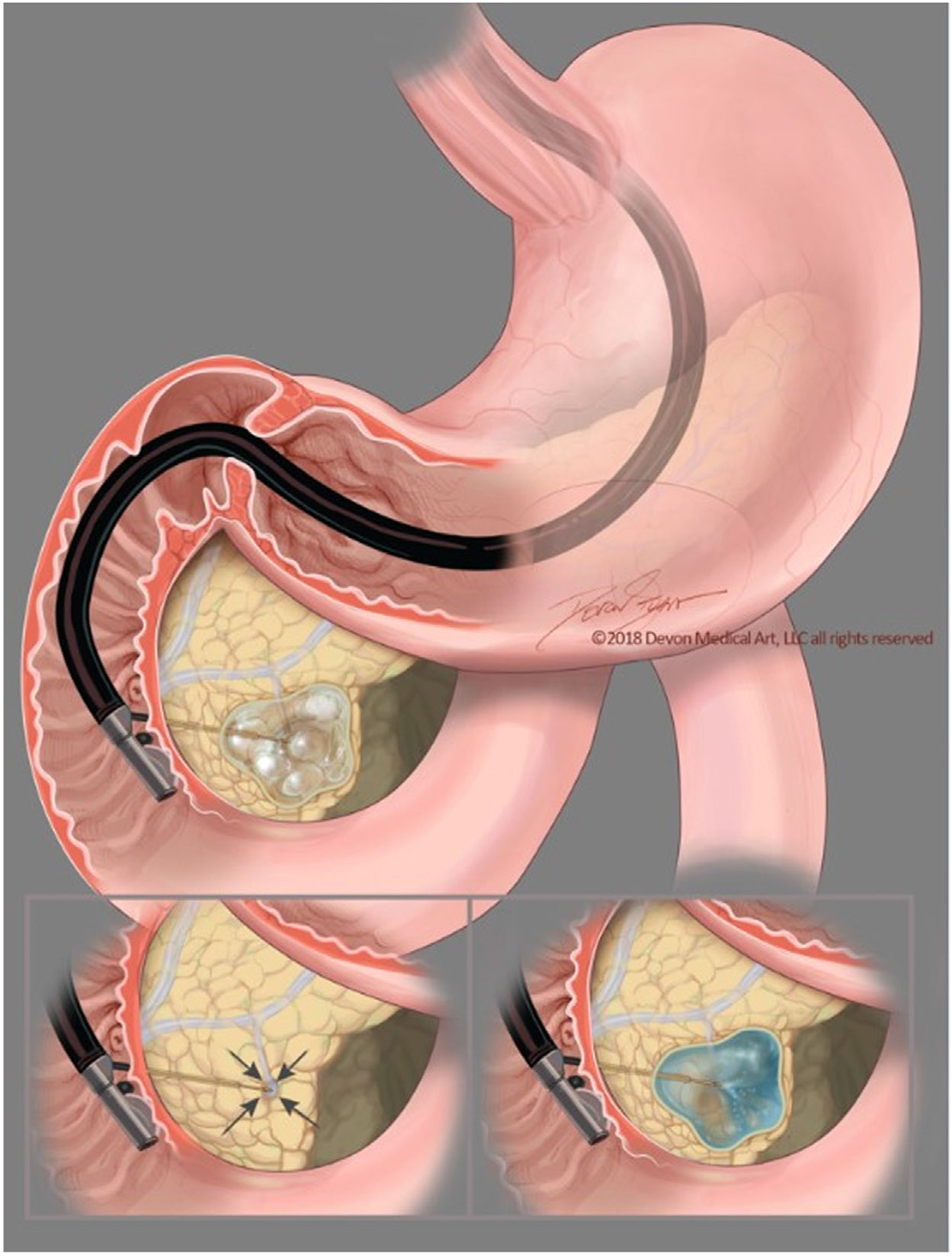
A step-wise representation of the alcohol-free, endoscopic ultrasound–guided, chemoablation process showing the fine-needle injection needle carefully introduced into the direct center of the cystic tumor. After near complete aspiration of the mucinous cystic fluid from all compartments, the chemoablation admixture is then infused, refilling the cyst using an volume of chemotherapy equal to the cyst fluid originally. This reconstitutes the cyst to its original size and dimensions.
An important metric of alcohol-free pancreatic cyst chemoablation is the treatment durability over time, and 2 trials have shown the long-term durability of this approach. In 2017, Choi et al15 reported the prospective long-term follow-up of 164 patients treated with EUS-guided ablation with alcohol lavage followed by paclitaxel infusion. In that study, when patients achieved complete EUS-guided pancreatic cyst ablation, 98% remained in remission at 6 years, demonstrating a durable response after ablative therapy. In 2021, the long-term follow-up of the randomized ChARM trial showed that 87% of patients who achieved complete ablation maintained that ablation through 3 to 5 years of follow-up. In addition, 31% of patients who did not achieve complete response at 1 year reached complete ablation at long-term follow-up, compatible with a delayed treatment response.16 In neither of those trials did any treated pancreatic cysts develop high-grade pathology or malignant transformation. Overall, these prospective trials demonstrate the increasing appeal of alcohol-free EUS-guided ablation as a safe, low-cost, minimally invasive approach that provides long-term control in appropriately selected pancreatic mucinous cysts. Although there are currently no long-term prospective data to make the claim that EUS-guided chemoablation prevents pancreatic adenocarcinoma, these results demonstrate that chemoablation effectively treats individual cystic tumors and can prevent the need for major pancreatic surgery. Patient selection, indications, contraindications, procedure technique, quality assurance, and recommendations for follow-up for this procedure are reviewed elsewhere.11-13
In the literature, there are currently no prospective studies to guide pancreatic cyst surveillance or to determine whether surveillance alters long-term outcomes or mortality. However, multiple guidelines strongly suggest surveillance for mucinous cysts, owing to strong direct evidence that IPMNs and MCNs progress to pancreatic cancer over a number of years. This presents the opportunity to identify high-grade dysplasia or overt pancreatic cancer early, hopefully improving survival rates through early detection and intervention.5-7 EUS-guided alcohol-free pancreatic cyst ablation offers an attractive, minimally invasive treatment option in appropriately selected mucinous cysts. This approach could add significant additional clinical value if reduced radiographic surveillance can also be offered in the postablation period. In the present study, we report the long-term follow-up from 2 randomized prospective clinical trials of EUS-guided chemoablation that use a reduced radiographic surveillance intensity after successful chemoablation. Compared with standard surveillance protocols for similar cysts, the potential economic and logistical savings could be significant. In addition, the patient perspective on the surveillance process for cystic pancreatic lesions is unclear. There is a paucity of data regarding the psychologic effects of MRI and EUS surveillance in this population and what, if any, relief could be offered via reduced surveillance. The investigators looked to evaluate this aspect with a patient questionnaire addressing their perspectives on surveillance and how attractive a reduction in surveillance would be if this could be offered. Reduced financial burden, patient stress, ionizing radiation, and contrast exposure in the postablation patient population would be of benefit if a reduction in imaging surveillance could be accomplished without increased oncologic risk.
METHODS
Selection criteria
The study protocol was approved by the Penn State University Institutional Review Board on January 23, 2023 (STUDY00021664). Just as in the first long-term follow-up of the ChARM trial,16 36 of the 39 treated patients who successfully completed the trial have continued to be observed. In addition, 16 patients in the currently ongoing randomized prospective multicenter ChARM II trial who have completed at least 2 years of follow-up were included. In total, 52 patients were included in this long-term follow-up, and the most recent images and outcome data were used for this report.
Clinical long-term post-treatment surveillance
The complete study design and eligibility criteria of the ChARM trial have been reported elsewhere.14 This is our first opportunity to describe the multicenter ChARM II trial. Briefly, expanding on ChARM, ChARM II randomizes patients with eligible mucinous cysts to either ethanol or saline lavage followed by EUS-guided chemoablation using a chemotherapeutic mixture of paclitaxel + gemcitabine with clinical follow-up and MRI imaging 1 year and 2 years after treatment. A fellowship-trained co-investigator radiologist with 15 years of experience read all baseline and follow-up images for each of the included patients, and cysts were measured in at least 2 dimensions with cyst volume calculated as , where is the mean cyst radius. Treatment response was defined according to percentage reduction in cyst volume from baseline as follows: complete response, ≥95% reduction in cyst volume; partial response, 94% to 75% reduction; and nonresponse, <75% reduction which is in accordance with previous trials and an international position paper on the subject.13 Patients were seen in a specialized pancreatic cyst clinic with an MRCP at 1-year intervals after treatment, and patients with complete response and at least 2 annual surveillance visits were offered transition to 2-year intervals if otherwise clinically appropriate.
Economic comparison
Three possible cyst management strategies were compared for overall costs over 5 years for a hypothetical 65-year-old woman with American Society of Anesthesiologists class III comorbidities, a good 5 to 10 years of life expectancy, with a 3.2 cm unilocular IPMN that has grown 3 mm over the past calendar year. As a control strategy, a 2.5-cm unilocular IPMN with the same growth and duration of surveillance was also tabulated. All costs were calculated using 2023 Medicare allowable hospital and professional charges for EUS-guided fine-needle injection (FNI) as well as MRCP and associated ambulatory clinic visit. Of note, the EUS-FNI costs include a professional and hospital anesthesia charge component.
First arm.
As recommended on page 4 of the Fukuoka guidelines,5 MRI was alternated with EUS surveillance every 3 to 6 months. For this, associated Medicare actuary costs17,18 were calculated as MRI alternating with EUS every 4.5 months over the entire 5 years.
Second arm.
American College of Gastroenterology (ACG) surveillance was taken from page 469 of the ACG guidelines.6 As recommended by these guidelines, MRI was alternated with EUS every 6 months for 3 years, then MRI alternating with EUS annually for the remainder of the 5-year study period.
Third arm.
Per our standard ChARM treatment algorithm, appropriate patients undergo EUS-guided pancreatic cyst chemoablation under monitored anesthesia care at time point 0 (ChARM II patients had a second EUS-FNI at 3 months). Patients then undergo MRCP at 1 and 2 years after the first ablation and then annually for 3 to 5 years, and can go to every 2 years if there is a complete response and it is stable for at least 2 years.
Control arm.
As recommended on page 4 of the Fukuoka guidelines,5 EUS was used in 3 to 6 months, then the interval lengthened to “up to 1 year,” alternating MRI with EUS as appropriate. In this case, an interval of 9 months was assumed.
The patient experience
To evaluate the patient experience and satisfaction with long-term radiographic surveillance, we constructed a 4-question Likert-type scale (1 strongly disagree to 5 strongly agree) questionnaire. Patients were queried on 4 topics: the emotional aspects of long-term pancreatic cyst surveillance, any dislike of the surveillance MRI itself, any financial burden of long-term pancreatic cyst surveillance, and the patient’s interest in reduction in post-chemoablation surveillance if that was possible. The questionnaire was sent to patients who were currently enrolled in an active long-term pancreatic cyst surveillance program at the Penn State Pancreatic Cyst Clinic and who had undergone at least 2 years of surveillance.
RESULTS
Among the 52 patients included in this long-term follow-up, baseline mean mucinous cyst diameter was 25.6 mm and most cysts were clinically diagnosed as IPMN (69.2%) or MCN (17.3%). Demographics and characteristics of the patients evaluated are presented in Table 1.
TABLE 1.
Baseline demographics and cyst characteristics (n = 52)
| Total | Entire cohort | ChARM patients | ChARM II patients |
|---|---|---|---|
| Female | 32 (61.5) | 22 | 10 |
| Mean age, y | 70.0 ± 10.8 (44.0-84.0) | 70.0 | 70.0 |
| Mean maximal cyst diameter, mm | 28.7 ± 7.7 (15.5-49.0) | 27.6 | 31.2 |
| Mean cyst volume, mL | 11.0 ± 9.3 (1.9-38.8) | 10.5 | 12.2 |
| Cyst location | |||
| Head | 21 (40.4) | 17 | 4 |
| Body | 22 (42.3) | 16 | 6 |
| Tail | 5 (9.6) | 1 | 4 |
| Neck | 4 (7.7) | 2 | 2 |
| Presumed diagnosis | |||
| MCN | 9 (17.3) | 8 | 1 |
| IPMN | 36 (69.2) | 25 | 11 |
| Indeterminate | 7 (13.5) | 3 | 4 |
| Multilocular | 13 (25.0) | 9 | 4 |
Values are n (%) or mean ± SD (range).
IPMN, Intraductal papillary mucinous neoplasms; MCN, mucinous cystic neoplasms.
Long-term follow-up and efficacy
Of the 52 patients included in this study, the 36 ChARM patients had been followed a mean of 5.25 years (range, 2-9 years), and the 16 included ChARM II patients had completed their 2-year follow-up protocol period. At 1 year, 26 (50.0%) of all 52 patients achieved complete ablation and 29 of 47 patients (61.7%) were in complete response at 2-year follow-up. At the most recent follow-up of the 52 patients, 36 (69.2%) achieved complete response, 11 (21.2%) showed partial response, and only 5 (9.6%) showed nonresponse. By presumed diagnosis and at most recent follow-up, 25 of the 36 IPMNs achieved complete response, 6 showed partial response, and 5 showed nonresponse. Of the 9 MCNs, 6 achieved complete response and 3 showed partial response. All treatment responses from baseline to the most recent surveillance are presented in Figure 2.
Figure 2.
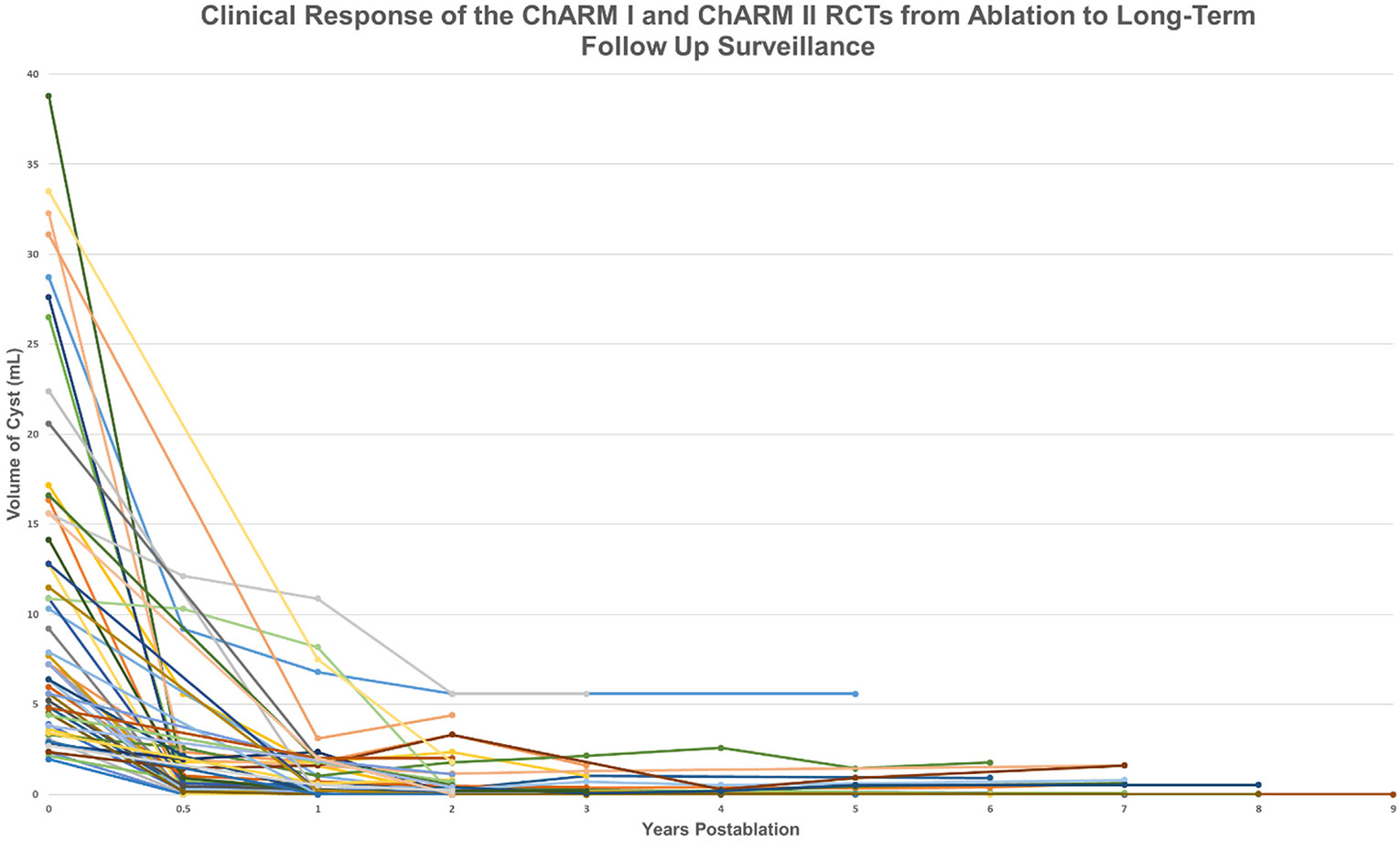
Long-term follow-up of the randomized prospective ChARM and ChARM II trials showing the durability of endoscopic ultrasound (EUS)–guided chemoablation. Shown are the volume calculations from baseline to long-term follow-up after EUS-guided chemoablation treatment using a mixture of gemcitabine + paclitaxel.
When separated into the specific trials, 28 (77.8%) of the 36 ChARM patients achieved complete response at any follow-up time point, 5 (13.9%) achieved partial response, and 3 (8.3%) showed nonresponse. Several initially persistent cysts proceeded toward resolution throughout the follow-up period: 11 cysts (30.6%) initially classified as partial response or nonresponse achieved complete response at their latest follow-up, and 2 additional cysts proceeded from nonresponse to partial response. One cyst regressed from complete response to partial response.
From the ChARM II cohort of 16 patients, at 1 year 12 (75.0%) showed either complete or partial response, which became 15 (93.8%) at 2 years, with 0 responders regressing to nonresponse and 3 of the nonresponses at 1 year proceeding toward partial response. The full distribution of treatment response from baseline to longest and most recent follow-up is presented in Table 2.
TABLE 2.
Treatment response at serial years post ablation and most recent follow-up imaging
| Years after ablation | Follow-up, n | Treatment response | ||
|---|---|---|---|---|
| Complete response | Partial response | Nonresponse | ||
| 0.5 | 35 | 17 (48.6) | 7 (20.0) | 11 (31.4) |
| 1 | 52 | 27 (51.9) | 15 (28.9) | 10 (19.2) |
| 2 | 47 | 29 (61.7) | 12 (25.5) | 6 (12.8) |
| 3 | 27 | 19 (70.4) | 5 (18.5) | 3 (11.1) |
| 4 | 15 | 11 (73.3) | 3 (20.0) | 1 (6.7) |
| 5 | 11 | 7 (63.6) | 2 (18.2) | 2 (18.2) |
| 6 | 7 | 5 (71.4) | 1 (14.3) | 1 (14.3) |
| 7 | 9 | 6 (66.7) | 2 (22.2) | 1 (11.1) |
| 8 | 4 | 3 (75.0) | 1 (25.0) | 0 (0.0) |
| 9 | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) |
| Most recent | 52 | 36 (69.2) | 11 (21.2) | 5 (9.6) |
Values are n (%).
No treated mucinous cyst developed high-grade pathology or required surgery, and no treated patient developed pancreatic cancer. Of the overall sample of 52 patients, 2 patients were considered lost to follow-up and 5 patients died by the time of this long-term evaluation from other malignancies (n = 3), cardiovascular disease (n = 1), or other cause (n = 1).
Economic performance comparison
Using a hypothetical 65-year-old patient with ASA III level comorbidities and a 3.2-cm unilocular side-branch IPMN with 3 mm of cyst diameter growth over the past year, we compared the financial costs of the ChARM Post-treatment Reduced Radiographic Surveillance Protocol with the same patient continuing with surveillance alone as recommended by the 2 most commonly used pancreatic cyst surveillance guidelines as determined by the latest 2023 costs found in the Physician Fee Schedule and Procedure Price Lookup from the Centers for Medicare and Medicaid Services.17,18 If this hypothetical patient underwent surveillance using the recommendations of the Fukuoka Guidelines, she would incur $18,388.70 of Medicare costs over 5 years. If she underwent surveillance using the recommendations of the ACG guidelines, she would incur $11,872.36 of charges over 5 years. If she underwent EUS-guided chemoablation and was then followed up under the ChARM protocol for 5 years, the patient would incur costs of $7209.00.17,18 For comparison, a control arm describing a cyst 2.5 cm in size was tabulated for surveillance following the Fukuoka guidelines of a cyst in the 2- to 3-cm size range, which resulted in costs of $9518.18 over the same duration as the other proposed arms. A breakdown of the patient’s surveillance schedule and costs (by guideline) are presented in Table 3 and Figure 3.
TABLE 3.
Representative 65-year-old American Society of Anesthesiologists III patient with a 3.1-cm unilocular side-branch traductal papillary mucinous neoplasm with 3 mm growth over the past year followed for 5 years using the Fukuoka, American College of Gastroenterology (ACG), and ChARM Post-treatment Reduced Radiographic Surveillance protocols
| Unit | Unit cost, $ | Total, $ |
|---|---|---|
| Arm 1—following Fukuoka guidelines | ||
| 6 EUS | 2309.18 | 13,855.08 |
| 7 MRI | 472.87 | 3310.09 |
| Total imaging | 17,165.17 | |
| 7 clinic visits | 174.79 | 1223.53 |
| Total | 18,388.70 | |
| Arm 2—following ACG guidelines | ||
| 4 EUS | 2309.18 | 9236.72 |
| 4 MRI | 472.87 | 1891.48 |
| Total imaging | 11,128.20 | |
| 4 clinic visits | 174.79 | 699.16 |
| Total | 11,827.36 | |
| Arm 3—following ChARM protocol | ||
| 1 EUS-FNI | 2309.18 | 4618.36 |
| 4 MRI | 472.87 | 1891.48 |
| Total imaging | 6509.84 | |
| 4 clinic visits | 174.79 | 699.16 |
| Total | 7209.00 | |
| Control arm—following Fukuoka guidelines for 2-3 cm | ||
| 3 EUS-FNI | 2309.18 | 6927.54 |
| 4 MRI | 472.87 | 1891.48 |
| Total imaging | 8819.02 | |
| 4 clinic visits | 174.79 | 699.16 |
| Total | 9518.18 |
Control arm assumes a 2.5-cm cyst followed under the corresponding Fukuoka guidelines for a cyst of 2-3 cm.
EUS-FNI, Endoscopic ultrasound–guided fine-needle injection; MRI, magnetic resonance imaging.
Figure 3.
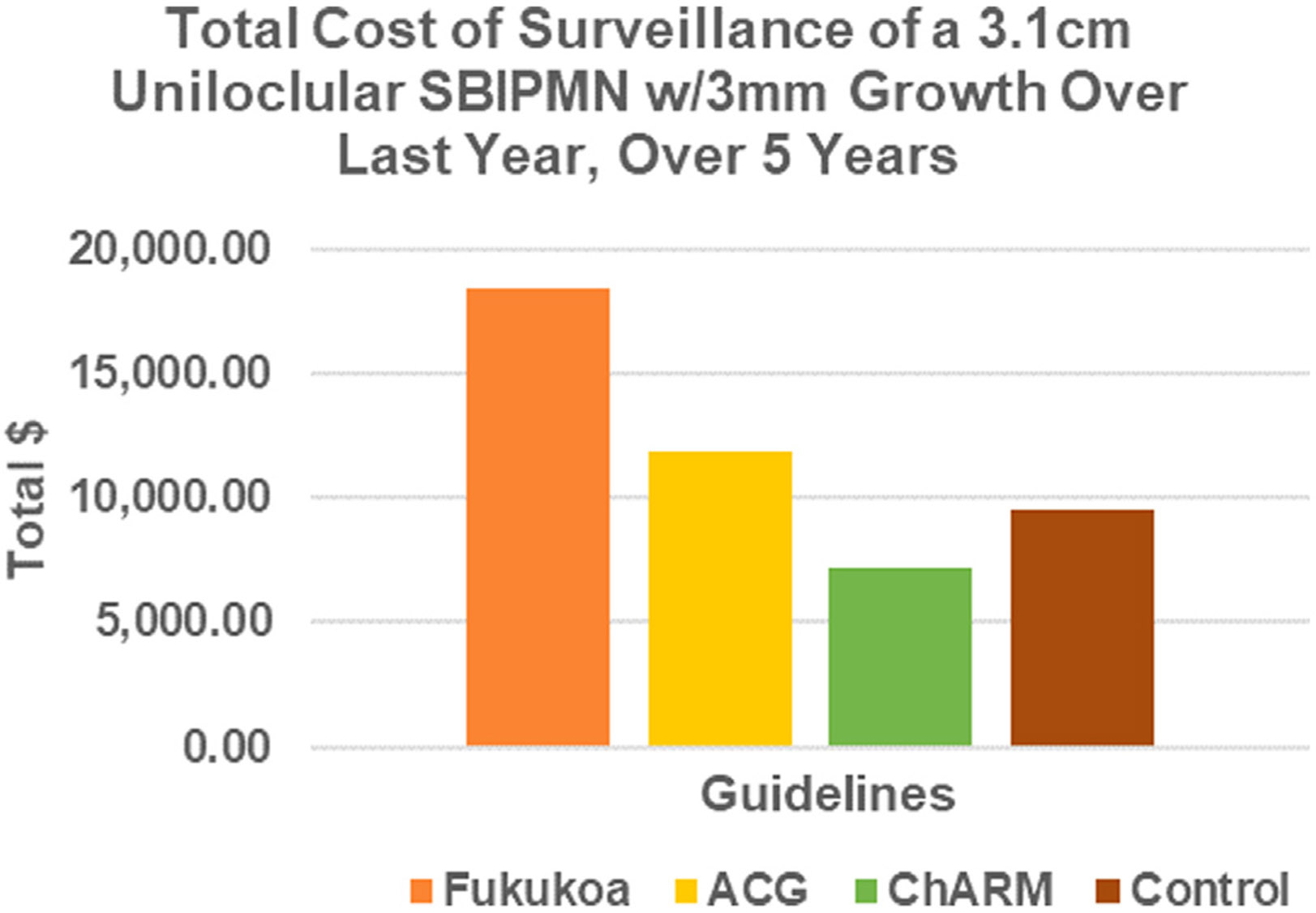
Economic comparison among 3 treatment options and control. ACG, American College of Gastroenterology; ChARM, Chemotherapy for Ablation and Resolution of Mucinous Pancreatic Cysts. sbIPMN, side-branch intraductal papillary mucinous neoplasm.
The patient experience
A total of 49 complete patient questionnaires were returned. The average Likert responses on the topics of emotional burden, surveillance dislike, financial burden, and reduction interest were 2.2 ± 0.9, 2.5 ± 1.0, 1.75 ± 1.1, and 2.9 ± 1.2, respectively; full response numbers are presented in Figure 4. The majority of patients reported mild to moderate emotional distress caused by MRI surveillance and moderate dislike of the MRI test, with a smaller number of patients reporting marked dislike, noting anxiety and claustrophobia. The majority of patients reported that they would be very interested in reducing the frequency of MRI-based pancreatic cyst surveillance after successful ablation. Interestingly, the majority of patients reported little to no financial distress due to MRI surveillance.
Figure 4.
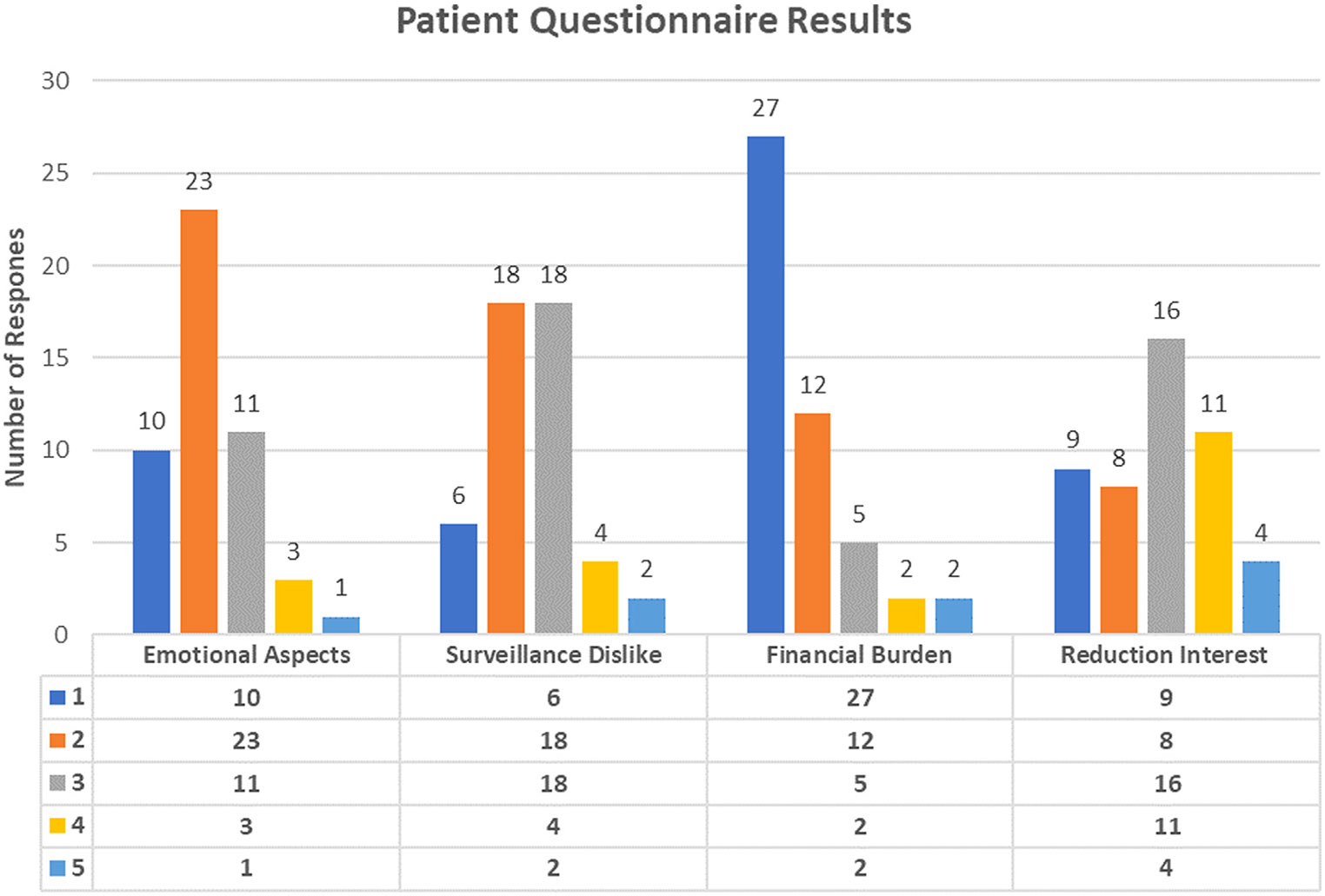
Results from patient questionnaire, a 4-question Likert-type scale scored in the areas of emotional aspects, “How much of an emotional burden is MRI surveillance of your pancreatic cyst?”; surveillance dislike, “How much do you dislike undergoing the MRI test for the surveillance of your pancreatic cyst?”; financial burden, “How much of a financial burden is MRI surveillance of your pancreatic cyst?”; and reduction interest, “If we could safely reduce the frequency of your radiographic surveillance after effective EUS-guided chemoablation how important would this be to you and your emotional well-being?”
DISCUSSION
Pancreatic cyst surveillance is hampered by uncertainty, and no current prospective studies have established a decrease in mortality with pancreatic cyst surveillance or a reliable surveillance interval that best balances efficacy with costs, efficiency, and a proper number needed to treat. Prospective data have illustrated that alcohol-free EUS-guided chemoablation offers an effective and safe treatment option with long-term durability in appropriately selected patients with mucinous pancreatic cysts.11,12,16,19,20 However, the appropriate evidence-based surveillance after chemoablation has been unknown. The present study reports a long-term surveillance strategy report based on 2 randomized prospective trials and demonstrates the important finding that the intensity of image-based surveillance can be safely and significantly reduced after successful EUS-guided chemoablation. This may represent significant cost savings to patients undergoing successful EUS-guided chemoablation for appropriate indications (most notably for cysts ≥3 cm). Although reduced ongoing surveillance appears to be appropriate, even if complete ablation is achieved, patients with IPMNs (not MCNs) maintain a risk of developing synchronous pancreatic malignancy throughout the pancreas estimated to be in the 2% to 4% range, so ongoing surveillance is necessary as long as retreatment or surgery remains a viable option.5,6,20,21
Our survey results indicate that the majority of subjects are enthusiastic regarding the prospects of reducing imaging surveillance if it can be done without a significant increase in disease risk. The majority of patients surveyed also reported mild to moderate emotional distress and moderate dislike of MRI surveillance, with some specifically commenting on anxiety and claustrophobia. The findings suggest that a safe reduction in radiographic surveillance frequency could be of significant emotional benefit to patients. Surveillance data based on prospective data are sparse. As such, these results should be considered at high-volume centers of excellence when developing a multidisciplinary EUS-guided pancreatic cyst ablation program.
There are limitations to this study, including a relatively small number of patients in a single-center setting. It should be acknowledged that serious adverse events associated with EUS-guided chemoablation, though rare, can occur; consequently, if an adverse event were to occur this would naturally add to the cost of the chemoablation arm in these scenarios. It should also be acknowledged that the economic comparisons profiled in this study are more proof-of-concept in nature than a comprehensive financial analysis of all patient scenarios possible between the strategies. We appreciate that our analysis limits the scope of our conclusions to cysts ≥3 cm. In addition, the cost savings proposed here may only be applicable over the 5 years evaluated in the study and may not reflect more long-term results. The proposed incurred costs were Medicare allowable charges and may vary significantly depending on an institution’s facility fees and additional charges. Finally, it bears mentioning that these long-term results and clinical management come from a specialized center with a highly developed and mature pancreatic cyst ablation program with interventionists, surgeons, and radiologists who are familiar with pancreatic cystic lesions, chemoablation, and interpretation of relevant imaging. Other high-volume centers of excellence considering adopting this approach are advised to review the article and chapter by Moyer et al11,12 and the position statement by Teoh et al13 for full description of patient selection, indications, contraindications, and recommendations on best practices for the technique, team structure, quality assurance, and management of adverse events.11-13
ACKNOWLEDGMENTS
This investigation was made possible through the generous support of the National Institutes of Health (R01 CA222648-01A1) and the Margo T. Walrath Career Development Award. At no time were these sponsors involved with the interpretation or reporting of the data.
Abbreviations:
- EUS
endoscopic ultrasound
- FNI
fine-needle injection
- IPMN
intraductal papillary mucinous neoplasm
- MCN
mucinous cystic neoplasm
- MRI
magnetic resonance imaging
Footnotes
DISCLOSURE
All authors disclosed no financial relationships.
REFERENCES
- 1.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039–49. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures 2018. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. Accessed March 1, 2024.
- 3.Fernandez–del Castillo C, Tanaka M. Management of pancreatic cysts: the evidence is not here yet. Gastroenterology 2015;148:685–7. [DOI] [PubMed] [Google Scholar]
- 4.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806–11. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Fernandez–del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17:738–53. [DOI] [PubMed] [Google Scholar]
- 6.Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol 2018;113:464–79. [DOI] [PubMed] [Google Scholar]
- 7.Expert Panel on Gastrointestinal Imaging; Fábrega-Foster K, Kamel IR, Horowitz JM, et al. ACR Appropriateness Criteria pancreatic cyst. J Am Coll Radiol 2020;17(5 Suppl):S198–206. [DOI] [PubMed] [Google Scholar]
- 8.Clancy TE. Surgery for pancreatic cancer. Hematol Oncol Clin North Am 2015;29:701–16. [DOI] [PubMed] [Google Scholar]
- 9.Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in hospital volume and failure to rescue for pancreatic surgery. J Gastrointest Surg 2015;19:1581–92. [DOI] [PubMed] [Google Scholar]
- 10.Canakis A, Law R, Baron T. An updated review on ablative treatment of pancreatic cystic lesions. Gastrointest Endosc 2020;91:520–6. [DOI] [PubMed] [Google Scholar]
- 11.Moyer MT, Maranki JL, DeWitt JM. EUS-guided pancreatic cyst ablation: a clinical and technical review. Curr Gastroenterol Rep 2019;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyer MT, Lakhtakia S. EUS-guided pancreatic cyst chemoablation, EUS-guided radiofrequency ablation, and EUS-guided fine-needle injection. In: Varadarajulu S, Fockens P, Hawes RH, editors. Endosonography. 5th ed. Amsterdam: Elsevier; 2023. p. 345–55. [Google Scholar]
- 13.Teoh AY, Seo DW, Brugge W, et al. Position statement on EUS-guided ablation of pancreatic cystic neoplasms from an international expert panel. Endosc Int Open 2019;7:e1064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer MT, Sharzehi S, Mathew A, et al. The safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology 2017;153:1295–303. [DOI] [PubMed] [Google Scholar]
- 15.Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy 2017;49: 866–73. [DOI] [PubMed] [Google Scholar]
- 16.Lester C, Walsh L, Hartz KM, et al. The durability of EUS-guided chemoablation of mucinous pancreatic cysts: a long-term follow-up of the ChARM trial. Clin Gastroenterol Hepatol 2022;20:e326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medicare. Procedure price lookup. Available at: https://www.medicare.gov/procedure-price-lookup/. Accessed March 1, 2024.
- 18.Centers for Medicare and Medicaid Services. Search the Physician Fee Schedule. Available at: https://www.cms.gov/medicare/physician-fee-schedule/search. Accessed March 1, 2024.
- 19.DeWitt JM, Arain M, Chang KJ, et al. Interventional endoscopic ultrasound: current status and future directions. Clin Gastroenterol Hepatol 2021;19:24–40. [DOI] [PubMed] [Google Scholar]
- 20.Tanno S, Nakano Y, Koizumi K, et al. Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 2010;39:36–40. [DOI] [PubMed] [Google Scholar]
- 21.Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas 2011;40:364–70. [DOI] [PubMed] [Google Scholar]


