Abstract
Objective
This paper investigated the effects of prenatal drug exposure (PDE), childhood trauma (CT), and their interactions on the neurobiological markers for emotion processing.
Method
Here, in a non-clinical sample of pre-adolescents (9-10 years of age) from the Adolescent Brain Cognitive Development (ABCD) Study (N = 6,146), we investigate the impact of PDE to commonly used substances (ie, alcohol, cigarettes, and marijuana), CT, and their interaction on emotion processing. From the Emotional N-back functional magnetic resonance imaging task data, we selected 26 regions of interests, previously implicated in emotion processing, and conducted separate linear mixed models (108 total) and accounted for available environmental risk factors.
Results
PDE was associated with reductions in response bias related to the processing of fearful compared to happy faces in widespread cortical regions (including the superior frontal and fusiform gyri and the inferior parietal lobule). Reduced response bias in the superior frontal gyrus emerged as PDE driven and was present regardless of CT status, but correlated with several items on the Child Behavior Checklist only in those children with both PDE and CT. The lower response bias of the left inferior parietal lobule, on the other hand, was observed only in children with both PDE and CT, and correlated with internalizing and externalizing behaviors.
Conclusion
The study’s results support the diathesis–stress model, and suggest that PDE may confer vulnerability to the effects of later CT through altered neurodevelopment. Children experiencing these “double-hit” conditions may represent at-risk individuals who could benefit from early interventions to mitigate the onset of psychopathology. Because of limitations in the way that PDE was reported in the ABCD Study, including lack of severity measures and retrospective reporting, results are not sufficient for making recommendations or dictating policy for pregnant persons. Nevertheless, this study is a necessary first step in examining the interactive effects of prenatal and early-life exposures, as well as many aspects of the sociodemographic and psychological environment.
Key words: prenatal drug exposure, childhood trauma, adolescents, emotion processing, ABCD Study
Plain language summary
This study looked at how prenatal drug exposure of commonly used substances (alcohol, cigarettes, and cannabis) and childhood trauma affect brain activity related to processing emotions in children from the Adolescent Brain Cognitive Development (ABCD) Study. Using brain imaging data from 6,146 children aged 9-10, the study found that prenatal drug exposure was associated with differing brain activity to emotional faces in several brain regions involved in emotion processing. Children who experienced both prenatal drug exposure and childhood trauma showed altered brain activity patterns that correlated with greater behavioral problems reported by parents. These findings suggest prenatal drug exposure may make children more vulnerable to the negative effects of childhood trauma on brain development and mental health.
Inconsistent scientific evidence regarding the effects of substance consumption on fetal development creates uncertainty surrounding a person’s choices during pregnancy.1,2 Most studies have focused on gestational exposure to illicit drugs (eg, cocaine)3 and high quantities (eg, binging, substance use disorders, etc) of legal substances.4 In contrast, even with the rising prevalence of moderate use of common psychoactive substances during pregnancy, its effect on the neurobiology and behavior of gestationally exposed children remains relatively unexplored.5 For instance, the rate of prenatal cannabis use in the United States increased from 6.75% to 8.14% from before to after the COVID-19 pandemic.6 Tobacco use during the second trimester of pregnancy in the United States between 2011 and 2018 was 6.4%.7 These increasing trends and relatively high rates of prenatal drug exposure (PDE) would be concerning if subclinical exposures are similarly linked to poor child health outcomes. It remains unclear whether moderate use of these common substances (eg, alcohol, cannabis, and tobacco) poses true risk. For example, almost 10% of women globally use alcohol during pregnancy, and 0.15% of live births have fetal alcohol spectrum disorder (FASD).8 There may still be important but less severe outcomes, such as increased externalizing and internalizing behaviors in children with PDE.9,10
Few studies in human beings have directly examined the effects of PDE on key brain functions, such as emotion processing. Although intact emotion processing in children has shown to confer resilience to trauma and to promote effective conflict resolution abilities,11,12 emotion processing difficulty is associated with later onset of psychiatric disorders including anxiety, depression, and eating disorders, as well as subclinical functional impairments.13 Across response inhibition tasks (Go/No-Go and Stop Signal tasks), children with exposure to legal substances compared to those without have demonstrated consistent hyperactivations in the anterior cingulate gyrus, fusiform gyrus, lateral orbitofrontal cortex, superior frontal gyrus, superior temporal gyrus, middle temporal gyrus, and inferior parietal cortex.14, 15, 16, 17, 18, 19 Hypoactivations have been observed in the medial orbitofrontal cortex and insula during the same response-inhibition tasks and contrasts.20 Although studies specific to emotion processing are minimal, these response inhibition tasks correlate with implicit and explicit emotion regulation.21 In addition, a smaller surface area in the anterior cingulate cortex in prenatal alcohol- and/or tobacco-exposed groups have been observed and linked to worse behavioral outcomes.22,23 Such observations indicate that in prenatally exposed children, a greater demand is placed on regions involved in executive control when regions typically involved in salience and affect recognition24, 25, 26 are less developed or underrecruited during task-related response inhibition. In the context of high doses of prenatal alcohol exposure, alcohol-related neurodevelopmental disorders and FASD are associated with attachment disorders, conduct disorder, post-traumatic stress disorder (PTSD), suicidality, and attention-deficit/hyperactivity disorder (ADHD).27 These studies report affect regulation impairment, fewer prosocial behaviors, and increased engagement in antisocial behaviors.28 Moreover, high-dose prenatal tobacco exposure increases the risk for externalizing behavior problems,29 conduct disorder,30 and substance use problems.31
Studies using data from the Ottawa Prenatal Prospective Study (OPPS) and the Maternal Health Practices and Child Development Project have shown that moderate to heavy prenatal cannabis exposure was associated with attentional problems as well as parent-reported impulsivity and hyperactivity at 6 and 10 years of age, respectively.32,33 More recent studies from the Generation R cohort found significant associations between prenatal cannabis exposure and externalizing problems at ages 7 to 9 years of age; however, this was found to be related to both maternal and paternal cannabis use during pregnancy, suggesting familial or genetic confounding factors.34 A study of prenatal cannabis exposure in the Adolescent Brain Cognitive Development (ABCD) Study cohort found differences in attention, externalizing, and total problem scores, but did not find differences on functional magnetic resonance imaging (fMRI) in either task performance or blood oxygen level–dependent (BOLD) activation.35 In a small follow-up cohort from the OPPS cohort at age 18 years, an increased amount of prenatal cannabis exposure was associated with decreased cerebellar activity and increased bilateral PFC activity on a Go/NoGo task.36 Notably, these foundational studies have not examined differences between light and no prenatal exposure to cannabis, and often combine these into a single group.
Childhood trauma (CT) is associated with changes in emotion processing. In adults, history of CT is associated with a bias toward interpreting valence as negative as well as enhanced selective attention to angry facial expressions.37 Children with a greater cumulative number of adverse childhood experiences demonstrate greater hyperactivation in the orbitofrontal cortex, ACC, and amygdala, as well as hypoactivation in the medial prefrontal cortex, in the face of fearful stimuli.38,39 In response to negatively valenced stimuli, youth with CT show reduced connectivity between the medial prefrontal cortex and both the amygdala and the hippocampus. This reduced connectivity is associated with the development of internalizing psychiatric symptoms.40 Many studies present an inverse relationship between cumulative number of adverse childhood experiences and functional connections among regions involved in executive function, affect regulation, memory, and reward pathways. These include the amygdala, left ventral ACC, ventral anterior superior frontal gyrus, rostral anterior cingulate cortex, precuneus, ventromedial prefrontal cortex, left anterior middle temporal gyrus, orbitofrontal gyrus, and right middle frontal gyrus.39,41, 42, 43 In addition, children with CT demonstrate reductions in the volumes of the ventromedial prefrontal cortex, right lateral orbitofrontal cortex, right inferior frontal gyrus, bilateral parahippocampal gyrus, left temporal pole, and superior temporal gyri.44
Although existing studies have investigated the effects of PDE and CT on emotion processing separately, this approach leaves a considerable gap in the current literature about their possible interactive effects in youth with both PDE and CT.45 One study on this interaction reported a diathesis–stress pattern in 363 adolescents with prenatal cocaine exposure (longitudinal study taken at 15 and 17 years of age), and showed greater emotional reactivity and poorer use of coping strategies in youth with both PDE and CT.46
Moreover, it is not known whether prenatal exposure to widely used, recreational substances (eg, alcohol, cannabis, and tobacco) confers a similar vulnerability to the effects of trauma. Such investigations are rare because of the difficulty in identifying and recruiting a large and diverse sample of youth who present with both prenatal exposure to recreational or legal substances, and early life trauma, along with comprehensive clinical, behavioral, and neuroimaging data to evaluate the effects of PDE and CT on emotion processing.
To address this knowledge gap, we used data from the ABCD Study, which presents a unique opportunity to study the independent and interactive influences of PDE and CT on emotion processing. It is important to note that in this nonclinical sample, prenatal and postnatal exposure data are retrospectively reported and represent a broad and heterogeneous range of type, intensity, severity, and cumulative number of drugs and traumatic experiences. To further test the diathesis–stress or 2-hit model, we hypothesize blunted response of the prefrontal cortical regions associated with emotion regulation and heightened response of subcortical brain regions involved in emotional reactivity in children with both PDE and CT, as compared to only 1, or neither, of the 2 exposures.
Method
Participants
The study sample for our analyses was taken from baseline and year 1 follow-up data of the ABCD Study (Release 3.0). The ABCD Study acquires data from 11,875 children 9 to 10 years of age, from 21 sites across the United States, tracking multiple domains of development through childhood to young adulthood. This study includes a comprehensive set of psychosocial data along with neuroimaging, behavioral, and clinical information. The sample used in this set of analyses consisted of participants with complete data for all relevant variables, including neuroimaging data and sociodemographic covariates (Table 1; see Figure S1, available online). The final sample consisted of 6,146 participants. The cohort was stratified into groups based on PDE and CT exposures.
Table 1.
Sociodemographic Characteristics of Exposure Subgroups With Statistical Comparison by Main Effect and Interaction
| PDE–/ CT– n = 4,780 | PDE–/ CT+ n = 972 | PDE+ /CT– n = 270 | PDE+/ CT+ n = 124 | PDE main effect | CT main effect | Interaction | |
|---|---|---|---|---|---|---|---|
| Subject agea | 9.93 ± 0.62 | 9.95 ± 0.62 | 9.99 ± 0.64 | 9.92 ± 0.63 | –1.06 | –0.86 | 1.71 |
| Sex (F) | 2,334 (50) | 485 (50) | 143 (53) | 66 (53) | 0.59 | 0.09 | 2.27 |
| Subject grade | 4.86 | 9.83 | 19.2 | ||||
| Second grade or below | 11 (0.2) | 6 (1) | 1 (4) | 2 (2) | |||
| Third grade | 800 (17) | 140 (14) | 48 (12) | 22 (18) | |||
| Fourth grade | 2,124 (44) | 432 (44) | 123 (46) | 56 (45) | |||
| Fifth grade | 1,696 (35) | 362 (37) | 85 (31) | 42 (34) | |||
| Sixth grade | 149 (3) | 32 (3) | 13 (5) | 2 (2) | |||
| Seventh grade or above | 1 (0.02) | 0 (0) | 0 (0) | 0 (0) | |||
| Race | 20.29∗∗ | 47.9∗∗∗ | 67.4∗∗∗ | ||||
| AIAN/NHPI | 14 (0.3) | 11 (1) | 1 (4) | 1 (1) | |||
| Asian | 103 (2) | 14 (1) | 1 (4) | 0 (0) | |||
| Black | 512 (11) | 149 (15) | 37 (14) | 23 (19) | |||
| White | 3,462 (72) | 640 (66) | 202 (75) | 78 (63) | |||
| Mixed | 505 (11) | 126 (13) | 27 (10) | 21 (17) | |||
| Other | 184 (4) | 32 (3) | 2 (7) | 1 (1) | |||
| Hispanic or Latino | 861 (18) | 266 (22) | 31 (11) | 21 (17) | 6.61∗ | 2.33 | 10.39∗ |
| Household income | 58.82∗∗∗ | 109.47∗∗∗ | 169.4∗∗∗ | ||||
| <50k | 1,040 (22) | 327 (34) | 83 (31) | 76 (61) | |||
| ≥50k to <100k | 1,390 (29) | 271 (28) | 78 (29) | 29 (23) | |||
| ≥100k | 2,350 (49) | 374 (38) | 109 (40) | 19 (15) | |||
| Financial insecurity | 633 (13) | 264 (27) | 70 (26) | 65 (52) | 92.0∗∗∗ | 167.0∗∗∗ | 246.7∗∗∗ |
| Parent marital status | |||||||
| Divorced | 363 (8) | 128 (13) | 26 (10) | 17 (14) | 99.5∗∗∗ | 180.5∗∗∗ | 270.0∗∗∗ |
| Living with partner | 177 (4) | 73 (8) | 24 (9) | 14 (11) | |||
| Married | 3,702 (77) | 577 (59) | 163 (60) | 46 (37) | |||
| Never married | 381 (8) | 132 (14) | 39 (14) | 38 (31) | |||
| Separated | 128 (3) | 54 (6) | 14 (5) | 8 (6) | |||
| Widowed | 29 (1) | 8 (1) | 4 (2) | 1 (1) | |||
| Parent employment | 33.9∗∗∗ | 34.4∗∗∗ | 79.4∗∗∗ | ||||
| Working | 3,543 (74) | 695 (72) | 207 (77) | 73 (59) | |||
| Not working | 202 (4) | 59 (6) | 13 (5) | 13 (10) | |||
| Stay at home parent | 816 (17) | 154 (16) | 31 (11) | 21 (17) | |||
| Student | 70 (1) | 24 (5) | 8 (3) | 5 (4) | |||
| Disabled | 69 (1) | 25 (3) | 8 (3) | 11 (9) | |||
| Other | 80 (2) | 15 (2) | 3 (1) | 1 (1) | |||
| Highest education | 74.3∗∗∗ | 87.4∗∗∗ | 169.1∗∗∗ | ||||
| < High school | 141 (3) | 33 (4) | 8 (3) | 5 (4) | |||
| High school/GED | 316 (7) | 63 (6) | 44 (16) | 14 (11) | |||
| Some college | 1,018 (21) | 302 (31) | 68 (25) | 65 (52) | |||
| Bachelor’s degree | 1,367 (29) | 273 (28) | 61 (23) | 28 (22) | |||
| Post-graduate degree | 1,938 (41) | 301 (31) | 89 (33) | 12 (10) | |||
| Handedness | 16.2∗∗∗ | 2.93 | 18.5∗∗ | ||||
| Left | 318 (7) | 67 (7) | 27 (10) | 14 (11) | |||
| Mixed | 596 (12) | 137 (14) | 47 (17) | 21 (17) | |||
| Right | 3,866 (81) | 768 (79) | 196 (73) | 89 (72) | |||
| Parent psychiatric history | 1,904 (34) | 1,590 (61) | 169 (63) | 110 (89) | 112.24∗∗∗ | 189.38∗∗∗ | 277.7∗∗∗ |
| History of being bullied | 859 (18) | 200 (21) | 46 (17) | 48 (39) | 34.19∗∗∗ | 83.03∗∗∗ | 118.9∗∗∗ |
| Family aggression scoreb | 1.90 ± 1.89 | 2.2 ± 2.1 | 1.95 ± 1.96 | 2.4 ± 2.0 | 1092038∗∗ | 25151611∗∗∗ | 18.3∗∗∗ |
| Neighborhood safety scoreb | 4.14 ± 1.0 | 3.98 ± 1.1 | 4.02 ± 1.1 | 3.66 ± 1.4 | 1225460∗ | 2977460∗∗∗ | 36.52∗∗∗ |
| Total life events | 15.69∗∗∗ | 75.03∗∗∗ | 85.51∗∗∗ | ||||
| High TLE | 2,297 (48) | 599 (62) | 149 (55) | 90 (73) | |||
| Low TLE | 2,483 (52) | 373 (38) | 121 (45) | 34 (27) | |||
| DSM-5 V current diagnosis | 426 (9) | 186 (19) | 33 (12) | 34 (27) | 15.2∗∗∗ | 110.56∗∗∗ | 121.09∗∗∗ |
Note: The left side of the table displays descriptive characteristics of the sample and are separated by exposure group. Continuous variables are reported as mean ± SD. The right side displays between-group comparisons with test statistic (p value). Categorical variables were analyzed via the χ2 test for independence for both main and interaction effects.
AIAN/NHPI = American Indian and Alaska Native/Native Hawaiian and Pacific Islander; CT = childhood trauma; PDE = prenatal drug exposure; TLE = total life events.
∗p < .05; ∗∗p < .01; ∗∗∗p < .001.
Group differences for parametric continuous variables were analyzed via independent t test for main effects and 1-way analysis of variance for interaction effects.
Group differences for non-parametric continuous variables were analyzed via Mann–Whitney U test for main effects and 1-way analysis of variance for interaction effects.
Prenatal Drug Exposure
PDE was defined by prenatal exposure to alcohol, cannabis, and tobacco after the mother became aware of the pregnancy, as assessed by the mother’s self-report on the Developmental History Questionnaire.47 Mothers were asked “once you knew you were pregnant, were you using any of the following?” individually for specific drugs: tobacco, alcohol, marijuana, cocaine, heroin/morphine, oxycontin, or “any other drug.” If a participant responded “yes” to any of these questions, they were asked about the frequency (“how many times per day?”) and amount (“how much each time?”) of use. Given our objective of examining the impact of prenatal exposure to legal substances, we included participants who had prenatal exposure to alcohol, tobacco, and marijuana only. Previously, patterns of alcohol exposure severity in the ABCD cohort have been explored.48 Here, the PDE variable was agnostic to substance use before the knowledge of pregnancy but meant that the mother was using substances after the pregnancy was known. Otherwise, there was no information available regarding in which month or trimester the substance use was occurring. These analyses are focused on the period of pregnancy when exposures are thought to be most relevant to fetal neurodevelopment (ie, after implantation).49 The resulting PDE variable was binary (94% PDE– /6% PDE+). Because of relatively low counts of each individual substance exposure and their combinations (see Table S1, available online), and even smaller counts when divided by the presence or absence of CT, PDE was kept as a single binary variable rather than divided into individual substances or the amount of use.
Childhood Trauma
CT was defined as binary lack of (82% CT–) or exposure to (18% CT+) 1 or more traumatic events from the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) PTSD-module.47 The item “received news of a loved one passing away” was excluded from the scoring because the item was overrepresented in the sample compared with other items (see Table S2, available online). Because of the limited sample size (n = 124) of the group of interest (the PDE+/CT+ “double-hit” group), CT was not separated into categories of trauma type (eg, interpersonal trauma, natural disaster, etc). Counts of each trauma type can be found in Table S3, available online. CT as a continuous cumulative risk variable (total number of CT exposures) was additionally explored.
The sample was further filtered by non-missingness of the covariates (described below). The final sample consisted of 6,146 participants. In this sample, the 394 (6.4%) youth had PDE and 1,096 (17.8%) had CT. Groups were further stratified into four subgroups: PDE–/CT– (non-exposed control; n=4,780), PDE–/CT+ (n=972), PDE+/CT– (n=270), and PDE+/CT+ (the “double-hit” group; n=124).
Covariates
Demographic variables including grade, race, and ethnicity were treated as covariates. Social risk factors common in PDE and CT were included because of their potential influence on neurodevelopment of emotion regulation (see Supplement 1, available online). Table 1 shows the distribution of these variables for PDE and CT, as well as their interaction groups. Variables considered but ultimately excluded from the models because of high variation inflation factor (ie, VIF > 5) included puberty score and crystallized IQ.
fMRI Task
The ABCD Emotional N-back (EN-back) task, summarized in the supplemental material (see Supplement 2, available online) was adapted from the N-back task used in the Human Connectome Project.50 This consisted of high (2-back) and low (0-back) memory load conditions that included happy, fearful, and neutral faces from the NimStim emotional stimulus set and the Racially Diverse Affective Expressions (RADIATE) set of stimuli as well as neutral, non-social, stimuli (picture of houses) from the Human Connectome Project.51 In this modified task, the face trials serve as both the working memory probe and emotional interference test. Following the convention of other published ABCD studies that use the EN-back task, the data included averages from both runs, and both 0-back and 2-back conditions.
Two contrasts of interest were defined: (1) valence (ie, the contrast of fearful [negative] minus happy [positive] faces); and (2) arousal (ie, the contrast of emotional [the mean of fearful and happy faces] minus neutral faces). Valence and arousal contrasts required only fearful, positive, and neutral faces, and did not use the neutral, non-social, stimuli. The selection of fearful vs happy trials representing valence is consistent with literature studying valence across fMRI and electroencephalographic modalities that use positive vs negative contrasts.52, 53, 54, 55 Our analyses were based on 120 trials with 40 trials per stimulus, consistent with fMRI studies involving emotion processing.56, 57, 58, 59, 60, 61, 62, 63, 64, 65
To further investigate the results, the significant valence findings were then decomposed into the fearful (negative) vs neutral condition and the happy (positive) vs neutral condition, to test whether a particular valence was driving the effect.
The behavioral outcome variables for emotional arousal and valence conditions were mean accuracy rate and mean reaction time (see Supplement 3, available online).
MRI Image Acquisition and Processing
fMRI 3.0T scans were taken in a fixed order beginning with a localizer, 3-dimensional (3D) T1-weighted images, 2 runs of resting state fMRI, diffusion weighted images, 3D T2-weighted images, then final runs of resting state. MRI assessments were reviewed by a neuroradiologist for incidental clinical findings. As part of the ABCD data processing workflow, the dataset was quality controlled for problems such as acquisition protocol compliance, imaging artifacts, or motion or file corruption. Furthermore, average and maximum framewise displacement, framewise translation, and framewise rotation were included in the model to account for head motion. The ABCD Data Analysis, Informatics and Resource Center (DAIRC) performed centralized initial quality control and processed the fMRI data. fMRI beta-weights are used for contrasts, and parcellations are from the Desikan and Destrieux atlases. The full details of the imaging acquisition and preprocessing protocol were previously described in Hagler et al.66 and outlined in the supplemental material (see Supplement 4, available online).
Regions of Interest
Regions of interest (ROIs) were selected from previous neuroimaging studies of emotion recognition, reactivity, and regulation. The amygdala was selected for its role in emotion reactivity and regulation, especially in judging both negatively and positively valenced facial expressions.67 Other regions, including the orbitofrontal cortex, rostral anterior cingulate cortex (rACC), and hippocampus were selected because they have been implicated in emotion processing and because of their connectivity with the amygdala.14, 15, 16, 17, 18, 19,68, 69, 70 Regions from the Picture Induced Negative Emotion Signature (PINES) network (eg, insula [Ins], posterior cingulate cortex [PCC], superior temporal gyrus [sTG], temporoparietal junction [TPJ], and occipital cortex) were also considered and included in our selection.71 The superior frontal gyrus (sFG) was selected for its role in top-down regulation of the amygdala via prefrontal regions.72 The inferior parietal lobule (iPL) was included because it was involved in implicit emotional regulation.73 Thus, 13 bilateral ROIs (26 total) were selected for these analyses. A supplemental figure (see Figure S2, available online) is provided showing the labeled cortical (top panel) and subcortical (bottom panel) ROIs selected for analyses.
Clinical Measures
Data from the ABCD Study’s psychosocial battery were included to determine the clinical and functional relevance of brain and behavioral data. This includes the Child Behavior Checklist (CBCL), the behavioral inhibition system and behavioral activation system (BIS/BAS) scale, the Urgency, Premeditation, Perseverance, Sensation seeking, and Positive urgency (UPPS-P) scale, and the Youth Prosocial Behavior Survey (PBS). The CBCL is completed by the participants’ caregivers and characterizes 8 behavioral and emotional syndromes in children and adolescents.74 The BIS/BAS, completed by the child, measures motivational systems: the behavioral inhibition system (BIS), corresponding to motivation to avoid aversive outcomes; and the behavioral activation system (BAS), corresponding to motivation to approach goal-oriented outcomes.75 The UPPS-P measures 5 domains of impulsivity, and is completed by the child. From the PBS, a summary score for prosocial behavior was included.
Statistical Analyses
For demographic data, the χ2 test for independence was used to determine group differences in categorical variables. For continuous variables, the Mann–Whitney U test was used to determine between-group effects, and 1-way analysis of variance (ANOVA) as used for interaction effects.
All brain and behavioral measures were analyzed in R (http://www.r-project.org/). Mixed linear models were used to analyze behavioral and fMRI models using the “lme4” package (https://cran.r-project.org/web/packages/lme4/lme4.pdf). In these models, independent variables included PDE, CT, and their interactions; dependent variables were task behavior (mean reaction time and accuracy), and beta weights of each ROI for both arousal and valence contrasts. A total of 108 models were run, given the number of contrasts (valence and arousal), main and interaction effects (PDE or CT, and their interaction), and outcome variables (reaction time and 13 bilateral ROIs). In these linear models, the effects of PDE and CT on valence and arousal contrasts are referred to as the “main effects of PDE” and “main effects of CT” for each of these contrasts, respectively. However, the examination of differences among the 4 groups (PDE–/CT–, PDE+/CT–, PDE–/CT+, and PDE+/CT+) is referred to as the “interaction effect” on the ROI or task behavior. Site and family ID were included in the models as random effect variables. False discovery rate (FDR) correction was performed across all models that were significant, to account for multiple comparisons and to minimize type I error. Our specific mixed-effects model formulas can be seen in the supplemental material (see Supplement 5, available online).
To determine the statistical power of our significant models, we used the SIMR package in R, which uses Monte Carlo simulations to estimate statistical power from mixed-effects linear regressions.76 We conducted 100 simulations to determine power as well as an associated 95% confidence interval.
In addition, with 24 unique covariates in our models, we determined the degree of overfit in our significant models by conducting a complexity-vs-generalization tradeoff analysis. We conducted a forward selection process, adding the covariate with the highest marginal R2 values at each step until we added all 24 possible covariates. In each step, we conducted cross-validation that was stratified by PDE, presence of CT, site ID, and family ID because of imbalances in these binary/categorical variables across participants. To assess overfit, we visually observed whether the test data performance distribution demonstrated consistent increases in its root mean squared error (RMSE) as the number of covariates increased, demonstrating poorer generalizability across increased model complexity.
Given the skewed distribution of all CBCL, BIS/BAS, UPPS-P, and prosocial behavior score variables, exploratory Spearman rank correlations were performed separately within each group to test for their associations with brain results. FDR correction was performed to account for multiple comparisons to minimize type I error.
Results
Participant Characteristics
Demographic and other characteristics of the included ABCD sample, separated by groups, are presented in Table 1. Groups were characterized both main effects: PDE– (n = 5,752), PDE+ (n = 394) and CT– (n = 5,050), CT+ (n = 1,096). To study the interaction between PDE and CT, the sample was stratified into 4 groups: PDE–/CT– (n = 4,780), PDE–/CT+ (n = 972), PDE+/CT– (n = 270), and PDE+/CT+ (n = 124). In the supplemental material, we provide counts of each drug or combination of drugs (alcohol, cannabis, tobacco) (see Table S1, available online), and counts and types of traumatic experience on the KSADS in this study cohort (see Table S2, available online).
Table 1 provides details about group differences in sociodemographic risk factors. These risk factors were most prevalent in the double-hit group (PDE+/CT+), with single-hit groups (either PDE+/CT– or PDE–/CT+) as moderately affected and the wholly unexposed group (PDE–/CT–) least affected. This is consistent with studies that informed our covariates in which adverse childhood experiences and prenatal exposure to psychoactive substances are more prevalent in individuals with less education, lower income, and unemployment (see Supplement 1, available online).
Regression Analysis
Task Behavior
Emotion processing was assessed using the EN-back, by examining contrasts of emotional trials across averaged 0- and 2-back conditions. Linear mixed models showed no significant main effects for PDE, CT, or their interaction on accuracy and mean reaction time, for both valence and arousal (CE < –4.66, p > .96) (see Table S4, available online).
Task-Related Brain Activation
Significant results are summarized in Table 2. In additional, results from all ROI models are summarized in the supplemental material (see Table S5, available online).
Table 2.
Region of Interest (ROI) Linear Mixed Models
| Valence contrast | |||
|---|---|---|---|
| Main effects | |||
| ROI | CE | CI | pFDR |
| PDE+ | |||
| Superior frontal gyrus (L) | –0.077 | –0.13, –0.03 | .001 |
| Superior frontal gyrus (R) | –0.079 | –0.13, –0.03 | .037 |
| Fusiform (L) | –0.060 | –0.11, –0.01 | .027 |
| Fusiform (R) | –0.067 | –0.13, –0.01 | .025 |
| Insula (L) | –0.041 | –0.078, 0.00 | .035 |
| Insula (R) | –0.043 | –0.08, 0.00 | .036 |
| Rostral anterior cingulate cortex (L) | –0.074 | –0.014, –0.010 | .037 |
| Inferior parietal lobule (R) | –0.055 | –0.10, –0.01 | .017 |
| Hippocampus (R) | –0.044 | 0.03, 0.12 | .047 |
| CT+ | |||
| Amygdala (L) | 0.039 | 0.00, 0.08 | .046 |
| Interaction effects | |||
| PDE+/CT– | |||
| Superior frontal gyrus (L) | –0.073 | –0.13, –0.02 | .014 |
| Superior frontal gyrus (R) | –0.073 | –0.13, –0.01 | .014 |
| Fusiform (L) | –0.063 | –0.13, 0.00 | .05 |
| Fusiform (R) | –0.073 | –0.14, 0.00 | .043 |
| Isthmus cingulate cortex (R) | –0.068 | –0.13, 0.00 | .04 |
| Arousal contrast | |||
| Main effects | |||
| PDE+ | |||
| Fusiform (R) | 0.053 | 0.00, 0.10 | .045 |
| Interaction effects | |||
| PDE+/CT+ | |||
| Fusiform (R) | 0.103 | 0.01, 0.19 | .02 |
Note: Summary table of the observed significant activations for main effects (PDE and CT) and interaction effects (PDE/CT). CE = contrast estimate; CT = childhood trauma; PDE = prenatal drug exposure; pFDR = false discovery rate−corrected p value.
Valence Contrast
The valence contrast was the difference between negative and positive image (ie, fearful minus happy faces) trials, and, for significant findings, was split into models of negative vs neutral and positive vs neutral faces (see Table S6, available online).
Main Effect
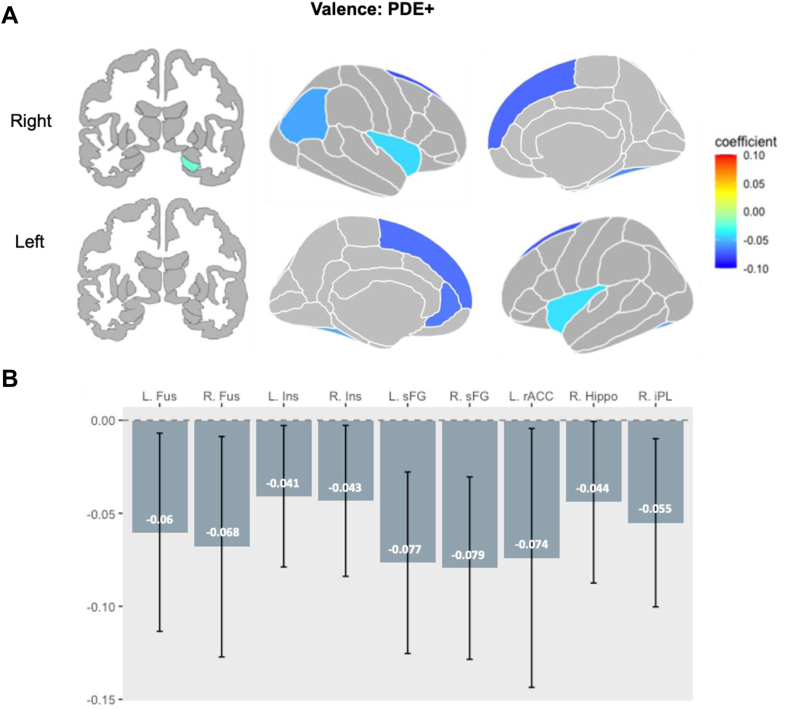
PDE (PDE+ vs PDE–): Linear mixed models revealed that the PDE+ group showed blunted response compared to the PDE– group in the following regions: bilateral sFG (left: CE = –0.077, pFDR = .010, CI = –0.13, –0.03; right: CE = –0.079, pFDR = .010, CI = –0.13, –0.03), bilateral Fus (left: CE = –0.060, pFDR = .046, CI =–0.11, –0.01; right: CE = –0.067, pFDR = .046, CI = –0.13, –0.01), bilateral Ins (left: CE = –0.041, pFDR = .046, CI = –0.078, 0.00; right: CE = –0.043, pFDR = .046, CI = –0.08, 0.00), left rACC (CE = –0.074, pFDR = .046, CI = –0.014, –0.010), right iPL (CE = –0.055, pFDR = .046, CI = –0.10, –0.01), and right hippocampus (CE = –0.044, pFDR = .047, CI = 0.03, 0.12) (Figure 1A and B). This model demonstrated sufficient statistical power in the bilateral sFG; left: 1 – β = 0.83, CI = 0.7418, 0.8977; right: 1 – β = 0.87, CI = 0.788, 0.9289) (see Table S7, available online).
Figure 1.
Main Effect of Prenatal Drug Exposure on Region of Interest (ROI) Activation in the Valence Condition
Note:In the main effect model, during the valence condition (A) PDE+ was associated with widespread reductions in activity across ROIs involved in emotion processing. Gray represents both unexplored and statistically non-significant regions. Coefficient describes the effect size and direction of the effect. (B) Bars represent the effect size in each ROI for PDE+ compared to PDE– (dotted line), and whiskers represent confidence interval. All depicted reductions in activity (cold-colored regions) were statistically significant for the PDE main effect (PDE+ < PDE–). PDE = prenatal drug exposure.
When these significant findings were further deconstructed, the negative vs neutral condition drove the PDE-associated findings in the bilateral sFG (left: CE = –0.08, p = .003, CI = –0.14, –0.02; right: CE = –0.08, p = .003, CI = –0.14, –0.02) and bilateral Fus (left: CE = –0.08, p = .004, CI = –0.02, –0.14; right: CE = 0.06, p = .03, CI = 0.001, 0.12).
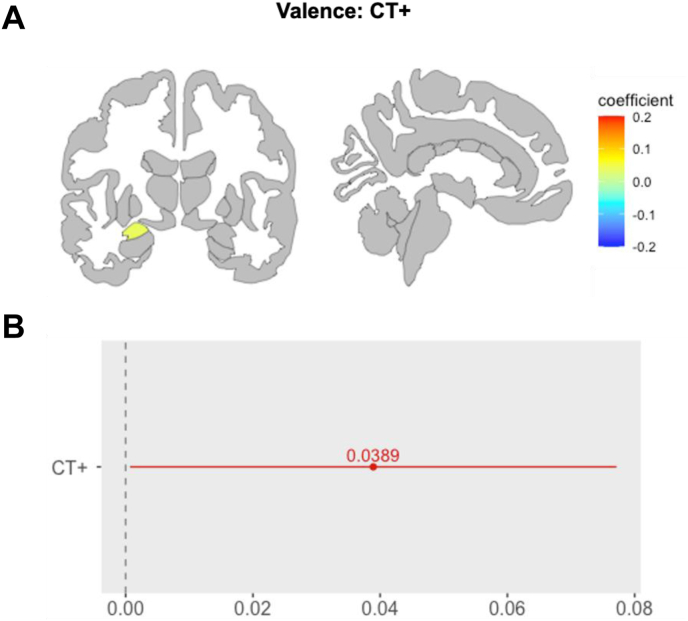
CT (CT+ vs CT–): CT+ youth showed heightened response in the left amygdala (CE = 0.039, pFDR = .046, CI = 0.00, 0.08), compared to CT– youth (Figure 2A and B).
Figure 2.
Main Effect of Childhood Trauma on Region of Interest (ROI) Activation in the Valence Condition
Note:In the main effect model, during the valence condition (A) CT+ was associated with greater activity in the left amygdala. Gray represents both unexplored and statistically non-significant regions. The coefficient describes the effect size and direction of the effect. (B) The red line shows the variation in effect size for CT+ compared to CT– (0-line). CT = childhood trauma.
This finding was driven by the negative vs neutral condition (CE = 0.04, p = .05, CI = 0.0008, 0.08). This model did not exhibit sufficient statistical power (amygdala; left: 1 – β = 0.36, CI = 0.2664, 0.4621) (see Table S7, available online).
Single-valence results that did not meet the significant threshold of p < .05 are catalogued in the supplemental material.
Interaction Effect
The interaction mixed models revealed reduced activity in a widespread pattern that was unique for different groups.
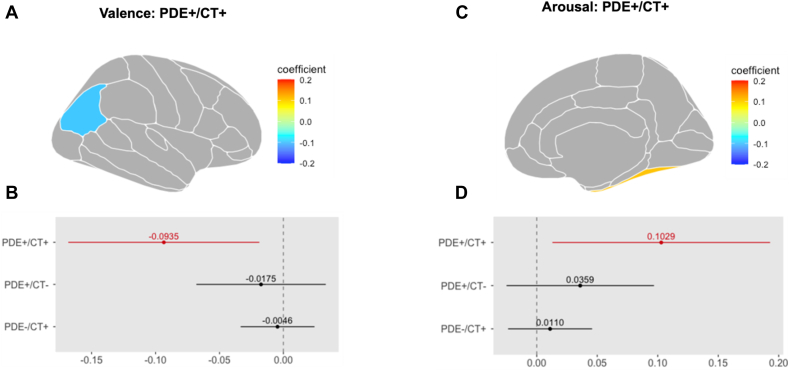
(PDE+/CT+ vs PDE–/CT–): There was reduced activity in the bilateral sFG (left: CE = –0.093, pFDR = .048, CI = –0.18, 0.01; right: CE = –0.099, pFDR = .045, CI = –0.12, –0.01), and in the left iPL (CE = –0.094, pFDR = .042, CI = –0.17, –0.02), compared to the PDE–/CT– control group (Figure 3A and B).
Figure 3.
Interaction Effects of Prenatal Drug Exposure and Childhood Trauma on Region of Interest (ROI) Activation in the Valence Condition
Note:(A) A double-hit specific decrease in the left inferior parietal lobule activity was revealed in the interaction models during the valence condition. Gray represents both unexplored and statistically non-significant regions. The coefficient describes the effect size and direction of the effect. (B) Whereas PDE+/CT+ was significantly associated with this effect, other subgroups were not. The dashed 0-line represents the PDE–/CT– reference group. (C) A double-hit specific increase in the right fusiform gyrus activity was revealed in the interaction models during the arousal condition. Gray represents both unexplored and statistically non-significant regions. The coefficient describes the effect size and direction of the effect. (D) Whereas PDE+/CT+ was significantly associated with this effect, other subgroups were not. The dashed 0-line represents the PDE–/CT– (reference) group. CT = childhood trauma; PDE = prenatal drug exposure.
This model was sufficiently powered in the right sFG: 1 – β = 0.81, CI = 0.7193, 0.8816 (see Table S7, available online).
(PDE+/CT– vs PDE–/CT–): There was reduced activity in the bilateral sFG (left: CE = –0.073, pFDR = .042, CI = –0.13, –0.02; right: CE = –0.073, pFDR = .042, CI = –0.13, –0.01), and also in the bilateral Fus (left: CE = –0.063, pFDR = .05, CI = –0.13, 0.00; right: CE = –0.073, pFDR = .048, CI = –0.14, 0.00) and in the right isthmus cingulate cortex (iCC: CE = –0.068, pFDR = .048, CI = –0.13, 0.00), compared to those in the PDE–/CT– control group.
The bilateral sFG findings were driven by the negative vs neutral condition (left: CE = –0.08, p = .03, CI = –0.1584, –0.0016; right: left: CE = –0.08, p = 0.03, CI = –0.1584, –0.0016).
(PDE–/CT+ vs PDE–/CT–): No significant activations were found for the PDE–/CT+ group.
These results suggest that the reduced response during the valence contrast in the bilateral sFG are primarily accounted for by the PDE main effect. In contrast, the blunted response in the left iPL was indexed in the PDE+/CT+ group, but not in the PDE+/CT– group, suggesting that this effect is specific to the double-hit group. In contrast, the blunted activity in the bilateral Fus, which was also observed in the PDE main effect and was absent in the double-hit (PDE+/CT+) group, was likely specific to the PDE+/CT– group.
None of these models exhibited sufficient statistical power (see Table S7, available online).
Arousal Contrast
The arousal contrast was the difference between the average of negative and positive relative to neutral trials (ie, fearful and happy minus neutral faces).
Main Effect
PDE (PDE+ vs PDE–): The PDE main effect model revealed significantly greater activity in the right Fus (CE = 0.053, pFDR = .047, CI = 0.00, 0.10) in the PDE+ compared to the PDE– groups. This model was not sufficiently powered (Fus; right: 1 – β = 0.50, CI = 0.3983, 0.6017) (see Table S7, available online).
CT (CT+ vs CT–). No significant activations were found for this comparison.
Interaction Effect (PDE+/CT+ vs PDE–/CT–): The interaction mixed models revealed that the double-hit group showed greater activity in the right Fus (CE = 0.103, pFDR = .045, CI = 0.01, 0.19) compared to the PDE–/CT– group (Figure 3C and D). All other effects did not yield statistically significant activation patterns.
This model was not sufficiently statistically powered (Fus; right: 1 – β = 0.48, CI = 0.3790, 0.5822) (see Table S7, available online).
(PDE+/CT– vs PDE–/CT–): No significant activations were found for this comparison.
Overfitting Analysis
From visual inspection, we did not observe any noticeable increase in RMSE across the cross-validation test set performances (see Figure S3, available online).
Additional Analyses With Main Effects as Continuous Variables
For mothers who reported marijuana and tobacco use after knowing that they were pregnant, we curated the frequencies of the number of times per day that they used these substances (see Figure S4, available online). Frequency data were not available for alcohol. The models examining the association between the significant ROIs and PDE and CT severity/cumulative risk did not yield statistically significant results.
Correlation Analysis
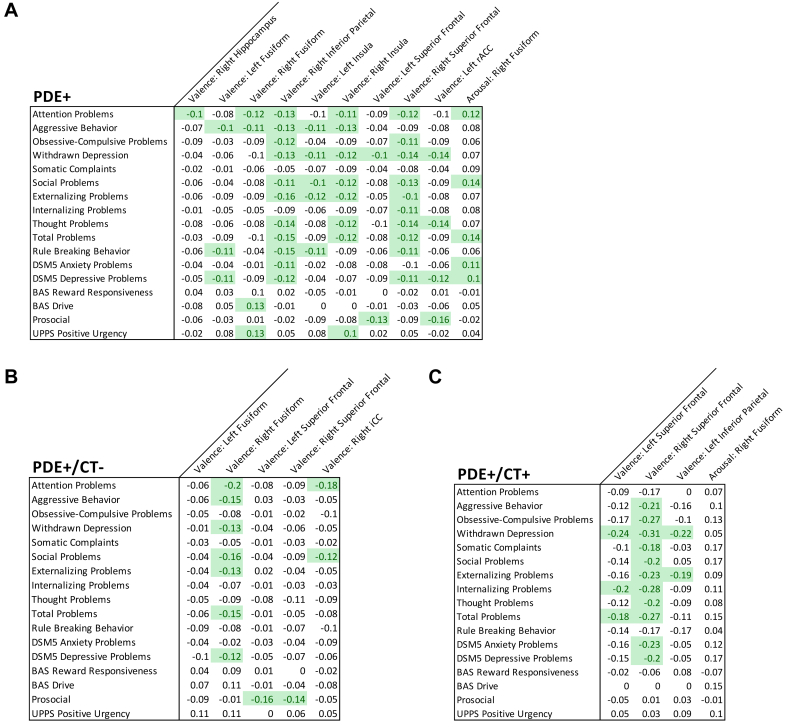
We performed correlational analyses between the significant ROI findings of main effects and behavioral measures from the CBCL, BIS BAS, the UPPS-P, and the PBS to explore whether the observed differences in brain function may be linked to behavioral problems. We found that reduced cortical activity in the valence contrast in the PDE+ group, specifically in the sFG, were correlated with higher scores on several behavioral problems (Figure 4A). Correlations with PDE subgroups revealed that such reduced activity was correlated with higher behavioral problems in the double-hit group (Figure 4C) but not in the PDE+/CT– group (Figure 4B).
FIGURE 4.
Correlations Between Subclinical Problematic Behaviors and Beta Contrasts of Significant Region of Interest (ROI) Activations
Note:Matrices illustrate correlations between ROIs significant in mixed models with clinical symptoms from scales including CBCL, UPPS, BIS BAS, and Youth Prosocial Behavior Survey for (A) PDE+ children, and interaction subgroups (B) PDE+/CT– and(C)PDE+/CT+ children. Green-shaded cells are significant at p < .05 after having been false discovery rate corrected for multiple comparisons. CBCL = Child Behavior Checklist; BIS BAS = behavioral inhibition system and behavioral activation system; CT = childhood trauma; PDE = prenatal drug exposure.
Discussion
The aim of this cross-sectional study was to evaluate a large sample of children (N = 6,146) enrolled in the ABCD Study for independent and interactive impacts of prenatal exposure to commonly used substances (alcohol, cannabis, and tobacco) and traumatic experiences on emotion processing. The results show that PDE was associated with lower activity in widespread cortical regions while youth were viewing fearful relative to happy faces in the EN-back task. Within the PDE subgroups, reduced activity in the bilateral sFG was found to be specific to PDE, irrespective of CT. Reduced activity in the left iPL was unique to the double-hit group, as it occurred in PDE youth who also had CT, and not in those without CT. In addition, youth with CT showed heightened activity in the left amygdala when viewing fearful relative to happy faces and fearful relative to neutral faces, a finding largely in line with existing literature.77 However, while viewing any emotional (fearful or happy) relative to neutral faces, youth in the double-hit group showed significantly higher activity in the right Fus. Finally, reduced cortical activity in the PDE group as well as in the double-hit group while viewing fearful relative to happy faces was associated with greater behavioral problems.
The finding of PDE associated with lower activity in the sFG, Fus, insular, and parietal cortices while viewing fearful relative to happy faces is novel. However, corroborating evidence from preclinical studies demonstrated decreased c-fos mRNA expression (a marker of lower activity) in the lateral and central nuclei of the amygdala as well as the ACC in rats that were prenatally exposed to alcohol.78 In addition, reduced valence-related activity in the rACC is also novel, but is in line with findings from prior studies showing smaller rACC volumes in children with prenatal alcohol exposure, which in turn have been associated with slower behavioral inhibition.22 Reduced activity in the hippocampus is also consistent with a previous study in which maternal urine tetrahydrocannabinol (THC) positivity was associated with decreased fetal hippocampal connectivity to nodes in the insular, frontal, cingulate, and temporal cortices. In turn, this decreased connectivity was associated with worse behavioral outcomes at age 5 years.79 CT was associated with greater activity in the left amygdala elicited by fearful relative to neutral faces, which is consistent with a finding seen extensively in the literature that trauma-exposed youth demonstrate amygdala hyperactivation to negative stimuli.77
The double-hit group showed blunted activity in the iPL while viewing fearful compared to happy faces. The iPL is a part of the TPJ and is involved in implicit emotion regulation and modulation of interpersonal emotions.80 In a recent study of the entire ABCD sample, the iPL was shown to be involved in implicit emotion regulation during the same EN-back task, with its activation correlated with the number of close friends, suggesting a role of the iPL in social behavior.73 However, we did not find such an association within in the double-hit group, perhaps due to the difference in contrast for the selected ROI activation. The iPL has also been implicated in stimulus-driven reorientation of attention,81 and therefore blunted activity in the iPL while viewing fearful compared to happy faces might suggest lower allocation of attentional resources to negative as compared to positive stimuli. In addition, studies have reported significantly reduced gray matter volume in the iPL between the participants who had experienced CT compared to those who did not, further suggesting that both PDE and CT are associated with changes to structure and function of iPL.82
Also in the double-hit group, we observed greater activity in the right Fus during the arousal condition. Since the EN-back involved facial stimuli, such heightened activity in the arousal condition may suggest that youth in the double-hit group required greater recruitment of the Fus in response to emotional (negative and positive) as compared with neutral face stimuli to achieve the same performance. These findings are consistent with prior reports of greater activity in the fusiform gyri when viewing emotionally arousing pictures83 in individuals with CT compared to those without CT.84 Moreover, prenatal alcohol exposure has been positively correlated with cortical volume in the right Fus.85 Together, these findings suggest that in children with PDE and CT may have structural and functional changes in the Fus.
The finding in the bilateral sFG supports the hypothesized interaction between PDE and CT, such that, regardless of later CT exposure, PDE is associated with reduced activity of the bilateral sFG while viewing fearful relative to happy faces as well as fearful faces relative to neutral faces. Further examination of this interaction showed that within youth exposed to CT, those who also had PDE showed exacerbated reduction in the sFG activity, as compared to those without PDE. Yet, importantly, task behavior was comparable across groups, highlighting that differing activation patterns emerged to achieve the same performance.
Although the sample is largely non-clinical, reduced sFG activity was significantly associated with behavioral problems in the PDE subgroup with CT (the double-hit group) and not in other subgroups. Thus, exposure to PDE is associated with lower response bias in the sFG in response to fearful relative to happy faces, irrespective of traumatic experiences, and the association with behavioral symptoms emerges only when there is this second “hit” (ie, PDE youth with CT). These observations suggest possible cumulative effect of these 2 environmental factors on cortical activation during a critical developmental period. This may reflect attenuation of top-down emotion regulation during a traumatic experience, which is known to be a risk factor for the development of psychiatric symptoms and post-traumatic stress disorder (PTSD).86
The current study had several limitations. First, our results yielded small effect sizes, which is consistent with findings from other studies conducted with ABCD’s diverse sample.87 Nevertheless, because sampling error is often minimal in these cases, small effect sizes may still have clinical significance.88 Another consideration of the small effect sizes is related to the use of large heterogeneous pre-selected ROI areas. Future studies might use a whole-brain or network-based approach to examine more precise neural substrates.89
Another limitation is the use of parent self-report questionnaires for defining both PDE and CT. Indeed, retrospective and self-report assessments of substance use are less robust methods compared to testing biospecimens.90 Furthermore, self-report of substance use is subject to underreporting bias, particularly in a research setting with substances that are associated with strong social stigma.91 This mirrors the risk that birthing parents face when disclosing substance use, with punitive legal action or family separation as a possible outcome.92 Recall of any event 10 years after it occurs is likely poor. Moreover, the KSADS-PTSD module parent report is likely not the optimal assessment of the child’s traumatic experiences, as items include parent or adult-driven violence. This could limit parent reporting, especially to a study personnel without a strong established therapeutic relationship. This presents a limitation that traumas in the home may not be fully represented.
Another limitation of this study is the inability to differentiate between patterns of substance use during pregnancy, and between type, severity, and level of exposure to trauma. In both cases, the lack of granularity may lead to overgeneralization about the effect of the exposure on the developing brain. Although supplementary analyses were performed to treat PDE as continuous rather than binary, this observational cohort was not designed to study severity, and no significant results were found. The frequency and quantity of substance exposure are key next steps in translating PDE findings to functional guidelines.93 Furthermore, alcohol, cannabis, and tobacco have distinct pharmacodynamic and pharmacokinetic properties, and larger sample sizes are needed to characterize the overlapping and independent effects on development. In addition, although we know that exposure during critical periods of gestation poses an increased risk to the fetus, the only specific information known about timing of exposure was that it occurred after the pregnancy was known.49 Thus, although these results provide rationale for further research on the impact of prenatal exposure on the developing brain, these results are not sufficient for making clinical or behavioral recommendations, or dictating policy for pregnant women and their families.
Finally, the study was also limited because of the insufficient power across the ROIs, except for that involving the sFG, our main finding, which were adequately powered.
Nevertheless, this study is a necessary first step in examining the interactive effects of prenatal and early-life exposures and accounting for many aspects of the sociodemographic and psychological environment. Notable were the differences in environmental variables (eg, race, household income, neighborhood safety, parent psychiatric history, etc) (Table 1) between the CT and PDE groups, which are typically not examined in studies of PDE.
There are also important considerations regarding the ABCD EN-back task, such that the emotional task is embedded within a working memory task, and there are many variations in task design, such as 1 study placing emotional distractors between memory-based stimuli.94 In addition, there are varying analytic approaches, with some studies including both 0-back and 2-back conditions in emotion contrasts, and other studies using memory-load specific emotion contrasts.95, 96, 97 Prior literature suggests that implicit regulation is required for goal-directed behavior in similar tasks, so our results are interpreted within the context of implicit emotional conflict regulation.98 Replication with a well-validated emotion regulation task is required to make rigorous conclusions regarding the effects of PDE and CT on emotion regulation.
Finally, because PDE and CT were nested and highly collinear, their interaction could not be assessed in the same statistical model as the main effects. Therefore, separate mixed models had to be used to investigate the main effects and the interactive effects on brain and behavior. Model choice was limited by the need to account for random effects in the sample.
Taken together, the unique independent and interactive effects of PDE and CT on brain activation during an emotion processing task highlight the potential impact of the prenatal (ie, effects of exposure to widely used legal substances during pregnancy) and postnatal (ie, early life adversity) environments on brain development. In addition to showing that these influences are associated with differential neural mechanisms underlying emotion processing in affected children, we also showed that these differences in brain activity are linked to higher internalizing and externalizing symptoms in this largely subclinical, nationally representative sample of youth. Furthermore, although the breadth of data collected by the ABCD Study allowed for contextualizing exposures such as PDE and CT in a broader biopsychosocial picture, these results make the case for future longitudinal large cohort studies, such as the HEALthy Brain and Child Development (HBCD) study. An important future direction will be to prospectively collect data using objective measures of prenatal drug exposure to better understand whether low-to-moderate doses, and of which substances, have an impact on fetal development. In addition, it is imperative to develop more sophisticated approaches for distinguishing between the impact of social factors and the impact attributed to substance or trauma exposure, so that evidence-based policy may be shaped to support families. Given the longitudinal nature of the ABCD Study, following these children identified as being at-risk will improve understanding of vulnerability vs resilience to the development of clinical syndromes. Efforts in these areas will certainly facilitate the development of holistic preventive strategies and treatment interventions.
In summary, we showed a widespread reduction in cortical response bias to negative relative to positive stimuli in youth with PDE compared to those without PDE. Such reduced response bias, specifically in the bilateral sFG and Fus as well as the right isthmus cingulate, appear to be primarily accounted for by the PDE. In contrast, reduced response bias to negative compared to positive stimuli in the left iPL was present only in the double-hit PDE+/CT+ group. In addition, CT+ was associated with a heightened response bias to emotional relative to non-emotional stimuli in the left amygdala. We further showed that the PDE- and PDE/CT-related reductions in response bias to negative relative to positive stimuli in cortical regions were associated with elevated scores on problematic behavior inventories. These findings may be useful for guiding future longitudinal gestational and developmental studies, for example, the National Institutes of Health–funded HEALthy Brain and Child Development study. Although that study has a special interest in birthing parents with opioid use disorders, our findings call for further investigation of prenatal cannabis, tobacco, and alcohol exposure, including when parental use is at non-clinical, population levels.
CRediT authorship contribution statement
Lauren Lepow: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Ariella Wagner: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization. Siddhartha Peri: Writing – review & editing, Data curation. Faith Adams: Writing – review & editing, Formal analysis, Data curation. Srinivasan Anantha Ramakrishnan: Writing – review & editing, Data curation. Md Ashad Alam: Supervision, Methodology, Formal analysis. Riaz B. Shaik: Writing – review & editing, Conceptualization. Nicholas A. Hubbard: Writing – review & editing. Harold W. Koenigsberg: Writing – review & editing. Yasmin Hurd: Writing – review & editing. Susan F. Tapert: Writing – review & editing. Iliyan Ivanov: Writing – review & editing, Supervision, Conceptualization. Muhammad A. Parvaz: Writing – review & editing, Writing – original draft, Supervision, Resources, Investigation, Funding acquisition, Conceptualization.
Footnotes
This article is part of a special series devoted to the subject of substance use, featuring topics relevant to child and adolescent behavioral health, including genetics, neuroscience, epidemiology, measurement, prevention, and treatment. This special series is edited by Guest Editor Kevin M. Gray, MD, JAACAP Open Deputy Editor Kara S. Bagot, MD, JAACAP Deputy Editor Mary Fristad, PhD, ABPP, JAACAP and JAACAP Open Associate Editor Robert R. Althoff, MD, PhD, JAACAP Open Editor Manpreet K. Singh, MD, MS, and Editor-in-Chief Douglas K. Novins, MD.
This work was supported by the National Institute on Aging T32AG049688 and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) F31AA031437 to Faith Adams; the Brain and Behavior Research Foundation and the National Institute of General Medical Sciences P20GM130461-6206 to Nicholas A. Hubbard; the National Institute of Mental Health (NIMH) R01MH123069 and R61MH125130 to Harold W. Koenigsberg; the National Institute on Drug Abuse (NIDA) P01DA047233, R01DA055434, R01DA051191, and R01DA048613 to Yasmin L. Hurd; the National Institute on Alcohol Abuse and Alcoholism (NIAAA) U24AA021695 and U01AA021692 and NIDA U01DA041089 to Susan F. Tapert; and NIDA K01DA043615, R01DA058039, and R61DA056779 to Muhammad A. Parvaz.
Dr. Alam served as the statistical expert for this research.
The authors wish to thank Rachel Yehuda, PhD, of the Icahn School of Medicine at Mount Sinai, for her support with conceptualization.
Disclosure: Dr. Lepow has reported being a Bob and Renee Parsons Fellow at the Center for Psychedelic Therapy Research. Drs. Alam, Shaik, Hubbard, Koenigsberg, Hurd, Tapert, Ivanov, and Parvaz, Ms. Wagner, Mr. Peri, Ms. Adams, and Mr. Ramakrishnan have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Alati R., MacLeod J., Hickman M., et al. Intrauterine exposure to alcohol and tobacco use and childhood IQ: findings from a parental-offspring comparison within the Avon Longitudinal Study of Parents and Children. Pediatr Res. 2008;64(6):659–666. doi: 10.1203/PDR.0b013e318187cc31. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary C.M., Bower C. Guidelines for pregnancy: what’s an acceptable risk, and how is the evidence (finally) shaping up? Drug Alcohol Rev. 2012;31(2):170–183. doi: 10.1111/j.1465-3362.2011.00331.x. [DOI] [PubMed] [Google Scholar]
- 3.Morie K.P., Zhai Z.W., Potenza M.N., Mayes L.C. Alexithymia, emotion-regulation strategies, and traumatic experiences in prenatally cocaine-exposed young adults. Am J Addict. 2020;29(6):492–499. doi: 10.1111/ajad.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindinger N.M., Jacobson J.L., Dodge N.C., et al. Stability and change in the interpretation of facial emotions in fetal alcohol spectrum disorders from childhood to adolescence. Alcohol Clin Exp Res. 2022;46(7):1268–1281. doi: 10.1111/acer.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson M., Oddy W., McLean N., et al. Low-moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study: low-moderate prenatal alcohol and behavioural development. Br J Obstet Gynecol. 2010;117(9):1139–1152. doi: 10.1111/j.1471-0528.2010.02596.x. [DOI] [PubMed] [Google Scholar]
- 6.Young-Wolff K.C., Ray G.T., Alexeeff S.E., et al. Rates of prenatal cannabis use among pregnant women before and during the COVID-19 pandemic. JAMA. 2021;326(17):1745. doi: 10.1001/jama.2021.16328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B., Xu G., Sun Y., et al. Maternal cigarette smoking before and during pregnancy and the risk of preterm birth: a dose–response analysis of 25 million mother–infant pairs. PLoS Med. 2020;17(8) doi: 10.1371/journal.pmed.1003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popova S., Lange S., Probst C., Gmel G., Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(3):e290–e299. doi: 10.1016/S2214-109X(17)30021-9. [DOI] [PubMed] [Google Scholar]
- 9.Ashford J., Smit F., van Lier P.A.C., Cuijpers P., Koot H.M. Early risk indicators of internalizing problems in late childhood: a 9-year longitudinal study. J Child Psychol Psychiatry. 2008;49(7):774–780. doi: 10.1111/j.1469-7610.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 10.Tien J., Lewis G.D., Liu J. Prenatal risk factors for internalizing and externalizing problems in childhood. World J Pediatr. 2020;16(4):341–355. doi: 10.1007/s12519-019-00319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Southwick S.M., Bonanno G.A., Masten A.S., Panter-Brick C., Yehuda R. Resilience definitions, theory, and challenges: interdisciplinary perspectives. Eur J Psychotraumatol. 2014;5(1) doi: 10.3402/ejpt.v5.25338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halperin E. emotion, emotion regulation, and conflict resolution. Emot Rev. 2014;6(1):68–76. doi: 10.1177/1754073913491844. [DOI] [Google Scholar]
- 13.Zafar H., Debowska A., Boduszek D. Emotion regulation difficulties and psychopathology among Pakistani adolescents. Clin Child Psychol Psychiatry. 2021;26(1):121–139. doi: 10.1177/1359104520969765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware A.L., Infante M.A., O’Brien J.W., et al. An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behav Brain Res. 2015;278:137–146. doi: 10.1016/j.bbr.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien J.W., Norman A.L., Fryer S.L., et al. Effect of predictive cuing on response inhibition in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2013;37(4):644–654. doi: 10.1111/acer.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fryer S.L., Tapert S.F., Mattson S.N., Paulus M.P., Spadoni A.D., Riley E.P. Prenatal alcohol exposure affects frontal–striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 17.Longo C.A., Fried P.A., Cameron I., Smith A.M. The long-term effects of prenatal nicotine exposure on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol. 2013;39:9–18. doi: 10.1016/j.ntt.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Bennett D.S., Mohamed F.B., Carmody D.P., et al. Response inhibition among early adolescents prenatally exposed to tobacco: an fMRI study. Neurotoxicol Teratol. 2009;31(5):283–290. doi: 10.1016/j.ntt.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith A.M., Fried P.A., Hogan M.J., Cameron I. Effects of prenatal marijuana on response inhibition: an fMRI study of young adults. Neurotoxicol Teratol. 2004;26(4):533–542. doi: 10.1016/j.ntt.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Kodali V.N., Jacobson J.L., Lindinger N.M., et al. Differential recruitment of brain regions during response inhibition in children prenatally exposed to alcohol. Alcohol Clin Exp Res. 2017;41(2):334–344. doi: 10.1111/acer.13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomew M.E., Heller W., Miller G.A. Inhibitory control of emotional processing: theoretical and empirical considerations. Int J Psychophysiol. 2021;163:5–10. doi: 10.1016/j.ijpsycho.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Migliorini R., Moore E.M., Glass L., et al. Anterior cingulate cortex surface area relates to behavioral inhibition in adolescents with and without heavy prenatal alcohol exposure. Behav Brain Res. 2015;292:26–35. doi: 10.1016/j.bbr.2015.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall A.T., Bodison S.C., Uban K.A., et al. The impact of prenatal alcohol and/or tobacco exposure on brain structure in a large sample of children from a South African birth cohort. Alcohol Clin Exp Res. 2022;46(11):1980–1992. doi: 10.1111/acer.14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markett S., Heeren G., Montag C., Weber B., Reuter M. Loss aversion is associated with bilateral insula volume. A voxel based morphometry study. Neurosci Lett. 2016;619:172–176. doi: 10.1016/j.neulet.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Gray M.A., Harrison N.A., Wiens S., Critchley H.D. Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One. 2007;2(6) doi: 10.1371/journal.pone.0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Protopopescu X., Pan H., Altemus M., et al. Orbitofrontal cortex activity related to emotional processing changes across the menstrual cycle. Proc Natl Acad Sci USA. 2005;102(44):16060–16065. doi: 10.1073/pnas.0502818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Temple V.K., Cook J.L., Unsworth K., Rajani H., Mela M. Mental health and affect regulation impairment in fetal alcohol spectrum disorder (FASD): results from the Canadian National FASD Database. Alcohol Alcohol. 2019;54(5):545–550. doi: 10.1093/alcalc/agz049. [DOI] [PubMed] [Google Scholar]
- 28.De Water E., Rockhold M.N., Roediger D.J., et al. Social behaviors and gray matter volumes of brain areas supporting social cognition in children and adolescents with prenatal alcohol exposure. Brain Res. 2021;1761 doi: 10.1016/j.brainres.2021.147388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark C.A.C., Espy K.A., Wakschlag L. Developmental pathways from prenatal tobacco and stress exposure to behavioral disinhibition. Neurotoxicol Teratol. 2016;53:64–74. doi: 10.1016/j.ntt.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornelius M., Goldschmidt L., DeGenna N., Day N. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9(7):739–750. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Genna NM., Richardson G.A., Goldschmidt L., Day N.L., Cornelius M.D. Prenatal exposures to tobacco and cannabis: associations with adult electronic cigarette use. Drug Alcohol Depend. 2018;188:209–215. doi: 10.1016/j.drugalcdep.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried P.A., Watkinson B., Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicol Teratol. 1992;14(5):299–311. doi: 10.1016/0892-0362(92)90036-A. [DOI] [PubMed] [Google Scholar]
- 33.Day N.L., Leech S.L., Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33(1):129–136. doi: 10.1016/j.ntt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Marroun H., Bolhuis K., Franken I.H.A., et al. Preconception and prenatal cannabis use and the risk of behavioural and emotional problems in the offspring; a multi-informant prospective longitudinal study. Int J Epidemiol. 2019;48(1):287–296. doi: 10.1093/ije/dyy186. [DOI] [PubMed] [Google Scholar]
- 35.Cioffredi L.A., Anderson H., Loso H., et al. Prenatal cannabis exposure predicts attention problems, without changes on fMRI in adolescents. Neurotoxicol Teratol. 2022;91 doi: 10.1016/j.ntt.2022.107089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larsen S.F., Johnson A.J., Dager S.R., Kleinhans N.M. Cannabis Use, Neurobiology, Psychology, and Treatment. Elsevier; 2023. The impact of prenatal cannabis exposure: an overview; pp. 55–69. [DOI] [Google Scholar]
- 37.Young J.C., Widom C.S. Long-term effects of child abuse and neglect on emotion processing in adulthood. Child Abuse Neglect. 2014;38(8):1369–1381. doi: 10.1016/j.chiabu.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wymbs N.F., Orr C., Albaugh M.D., et al. Social supports moderate the effects of child adversity on neural correlates of threat processing. Child Abuse Neglect. 2020;102 doi: 10.1016/j.chiabu.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S.W., Kim S., Lee S.J., et al. Effects of emotional maltreatment on semantic network activity during cognitive reappraisal. Brain Imaging Behav. 2021;15(3):1181–1190. doi: 10.1007/s11682-020-00318-2. [DOI] [PubMed] [Google Scholar]
- 40.Herringa R.J., Birn R.M., Ruttle P.L., et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zielinski M.J., Privratsky A.A., Smitherman S., Kilts C.D., Herringa R.J., Cisler J.M. Does development moderate the effect of early life assaultive violence on resting-state networks? An exploratory study. Psychiatry Res Neuroimaging. 2018;281:69–77. doi: 10.1016/j.pscychresns.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassiers L.L.M., Sabbe B.G.C., Schmaal L., Veltman D.J., Penninx B.W.J.H., Van Den Eede F. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: a systematic review of neuroimaging findings. Front Psychiatry. 2018;9:329. doi: 10.3389/fpsyt.2018.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu B., Wei S., Yin X., Jin X., Yan S., Jia L. The relationship between childhood emotional neglect experience and depressive symptoms and prefrontal resting functional connections in college students: the mediating role of reappraisal strategy. Front Behav Neurosci. 2023;17 doi: 10.3389/fnbeh.2023.927389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gold A.L., Sheridan M.A., Peverill M., et al. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Child Psychol Psychiatry. 2016;57(10):1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamner M.B., Lorberbaum J.P., George M.S. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety. 1999;9(1):1–14. [PubMed] [Google Scholar]
- 46.Min M.O., Minnes S., Kim J.Y., Yoon M., Singer L.T. Association of prenatal cocaine exposure, childhood maltreatment, and responses to stress in adolescence. Drug Alcohol Depend. 2017;177:93–100. doi: 10.1016/j.drugalcdep.2017.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barch D.M., Albaugh M.D., Avenevoli S., et al. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Dev Cogn Neurosci. 2018;32:55–66. doi: 10.1016/j.dcn.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lees B., Mewton L., Jacobus J., et al. Association of prenatal alcohol exposure with psychological, behavioral, and neurodevelopmental outcomes in children from the Adolescent Brain Cognitive Development Study. Am J Psychiatry. 2020;177(11):1060–1072. doi: 10.1176/appi.ajp.2020.20010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Organization of Teratology Information Specialists (OTIS) Mother To Baby. Fact Sheets; 1994. Critical Periods of Development.http://www.ncbi.nlm.nih.gov/books/NBK582659/ [PubMed] [Google Scholar]
- 50.Barch D.M., Burgess G.C., Harms M.P., et al. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage. 2013;80:169–189. doi: 10.1016/j.neuroimage.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casey B.J., Cannonier T., Conley M.I., et al. The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kauschke C., Bahn D., Vesker M., Schwarzer G. The role of emotional valence for the processing of facial and verbal stimuli—positivity or negativity bias? Front Psychol. 2019;10:1654. doi: 10.3389/fpsyg.2019.01654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballotta D., Maramotti R., Borelli E., Lui F., Pagnoni G. Neural correlates of emotional valence for faces and words. Front Psychol. 2023;14 doi: 10.3389/fpsyg.2023.1055054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Citron F.M.M., Gray M.A., Critchley H.D., Weekes B.S., Ferstl E.C. Emotional valence and arousal affect reading in an interactive way: neuroimaging evidence for an approach-withdrawal framework. Neuropsychologia. 2014;56:79–89. doi: 10.1016/j.neuropsychologia.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayer M., Schacht A. Event-related brain responses to emotional words, pictures, and faces: a cross-domain comparison. Front Psychol. 2014;5 doi: 10.3389/fpsyg.2014.01106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gingnell M., Bannbers E., Moes H., et al. Emotion reactivity is increased 4-6 weeks postpartum in healthy women: a longitudinal fMRI study. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lungu O., Potvin S., Tikàsz A., Mendrek A. Sex differences in effective fronto-limbic connectivity during negative emotion processing. Psychoneuroendocrinology. 2015;62:180–188. doi: 10.1016/j.psyneuen.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 58.Donegan N.H., Sanislow C.A., Blumberg H.P., et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54(11):1284–1293. doi: 10.1016/S0006-3223(03)00636-X. [DOI] [PubMed] [Google Scholar]
- 59.Goodman M., Carpenter D., Tang C.Y., et al. Dialectical behavior therapy alters emotion regulation and amygdala activity in patients with borderline personality disorder. J Psychiatr Res. 2014;57:108–116. doi: 10.1016/j.jpsychires.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guitart-Masip M., Pascual J.C., Carmona S., et al. Neural correlates of impaired emotional discrimination in borderline personality disorder: an fMRI study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(8):1537–1545. doi: 10.1016/j.pnpbp.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Koenigsberg H.W., Fan J., Ochsner K.N., et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry. 2009;66(9):854–863. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koenigsberg H.W., Siever L.J., Lee H., et al. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res Neuroimaging. 2009;172(3):192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minzenberg M.J., Fan J., New A.S., Tang C.Y., Siever L.J. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res Neuroimaging. 2007;155(3):231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niedtfeld I., Schulze L., Kirsch P., Herpertz S.C., Bohus M., Schmahl C. Affect regulation and pain in borderline personality disorder: a possible link to the understanding of self-injury. Biol Psychiatry. 2010;68(4):383–391. doi: 10.1016/j.biopsych.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 65.Schnell K., Herpertz S.C. Effects of dialectic-behavioral-therapy on the neural correlates of affective hyperarousal in borderline personality disorder. J Psychiatr Res. 2007;41(10):837–847. doi: 10.1016/j.jpsychires.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Hagler D.J., Hatton SeanN., Cornejo M.D., et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White S.F., Adalio C., Nolan Z.T., Yang J., Martin A., Blair J.R. The amygdala’s response to face and emotional information and potential category-specific modulation of temporal cortex as a function of emotion. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M., Tsai S.J., Li C.S.R. Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: a meta-analysis of the effects of valence of emotion and passive exposure vs active regulation. Brain Struct Funct. 2020;225(1):345–363. doi: 10.1007/s00429-019-02007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed S.P., Bittencourt-Hewitt A., Sebastian C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev Cogn Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang L.J., Gianaros P.J., Manuck S.B., Krishnan A., Wager T.D. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13(6) doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koush Y., Pichon S., Eickhoff S.B., Van De Ville D., Vuilleumier P., Scharnowski F. Brain networks for engaging oneself in positive-social emotion regulation. Neuroimage. 2019;189:106–115. doi: 10.1016/j.neuroimage.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 73.Geckeler K.C., Barch D.M., Karcher N.R. Associations between social behaviors and experiences with neural correlates of implicit emotion regulation in middle childhood. Neuropsychopharmacology. 2022;47(6):1169–1179. doi: 10.1038/s41386-022-01286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Achenbach T.M. In: The use of psychological testing for treatment planning and outcomes assessment. 2nd ed. Maruish M.E., editor. Lawrence Erlbaum Associates Publishers; 1999. The Child Behavior Checklist and related instruments; pp. 429–466. [Google Scholar]
- 75.Hewig J., Hagemann D., Seifert J., Naumann E., Bartussek D. The relation of cortical activity and BIS/BAS on the trait level. Biol Psychol. 2006;71(1):42–53. doi: 10.1016/j.biopsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Green P., MacLeod C.J. SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol. 2016;7(4):493–498. doi: 10.1111/2041-210X.12504. [DOI] [Google Scholar]
- 77.Jenness J.L., Peverill M., Miller A.B., et al. Alterations in neural circuits underlying emotion regulation following child maltreatment: a mechanism underlying trauma-related psychopathology. Psychol Med. 2021;51(11):1880–1889. doi: 10.1017/S0033291720000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lam V.Y.Y., Raineki C., Takeuchi L.E., Ellis L., Woodward T.S., Weinberg J. Chronic stress alters behavior in the forced swim test and underlying neural activity in animals exposed to alcohol prenatally: sex- and time-dependent effects. Front Behav Neurosci. 2018;12:42. doi: 10.3389/fnbeh.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomason M.E., Palopoli A.C., Jariwala N.N., et al. Miswiring the brain: human prenatal Δ9-tetrahydrocannabinol use associated with altered fetal hippocampal brain network connectivity. Dev Cogn Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grecucci A., Giorgetta C., Bonini N., Sanfey A.G. Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Front Hum Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Igelström K.M., Graziano M.S.A. The inferior parietal lobule and temporoparietal junction: a network perspective. Neuropsychologia. 2017;105:70–83. doi: 10.1016/j.neuropsychologia.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Fan J., Liu W., Xia J., et al. Childhood trauma is associated with social anhedonia and brain gray matter volume differences in healthy subjects. Brain Imaging Behav. 2022;16(5):1964–1972. doi: 10.1007/s11682-022-00666-1. [DOI] [PubMed] [Google Scholar]
- 83.Mather M., Mitchell K.J., Raye C.L., Novak D.L., Greene E.J., Johnson M.K. Emotional arousal can impair feature binding in working memory. J Cogn Neurosci. 2006;18(4):614–625. doi: 10.1162/jocn.2006.18.4.614. [DOI] [PubMed] [Google Scholar]
- 84.Edmiston E.K., Blackford J.U. Childhood maltreatment and response to novel face stimuli presented during functional magnetic resonance imaging in adults. Psychiatry Res Neuroimaging. 2013;212(1):36–42. doi: 10.1016/j.pscychresns.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uban K.A., Jonker D., Donald K.A., et al. Associations between prenatal alcohol and tobacco exposure and cortical and subcortical brain measures in South African Children: a pilot study. Pediatrics. 2022 doi: 10.1101/2022.06.07.22276078. [DOI] [Google Scholar]
- 86.Nicholson A.A., Friston K.J., Zeidman P., et al. Dynamic causal modeling in PTSD and its dissociative subtype: bottom-up vs top-down processing within fear and emotion regulation circuitry. Hum Brain Mapp. 2017;38(11):5551–5561. doi: 10.1002/hbm.23748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Owens M.M., Potter A., Hyatt C.S., et al. Recalibrating expectations about effect size: a multi-method survey of effect sizes in the ABCD Study. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verbeek J., Hoving J., Boschman J., Chong L.Y., Livingstone-Banks J., Bero L. Systematic reviews should consider effects from both the population and the individual perspective. Am J Public Health. 2021;111(5):820–825. doi: 10.2105/AJPH.2020.306147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hubbard N.A., Auerbach R.P., Siless V., et al. Connectivity patterns evoked by fearful faces demonstrate reduced flexibility across a shared dimension of adolescent anxiety and depression. Clin Psychol Sci. 2023;11(1):3–22. doi: 10.1177/21677026221079628. [DOI] [Google Scholar]
- 90.Charness M.E., Riley E.P., Sowell E.R. Drinking during pregnancy and the developing brain: is any amount safe? Trends Cogn Sci. 2016;20(2):80–82. doi: 10.1016/j.tics.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khalili P., Nadimi A.E., Baradaran H.R., et al. Validity of self-reported substance use: research setting vs primary health care setting. Subst Abuse Treat Prev Policy. 2021;16(1):66. doi: 10.1186/s13011-021-00398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barber C.M., Terplan M. Principles of care for pregnant and parenting people with substance use disorder: the obstetrician gynecologist perspective. Front Pediatr. 2023;11 doi: 10.3389/fped.2023.1045745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singer L.T., Chambers C., Coles C., Kable J. Fifty years of research on prenatal substances: lessons learned for the opioid epidemic. Adv Res Sci. 2020;1(4):223–234. doi: 10.1007/s42844-020-00021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z., Coles C.D., Lynch M.E., et al. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol Teratol. 2009;31(6):342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Brien K.J., Barch D.M., Kandala S., Karcher N.R. Examining specificity of neural correlates of childhood psychotic-like experiences during an emotional n-back task. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(6):580–590. doi: 10.1016/j.bpsc.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Demidenko M.I., Ip K.I., Kelly D.P., Constante K., Goetschius L.G., Keating D.P. Ecological stress, amygdala reactivity, and internalizing symptoms in preadolescence: is parenting a buffer? Cortex. 2021;140:128–144. doi: 10.1016/j.cortex.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosenberg M.D., Martinez S.A., Rapuano K.M., et al. Behavioral and neural signatures of working memory in childhood. J Neurosci. 2020;40(26):5090–5104. doi: 10.1523/JNEUROSCI.2841-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn Emot. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.