Abstract
Objective
Conflicting results have arisen regarding the association between prenatal cannabis exposure and risk of parent-reported developmental delay in infancy. In certain instances, this literature has become outdated or failed to adjust for confounding variables. The current study aimed to determine if prenatal cannabis exposure was associated with a greater likelihood of risk of parent-reported developmental delay at 12 months of age in a contemporary cohort, while adjusting for common confounding variables.
Method
Participants (n = 10,695) were part of the Pregnancy During the COVID-19 Pandemic (PdP) study. A subset of the sample (n = 3,742) provided a parent-report developmental assessment, the Ages and Stages Questionnaire, Third Edition (ASQ-3), of their infant at 12 months old. Sociodemographic differences between participants who reported cannabis use (CU+ group) and those who did not (CU− group) were analyzed. To address potential heterogeneity between CU+ and CU− groups, propensity score weighting was used. G-computations were performed to analyze the association between outcome variables (gestational age, birth weight, and risk of parent-reported developmental delay) and prenatal cannabis exposure. Weighted linear or quasi-binominal logistic regression models were used, with differences of averages and odds ratios reported.
Results
Participants in CU+ and CU− groups significantly differed on all sociodemographic variables. Prenatal cannabis exposure was not associated with any birth outcomes (ps > .05). Prenatal cannabis exposure was significantly associated with risk of parent-reported developmental delay on the communication domain (p = .02). This finding was not significant after adjusting for multiple comparisons. No additional domains were significantly associated (ps > .05).
Conclusion
Prenatal cannabis exposure was associated with increased odds of delay on the communication domain before adjusting for multiple comparisons. No other domains were significantly associated with increased odds of delay. These findings should not be interpreted as suggesting that consuming cannabis products during pregnancy is safe for infant development. Further, the analysis was performed using data from a longitudinal sample that was not specifically created to address this question, but was leveraged to explore these outcomes. Additional studies that are specifically designed to examine these outcomes are needed.
Diversity & Inclusion Statement
We worked to ensure that the study questionnaires were prepared in an inclusive way. We worked to ensure race, ethnic, and/or other types of diversity in the recruitment of human participants. The author list of this paper includes contributors from the location and/or community where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work. One or more of the authors of this paper self-identifies as a member of one or more historically underrepresented sexual and/or gender groups in science.
Key words: Ages and Stages Questionnaire, birth outcomes, cannabis, infant development, pregnancy
Plain language summary
Prior research has been conflicting regarding the link between prenatal cannabis exposure and developmental delays. Secondary analysis of data from the cross-Canada Pregnancy During the COVID-19 Pandemic (PdP) study showed significant sociodemographic differences between individuals who did and did not use cannabis during pregnancy. However, prenatal cannabis exposure was not associated preterm birth or birth weight. Prenatal cannabis exposure was nonsignificantly associated with risk of parent-reported delays in communication. Future research should address these questions and include considerations of quantity of cannabis used.
Cannabis, commonly known as marijuana, is a controlled substance that is consumed worldwide for medicinal (ie, to treat medical symptoms) and recreational (ie, for pleasure, amusement, spiritual, or lifestyle reasons) purposes.1,2 Approximately 192 million people report using cannabis worldwide, rendering cannabis the third most consumed drug, after alcohol and tobacco.2 Despite warnings from governing health bodies advising against the use of cannabis products during preconception, pregnancy, and breastfeeding, cannabis continues to be consumed by some pregnant women.3, 4, 5, 6, 7 Pregnant women commonly report using cannabis for medical purposes, such as treating symptoms of nausea and vomiting associated with pregnancy,8, 9, 10, 11 with some viewing cannabis as an alternative to prescription medications that treat nausea.10 Additional reasons for consuming cannabis during pregnancy include lack of appetite, pain, insomnia, fatigue, stress relief, and symptoms of anxiety and depression.9,11,12 The use of cannabis to treat symptoms of stress, anxiety, and depression may be related to inadequate access to high-quality mental health care for some perinatal women, potentially highlighting a broader societal issue that warrants further investigation.
Approximately 2% to 5% of individuals self-report cannabis use (CU) during pregnancy; however, this statistic likely underestimates actual use.8,13 Recent estimates of prenatal CU across North America also suggest that the prevalence of use is increasing, with a recently published systematic review suggesting that legalization is likely associated with increases in use.14 National reports of use among pregnant women in the United States have significantly increased, rising from 3.4% in 2002/2003 to 7.0% in 2016/2017.15 In a Canadian population-based study, 6.5% of participants between the ages of 15 and 24 reported using cannabis during their pregnancy, significantly increasing from the 4.9% who reported use in 2012.4
The average concentration of tetrahydrocannabinol in seized cannabis samples has also substantially increased over time, from 8.9% in 2008 to 17.1% in 2017.16 Further, analysis of cannabis products sold legally found that the average tetrahydrocannabinol concentration of the cannabis extracts was more than triple that of the cannabis flower, suggesting a desire of consumers to purchase products of higher potency.17 Further, there was a significant increase in purchases of cannabis extracts for inhalation between 2014 and 2016,17 which are often of even higher potency compared with cannabis flower. Increases in frequency of use and potency of cannabis products have led to speculation that effects on infants exposed to cannabis prenatally may now be greater than previously estimated.8,18 With the legalization of cannabis in Canada and increasing prenatal cannabis exposure, in both frequency and potency, it is essential to understand how CU during pregnancy is associated with infant developmental outcomes to provide an evidence-based rationale for prevention and intervention measures.
CU during pregnancy is associated with various sociodemographic and biopsychosocial factors. Reports of prenatal CU are most common among young individuals, with estimates that more than half of North Americans who reported use were between the ages of 15 and 24.4,19 Prenatal CU is also more prevalent among individuals who are non-Hispanic White,12,19,20 who are nonmarried,12,20 and who completed less than 12 years of education.10,19,20 Prenatal CU is also associated with lower household income4,12 and concurrent use of other substances.10,12 Previous analysis of the Pregnancy During the COVID-19 Pandemic (PdP) study (overlapping with this sample) revealed that half of the 4.3% of participants who reported using cannabis during pregnancy were also using alcohol, tobacco, and/or other illicit drugs.21
Previous research on prenatal cannabis exposure has explored associations with fetal outcomes at birth and psychosocial developmental trends throughout childhood and adolescence. Conflicting results have arisen regarding the association between prenatal cannabis exposure and birth weight. Whereas some studies found that prenatal cannabis exposure was related to lower birth weight,22,23 others did not find this association.19,24,25 Similarly conflicting results have arisen for preterm birth: while some studies have shown no association,26,27 others found prenatal cannabis exposure to be associated with increased rates of preterm birth.4,7,28
Studies have identified adverse cognitive and behavioral consequences associated with prenatal cannabis exposure throughout infancy, childhood, and adolescence. Female infants prenatally exposed to cannabis exhibited increased rates of aggression and inattention at 18 months of age29 relative to healthy comparison unexposed infants. Between the ages of 4 and 9 years, exposed children across various cohorts showed poorer performance on verbal reasoning and short-term memory tasks, poorer academic achievement in reading and spelling, delays in language comprehension, and increased hyperactivity and inattention.8,18,24,30,31 Children exposed to cannabis prenatally have also reported significantly higher rates of anxiety and depressive symptoms at age 10.30,32 Animal models have also identified various neurodevelopmental consequences of prenatal cannabis exposure, with several studies finding decreased social behavior, increased anxiety-like behavior, altered approach behaviors toward drug-related stimuli, and long-term memory disruption in rodent offspring prenatally exposed to cannabis.33, 34, 35 These potential neurodevelopmental consequences of prenatal cannabis exposure have recently been captured prenatally in human offspring, with differences in fetal functional brain connectivity identified using fetal functional magnetic resonance imaging.36
Although various significant associations have been identified, the interpretation of these findings must be considered in their specific context. Some prior clinical studies have failed to adjust for important confounders, such as concurrent tobacco exposure, socioeconomic status, or parental mental health.8,37 In light of these limitations, further research is needed to explore the potential cognitive and neurodevelopmental consequences of prenatal cannabis exposure. For example, recent work by Kharbanda et al.27 found no significant association between risk of parent-reported developmental delay at 9 or 12 months of age after accounting for race/ethnicity, maternal age, and tobacco consumption during pregnancy. These findings highlight the need for further investigation regarding the consequences of prenatal cannabis exposure, while considering other important contextual factors that may impact child development. Further, some cohort studies examining associations between child development and prenatal cannabis exposure were initiated in the late 1970s and early 1980s,38 rendering the results outdated.
To address these limitations, the current study used a large cohort spanning across Canada to describe differences in sociodemographic characteristics of participants who reported CU during pregnancy and those who did not and examine the association between prenatal cannabis exposure and instances of preterm birth, differences in birth weight, and risk of parent-reported developmental delay in infants, after adjusting for commonly confounding variables.
Method
Study Design
The current study used data from the PdP39 study, a prospective longitudinal cohort of individuals residing in Canada who were pregnant during the COVID-19 pandemic. Detailed information on study design and data collection is described elsewhere.39 The current study used data from the initial baseline survey (completed between April 5, 2020, and April 29, 2021), follow-up surveys completed during pregnancy (completed between May 8, 2020, and February 2, 2022), and the 12-month postnatal survey (completed between May 28, 2021, and December 14, 2022).
Participants
Participant recruitment for the PdP study occurred between April 5, 2020, and April 9, 2021.39 Participants were recruited using social media advertising and then completed surveys in REDCap (Research Electronic Data Capture). Specific recruitment strategies are described elsewhere.39 To meet the eligibility criteria for enrollment in the PdP study, participants had to be pregnant with a gestational age of ≤35 weeks at enrollment, 17 years of age or older, currently living in Canada, and able to read and write in English or French. A total of 10,695 French and English participants consented to participate in the PdP study, of whom 10,542 provided information on their substance-using behaviors during pregnancy in the initial baseline survey or a follow-up survey during pregnancy. As of August 2023, 3,786 participants completed the Ages and Stages Questionnaire, Third Edition (ASQ-3) portion of the 12-month postpartum survey to evaluate infant development. Only singleton births were included in the final analysis.
Attrition
Participants were initially invited to complete the baseline survey and later invited to complete follow-up surveys. As such, there was attrition between the baseline survey and the 12-month postnatal survey. Of the 10,695 original participants in the cohort, 6,273 provided information on a delivery survey (ie, gestational age, birth weight, child sex), and 3,572 provided information on developmental milestones in the 12-month postnatal survey. Participants who continued in the study were more likely to be older, to be married, to have higher income, to report less severe depression and anxiety symptoms, and to be less likely to report CU during pregnancy. Comparisons are summarized in Table S1, available online. The sociodemographic characteristics of participants who dropped out before the 12-month postnatal survey were identified as risk factors for prenatal CU within the sample; therefore, results pertaining to the association between prenatal cannabis exposure and risk of parent-reported developmental delay may be underestimating the association.
Variables
Risk of Parent-Reported Developmental Delay
Risk of developmental delay was evaluated using the ASQ-340 in the 12-month postnatal survey. The ASQ-3 is a 30-item, parent-reported measure that screens for developmental milestones across 5 domains: communication, gross motor, fine motor, problem-solving, and personal social skills. Parents rate their infant’s current ability with 3 options: “Not yet” (scored 0), “Sometimes” (scored 5), or “Yes” (scored 10). Before questionnaire completion, the child’s birth date and gestational age are collected. If necessary, the child’s age is adjusted to account for prematurity, as per ASQ-3 guidelines.40 The ASQ-3 provides multiple forms to assess for developmental milestones for various age bands. Forms ranging from 12 months to 18 months were administered based on the child’s age at the 12-month postnatal survey, with the majority of participants completing the 12-month form. Sum scores are produced for each developmental domain. Missing responses within a domain are either adjusted for (if 1 or 2 items are missing responses) or nullified if 3 or more items are missing responses. Scores that fall below the predetermined cutoff score, defined as 2 SDs below the normed mean, indicate a risk of developmental delay, and further assessment with a professional is advised.40 Infants were categorized as at risk of developmental delay or not at risk based on this cutoff score. The ASQ-3 has strong reliability (0.93 interrater reliability; 0.92 test-retest reliability)41 and is endorsed by the American Academy of Pediatrics to screen for delayed developmental milestones in children.42
Risk Factors Associated With Developmental Delay
Several risk factors that may contribute to a child experiencing a developmental delay have been identified.43 To capture these risks and isolate them away from any potential impact of CU on developmental risk, we gathered additional sociodemographic and substance use information from participants in the baseline, prenatal follow-up, and postdelivery questionnaires.
At baseline, participants were asked to declare their age, ethnicity, household income before tax, and relationship status. Income was stratified into sample quintiles, and relationship status was stratified into married, in a marriage-like relationship, and single (including divorced, separated, and widowed). At baseline, participants were also asked to report if they had used cannabis, alcohol, or tobacco after acknowledgment of their pregnancy. Prenatal follow-up surveys queried if participants had used cannabis, alcohol, or tobacco in the past month, while pregnant. Prenatal cannabis, alcohol, and tobacco use was defined as positive self-report of use that occurred after acknowledgment of pregnancy, but before birth, on either the baseline survey or a prenatal follow-up survey.
Self-reported mental health symptoms were assessed with the Edinburgh Postnatal Depression Scale (EPDS)44 and the Patient-Reported Outcomes Measurement Information System (PROMIS) Anxiety.45 The EPDS comprises 10 items that assess maternal depression symptoms in the past week. It has been validated for use in both the prenatal and the postpartum periods.46,47 Participant scores were dichotomized based on previously identified cutoff score ≥13, with scores below this cutoff indicating no symptoms or minor symptoms of depression and scores ≥13 identifying individuals with clinically concerning symptoms of major depression.44 The PROMIS Anxiety comprises 7 items to assess symptoms of general anxiety experienced in the past week.45 Participant scores were diagnostically classified using the general US population reference sample for norming. T-scores of 36 to 54 indicate symptoms within normal limits, T-scores of 55 to 59 indicate clinically mild symptoms, and T-scores of 60 to 69 indicate clinically moderate anxiety symptoms. T-scores of ≥70 indicate symptoms of severely elevated anxiety and may indicate the presence of an anxiety disorder.48
Birth Outcomes
Gestational age (in weeks), birth weight (in grams), and child sex assigned at birth were reported by participants in the postdelivery survey. Infants were defined as preterm if gestational age was less than 37 weeks at birth and defined as low birth weight (LBW) if they weighed less than 2,500 g at birth.49
Statistical Analysis
R programming language (R Foundation for Statistical Computing, Vienna, Austria) was used for analysis. First, the association between the sociodemographic characteristics of participants and whether they reported CU during pregnancy was examined. Continuous variables were analyzed using Wilcoxon rank sum test. Categorical variables were assessed using either χ2 test for independence or Fisher exact test when an observed cell count was less than 5.
It is assumed that the subpopulations of participants who used cannabis during pregnancy (CU+ group) and those who did not (CU− group) will be largely heterogeneous. To account for potential confounders when investigating gestational age, birth weight, and risk of parent-reported developmental delay measured by the ASQ-3, the data were analyzed using propensity score weighting. Sociodemographic variables are used to estimate propensity scores through a logistic regression of the reported CU during pregnancy variable. Scores are used to conduct an optimal full matching of the data into a series of strata, with the number of strata chosen to minimize the absolute distances between the propensity scores of observations within each stratum, known as the absolute within-class distance.50 These strata are used to assign propensity weights to each individual, where every participant in the CU+ group receives a propensity weight of 1, and every participant in the CU− group receives a weight equal to the inverse of a stratum propensity score. Analysis was conducted to determine whether the balance of covariates improves between the unweighted and weighted data. Implementation of this matching algorithm was from the MatchIt software package.51
The association between the outcome variables (gestational age, birth weight, and odds of a child falling below an ASQ cutoff score) and reports of CU during pregnancy were studied through a series of g-computations.52 A method increasingly used in epidemiology for causal inference, g-computations involve simulating outcomes under different exposure scenarios to enable unbiased estimation of causal effects.52 In g-computation, a regression model is fitted to estimate the average differences between exposed and unexposed groups. In this case, we fit either a linear or a quasi-binomial logistic regression model to capture the association between the outcome variables, CU classification, and covariates of COVID-19 infection during pregnancy and child assigned sex at birth. These models are then weighed using inverse propensity scores, which are used to estimate the average differences between CU+ and CU− groups as either a difference of means (continuous outcomes) or an odds ratio (OR) (dichotomous outcomes). We used cluster-robust variance estimation to obtain standard errors, with propensity stratum serving as the clustering variable.53 Computation enables unbiased estimation of causal effects even when certain exposures are unobserved. The 95% CI and p value for these contrasts are reported for all statistics.
Results
Of the 10,542 participants who provided information on their CU on the initial or follow-up survey, 596 (5.6%) reported consuming cannabis after acknowledgment of their pregnancy. Of these participants, 437 reported CU on the initial survey only, 61 participants reported CU on a follow-up survey only, and 98 participants reported CU on both the enrollment survey and on a least 1 follow-up survey. Sociodemographic characteristics and detailed self-reported substance use after acknowledgment of pregnancy at enrollment provided in Tables S2 and S3, respectively, available online.
Sociodemographic Differences Between CU+ and CU− Groups
Sociodemographic characteristics of the total sample and group differences by reported CU during pregnancy are summarized in Table 1. Several significant differences between participants in CU+ and CU− groups were identified. Using Wilcoxon signed rank test, the median maternal age was determined to be significantly lower among participants in the CU+ group (median = 30.6) compared with the CU− group (median = 31.8) (p < .001). Fisher exact test indicated a significant difference between the proportions of ethnic identities within each group (p < .001). Disparities in income were identified with a χ2 test of independence, with participants in the CU+ group more likely to report an annual income of less than CAD $70,000 (46.5% vs 20.4% of CU− group) (p < .001). Mental health symptoms also significantly differed between the subpopulations, with participants in the CU+ group more likely to exhibit clinically elevated depression symptoms (55.0% vs 32.2% of CU− group) (p < .001) and clinically severe anxiety symptoms (14.3% vs 6.6% of CU− group) (p <.001). A significant difference in relationship status was identified, with a higher proportion of participants in the CU+ groups reporting a marriage-like relationship (49.3% vs 32.9% of CU− group) compared with the CU− group (p < .001). Finally, higher rates of reported alcohol (29.0% for CU+ vs 12.0% for CU−) and tobacco (29.0% CU+ and 4.2% for CU−) use was observed among participants in the CU+ group (ps < .001).
Table 1.
Sociodemographic Characteristics and Group Differences
| Total sample (N = 10,695 |
CU− group (n = 10,099) |
CU+ group (n = 596) |
p | ||||
|---|---|---|---|---|---|---|---|
| Median | (Q1, Q3) | Median | (Q1, Q3) | Median | (Q1, Q3) | ||
| Maternal age, y | 31.8 | (28.9, 34.8) | 31.8 | (29.0, 34.8) | 30.6 | (27.1, 34.1) | <.001 |
| n | n | n | |||||
| Missing | 113 | 109 | 4 | ||||
| n | (%) | n (%) | (%) | n (%) | (%) | ||
| Ethnicity | |||||||
| Biracial | 254 | (2.4) | 237 | (2.4) | 17 | (2.9) | <.001 |
| Black | 131 | (1.3) | 124 | (1.3) | 7 | (1.2) | |
| East or Southeast Asian | 333 | (3.2) | 324 | (3.3) | 9 | (1.5) | |
| Hispanic | 212 | (2.0) | 202 | (2.1) | 10 | (1.7) | |
| Indigenous | 297 | (2.9) | 252 | (2.6) | 45 | (7.7) | |
| South Asian | 276 | (2.7) | 273 | (2.8) | 3 | (0.5) | |
| West Asian | 110 | (1.1) | 108 | (1.1) | 2 | (0.3) | |
| White | 8,775 | (84.4) | 8,283 | (84.4) | 492 | (84.1) | |
| Missing | 307 | 296 | 11 | ||||
| Income, CAD | <.001 | ||||||
| <$70,000 | 2,286 | (22.0) | 2,013 | (20.4) | 273 | (46.5) | |
| $70,000-$99,999 | 2,080 | (19.9) | 1,963 | (19.9) | 117 | (19.9) | |
| $100,000-$124,999 | 1,946 | (18.6) | 1,867 | (18.9) | 79 | (13.5) | |
| $125,000-$174,999 | 2,484 | (23.8) | 2,400 | (24.4) | 84 | (14.3) | |
| ≥$175,000 | 1,646 | (15.8) | 1,612 | (16.4) | 34 | (5.8) | |
| Missing | 253 | 244 | 9 | ||||
| Relationship status | <.001 | ||||||
| Marriage-like | 3,560 | (33.9) | 3,268 | (32.9) | 292 | (49.3) | |
| Married | 6,478 | (61.6) | 6,247 | (63.0) | 231 | (39.0) | |
| Single | 476 | (4.5) | 407 | (4.1) | 69 | (11.7) | |
| Missing | 181 | 177 | 4 | ||||
| Symptoms of depression | <.001 | ||||||
| Clinically elevated | 3,190 | (33.5) | 2,901 | (32.2) | 289 | (55.0) | |
| Not elevated | 6,334 | (66.5) | 6,098 | (67.8) | 236 | (45.0) | |
| Missing | 1,171 | 1,100 | 71 | ||||
| Symptoms of anxiety | <.001 | ||||||
| Clinically severe | 663 | (7.0) | 588 | (6.6) | 75 | (14.3) | |
| Clinically moderate | 3,804 | (40.1) | 3,534 | (39.4) | 270 | (51.6) | |
| Clinically mild | 1,981 | (20.9) | 1,915 | (21.4) | 66 | (12.6) | |
| Not elevated | 3,044 | (32.1) | 2,932 | (32.7) | 112 | (21.4) | |
| Missing | 1,203 | 1,130 | 73 | ||||
| Alcohol use during pregnancy | 1,341 | (13.0) | 1,169 | (12.0) | 172 | (29.0) | <.001 |
| Tobacco use during pregnancy | 599 | (5.6) | 424 | (4.2) | 175 | (29.0) | <.001 |
Note: CU+ = participants who reported cannabis use during pregnancy; CU− = participants who did not report cannabis use during pregnancy; Q1 = first quartile (25th percentile); Q2 = third quartile (75th percentile. Differences in age were assessed with Wilcoxon signed rank test, and differences in ethnicity were assessed with Fisher exact test; all other differences were assessed with χ2 test of independence. p values exclude missing observations.
Propensity Score Matching
A total of 9,399 participants provided the necessary data for propensity score matching on maternal age, ethnicity, income, relationship status, depression symptoms, anxiety symptoms, and alcohol and tobacco use. Results of matching are summarized in Table 2. Optimized full matching was conducted, which produced a sample of 519 participants for the CU+ group (effective sample size = 519) and 8,880 participants for the CU− group (effective sample size = 820.6). Table 2 summarizes the absolute standardized mean differences between the CU+ and CU− groups before and after weighing. After weighing, the matched variables between each group were smaller than 0.1 standardized mean difference. The distributions of both COVID-19 infection status and infant sex assigned at birth between CU+ and CU− groups also decreased in standardized mean difference after balancing, providing strong evidence that the balanced data can be used to explore the association between CU during pregnancy and birth and developmental outcomes while avoiding obvious sources of confounding.
Table 2.
Propensity Score Matching
| Mean before matching |
Mean after matching |
|||||
|---|---|---|---|---|---|---|
| CU+ Group (n = 519) | CU− Group (n = 8,880) | SMD | CU+ Group (ESS = 519, n = 519) | CU− Group (ESS = 820.6, n = 8,880) | SMD | |
| Distance | 0.153 | 0.050 | 0.712 | 0.153 | 0.153 | 0.000 |
| Mean maternal age, y | 30.573 | 31.979 | −0.291 | 30.57 | 30.595 | −0.005 |
| Ethnicity | ||||||
| Biracial | 0.027 | 0.025 | 0.010 | 0.027 | 0.028 | −0.007 |
| Black | 0.012 | 0.012 | −0.002 | 0.012 | 0.010 | 0.018 |
| East or Southeast Asian | 0.017 | 0.033 | −0.122 | 0.017 | 0.010 | 0.060 |
| Hispanic | 0.017 | 0.019 | −0.016 | 0.017 | 0.021 | −0.028 |
| Indigenous | 0.077 | 0.024 | 0.198 | 0.077 | 0.065 | 0.046 |
| South Asian | 0.004 | 0.028 | −0.987 | 0.004 | 0.005 | −0.017 |
| West Asian | 0.004 | 0.011 | −0.109 | 0.004 | 0.002 | 0.029 |
| White | 0.842 | 0.848 | −0.015 | 0.842 | 0.860 | −0.049 |
| Income, CAD | ||||||
| <$70,000 | 0.459 | 0.192 | 0.535 | 0.459 | 0.441 | 0.035 |
| $70,000-$99,999 | 0.195 | 0.197 | −0.007 | 0.195 | 0.213 | −0.047 |
| $100,000-$124,999 | 0.139 | 0.192 | −0.154 | 0.139 | 0.153 | −0.041 |
| $125,000-$174,999 | 0.146 | 0.249 | −0.290 | 0.146 | 0.149 | −0.008 |
| ≥$175,000 | 0.062 | 0.170 | −0.449 | 0.062 | 0.043 | 0.076 |
| Relationship status | ||||||
| Marriage-like | 0.484 | 0.322 | 0.324 | 0.484 | 0.509 | −0.051 |
| Married | 0.407 | 0.639 | −0.474 | 0.407 | 0.377 | 0.060 |
| Single | 0.110 | 0.039 | 0.226 | 0.110 | 0.114 | −0.013 |
| Symptoms of depression | ||||||
| Clinically elevated | 0.547 | 0.321 | 0.455 | 0.547 | 0.535 | 0.025 |
| Not elevated | 0.453 | 0.679 | −0.455 | 0.453 | 0.465 | −0.025 |
| Symptoms of anxiety | ||||||
| Clinically severe | 0.143 | 0.065 | 0.221 | 0.143 | 0.116 | 0.076 |
| Clinically moderate | 0.516 | 0.394 | 0.244 | 0.516 | 0.522 | −0.012 |
| Clinically mild | 0.125 | 0.214 | −0.269 | 0.125 | 0.123 | 0.006 |
| Not elevated | 0.216 | 0.326 | −0.268 | 0.216 | 0.238 | −0.055 |
| Alcohol use during pregnancy | 0.299 | 0.118 | 0.394 | 0.299 | 0.303 | −0.010 |
| Tobacco use during pregnancy | 0.281 | 0.041 | 0.535 | 0.281 | 0.286 | −0.010 |
| Child sexa | ||||||
| Female | 0.224 | 0.290 | −0.067 | 0.224 | 0.227 | −0.003 |
| Male | 0.260 | 0.316 | −0.056 | 0.260 | 0.264 | −0.004 |
| Other | 0.002 | 0.000 | 0.002 | 0.002 | 0.000 | 0.002 |
| Not reported | 0.514 | 0.393 | 0.121 | 0.514 | 0.509 | 0.005 |
| COVID-19 infectiona | ||||||
| Confirmed negative | 0.073 | 0.095 | −0.022 | 0.073 | 0.076 | −0.003 |
| Confirmed positive | 0.004 | 0.005 | −0.001 | 0.004 | 0.001 | 0.003 |
| Not reported | 0.923 | 0.900 | 0.023 | 0.923 | 0.923 | 0.000 |
Note: CU+ = Participants who reported cannabis use during pregnancy; CU− = participants who did not report cannabis use during pregnancy; ESS = effective sample size; SMD = standard mean difference.
Participants were not matched on this variable.
Associations Between Prenatal Cannabis Exposure and Birth Outcomes
Preterm birth occurred in 6.2% (n = 397) of unexposed infants and 7.4% (n = 23) of infants exposed to cannabis prenatally. The mean (SD) birth weight for infants not exposed to cannabis prenatally was 3,408.94 (546.30) g and 3,384.78 (612.15) g for infants who were exposed. Table 3 summarizes the results of all g-computations for the analysis of the association between prenatal cannabis exposure and birth outcomes. No significant associations were revealed regarding prenatal cannabis exposure and either the gestational age of the child at birth (mean = −0.01 week) (95% CI −0.25 weeks to 0.23 weeks, p = .94) or the odds of being born preterm (ie, less than 37 gestational weeks at birth) (OR 0.96, 95% CI 0.55 to 1.69, p = .89). Similarly, no significant associations were revealed regarding prenatal cannabis exposure and either birth weight (mean = 1.91 g) (95% CI −84.10 g to 87.92 g, p = .97) or the odds of being born with LBW (ie, less than 2500 g) (OR 0.99, 95% CI 0.50 to 1.69, p = .98).
Table 3.
Effect Estimates
| Metric | n | Estimate | p | s value | 95% CI | |
|---|---|---|---|---|---|---|
| Gestational age | ||||||
| Continuous | Difference of means | 6,273 | −0.01 | .94 | 0.09 | −0.25 to 0.23 |
| <37 wk | Odds ratio | 6,273 | 0.96 | .89 | 0.17 | 0.55 to 1.69 |
| Birth weight | ||||||
| Continuous | Difference of means | 5,624 | 1.91 | .97 | 0.05 | −84.10 to 87.92] |
| < 2500 g | Odds ratio | 5,624 | 0.99 | .98 | 0.03 | 0.50 to 1.98 |
| Below cutoff on ASQ | ||||||
| At least 1 domain | Odds ratio | 3,572 | 0.71 | .25 | 1.98 | 0.39 to 1.28 |
| Communication | Odds ratio | 3,568 | 3.92 | .02 | 5.90 | 1.28 to 12.01 |
| Gross motor | Odds ratio | 3,568 | 1.00 | .99 | 0.00 | 0.40 to 2.52 |
| Fine motor | Odds ratio | 3,559 | 0.78 | .77 | 0.39 | 0.15 to 3.99 |
| Problem solving | Odds ratio | 3,546 | 0.80 | .67 | 0.57 | 0.29 to 2.22 |
| Personal social | Odds ratio | 3,546 | 0.42 | .12 | 3.03 | 0.14 to 1.26 |
Note: Boldface indicates significant results. ASQ = Ages and Stages Questionnaire.
Associations Between Prenatal Cannabis Exposure and Risk of Parent-Reported Developmental Delay
A model was developed to assess the impact of prenatal cannabis exposure, child sex, and COVID-19 infection during pregnancy on a child’s propensity to be identified as risk of developmental delay as measured by the ASQ-3. The outcome of being identified on at least 1 ASQ domain was analyzed first. No significant evidence was found to suggest that prenatal cannabis exposure was associated with the odds of being identified at risk of parent-reported developmental delay on at least 1 ASQ domain (OR 0.71, 95% CI 0.39 to 1.28, p = .25). Frequency of CU was not associated with being identified as at risk of parent-reported developmental delay; results are reported in Table S4, available online.
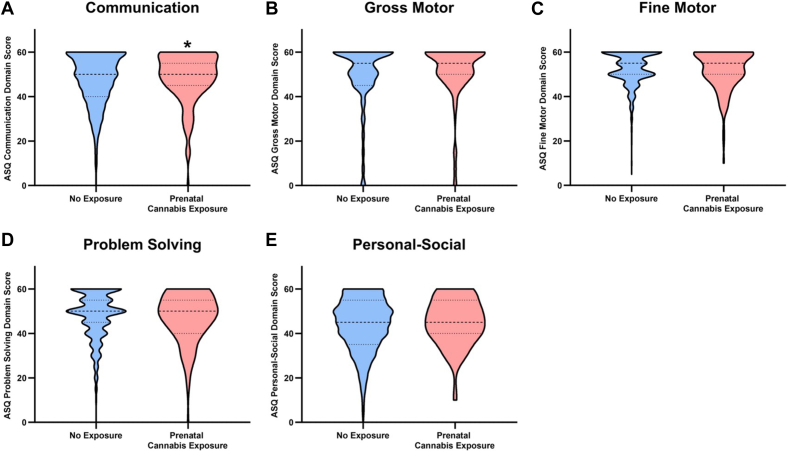
Subsequently, similar models were applied to each developmental domain of the ASQ to determine whether prenatal cannabis exposure was associated with being identified at risk of parent-reported developmental delay within a specific area. A weak association was identified in which infants exposed to cannabis prenatally were more likely to be identified as at risk of developmental delay on the communication domain of the ASQ (OR 3.92, 95% CI 1.28 to 12.01, p = .02). When adjusting for multiple comparisons, this association did not remain significant. No additional evidence was found to suggest that prenatal cannabis exposure was associated with risk of parent-reported developmental delay across other specific domains. Full results are summarized in Table 3, with visual representation of the distribution of each ASQ domain by exposure presented in Figure 1. Descriptive statistics for each ASQ domain are available in Table S5s, available online.
Figure 1.
Violin plots of Ages and Stages Questionnaire (ASQ) Scores by Domain and Prenatal Cannabis Exposure
Note:Dashes represent the median score; dots represent the quartiles.
∗Difference of p < .05.
Discussion
In this recent cohort spanning across Canada, participants who used cannabis during pregnancy were significantly younger and less likely to be married than those who did not. They were also more likely to report an annual income of less than CAD $70,000, experience more severe symptoms of depression and anxiety, and use alcohol and tobacco during pregnancy. These predictors are consistent with previous findings.4,10,12,19,20 Differences in mental health symptoms have been less commonly explored among predictors of use; however, previous literature reports that pregnant women use cannabis products to aid in the treatment of depression11 and anxiety symptoms.9,11 Participants in the current study were not asked to provide reasons why they used cannabis during their pregnancy; however, our findings identified that participants who reported CU experienced more severe depression and anxiety symptoms than participants who reported no CU. Continued monitoring of these trends is vital for creating public health literature about CU during pregnancy. Identification of risk factors associated with prenatal CU provides valuable insight to health care researchers and practitioners who may be responsible for creating and distributing educational materials to at-risk groups.
Previous examinations of the association between prenatal cannabis exposure and birth outcomes have repeatedly found conflicting results. The current analysis adjusted for risk factors associated with adverse birth outcomes. No significant associations were identified between prenatal cannabis exposure and gestational age, increased rate of preterm birth, birth weight, or odds of being classified as LBW. Of note, the preterm birth rate of the sample was lower than the national average,54 and the mean birth weight of both infants exposed to cannabis prenatally and healthy comparison infants fell above LBW cutoff of <2,500 g.4 These factors may indicate that participants are a low-risk sample who may be less vulnerable to risk of developmental delay. Significant associations between preterm birth,4,7,28 lower birth weight,2,23 and prenatal cannabis exposure have been previously identified. Methodological nuances and the clinical significance of these findings should be considered when interpreting these findings. A meta-analysis examining the association between prenatal cannabis exposure and birth weight identified a mean difference between exposed infants and healthy unexposed infants of approximately 100 g.55 In comparison, an alternative meta-analysis found that after adjusting for tobacco exposure, prenatal cannabis exposure was not significantly associated with LBW.37 The clinical implications and developmental consequences of this difference in birth weight remain unclear, specifically when considering that associations between prenatal cannabis exposure and birth outcomes are often not significant in analyses that properly adjust for confounding factors.4 The present results did not find a significant association, further underscoring the importance of the methodological necessity of adjusting for commonly confounding variables when assessing this association.
Prenatal exposure to cannabis did not significantly predict the likelihood of failing to meet the cutoff score across any of the developmental domains measured by the ASQ-3 except for the communication domain. After adjusting for multiple comparisons, no significant differences were identified on any domains. Comparable studies examining prenatal cannabis exposure and infant development at or before 12 months of age have been limited, with little conclusive evidence that consequences of prenatal cannabis exposure emerge within this age range. Of 3 identified studies, two reported no significant associations with developmental delay,27,56 and one reported an association between one or more joints per day during the third trimester and lower mental development scores at 9 months of age; however, these effects did not persist at 19 months.57
Contextualized within past findings, signal surrounding greater odds of being identified as at risk within the communication domain speak to the potential neurodevelopmental consequences of prenatal cannabis exposure. The inconsistent pattern of findings in infancy also raises the question of whether impacts observed in relation to prenatal cannabis exposure are long lasting or temporary. Future research should prioritize measurement of timing, frequency, and amount of cannabis exposure to which infants are subjected to draw more specific conclusions regarding the early developmental risks of exposure as well as longitudinal follow-up to determine the longevity of observed effects.
Animal models, which can account for some of the confounds in clinical studies and speak more directly to causal attribution, also find conflicting results. Some studies have found reduced birth weight in offspring exposed to cannabis prenatally,58,59 and others have found no change in weight.33,60,61 As previously stated, several studies have found neurodevelopmental consequences related to prenatal cannabis exposure33, 34, 35; however, many of these studies use injection administration, which results in different pharmacokinetics and maternal-fetal transmission62 than inhalation or oral exposure (common administration routes in humans). Further, many studies use dosages surpassing typical levels in clinical populations or synthetic cannabinoids that are much more potent than phytocannabinoids. Several recent studies using inhalation or oral exposure of cannabis during pregnancy have shown no changes in anxiety-like behavior, memory acquisition, or social behavior,60,61,63 impairments in social interaction and behavioral flexibility only with high dosages,60 or improvements in discrimination and learning.63 These methodological differences may provide insight regarding the discrepancy seen in developmental delays between human and animal models in early life.
Finally, the majority of data available on outcomes related to CU during pregnancy have been collected in the United States, where the legalization of cannabis varies by state. Acceptability and commonality of use may differ between legalized states and countries; therefore, data collected in contexts where cannabis is legalized may provide key insights for future policy creation. Further, locations with legalization may also garner more accurate reporting due to absence of legal ramifications related to CU disclosure. Although limited, Canadian data suggest that CU among pregnant women has not significantly increased since legalization.64,65 Despite these findings, a recent analysis found notable changes to acute care related to CU during pregnancy since legalization, with the mean quarterly rate increasing from 11.0 per 100,000 pregnancies before legalization to 20.0 per 100,000 pregnancies after legalization.66 Increased CU and related hospital admissions following legalization in the United States were also identified in a systematic review of the literature.14 Such figures should also be considered when evaluating the consequences related to prenatal CU.
The findings of this study must be interpreted considering their limitations. The substance use measure did not capture the quantity of cannabis used (in grams or potency) or the specific timing of prenatal cannabis exposure. Direct comparison of the effects of dosage and trimester of exposure could not be completed to determine if higher potency or a specific trimester of exposure influenced the risk of parent-reported developmental delay. CU was measured only in the month before completion of follow-up surveys; therefore, it is possible that a participant may have used cannabis outside of this time frame and did not report it, resulting in possible misclassification. In addition, data analyzed in the current study were based on live birth figures, which cannot account for the potential impacts of prenatal cannabis exposure that does not result in live birth. Future research should prioritize data collection on spontaneous miscarriage, which may be done by requesting permission to link administrative birth records to participant data. Such procedures could aid in broadening the scope of analysis to include effects of prenatal CU on the developing fetus during the prenatal period.
Self-report online surveys were used for data collection. Although this method increases the ability to reach large samples, self-reports of substance use may not provide estimates as accurate as biochemical sampling,29 and parent-reported measures of development cannot be considered diagnostic tools or as optimally sensitive as formal neurocognitive assessments.27 Parent-report measures rely on observations from caregivers, and it is unknown if parents who reported CU report their child’s abilities in a systematically biased way compared with parents who did not report use (eg, overestimating or underestimating their child’s abilities). Despite these limitations, the PdP cohort provided a unique opportunity to monitor individuals from every province and territory, providing a more holistic view of trends relating to prenatal CU nationally. Previous Canadian studies that have assessed the association between prenatal cannabis exposure and adverse childhood development outcomes primarily used data collected before legalization and were limited in their geographical composition, with samples often consisting of individuals from a single province (eg, Corsi et al.,4 Luke et al.,7 Myran et al.66).
Data were collected during the COVID-19 pandemic, during which societal measures (eg, social distancing and quarantining) may have impacted the development of infants born during this time generally.67 Emerging analysis indicates that infants born during the COVID-19 pandemic may be lagging developmentally compared with historical cohorts68,69; therefore, cannabis-specific differences may have been more difficult to detect. Specific mean ASQ-3 scores reported in the current study may not be directly comparable to nonpandemic cohorts.
The lack of significant associations identified in the current study should not be misinterpreted to suggest that consuming cannabis products during pregnancy is safe. Previous research has found associations between prenatal cannabis exposure and adverse neonatal health outcomes, with prenatal cannabis exposure consistently associated with fetal growth.22,23 Further, increased risk of developmental delay emerges among some populations in toddlerhood (eg, El Marroun et al.29) and throughout childhood (eg, Goldschmidt et al.70); therefore, studies evaluating delays in development in infancy must be followed up longitudinally to determine if these measures change over time. Because the current analysis was performed using data from a longitudinal sample that was not specifically created to address this question, additional studies that are specifically designed examine these outcomes are needed.
CRediT authorship contribution statement
Dana Watts: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Catherine Lebel: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Data curation. Kathleen Chaput: Writing – review & editing, Supervision, Methodology. Gerald F. Giesbrecht: Writing – review & editing, Methodology. Kyle Dewsnap: Writing – review & editing, Writing – original draft, Formal analysis. Samantha L. Baglot: Writing – review & editing, Writing – original draft, Resources. Lianne Tomfohr-Madsen: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Footnotes
This article is part of a special series devoted to the subject of substance use, featuring topics relevant to child and adolescent behavioral health, including genetics, neuroscience, epidemiology, measurement, prevention, and treatment. This special series is edited by Guest Editor Kevin M. Gray, MD, JAACAP Open Deputy Editor Kara S. Bagot, MD, JAACAP Deputy Editor Mary Fristad, PhD, ABPP, JAACAP and JAACAP Open Associate Editor Robert R. Althoff, MD, PhD, JAACAP Open Editor Manpreet K. Singh, MD, MS, and Editor-in-Chief Douglas K. Novins, MD.
This project was funded by the Social Sciences and Humanities Research Council (435-2021-0464) and supported by funds from the Owerko Centre and the Alberta Children's Hospital Research Institute. Drs. Tomfohr-Madsen and Lebel are supported by Canada Research Chairs.
The research was performed with permission from the University of Calgary (Calgary, Alberta, Canada) Conjoint Health Research Ethics Board.
Consent has been provided for descriptions of specific patient information.
M. Dewsnap served as the statistical expert for this research.
The authors thank the participants of the Pregnancy During the COVID-19 Pandemic study for their time and effort.
Disclosure: Drs. Lebel, Chaput, Giesbrecht, and Tomfohr-Madsen, Ms. Watts, M. Dewsnap, and Ms. Baglot have reported no biomedical financial interests or potential conflicts of interest.
Supplemental Material
References
- 1.Clearing the smoke on cannabis: cannabis use during pregnancy and breastfeeding. Canadian Centre on Substance Use and Addiction; 2022. https://www.ccsa.ca/clearing-smoke-cannabis-cannabis-use-during-pregnancy-and-breastfeeding [Google Scholar]
- 2.UNODC world drug report 2020 . United Nations Office on Drugs and Crime; 2020. Global drug use rising; while COVID-19 has far reaching impact on global drug markets.https://www.unodc.org/unodc/press/releases/2020/June/media-advisory---global-launch-of-the-2020-world-drug-report.html [Google Scholar]
- 3.American College of Obstetricians and Gynecologists ACOG committee opinion summary: marijuana use during pregnancy and lactation. Obstet Gynecol. 2017;130(4):931–932. doi: 10.1097/AOG.0000000000002349. [DOI] [PubMed] [Google Scholar]
- 4.Corsi D.J., Hsu H., Weiss D., et al. Trends and correlates of cannabis use in pregnancy: a population-based study in Ontario, Canada from 2012 to 2017. Can J Public Health. 2019;110:76–84. doi: 10.17269/s41997-019-0148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Marroun H., Tiemeier H., Jaddoe V.W., et al. Demographic, emotional and social determinants of cannabis use in early pregnancy: the Generation R study. Drug Alcohol Depend. 2008;98(3):218–226. doi: 10.1016/j.drugalcdep.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Grant K.S., Conover E., Chambers C.D. Update on the developmental consequences of cannabis use during pregnancy and lactation. Birth Defects Res. 2020;112(15):1126–1138. doi: 10.1002/bdr2.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luke S., Hutcheon J., Kendall T. Cannabis use in pregnancy in British Columbia and selected birth outcomes. J Obstet Gynaecol Can. 2019;41(9):1311–1317. doi: 10.1016/j.jogc.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Badowski S., Smith G. Cannabis use during pregnancy and postpartum. Can Fam Physician. 2020;66(2):98–103. [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput K.H., Sanghera H., Sekandary S., et al. Contemporary social context and patterns of prenatal cannabis use in Canada following legalization: a secondary analysis of prospective cohort data. Preprint. Posted online June 22. 2022 doi: 10.1101/2022.06.20.22276670. medRxiv. [DOI] [Google Scholar]
- 10.Mark K., Gryczynski J., Axenfeld E., Schwartz R.P., Terplan M. Pregnant women’s current and intended cannabis use in relation to their views toward legalization and knowledge of potential harm. J Addict Med. 2017;11(3):211–216. doi: 10.1097/ADM.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 11.Westfall R.E., Janssen P.A., Lucas P., et al. Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ‘morning sickness’. Complement Ther Clin Pract. 2006;12(1):27–33. doi: 10.1016/j.ctcp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Ko J.Y., Farr S.L., Tong V.T., et al. Prevalence and patterns of marijuana use among pregnant and nonpregnant women of reproductive age. Am J Obstet Gynecol. 2015;213(2):201.e1–201.e10. doi: 10.1016/j.ajog.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roncero C., Valriberas-Herrero I., Mezzatesta-Gava M., et al. Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health. 2020;17(1):1–9. doi: 10.1186/s12978-020-0880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson S., Rhee S.H. Causal effects of cannabis legalization on parents, parenting, and children: a systematic review. Prev Med. 2022;156 doi: 10.1016/j.ypmed.2022.106956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow N.D., Han B., Compton W.M., et al. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167–169. doi: 10.1001/jama.2019.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra S., Radwan M.M., Majumdar C.G., et al. New trends in cannabis potency in USA and Europe during the last decade (2008–2017) Arch Psychiatry Clin Neurosci. 2019;269:5–15. doi: 10.1007/s00406-019-00983-5. [DOI] [PubMed] [Google Scholar]
- 17.Smart R., Caulkins J.P., Kilmer B., et al. Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state. J Addict. 2017;112(12):2167–2177. doi: 10.1111/add.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang A., Marshall R., Kelsberg G., et al. What effects—if any—does marijuana use during pregnancy have on the fetus or child? J Fam Pract. 2017;66(7):462–466. [PubMed] [Google Scholar]
- 19.van Gelder M.M., Reefhuis J., Caton A.R., et al. Characteristics of pregnant illicit drug users and associations between cannabis use and perinatal outcome in a population-based study. Drug Alcohol Depend. 2010;109(1-3):243–247. doi: 10.1016/j.drugalcdep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Crume T.L., Juhl A.L., Brooks-Russell A., et al. Cannabis use during the perinatal period in a state with legalized recreational and medical marijuana: the association between maternal characteristics, breastfeeding patterns, and neonatal outcomes. J Pediatr. 2018;197:90–96. doi: 10.1016/j.jpeds.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Kar P., Tomfohr-Madsen L., Giesbrecht G., et al. Alcohol and substance use in pregnancy during the COVID-10 pandemic. Drug Alcohol Depend. 2021;225 doi: 10.1016/j.drugalcdep.2021.108760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Marroun H., Tiemeier H., Steegers E.A., et al. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J Am Acad Child Adolesc Psychiatry. 2009;48(12):1173–1181. doi: 10.1097/CHI.0b013e3181bfa8ee. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez C.E., Sheeder J., Allshouse A.A., et al. Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. BJOG. 2019;126(12):1491–1497. doi: 10.1111/1471-0528.15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fried P.A. Marihuana use by pregnant women: neurobehavioral effects in neonates. Drug Alcohol Depend. 1980;6(6):415–424. doi: 10.1016/0376-8716(80)90023-X. [DOI] [PubMed] [Google Scholar]
- 25.Day N.L., Richardson G.A. Prenatal marijuana use: epidemiology, methodologie issues, and infant outcome. Clin Perinatol. 1991;18(1):77–91. doi: 10.1016/S0095-5108(18)30535-9. [DOI] [PubMed] [Google Scholar]
- 26.Baer R.J., Chambers C.D., Ryckman K.K., et al. Risk of preterm and early term birth by maternal drug use. J Perinatol. 2019;39(2):286–294. doi: 10.1038/s41372-018-0299-0. [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda E.O., Vazquez-Benitez G., Kunin-Batson A., et al. Birth and early developmental screening outcomes associated with cannabis exposure during pregnancy. J Perinatol. 2020;40(3):473–480. doi: 10.1038/s41372-019-0576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duko B., Dachew B.A., Pereira G., Alati R. The effect of prenatal cannabis exposure on offspring preterm birth: a cumulative meta-analysis. Addiction. 2023;118(4):607–619. doi: 10.1111/add.16072. [DOI] [PubMed] [Google Scholar]
- 29.El Marroun H., Hudziak J.J., Tiemeier H., et al. Intrauterine cannabis exposure leads to more aggressive behavior and attention problems in 18-month-old girls. Drug Alcohol Depend. 2011;118(2-3):470–474. doi: 10.1016/j.drugalcdep.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Leech S.L., Larkby C.A., Day R., et al. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. J Am Acad Child Adolesc Psychiatry. 2006;45(2):223–230. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- 31.McLemore G.L., Richardson K.A. Data from three prospective longitudinal human cohorts of prenatal marijuana exposure and offspring outcomes from the fetal period through young adulthood. Data Brief. 2016;9:753–757. doi: 10.1016/j.dib.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray K.A., Day N.L., Leech S., et al. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27(3):439–448. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Trezza V., Campolongo P., Cassano T., et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 34.Mereu G., Fà M., Ferraro L., et al. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100(8):4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubio P., Rodriguez De Fonseca F., Martín-Calderón J.L., et al. Maternal exposure to low doses of Δ9-tetrahydrocannabinol facilitates morphine-induced place conditioning in adult male offspring. Pharmacol Biochem Behav. 1998;61(3):229–238. doi: 10.1016/S0091-3057(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 36.Thomason M.E., Palopoli A.C., Jariwala N.N., et al. Miswiring the brain: human prenatal Δ9-tetrahydrocannabinol use associated with altered fetal hippocampal brain network connectivity. Dev Cogn Neurosci. 2021;51 doi: 10.1016/j.dcn.2021.1010000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conner S.N., Bedell V., Lipsey K., Macones G.A., Cahill A.G., Tuuli M.G. Maternal marijuana use and adverse neonatal outcomes. Obstet Gynecol. 2016;128(4):713–723. doi: 10.1097/AOG.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 38.Campolongo P., Trezza V., Palmery M., Trabace L., Cuomo V. Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterations. Int Rev Neurobiol. 2009;85:117–133. doi: 10.1016/S0074-7742(09)85009-5. [DOI] [PubMed] [Google Scholar]
- 39.Giesbrecht G.F., Bagshawe M., van Sloten M., et al. Protocol for the Pregnancy During the COVID-19 Pandemic (PdP) study: a longitudinal cohort study of mental health among pregnant Canadians during the COVID-19 pandemic and developmental outcomes in their children. JMIR Res Protoc. 2021;10(4) doi: 10.2196/25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Squires J., Bricker D. Paul H. Brooks Publishing Co.; Baltimore, MD: 2009. Ages & Stages Questionnaires, Third Edition (ASQ-3) [Google Scholar]
- 41.Rothstein A., Miskovic A., Nitsch K. Brief review of psychometric properties and clinical utility of the Ages and Stages Questionnaires, for evaluating pediatric development. Arch Phys Med Rehab. 2017;98(4):809–810. doi: 10.1016/j.apmr.2016.11.001. [DOI] [Google Scholar]
- 42.Sandler A.D., Brazdziunas D., Carl W., et al. Developmental surveillance and screening of infants and young children. J Pediatr. 2001;108(1):192–195. doi: 10.1542/peds.108.1.192. [DOI] [PubMed] [Google Scholar]
- 43.Beam M., Kaiser A., Paré E., Schellenbach C., Murphy M. Early developmental screening in high-risk communities: implications for research and child welfare policy. The Advanced Generalist. Social Work Research Journal. 2015;1(3/4):18–36. [Google Scholar]
- 44.Cox J.L., Holden J.M., Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 45.Pilkonis P.A., Choi S.W., Reise S.P., et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergink V., Kooistra L., Lambregtse-van den Berg M.P., et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 47.Kozinszky Z., Dudas R.B. Validation studies of the Edinburgh Postnatal Depression Scale for the antenatal period. J Affect Disord. 2015;176:95–105. doi: 10.1016/j.jad.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 48.Cella D., Riley W., Stone A., et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canadian Institute for Health Information . CIHI; Ottawa, ON: 2009. Too Early, Too Small: A Profile of Small Babies Across Canada. [Google Scholar]
- 50.Stuart E.A., Green K.M. Using full matching to estimate causal effects in nonexperimental studies: examining the relationship between adolescent marijuana use and adult outcomes. Dev Psychol. 2008;44(2):395. doi: 10.1037/0012-1649.44.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho D., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 52.Snowden J.M., Rose S., Mortimer K.M. Implementation of G-computation on a simulated data set: demonstration of a causal inference technique. J Epidemiol. 2011;173(7):731–738. doi: 10.1093/aje/kwq472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abadie A., Spiess J. Robust post-matching inference. J Am Stat Assoc. 2022;117(538):983–995. [Google Scholar]
- 54.Bartholomew S., Dzakpasu S., Deb-Rinker P. desLibris; 2013. Perinatal health indicators for Canada 2013: a report from the Canadian Perinatal Surveillance System. [Google Scholar]
- 55.Gunn J., Rosales C., Center K., et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4) doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fried P., Watkinson B. 12-and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol Teratol. 1988;10(4):305–313. doi: 10.1016/0892-0362(88)90032-3. [DOI] [PubMed] [Google Scholar]
- 57.Richardson G.A., Day N.L., Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicol Teratol. 1995;17(4):479–487. doi: 10.1016/0892-0362(95)00006-d. [DOI] [PubMed] [Google Scholar]
- 58.Natale B.V., Gustin K.N., Lee K., et al. Δ9-tetrahydrocannabinol exposure during rat pregnancy leads to symmetrical fetal growth restriction and labyrinth-specific vascular defects in the placenta. Sci Rep. 2020;10(1):544. doi: 10.1038/s41598-019-57318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fried P. Short and long-term effects of pre-natal cannabis inhalation upon rat offspring. Psychopharmacology. 1976;50:285–291. doi: 10.1007/BF00426846. [DOI] [PubMed] [Google Scholar]
- 60.Weimar H.V., Wright H.R., Warrick C.R., et al. Long-term effects of maternal cannabis vapor exposure on emotional reactivity, social behavior, and behavioral flexibility in offspring. Neuropharmacology. 2020;179 doi: 10.1016/j.neuropharm.2020.108288. [DOI] [PubMed] [Google Scholar]
- 61.Campolongo P., Trezza V., Cassano T., et al. Preclinical study: perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol. 2007;12(3-4):485–495. doi: 10.1111/j.1369-1600.2007.00074.x. [DOI] [PubMed] [Google Scholar]
- 62.Baglot S.L., VanRyzin J.W., Marquardt A.E., et al. Maternal-fetal transmission of delta-9-tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. J Neurosci Res. 2022;100(3):713–730. doi: 10.1002/jnr.24992. [DOI] [PubMed] [Google Scholar]
- 63.Sandini T.M., Onofrychuk T.J., Roebuck A.J., et al. Repeated exposure to high-THC Cannabis smoke during gestation alters sex ratio, behavior, and amygdala gene expression of Sprague Dawley rat offspring. eNeuro. 2023;10(11) doi: 10.1523/ENEURO.0100-23.2023. ENEURO.0100-0123.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bayrampour H., Asim A. Cannabis use during the pre-conception period and pregnancy after legalization. J Obstet Gynaecol Can. 2021;43(6):740–745. doi: 10.1016/j.jogc.2021.02.119. [DOI] [PubMed] [Google Scholar]
- 65.Drabkin M., Pudwell J., Smith G.N. Before and after legalization: cannabis use among pregnant patients at a tertiary care center in Ontario. J Obstet Gynaecol Can. 2022;44(7):808–812. doi: 10.1016/j.jogc.2002.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Myran D.T., Roberts R., Pugliese M., et al. Acute care related to cannabis use during pregnancy after the legalization of nonmedical cannabis in Ontario. CMAJ. 2023;195(20):E699–E708. doi: 10.1503/cmaj.230045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metz T.D. Is it exposure to the pandemic or to maternal SARS-CoV-2 infection that is adversely affecting early childhood neurodevelopment? JAMA Netw Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.15793. [DOI] [PubMed] [Google Scholar]
- 68.Giesbrecht G., Lebel C., Dennis C.-L., et al. Risk for developmental delay among babies born during the COVID-19 pandemic. J Dev Behav Pediatr. 2023;44(6):e412–e420. doi: 10.1097/DBP.0000000000001197. [DOI] [PubMed] [Google Scholar]
- 69.Shuffrey L.C., Firestein M.R., Kyle M.H., et al. Association of birth during the COVID-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal SARS-CoV-2 infection. JAMA Pediatr. 2022;176(6) doi: 10.1001/jamapediatrics.2021.5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldschmidt L., Day N.L., Richardson G.A. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325–336. doi: 10.1016/S0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.