Abstract
Background
In Europe, canine leishmaniasis is commonly caused by Leishmania infantum. Allopurinol is the main drug for long-term management of the disease, and clinical relapses of L. infantum infection treated with this drug are described. Resistance to allopurinol has been demonstrated in-vitro, but there is only little knowledge on in vivo resistance in dogs.
Findings
A two-year-old female spayed Akita Inu that was adopted from a breeding facility near Nice in France was initially diagnosed with primary immune-mediated hemolytic anemia. Immunosuppressive treatment was initiated, and the dog was referred for a second opinion to the Clinique Veterinaire Alliance in France. PCR testing for L. infantum was performed out of EDTA blood and IFA as well as ELISA testing out of serum. Resistance to allopurinol was associated with chromosome and gene copy number (CN) variations including a decrease in the S-adenosylmethionine synthetase (METK) gene CN.
Results
The dog showed pale mucous membranes, fever (39.1 °C), and a relapse of the anemia. The diagnosis of leishmaniasis was based on the cytological finding of Leishmania amastigotes (bone marrow, spleen, liver), positive PCR testing, and positive IFAT serology. The dog was treated with allopurinol over a period of 1316 days and additionally received two cycles of Glucantime® (meglumine antimoniate), before samples were submitted to the LABOKLIN laboratory to test for resistance against allopurinol. The laboratory work-up revealed mild thrombocytopenia, mild hyperproteinemia with hyperglobulinemia, a marked elevation of the c-reactive protein, and decreased iron concentration. Serum protein electrophoresis showed a polyclonal peak in the gamma globulins. Serology was positive in both ELISA (21.5 LE) and IFAT (1:1024). Quantitative PCR testing of blood was positive with low numbers of Leishmania (10/ml blood) at the timepoint of suspicion for resistance. The urinary protein-to-creatinine ratio was markedly elevated (2.5) and xanthine crystalluria was detected. A CN level of below 3 is considered suspicious for resistance, as revealed in the described Akita Inu dog.
Conclusions
Relapse of L. infantum infection after applying allopurinol for 1316 days due to resistance was suspected clinically. Positive PCR testing, consistent hematological and biochemistry abnormalities, and reduction in the METK gene CN backed up the clinical suspicion of resistance. Dogs infected with allopurinol resistant strains of L. infantum may represent a great risk for infection of naïve dogs, cats, and humans.
Graphical Abstract
Keywords: Vector-borne infection, Leishmaniasis, Treatment
Main text
Introduction
Leishmaniasis is a protozoal disease caused by Leishmania infantum in humans, dogs, and cats in Europe [1–4]. In general, leishmaniasis is considered a globally emerging disease, with phlebotomine sandflies as primary vectors and dogs as the main pathogen reservoir [1, 2]. In addition to vector transmission, blood transfusion [5–9], vertical transmission [10], and venereal transmission [11] have been proven as sources for canine L. infantum infections. The purine analog allopurinol acts as a xanthine oxidase inhibitor to reduce the serum urate concentration, and is used for the management of gout in human medicine [12]. In canine leishmaniasis, antileishmanial activity of allopurinol was shown by inhibition of the leishmanial enzyme hypoxanthine–guanine phosphoribosyl transferase, leading to a disruption in protein translation and selective death of leishmanial parasites [13, 14]. In veterinary medicine, allopurinol is the first-line drug of choice for long-term management of canine leishmaniasis, applied alone or in combination with pentavalent antimonials or miltefosine [15, 16]. While allopurinol is rarely used in human medicine for the treatment of human leishmaniasis, it is the only drug recommended for use in dogs by the World Health Organization (WHO) [1]. In several previous studies, no direct association between clinical disease relapse in dogs with leishmaniasis and drug resistance was demonstrated [17–19]. However, in 2016, a first detailed report of resistance to allopurinol in L. infantum parasites isolated from dogs and associated with clinical relapse of disease was published [20]. Resistance was demonstrated in three forms of L. infantum parasites [20]. Allopurinol resistance has been successfully induced in vitro in L. infantum isolates from dogs cultured under increasing drug pressure, and genetic modifications were identified in resistant isolates [21, 22].
Methods
TaqMan® real-time PCR testing for detection of L. infantum was performed at LABOKLIN (Bad Kissingen, Germany) using a LightCycler 96 (Roche Diagnostics, Mannheim, Germany). Cycle threshold (Cq) values below 35 were considered positive. Each PCR run included a negative and a positive control, as well as an extraction control in each sample, to check for nucleic acid extraction and PCR inhibition (DNA/RNA Process Control Detection Kit, Roche Diagnostics GmbH, Mannheim, Germany). EDTA-blood was used for PCR testing (target: kinetoplast minicircle DNA; primer: 5′-AAC TTT TCT GGT CCT CCG GGT AG-3′, 5′-ACC CCC AGT TTC CCG CC-3′; probe: 5′-FAM-AAA AAT GGG TGC AGA AAT-NFQMGB-3′ [21]). For serological detection of Leishmania spp., enzyme-linked immunosorbent assay (ELISA) testing (NovaTec VetLine Leishmania ELISA, Immundiagnostica GmbH, Dietzenbach, Germany, > 11 LE positive) and indirect fluorescent antibody (IFA) testing (MegaFLUO® LEISH, MegaCor Diagnostik GmbH, Hörbranz, Austria; > 1:64 positive) were used on serum.
Primers and probes specific for the METK and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, as reference gene) genes of L. infantum were designed and tested in ddPCR using Supermix, DropletGenerator, and Droplet Reader (BioRad, Feldkirchen, Germany) in the LABOKLIN laboratory (Bad Kissingen, Germany). Cross-reactivity with canine DNA could be ruled out by the absence of amplification of the METK gene in samples from dogs that tested negative for Leishmania. Accuracy was tested and confirmed by quantifying the copy number of METK in the WHO strain MHOM/TN/80/IPT-1, described as having only one CN of METK [22]. Samples were sent to NANO1HEALTH SL (Barcelona, Spain) for additional investigation of CNs of the METK gene.
Additionally, a complete blood count (Sysmex XN-V analyzer, Sysmex Deutschland, Norderstedt, Germany), a biochemical profile including CRP (Cobas 8000, Roche Deutschland Holding GmbH, Mannheim, Germany), and a serum protein capillary electrophoresis (Sebia Minicap, Sebia, Mainz, Germany) were performed in the LABOKLIN laboratory. Urinalysis was done on the Cobas u601 (Roche Deutschland Holding GmbH, Mannheim, Germany), with an additional microscopical examination of the urinary sediment and a urinary protein-to-creatinine ratio (Cobas 8000, Roche Deutschland Holding GmbH, Mannheim, Germany).
Case report
A 2-year-old female, spayed Akita Inu dog was adopted from a breeding facility near Nice located at the southeastern coast of France on the Mediterranean Sea. This part of France is endemic for L. infantum, with the highest reported incidences in humans and dogs in the country [23]. The dog was referred for a second opinion to the veterinary clinic Clinique Veterinaire Alliance in Bordeaux, France, with a diagnosis of a primary immune-mediated hemolytic anemia (IMHA). The dog had been treated for IMHA for 5 months, receiving cyclosporine 10 mg/kg once daily orally and prednisolone 0.8 mg/kg once daily orally, when tested for leishmaniasis (Table 1). At the time IMHA was diagnosed, a screening for additional vector-borne pathogens was carried out without revealing coinfections (PCR out of EDTA blood: Babesia spp., Borrelia spp., Ehrlichia canis, Anaplasma phagocytophilum, A. platys; SNAP 4Dx test [IDEXX laboratories, Inc., Maine, USA]: antibodies for Borrelia burgdorferi, A. phagocytophilum, A. platys, E. canis; and antigen testing for Dirofilaria immitis). The owners had decreased the dosage of the immunosuppressive treatment, and a relapse of the anemia occurred. Physical examination revealed pale mucous membranes and a body rectal temperature of 39.1 °C, plus a mild nonregenerative anemia (hematocrit 0.27 l/l, reference range [RR] 0.37–0.63 l/l), thrombocytopenia (121 × 109/l, RR 148–484 × 109/l), mild hyperproteinemia (87 g/l, RR 52–82 g/l) with hyperglobulinemia (52 g/l, RR 25–45 g/l), and albumin in the RR (25 g/l, RR 23–40 g/l, Table 2). A urinary protein-to-creatinine ratio (UPC) of 0.66 (RR < 0.3) was noted. Thoracic imaging was unremarkable, and a mottled parenchyma of the spleen, hyperechoic hepatomegaly, and small adrenal glands were noted on abdominal ultrasound. Fine needle aspirate cytology showed lymphoid hyperplasia, granulomatous inflammation, and numerous Leishmania sp. amastigotes in the spleen as well as in the bone marrow (Fig. 1) and, to a smaller extent, also in the liver. Additionally, the dog tested serologically positive for L. infantum by IFAT (1:640) in the Orbio laboratory (Bron, France) (Tables 1, 2), and treatment was started with the antileishmanial drugs allopurinol and meglumine antimonate.
Table 1.
Course of treatment in a 2-year-old female, spayed Akita Inu infected with Leishmania infantum
| Day | Treatment | Diagnosis | L. infantum status |
|---|---|---|---|
| −5 months |
Cyclosporine 10 mg/kg once daily orally Prednisolone 0.8 mg/kg once daily orally |
Primary immune-mediated hemolytic anemia | — |
| Day 0 (first presentation) |
Allopurinol 9.3 mg/kg twice daily orally Meglumine antimonate 92 mg/kg once daily subcutaneouslya Prednisolone 0.6 mg/kg once daily orally Stopped cyclosporine |
Leishmaniasis | Serology 1:640 (positive)b |
| Day 38 |
Allopurinol 9.3 mg/kg twice daily orally Meglumine antimonate 92 mg/kg once daily subcutaneously Prednisolone 0.4 mg/kg once daily orally |
— | |
| Day 73 |
Allopurinol 7.4 mg/kg twice daily orally Prednisolone 0.2 mg/kg once daily orally Meglumine antimonate finished |
— | |
| Day 143 |
Allopurinol 7.4 mg/kg twice daily orally Prednisolone stopped |
Serology 1:640 (positive)b | |
| Day 679 |
Allopurinol 7.4 mg/kg twice daily orally Meglumine antimonate 80 mg/kg once daily subcutaneously |
Serology 1:640 (positive)b PCR positiveb |
|
| Day 722 |
Allopurinol 7.4 mg/kg twice daily orally Meglumine antimonate finished |
— |
aGlucantime 1.5 g/5 ml, Sanofi-Aventis France, Gentilly Cedex
bOrbio laboratory, Bron, France
Table 2.
Body weight, complete blood count, biochemical parameters, and parasitological status in a 2-year-old female, spayed Akita Inu infected with Leishmania infantum from first presentation (day 0, D0) to day 1316 (D1316) recorded in the Clinique Veterinaire Alliance (Bordeaux, France)
| Parameter | RI | D0 | D38 | D73 | D143 | D244 | D351 | D416 | D625 | D679 | D736 | D875 | D1050 | D1270 | D1316 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW (kg) | — | 27.0 | 27.4 | — | 26.0 | 24.3 | 25.2 | 24.4 | — | 22.9 | 23.6 | 25.5 | 26.0 | 25.3 | 26.2 |
| Change in BW (%) | — | 100.0 | 101.5 | — | 96.3 | 90.0 | 93.3 | 90.4 | — | 84.8 | 87.4 | 94.4 | 96.3 | 93.7 | 97.0 |
| Complete blood counta | |||||||||||||||
| RBC (× 1012/l) | 5.65–8.87 | 5.08 | 6.93 | 7.35 | 7.14 | 8.12 | 8.31 | — | 7.22 | — | 7.29 | 7.53 | 8.3 | — | 8.29 |
| HGB (g/l) | 131–205 | 97 | 128 | 143 | 138 | 160 | 156 | — | 125 | — | 113 | 141 | 154 | — | 125 |
| HCT (l/l) | 0.37–0.62 | 0.27 | 0.37 | 0.39 | 0.39 | 0.46 | 0.46 | — | 0.37 | 0.35 | 0.42 | 0.47 | — | 0.38 | |
| WBC (× 1012/l) | 5.1–16.8 | 12.9 | 9.7 | 7.0 | 6.7 | 6.4 | 7.1 | — | 8.9 | — | 16.9 | 6.1 | 4.14 | — | 6.1 |
| Seg (× 109/l) | 3.0–11.6 | 10.2 | 7.8 | 4.0 | 4.3 | 3.2 | 3.8 | — | 5.8 | — | 12.9 | 2.9 | 1.9 | — | 3.4 |
| Lymphs (× 109/l) | 1.1–5.1 | 1.8 | 1.5 | 2.4 | 2.0 | 2.7 | 2.9 | — | 2.6 | — | 2.8 | 2.5 | 1.9 | — | 2.4 |
| Eos (× 109/l) | 0.06–1.23 | 0.01 | 0.01 | 0.20 | 0.15 | 0.24 | 0.19 | — | 0.14 | — | 0.25 | 0.41 | 0.19 | — | 0.09 |
| Monos (× 109/l) | 0.2–1.1 | 0.8 | 0.4 | 0.3 | 0.3 | 0.2 | 0.2 | — | 0.3 | — | 0.9 | 0.2 | 0.1 | — | 0.2 |
| CHr (pg) | 22.3–29.6 | 17.0 | 17.8 | 18.6 | 18.0 | 17.7 | 21.4 | — | 17.4 | — | 20.6 | 21.9 | 21.9 | — | 17.0 |
| THR (× 109/l) | 148–484 | 121 | 201 | 166 | 241 | 144 | 125 | — | 119 | — | 165 | 142 | 107 | — | 74 |
| Ret (× 109 /l) | 10.0–110.0 | 63.5 | 83.9 | 17.6 | 75.7 | 78.0 | 46.5 | — | 69.3 | — | 44.5 | 29.2 | 67.2 | — | 38.1 |
| Biochemistryb | |||||||||||||||
| TP (g/l) | 52–82 | 87 | 83 | 72 | — | 72 | 70 | — | 81 | 88 | 82 | 69 | 73 | 84 | 87 |
| Alb (g/l) | 23–40 | 25 | 40 | 36 | — | 33 | 32 | — | 30 | 28 | 29 | 32 | 34 | 28 | 29 |
| Glob (g/l) | 25–45 | 52 | 43 | 36 | — | 39 | 38 | — | 51 | 60 | 53 | 37 | 39 | 56 | 58 |
| A/G | — | 0.7 | 0.9 | 1.0 | — | 0.8 | 0.8 | — | 0.6 | 0.5 | 0.5 | 0.9 | 0.9 | 0.5 | 0.5 |
| Urea (g/l) | 0.15–0.57 | 0.32 | — | — | — | — | — | — | 0.18 | — | — | — | — | 0.09 | — |
| Crea (mg/l) | 5.0–18.0 | 8.7 | 6.0 | 10.2 | 12.0 | 15.0 | 18.1 | 15.2 | 14.4 | 23.3 | 19.4 | 16.7 | 16.5 | 15.0 | 18.7 |
| Bil (mg/l) | < 9.0 | 3.5 | — | — | — | — | — | — | 1.6 | — | — | — | — | 1.0 | — |
Bold indicates values outside reference intervals
A/G albumin-to-globulin ratio, Alb albumin, Bil bilirubin, BW body weight, CHr reticulocyte hemoglobin content, Crea creatinine, Eos eosinophilic granulocytes, Glob globulin, HCT hematocrit, HGB hemoglobin, Lymphs lymphocytes, Monos monocytes, RBCs red blood cells, Seg segmented neutrophilic granulocytes, Ret reticulocytes, THR platelets, TP total protein, WBCs white blood cells
aIDEXX ProCyte One Hematology Analyzer
bIDEXX Catalyst One Chemistry Analyzer
Fig. 1.
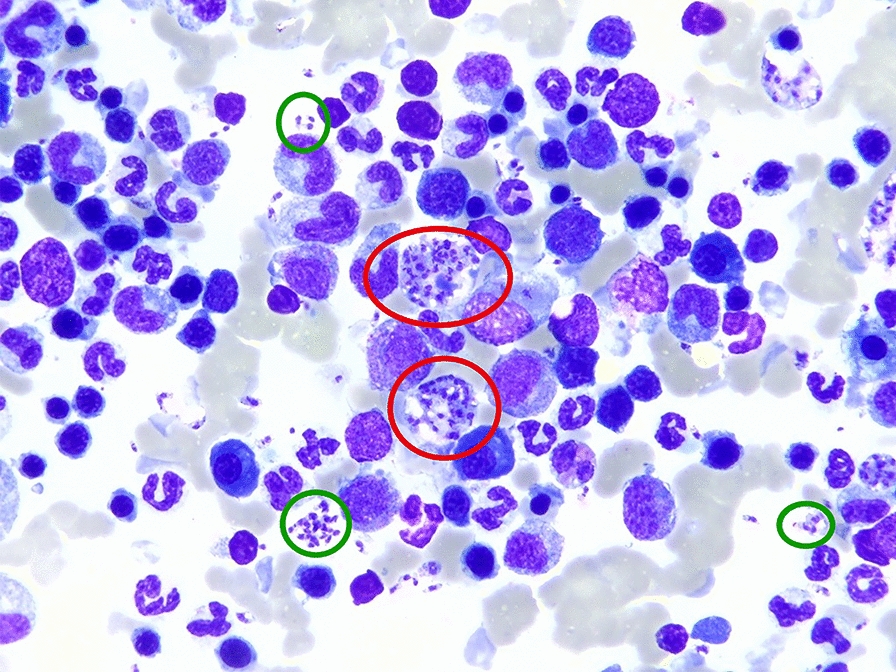
Cytology of the bone marrow of a 2-year-old female, spayed Akita Inu infected with Leishmania infantum with numerous intracellular (red circles) and extracellular amastigotes (green circles)
The dog’s course of treatment is shown in Table 1, and the results of the bloodwork in Table 2. The immunosuppressive therapy with cyclosporine was stopped as soon as leishmaniasis was diagnosed, while prednisolone dosage was tapered over 2 months before it was stopped as well (Table 1). Physical examination of the dog on day 38, after the first presentation in the veterinary clinic (Clinique Veterinaire Alliance), was unremarkable, indicating clinical improvement. On day 73, the urine sediment revealed a xanthine crystalluria in urinalysis, and a low purine diet was applied (Urinary U/C low purine, Royal Canin, Aimargues, France). Meglumine antimonate treatment was finished, and the dog continued to be managed on allopurinol (Table 1). On day 351 after the first presentation, the owner did not report any clinical abnormalities; however, mild azotemia (18.1 mg/l; RR 5.0–18.0) with a urinary specific gravity of 1.016 (RR > 1.025), a mild leukocyturia, and xanthine crystalluria were detected. No bacteria were evident in the urine. Two months later, a UPC of 0.04 (RR < 0.3) was measured. On day 625, a borderline hematocrit (0.37 l/l, RR 0.37–0.63), thrombocytopenia (119 × 109/l, RR 148–484 × 109/l), and hyperglobulinemia (51 g/l, RR 25–45 g/L) were present, comparable to the findings on day 0, except for the raise in hematocrit. On day 679, worsening hyperproteinemia (88 g/L, RR 52–82 g/L), hyperglobulinemia (60 g/l, RR 25–45 g/L), and azotemia, with a creatinine of 23.3 mg/l (RR 5–18 mg/l), were found. However, albumin levels were within normal limits (28 g/l, RR 23–40 g/l, Table 2). IFAT titer stayed at the same level (1:640) compared with day 0. PCR testing for Leishmania was performed on blood and bone marrow, and revealed a moderate and high parasite load, respectively. Besides allopurinol, meglumine antimoniate treatment was again initiated (Table 1). After almost 6 weeks on meglumine antimoniate, the dog was reevaluated on day 736 and its clinical parameters were within normal limits, but the blood tests still revealed a mild but improving azotemia with a creatinine level of 19.4 mg/l (RR 5–18 mg/l), a persisting mild anemia (0.35 l/l, RR 0.37–0.63), and improving hyperglobulinemia (53 g/l, RR 25–45 g/L) (Table 2). Quantitative PCR on blood and bone marrow was still positive.
On day 1322, the dog was still in good condition and maintained the same body weight. Physical examination revealed mild splenomegaly and prescapular, as well as popliteal, lymph adenomegaly. Hematology in the LABOKLIN laboratory revealed mild thrombocytopenia (93 × 109/l, RR 150–500 × 109/l), and serum biochemistry showed a mild hyperproteinemia (75.9 g/l, RR 54–75 g/l) with hyperglobulinemia (46.5 g/l, RR < 45 g/l), a marked elevation of the CRP (38.2 mg/l, RR < 15.0 mg/l), and decreased iron concentration (10.4 µmol/l, RR 15–45 µmol/l; Table 2). Serum protein electrophoresis showed an alpha-2 peak, a beta-gamma bridging, and a polyclonal peak in the gamma sections (Fig. 2). The UPC was significantly elevated at 2.5 (RR < 0.2), and a urinary specific gravity of 1.020 (RR 1.016–1.040) was noted in combination with xanthine crystalluria.
Fig. 2.
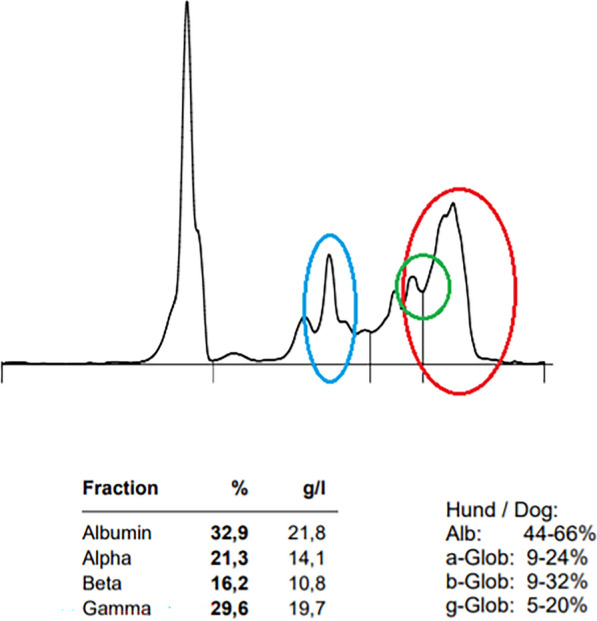
Serum protein capillary electrophoresis in a 2-year-old female, spayed Akita Inu infected with Leishmania infantum with polyclonal peaks in the alpha-2 (blue circle) and gamma sections (red circle), and beta–gamma bridging (green circle)
Leishmania ELISA serology was positive (21.5 LE, < 11 LE negative), as well as the IFAT (1:1024, ≤ 1:64 negative, Table 2). The quantitative PCR blood test was positive, with low concentrations of Leishmania (10 Leishmania/ml EDTA blood) (Table 2). Testing for resistance against allopurinol revealed a METK gene CN of below 3, confirmed by running the drug resistance panel LeishGenR (NANO1HEALTH SL, Bellaterra, Barcelona, Spain) at Nano1Health SL.
Discussion
This case report is the first to describe a potential correlation between the decrease in the CN of the Leishmania METK gene and the clinical findings suggestive of resistance in a dog with a relapse of canine leishmaniasis. The current treatment protocol [15] recommends treatment with pentavalent antimonials, to rapidly reduce parasite load and clinical signs, combined with prolonged allopurinol treatment, which has a mainly parasitostatic action. Studies have shown that the use of allopurinol reduces the frequency of relapses [24, 25]. In the case described herein, treatment with antimonials induced, as expected, a clear clinical improvement, but after months of treatment, clinical relapses occurred, indicating a lack of efficacy of allopurinol.
The use of PCR testing to reveal CNs is a fast, reliable, and cost-effective diagnostic method. Direct PCR testing of tissues represents a great advantage compared with testing resistance by culturing the parasite; this includes isolation of the parasite and growing it in increasing levels of allopurinol, which is a laborious, expensive, and lengthy procedure requiring months of work, and not suitable for providing fast results as needed in clinics.
The reliability of the determination of the CN of the METK gene is based on a critical level of L. infantum parasites in the sample tested. The quantification in the presented dog revealed a low parasitemia of 10 Leishmania/ml EDTA blood. Therefore, testing bone marrow instead of EDTA blood might have provided higher yields of L. infantum and therefore higher accuracy in the diagnostic method used for determination of the CN. Invasive bone marrow sampling was declined by the dog owners, although it is a common procedure in clinical practice. However, the clinical relapse of the disease accompanied by hematological and biochemical results, as well as the reported low CN of the METK gene, supported a strong suspicion of resistance to allopurinol in the presented dog.
Until recently, limited data regarding cutoff values for reduction in the METK gene CN and clinical suspicion of resistance were available. A reduction of the CN was demonstrated in two Leishmania isolates with clinical suspicion for resistance and in four isolates with experimentally induced resistant clonal strains, revealing an average relative quantity value of 1.02 ± 0.08, while the average for susceptible ones was 1.48 ± 0.04, with statistically significant differences between both groups (P = 0.017) [26]. These numbers are equivalent to two and three copies, which suggest a deletion of one copy of this locus in the resistant clones [26]. Therefore, a cutoff of < 3 CNs can be stated as suspicious for resistance, but further studies and clinical cases suspicious for resistance are needed for confirmation.
The diagnosis of L. infantum infection was based on the cytological finding of amastigotes in the spleen, bone marrow, and in the liver; a positive qPCR of blood and bone marrow; as well as a positive serology by IFAT and ELISA. Additionally, hematological and biochemical abnormalities consistent with a L. infantum infection were noted (Tables 2 , 3). In our dog, treatment of 1316 days with allopurinol was followed by a clinical relapse of L. infantum infection, verified by positive PCR testing of peripheral blood, positive IFAT, and ELISA testing. This diagnosis was accompanied by thrombocytopenia, hyperglobulinemia, elevation of CRP, and a decrease in iron concentration. All mentioned hematological and biochemical abnormalities are consistent with canine leishmaniasis [15, 27]. The Leishmania titer increased from 1:640 to 1:1024 following the clinical relapse. However, the increase must be interpreted with care because, owing to the measurement in two different diagnostic laboratories, making comparisons between the titers is challenging [27]. Additionally, the difference can be interpreted as tolerable interassay and interobserver variability. An ELISA titer of 22.1 LE represents a moderate elevation in the LABOKLIN laboratory [27].
Table 3.
Complete blood count, biochemical parameters, and parasitological status in a 2-year-old female, spayed Akita Inu infected with Leishmania infantum in the LABOKLIN laboratory (Bad Kissingen, Germany) on day 1322 after first presentation
| Parameter | Reference interval | Values |
|---|---|---|
| Complete blood counta | ||
| Red blood cells (× 1012/l) | 5.5–8.5 | 8.76 |
| Hemoglobin (g/l) | 150–190 | 132 |
| Hematocrit (l/l) | 0.44–0.52 | 0.47 |
| White blood cells (× 1012/l) | 6.0–12.0 | 7.1 |
| Segmented (× 109/l) | 3.0–9.0 | 3.4 |
| Bands (× 109/l) | < 0.6 | 0.0 |
| Lymphocytes (× 109/l) | 1.0–3.6 | 3.4 |
| Eosinophils (× 109/l) | 0.04–0.6 | 0.1 |
| Monocytes (× 109/l) | 0.04–0.5 | 0.3 |
| Hypochromasia | Negative | Negative |
| Anisocytosis | Negative | Negative |
| Platelets (× 109/l) | 150–500 | 93 |
| Biochemistryb | ||
| Alpha-amylase (U/l) | < 1,650.0 | 1,618 |
| DGGR-lipase (U/l) | < 120.0 | 31.8 |
| Glucose (mmol/l) | 3.05–6.1 | 5.2 |
| Fructosamine (µmol/l) | < 374 | 253 |
| Triglycerides (mmol/l) | < 3.9 | 0.95 |
| Cholesterol (mmol/l) | 3.1–10.1 | 7.2 |
| Bilirubin (µmol/l) | < 3.4 | < 0.1 |
| Alkaline phosphatase (U/l) | < 147 | 64 |
| Glutamate dehydrogenase (U/l) | < 8.0 | 2.0 |
| Gamma-glutamyl transferase (U/l) | < 10.0 | 1.7 |
| Alanine transaminase (U/l) | < 88.0 | 23.7 |
| Aspartate aminotransferase (U/l) | < 51.0 | 27.5 |
| Creatine kinase (U/l) | < 200.0 | 47.0 |
| Total protein (g/l) | 54.0–75.0 | 75.9 |
| Albumin (g/l) | 25.0–44.0 | 29.4 |
| Globulin (g/l) | < 45.0 | 46.5 |
| Urea (mmol/l) | 3.3–8.3 | 4.7 |
| Creatinine (µmol/l) | < 125 | 122 |
| Phosphorus (mmol/l) | 0.7–1.6 | 0.8 |
| Magnesium (mmol/l) | 0.6–1.3 | 0.9 |
| Calcium (mmol/l) | 2.3–3.0 | 2.4 |
| Sodium (mmol/l) | 140–155 | 141 |
| Potassium (mmol/l) | 3.5–5.1 | 4.3 |
| Iron (µmol/l) | 15–45 | 10.4 |
| C-reactive protein (mg/l) | < 15.0 | 38.2 |
| Leishmania testing | ||
| Leishmania IFATc | ≤ 1:64 | 1:1024 |
| Leishmania ELISAd | < 11 LE | 22.1 |
| Leishmania qPCR (/ml EDTA blood) | — | 10 (positive) |
Segmented segmented neutrophilic granulocytes, Bands banded neutrophilic granulocytes, EDTA ethylenediamine tetraacetic acid, ELISA enzyme-linked immunosorbent assay, IFAT immunofluorescence antibody test, qPCR quantitative polymerase chain reaction,
aSysmex XN-V analyzer, Sysmex Deutschland, Norderstedt, Germany
bCobas 8000, Roche Deutschland Holding GmbH, Mannheim, Germany
cMegaFLUO® LEISH, MegaCor Diagnostik GmbH, Hörbranz, Austria
dNovaTec VetLine Leishmania ELISA, Immundiagnostica GmbH, Dietzenbach, Germany
Bold values are outside the reference intervals of the commercial LABOKLIN laboratory (Bad Kissingen, Germany)
In the monitoring of canine leishmaniasis, special focus should be put on the renal and inflammatory status of the dog infected with Leishmania [15, 27]. The UPC is useful for evaluating the risk of chronic kidney disease [28], and proteinuria is a negative prognostic factor in canine leishmaniasis [29]. In our dog, an increase in the UPC was indicative of treatment failure and the suspicion of allopurinol resistance. For monitoring the inflammatory status, the CRP is a very reliable diagnostic tool. Usually, in dogs responding well to treatment, a decrease in CRP can already be noted within a timeframe of 2 weeks after starting antileishmanial therapy, with values in the reference ranges after about 4 weeks [30–32]. Owing to the relatively short half-life of this acute-phase protein, it reacts much quicker compared with serum protein electrophoresis and/or serological titers [27]. Serological titers usually start to decrease in a period of 30 days, if the dog responds well to therapy [33, 34]. However, some individual dogs do not show a decrease in antibodies even in a period of 6 months [35], and some treated infected dogs do not reach antibody titers that are in the reference range even after successful treatment and clinical control of disease [36]. Therefore, qPCR testing for Leishmania spp., ideally out of bone marrow, and determination of the METK CN are very helpful methods in dogs with suspected treatment failure and/or resistance against allopurinol, as shown in this case report.
Clinical relapse of canine leishmaniasis can be supported by the increase of Leishmania concentration revealed by qPCR testing, cytopenia (mono-, bi-, pancytopenia) in hematology, worsening hyperproteinemia with hyperglobulinemia, an increase in CRP, and monitoring of the UPC, as demonstrated in this case report. If PCR testing of bone marrow is not possible, lymph node aspirates and/or EDTA blood can be used. In EDTA blood, the sensitivity can be lower owing to smaller numbers of Leishmania amastigotes circulating in the peripheral blood [37].
Relapse of canine leishmaniasis after long-term application of allopurinol, either alone or combined with meglumine antimonate and/or miltefosine, has been described previously [35, 38–41]. However, an association between resistance to antileishmanial drugs and disease relapse with drug resistance substantiated by genetic testing of variation in gene CNs has rarely been documented [17–19], with only one study investigating L. infantum isolates from dogs with clinical relapse of leishmaniasis [20]. The reduction in the METK gene was found to be typical of resistance to allopurinol in L. infantum isolates from dogs with relapse, owing to resistance to this drug, and in L. infantum strains that were induced to develop resistance in a previous well-detailed study [26].
Owing to the suspicion of primary IMHA in the beginning, treatment with prednisolone was reduced stepwise and stopped on day 722, most likely not influencing the clinical relapse of leishmaniasis on day 1316, almost 2 years after the end of steroid therapy. Mild to moderate nonregenerative anemia is closely associated with canine leishmaniasis [15] and represents a negative prognostic factor for sick dogs [42]. The nonregenerative anemia found in the dog during its relapse was most likely secondary to leishmaniasis and was not primary immune-mediated, where a regenerative anemia with reticulocytosis is expected. Therefore, the anemia was most likely a consequence of the clinical relapse.
A lack of therapeutic effectiveness of antimonial drugs could not be demonstrated as described above. The relapses were related to the administration of allopurinol as the only prescribed drug for two periods of more than one and a half years, respectively. Owing to the results of the METK CN assay, the relapses were most likely associated with a lack of efficacy of allopurinol, and raised the clinical suspicion of resistance to it. Additionally, reinfection with a new L. infantum strain that caused the clinical relapse cannot be ruled out completely.
However, in conclusion, the consistency of clinical relapse of canine leishmaniasis, the hematological and biochemical results, the outcomes of L. infantum qPCR and serology, and the reduction in the CN of the METK gene were interpreted as highly suspicious for resistance against allopurinol in the presented dog. PCR testing is a cost-effective, fast, reliable, objective, and highly specific diagnostic tool for determination of CNs in the METK gene in dogs with clinical suspicion of resistance against allopurinol.
Resistance against antileishmanial drugs should be considered as a potential underlying cause in dogs with relapse of canine leishmaniasis, especially if allopurinol is used for long-term management. Dogs with clinical L. infantum infections are also highly infectious to sand flies [43], and therefore dogs infected with allopurinol-resistant strains may provide a great risk for infection of naïve dogs, cats, and humans.
Acknowledgements
The authors kindly thank Joan Marti and Marina Carrasco from NANO1HEALTH SL (Barcelona, Spain) for verifying the genetic studies.
Abbreviations
- CN
Copy number
- CRP
C-reactive protein
- ddPCR
Digital droplet polymerase chain reaction
- DNA
Deoxyribonucleic acid
- ELISA
Enzyme-linked immunosorbent assay
- EDTA
Ethylenediaminetetraacetic acid
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IFAT
Indirect fluorescent antibody test
- IgG
Immunoglobulin G
- IMHA
Primary immune-mediated hemolytic anemia
- LE
Laboratory specific units
- METK
S-adenosylmethionine synthetase
- PCR
Polymerase chain reaction
- qPCR
Quantitative polymerase chain reaction
- UPC
Urinary protein-to-creatinine ratio
Author contributions
The dog included in the manuscript was presented to M.F. M.F. and I.S. initiated the study, evaluated the data, and wrote the manuscript. A.K. and M.C. performed the molecular and resistance testing. Y.N.B. supported the resistance testing. G.B., L.F., E.M., and T.N. supervised the study. All authors reviewed the manuscript.
Funding
This study was partially funded by the Israeli Ministry of Agriculture grant no. 12-11-0025 entitled “Canine leishmaniasis in Israel: insights on its distribution, transmission and association with human visceral leishmaniasis.”
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The owner of the mentioned dog agreed to the publishing of this study, figures, and tables. Testing for resistance was indicated by the treating veterinarian M.F.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Control of the Leishmaniasis. Report of a WHO expert committee. World Health Organ Tech Rep Ser. 2010;949:1–186. [Google Scholar]
- 2.Moreno J, Alvar J. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 2002;18:399–405. [DOI] [PubMed] [Google Scholar]
- 3.Schäfer I, Schmidt A, Gräßer F, Schieszler A, Aupperle-Lellbach H, Loesenbeck G, et al. Feline leishmaniosis with focus on ocular manifestation: a case report. Parasit Vectors. 2023;16:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennisi MG, Hartmann K, Lloret A, Addie D, Belak S, Boucraut-Baralon C, et al. Leishmaniosis in cats: ABCD guidelines on prevention and management. J Feline Med Surg. 2013;15:638–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persichetti MF, Solano-Gallego L, Serrano L, Altet L, Reale S, Masucci M, et al. Detection of vector-borne pathogens in cats and their ectoparasites in southern Italy. Parasit Vectors. 2016;9:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attipa C, Papasouliotis K, Solano-Gallego L, Baneth G, Nachum-Biala Y, Sarvani E, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors. 2017;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brianti E, Falsone L, Napoli E, Gaglio G, Giannetto S, Pennisi MG, et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit Vectors. 2017;10:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diakou A, Di Cesare A, Accettura PM, Barros L, Iorio R, Paoletti B, et al. Intestinal parasites and vector-borne pathogens in stray and free-roaming cats living in continental and insular Greece. PLoS Negl Trop Dis. 2017;11:e0005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otranto D, Napoli E, Latrofa MS, Annoscia G, Tarallo VD, Greco G, et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: pathogen and vector circulation in a confined environment. Vet Parasitol. 2017;236:144–51. [DOI] [PubMed] [Google Scholar]
- 10.da Silva SM, Ribeiro VM, Ribeiro RR, Tafuri WL, Melo MN, Michalick MS. First report of vertical transmission of Leishmania (Leishmania) infantum in a naturally infected bitch from Brazil. Vet Parasitol. 2009;166:159–62. [DOI] [PubMed] [Google Scholar]
- 11.Silva FL, Oliveira RG, Silva TM, Xavier MN, Nascimento EF, Santos RL. Venereal transmission of canine visceral leishmaniasis. Vet Parasitol. 2009;160:55–9. [DOI] [PubMed] [Google Scholar]
- 12.Sivera F, Andres M, Carmona L, Kydd AS, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baneth G, Shaw SE. Chemotherapy of canine leishmaniosis. Vet Parasitol. 2002;106:315–24. [DOI] [PubMed] [Google Scholar]
- 14.Chawla B, Madhubala R. Drug targets in Leishmania. J Parasit Dis. 2010;34:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solano-Gallego L, Miro G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manna L, Corso R, Galiero G, Cerrone A, Muzj P, Gravino AE. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasit Vectors. 2015;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gramiccia M, Gradoni L, Orsini S. Decreased sensitivity to meglumine antimoniate (Glucantime®) of Leishmania infantum isolated from dogs after several courses of drug treatment. Ann Trop Med Parasitol. 1992;86:613–20. [DOI] [PubMed] [Google Scholar]
- 18.Carrio J, Portus M. In vitro susceptibility to pentavalent antimony in Leishmania infantum strains is not modified during in vitro or in vivo passages but is modified after host treatment with meglumine antimoniate. BMC Pharmacol. 2002;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ait-Oudhia K, Gazanion E, Sereno D, Oury B, Dedet JP, Pratlong F, et al. In vitro susceptibility to antimonials and amphotericin B of Leishmania infantum strains isolated from dogs in a region lacking drug selection pressure. Vet Parasitol. 2012;187:386–93. [DOI] [PubMed] [Google Scholar]
- 20.Yasur-Landau D, Jaffe CL, David L, Baneth G. Allopurinol resistance in Leishmania infantum from dogs with disease relapse. PLoS Negl Trop Dis. 2016;10:e0004341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francino O, Altet L, Sanchez-Robert E, Rodriguez A, Solano-Gallego L, Alberola J, et al. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006;137:214–21. [DOI] [PubMed] [Google Scholar]
- 22.Marti-Carreras J, Carrasco M, Gomez-Ponce M, Noguera-Julian M, Fisa R, Riera C, et al. Identification of Leishmania infantum epidemiology, drug resistance and pathogenicity biomarkers with nanopore sequencing. Microorganisms. 2022;10:2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hide M, Michel G, Legueult K, Pin R, Leonard S, Simon L, et al. Asymptomatic Leishmania infantum infection in dogs and dog owners in an endemic area in southeast France. Parasite. 2024;31:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gizzarelli M, Foglia Manzillo V, Inglese A, Montagnaro S, Oliva G. Retrospective long-term evaluation of miltefosine-allopurinol treatment in canine leishmaniosis. Pathogens. 2023;12:864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reguera RM, Moran M, Perez-Pertejo Y, Garcia-Estrada C, Balana-Fouce R. Current status on prevention and treatment of canine leishmaniasis. Vet Parasitol. 2016;227:98–114. [DOI] [PubMed] [Google Scholar]
- 26.Yasur-Landau D, Jaffe CL, David L, Doron-Faigenboim A, Baneth G. Resistance of Leishmania infantum to allopurinol is associated with chromosome and gene copy number variations including decrease in the S-adenosylmethionine synthetase (METK) gene copy number. Int J Parasitol-Drug. 2018;8:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäfer I, Müller E. Naucke TJ [Canine leishmaniosis: an update regarding diagnostics, therapeutic approaches, and monitoring]. Tierarztl Prax. 2022;50:431–45. [DOI] [PubMed] [Google Scholar]
- 28.Jacob F, Polzin DJ, Osborne CA, Neaton JD, Kirk CA, Allen TA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. 2005;226:393–400. [DOI] [PubMed] [Google Scholar]
- 29.Geisweid K, Mueller R, Sauter-Louis C, Hartmann K. Prognostic analytes in dogs with Leishmania infantum infection living in a non-endemic area. Vet Rec. 2012;171:399. [DOI] [PubMed] [Google Scholar]
- 30.Rossi G, Ibba F, Meazzi S, Giordano A, Paltrinieri S. Paraoxonase activity as a tool for clinical monitoring of dogs treated for canine leishmaniasis. Vet J. 2014;199:143–9. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Subiela S, Bernal LJ, Ceron JJ. Serum concentrations of acute-phase proteins in dogs with leishmaniosis during short-term treatment. Am J Vet Res. 2003;64:1021–6. [DOI] [PubMed] [Google Scholar]
- 32.Sasanelli M, Paradies P, de Caprariis D, Greco B, De Palo P, Palmisano D, et al. Acute-phase proteins in dogs naturally infected with Leishmania infantum during and after long-term therapy with allopurinol. Vet Res Commun. 2007;31:335–8. [DOI] [PubMed] [Google Scholar]
- 33.Solano-Gallego L, Di Filippo L, Ordeix L, Planellas M, Roura X, Altet L, et al. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasit Vectors. 2016;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliva G, Gradoni L, Cortese L, Orsini S, Ciaramella P, Scalone A, et al. Comparative efficacy of meglumine antimoniate and aminosidine sulphate, alone or in combination, in canine leishmaniasis. Ann Trop Med Parasitol. 1998;92:165–71. [DOI] [PubMed] [Google Scholar]
- 35.Torres M, Bardagi M, Roura X, Zanna G, Ravera I, Ferrer L. Long term follow-up of dogs diagnosed with leishmaniosis (clinical stage II) and treated with meglumine antimoniate and allopurinol. Vet J. 2011;188:346–51. [DOI] [PubMed] [Google Scholar]
- 36.Paltrinieri S, Solano-Gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M, et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1184–91. [DOI] [PubMed] [Google Scholar]
- 37.Chulay JD, Bryceson AD. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983;32:475–9. [DOI] [PubMed] [Google Scholar]
- 38.Ginel PJ, Lucena R, Lopez R, Molleda JM. Use of allopurinol for maintenance of remission in dogs with leishmaniasis. J Small Anim Pract. 1998;39:271–4. [DOI] [PubMed] [Google Scholar]
- 39.Cavaliero T, Arnold P, Mathis A, Glaus T, Hofmann-Lehmann R, Deplazes P. Clinical, serologic, and parasitologic follow-up after long-term allopurinol therapy of dogs naturally infected with Leishmania infantum. J Vet Intern Med. 1999;13:330–4. [DOI] [PubMed] [Google Scholar]
- 40.Slappendel RJ, Teske E. The effect of intravenous or subcutaneous administration of meglumine antimonate [Glucantime®] in dogs with leishmaniasis. A randomized clinical trial. Vet Quart. 1997;19:10–3. [DOI] [PubMed] [Google Scholar]
- 41.Manna L, Reale S, Vitale F, Picillo E, Pavone LM, Gravino AE. Real-time PCR assay in Leishmania-infected dogs treated with meglumine antimoniate and allopurinol. Vet J. 2008;177:279–82. [DOI] [PubMed] [Google Scholar]
- 42.Pereira MA, Santos R, Oliveira R, Costa L, Prata A, Goncalves V, et al. Prognostic factors and life expectancy in canine leishmaniosis. Vet Sci. 2020;7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinnell RJ, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136:1915–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analyzed during the current study.