Abstract
Background
In 2023, Tennessee replaced $6.2 M in US Centers for Disease Control and Prevention (CDC) human immunodeficiency virus (HIV) prevention funding with state funds to redirect support away from men who have sex with men (MSM), transgender women (TGW), and heterosexual Black women (HSBW) and to prioritize instead first responders (FR), pregnant people (PP), and survivors of sex trafficking (SST).
Methods
We used a simulation model of HIV disease to compare the clinical impact of Current, the present allocation of condoms, preexposure prophylaxis (PrEP), and HIV testing to CDC priority risk groups (MSM/TGW/HSBW); with Reallocation, funding instead increased HIV testing and linkage of Tennessee-determined priority populations (FR/PP/SST). Key model inputs included baseline condom use (45%–49%), PrEP provision (0.1%–8%), HIV testing frequency (every 2.5–4.8 years), and 30-day HIV care linkage (57%–65%). We assumed Reallocation would reduce condom use (−4%), PrEP provision (−26%), and HIV testing (−47%) in MSM/TGW/HSBW, whereas it would increase HIV testing among FR (+47%) and HIV care linkage (to 100%/90%) among PP/SST.
Results
Reallocation would lead to 166 additional HIV transmissions, 190 additional deaths, and 843 life-years lost over 10 years. HIV testing reductions were most influential in sensitivity analysis; even a 24% reduction would result in 287 more deaths compared to Current. With pessimistic assumptions, we projected 1359 additional HIV transmissions, 712 additional deaths, and 2778 life-years lost over 10 years.
Conclusions
Redirecting HIV prevention funding in Tennessee would greatly harm CDC priority populations while conferring minimal benefits to new priority populations.
Keywords: HIV, HIV prevention, Tennessee HIV prevention, HIV prevention resource allocation, Health policy
A microsimulation model projected that reallocating Centers for Disease Control and Prevention (CDC) HIV prevention funding in Tennessee from CDC priority populations to first responders, pregnant people, and survivors of sex trafficking would harm CDC priority populations, while conferring minimal benefits to the new priority populations.
In January 2023, the State of Tennessee announced that it would reject $6.2 million in annual funding from the US Centers for Disease Control and Prevention (CDC) [1]. The rejected federal funds were comprised of two grant contracts aimed to direct human immunodeficiency virus (HIV) prevention and surveillance resources to people at increased risk of acquiring HIV, including men who have sex with men (MSM), transgender women (TGW), heterosexual Black women (HSBW), and people who inject drugs (PWID), as defined in the CDC HIV surveillance report [2]. Tennessee healthcare leaders warned of the immediate and substantial adverse consequences of reducing provision of essential HIV services, such as condom distribution, funding for preexposure prophylaxis (PrEP), and HIV testing [3]. The State of Tennessee subsequently fully replaced the $6.2 million in federal funds forgone with equivalent state dollars, to prioritize populations at lower risk of HIV, such as pregnant people, first responders, and survivors of sex trafficking (SST) [4].
We sought to quantify the clinical and economic impact of these proposed changes both for the State of Tennessee as a whole and for subpopulations. Quantifying the potential impact of proposed policy changes is critical to understand the clinical implications of this type of state-level decision for Tennessee as well as other settings considering similar policy decisions [5]. Our objective was to use an established computer microsimulation model of HIV disease, prevention, and treatment to project the 10-year clinical and economic consequences of this HIV resource reallocation in Tennessee on people at risk for and diagnosed with HIV from 2023 to 2033 [6, 7].
METHODS
Analytic Overview
Using the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model, a microsimulation model of HIV disease and treatment, we simulated alternative resource allocation scenarios in Tennessee [6, 7]. We compared HIV transmissions, deaths, and life-years between 2 scenarios: (1) Current, with $6.2 million in funding directed to CDC priority populations including MSM, TGW, and HSBW [2], and (2) Reallocation, or reallocating $6.2 million to HIV testing in Tennessee-determined priority populations, including first responders, pregnant people, and SST. To capture the impact of reallocation, we simulated people with HIV (PWH) in whom HIV is not yet diagnosed and people who newly acquire HIV in Tennessee.
We aimed to provide a conservative estimate by deliberately understating the harms of reallocation. Thus, we did not model people already engaged in HIV care. We also did not consider the likely detrimental effects on linkage to care for the CDC priority populations under Reallocation. We only assessed primary transmissions, omitting in the results any secondary transmissions that would occur from an increased number of PWH with viremia. We excluded PWID and modeled only CDC priority populations in whom HIV transmission predominantly occurs through sexual contact. The impact of altering these assumptions was assessed in scenario analyses. While in the base case, we made assumptions that would lead to the least amount of harm for CDC priority populations (the most optimistic scenario), in scenario analyses, we assumed the worst possible correlation between parameters to understand the most “pessimistic” scenario. We report undiscounted economic outcomes in 2022 US dollars from a Tennessee Department of Health payer perspective. We describe model calibration in Supplementary Methods A.
Model Description
HIV Diagnosis and Treatment
At model initiation, people with undiagnosed HIV experience monthly probabilities of HIV diagnosis either through routine HIV screening or presentation to care with an opportunistic infection. Diagnosed individuals then face a probability of linking to HIV care. In the absence of care, individuals with HIV experience declining CD4 counts with increased risks of opportunistic infection and HIV-related mortality [6]. Individuals are prescribed antiretroviral therapy (ART) upon linkage to care. Viral suppression, when achieved, leads to increased CD4 count and decreased probabilities of HIV-related morbidity and mortality. Defined probability distributions are employed to assign each individual in care a risk of becoming lost to follow-up, discontinuing ART, and subsequently reengaging in care.
Modeled Population
We simulated PWH in whom HIV is not yet diagnosed and people who newly acquire HIV in Tennessee over 10 years. We simulated CDC priority risk groups (MSM, TGW, and HSBW) and populations prioritized by Tennessee's proposed funding reallocations: first responders, pregnant people, and SST (see Table 1 for details). We included pregnant people whose HIV was not diagnosed during their pregnancy under the current allocation (∼1 person each year in Tennessee) [12]. For SST, we included the people with HIV freed from sex trafficking in Tennessee (∼46/year), assuming HIV testing and linkage programs would not impact people actively being trafficked (victims of sex trafficking) [25]. Supplementary Methods D provides a detailed description of estimating population sizes for all subgroups. The simulated cohorts were modeled in a mutually exclusive way (Supplementary Methods B).
Table 1.
Select Model Input Parameters for an Analysis of the Impact of HIV Prevention Funding Reallocation in Tennessee
| Parameters | Populations | ||||||
|---|---|---|---|---|---|---|---|
| MSM | TGW | HSBW | First Responders | Pregnant People | Survivors of Sex Trafficking | Ref. | |
| Cohort characteristics | |||||||
| Overall population size, n | 73 639 | 11 858 | 614 218 | 23 826 | 89 412/year | NAa | [7–10] |
| Sex at birth, female/male, % | 0/100 | 0/100 | 100/0 | 27/73 | 100/0 | 93/7 | [11] |
| People with HIV, total, n | 12 680 | 1672 | 2159 | 83 | 71/y | NAa | [11, 12] |
| Prevalent undiagnosed HIV, n | 3216 | 1452 | 200 | 11 | 1/y | 46/y | |
| Incident HIV, year 2019, n | 501 | 56 | 45 | 3 | NAb | [12, 13] | |
| Age, undiagnosed population, mean (SD), y | 33 (12) | 26 (6) | 40 (14) | 35 (13) | 31 (6) | 23 (9) | [11, 12] |
| Age at HIV infection, mean (SD), y | 30 (12) | 23 (6) | 38 (14) | 32 (13) | NA | NA | |
| Mean 30-d linkage to care, % | 62 | 62 | 65 | 61 | 100 | 56 | [12] |
| CD4 at HIV infection, mean (SD), cells/μL | 667 (134) | 667 (134) | 667 (134) | 667 (134) | NAb | NAb | [6] |
| CD4 at model initiation, undiagnosed cohort, mean (SD), cells/μL | 436 (166) | 436 (166) | 436 (166) | 436 (166) | 436 (166) | 436 (166) | |
| Retention in HIV care, % in care at month 24 | 78 | 78 | 78 | 78 | 78 | 78 | [14] |
| Return to care, per 100 PY | 18.1 | 18.1 | 18.1 | 18.1 | 18.1 | 18.1 | [14] |
| ART efficacy for >91% adherence, %, range by regimen | 93–96c | 93–96c | 93–96c | 93–96c | 93–96c | 93–96c | [15, 16] |
| Intervention characteristics | |||||||
| PrEP efficacy, % | 75 | 75 | 75 | NA | NA | NA | [7] |
| Condom efficacy, % | 80 | 80 | 80 | NA | NA | NA | [17–19] |
| Baseline intervention use prevalence | |||||||
| HIV testing frequency, mean y | 4.8 | 4.8 | 2.5 | 4.4 | 4.4 | NAd | [20] |
| Condom use, % of sex acts | 45 | 45 | 49 | NA | NA | NA | [21] |
| PrEP use, % of population | 8 | 8 | 0.1 | NA | NA | NA | [22] |
| Changes in intervention use prevalence in Reallocatione | |||||||
| HIV testing frequency, % | −47 | −47 | −47 | +47 | 100f | Unchanged | [23, 24] |
| 30-d linkage to care mean, % | Unchanged | Unchanged | Unchanged | Unchanged | Unchanged | +61 | Assumed |
| Condom use, % | −4 | −4 | −4 | NA | NA | NA | [21] |
| PrEP use, % | −26 | −26 | −26 | NA | NA | NA | [22] |
Supplementary Table 1 details additional data sources and the ranges examined in sensitivity analyses. Race/ethnicity information was not provided in the references used to estimate Tennessee-specific population sizes.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; HSBW, heterosexual Black women; MSM, men who have sex with men; NA, not available; PrEP, HIV preexposure prophylaxis; PY, person-years; SD, standard deviation; TGW, transgender women.
a293 survivors of sex trafficking were rescued in Tennessee in 2019, as reported in National Human Trafficking Hotline, Tennessee report. See Supplementary Methods D for more details.
bIncident cohorts were not simulated for pregnant people or survivors of sex trafficking. For pregnant people, only those whose HIV was not diagnosed during their pregnancy under current allocation was included; for survivors of sex trafficking, only the yearly number of people with HIV freed from sex trafficking were included.
cART efficacy indicates mean percentage of virologically suppressed patients at 48 wk.
dSurvivors of sex trafficking were not simulated to receive regular HIV testing, assuming no testing took place before being freed from sex trafficking.
ePercent changes to intervention use prevalence in Reallocation denotes change in relation to intervention use prevalence in Current.
fPregnant people were simulated to be diagnosed immediately at model start in Reallocation.
Projecting the Impact of Condom and PrEP Use on HIV Transmission
The model incorporates 10 years of people with incident HIV in each simulated population. A new cohort with incident HIV is introduced yearly based on historical trends [12]. In Reallocation, we calculated the projected impact of reductions in condom and PrEP provision among MSM, TGW, and HSBW by incorporating the baseline prevalence of condom use and PrEP provision and change in prevalence because of reallocation and adjusting HIV transmissions in those CDC priority populations, as in prior work [26]. Because future trends are unknown, we assumed a constant prevalence of condom use and PrEP provision (see Supplementary Methods E and F).
Effects of Reallocation
The exact allocation of CDC funding toward condom distribution, PrEP provision, and HIV testing is unknown. Our assumptions were informed by published data and detailed budget management discussions with 2 community-based organizations in Tennessee [27–30]. The CDC funding supports PrEP ancillary services that allow community-based organizations to distribute PrEP. The funding does not directly support PrEP drug costs [29, 30]. In the base case, we assumed that the reallocation of $6.2 million in CDC funding would decrease condom use by 4% ($203 000), PrEP provision by 26% ($1 799 100), and HIV testing by 47% ($4 197 900) among the CDC priority populations (see Supplementary Methods C for details). We sought to estimate maximum possible gains among the Tennessee priority populations. We assumed that HIV testing would increase by 47% in first responders and HIV testing and linkage would increase to 100% in pregnant people and 90% in SST. All percentage changes were made relative to their levels in Current, except among pregnant people and SST. The changes in condom use and PrEP provision under Reallocation would directly impact HIV transmission, whereas the changes in HIV testing would impact diagnosing PWH (Supplementary Figure 1).
Model Input Data
Cohort Characteristics
We assigned Tennessee-specific demographic and clinical characteristics to simulated PWH according to their HIV diagnosis status (Table 1). Where Tennessee-specific data were unavailable, we consulted Tennessee community-based HIV service organizations or used national data to derive Tennessee-specific numbers [8, 13, 20] (Supplementary Methods D).
Incident HIV
The numbers of people with newly acquired HIV in year 1 of the simulation ranged from 477 MSM to 3 first responders [9, 12]. We assumed reallocation would not increase HIV transmissions because of occupational exposures for first responders [31]. In Reallocation, we assumed that redirecting funds would reduce condom use and PrEP provision, leading to a 1.6%–3.1% increase in HIV transmissions among MSM, TGW, and HSBW [26] (Supplementary Methods E).
HIV Diagnosis and Linkage to Care
The status quo frequency of HIV testing for Current was calibrated to Tennessee-specific time until HIV diagnosis for people who newly acquire HIV infection (ranging from 4.8 years among MSM and TGW to 2.5 years among HSBW; Table 1, Supplementary Methods A) [7]. Details of calibration of Tennessee-specific time from infection to diagnosis is presented in Supplementary Table 3. In Reallocation, the frequency of HIV testing declines by 47% compared with baseline levels in Current such that MSM/TGW and HSBW are tested every 9.1 and 4.8 years. We assume that all pregnant PWH would be diagnosed during pregnancy in Reallocation, compared with baseline HIV background testing rates under Current of 2%/month. We applied a constant rate of HIV testing uptake over 10 years. Tennessee Department of Health data informed the probability of HIV care linkage (Table 1) [12].
HIV Treatment
Upon linkage, PWH initiate an integrase strand transfer inhibitor-based ART regimen [32]. PWH in care also face adherence-stratified probabilities of becoming lost to follow-up monthly. Loss to follow-up and return to care rates were calibrated to published data (78% in care at 24 months [14, 33]).
Economic Outcomes
We report the cost per HIV transmission averted, death averted, HIV diagnosis, and life-year saved by dividing the 10-year CDC HIV prevention budget ($6.2 million/year) by the corresponding outcome under Current and Reallocation.
Sensitivity and Scenario Analyses
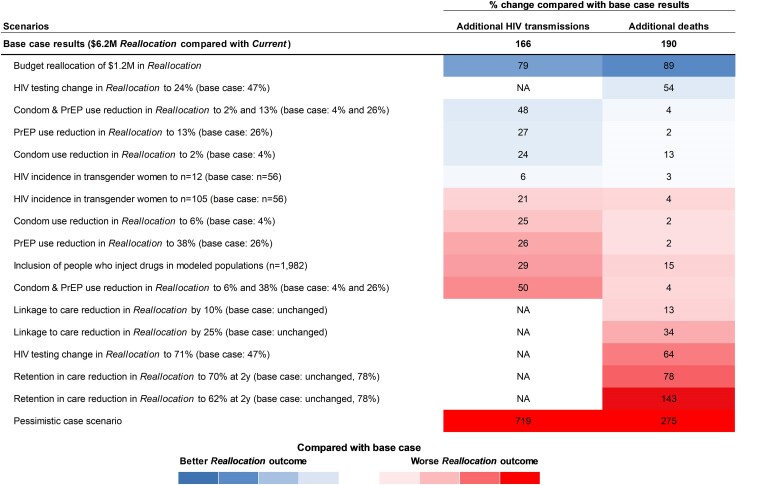
We used deterministic sensitivity analyses to understand the robustness of our findings in the face of parameter uncertainty. We conducted 1-way sensitivity analyses, varying influential parameters in Table 1 across their plausible ranges. We then conducted a series of scenario analyses based on variables identified as particularly influential, as well as input from Tennessee community-based HIV service organization leaders. Additionally, we conducted a scenario analysis to project the impact of reallocation on PWID in the modeled populations. A pessimistic case scenario analysis was conducted in which all influential parameter values were set to their least favorable values and in which secondary transmissions from additional PWH with viremia under Reallocation were incorporated into the annual projected number of people with newly acquired HIV (Supplementary Methods G). We report the percent change in total cumulative HIV transmissions and deaths over 10 years in the most influential scenario analyses in Figure 4. All parameter ranges assessed are presented in Supplementary Table 1 and all results of sensitivity analyses are presented in Supplementary Table 2.
Figure 4.
Change in key clinical outcomes across scenario analyses. This figure shows the impact of varying selected input parameters across their plausible ranges on estimated additional HIV transmissions, deaths, and life-years lost under Reallocation compared with Current. Each row is a single scenario analysis where either 1 parameter, or multiple parameters, were varied from their base case value. The numerical impact of the parameter change on the clinical outcomes is shown on the right, and the color gradient depicts whether the projected outcome under the given scenario analysis has better (blue) or worse (red) outcomes in that sensitivity analysis compared to the base case reallocation value. The pessimistic case scenario included changing 6 combined parameters: increase in transmissions from PWH with viremia, retention in care to 62% at 2 y in reallocation, HIV testing change to 71%, linkage to care reduction in reallocation by 25%, reduction in condom use to 6% and PrEP use to 38%, and condom and PrEP efficacy to 91% and 95%. Abbreviations: HIV, Human immunodeficiency virus; PrEP, preexposure prophylaxis; PWH, people with HIV.
RESULTS
HIV Transmissions
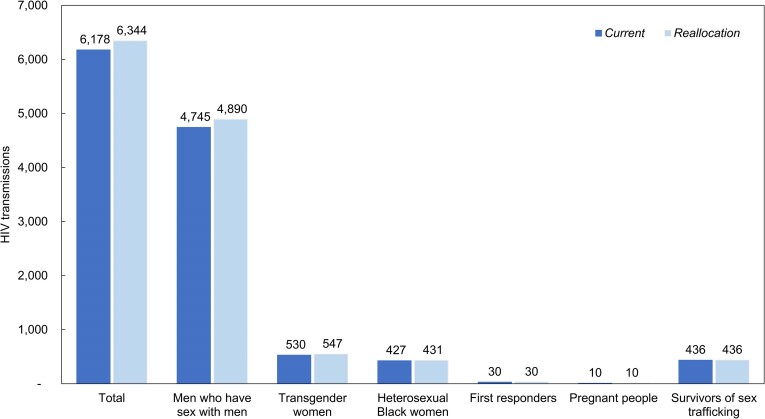
Over 10 years, Current would result in 6178 total transmissions, with 4745 in MSM (77%), 530 in TGW (8.6%), 427 in HSBW (6.9%), 30 in first responders (0.5%), 10 in pregnant people (0.2%), and 436 in SST (7.1%).
Reallocation would increase overall transmissions by 166 (6344 total transmissions) (Figure 1, Table 2). In Reallocation, CDC priority populations would have increased HIV transmissions (145 in MSM, 17 in TGW, and 4 in HSBW), whereas there would be no change in HIV transmissions for first responders, pregnant people, and SST. Under Reallocation, MSM would comprise most of the HIV transmissions (77%), followed by TGW (8.6%) and HSBW (6.8%). First responders would contribute 0.5%, pregnant people 0.2%, and SST 6.9% to the total HIV transmissions over 10 years.
Figure 1.
Cumulative HIV transmissions among the modeled populations at 10 y in the Current and Reallocation strategies. This figure depicts the number of HIV transmissions over 10 y resulting from the Current (dark shade) and Reallocation (light shade) strategies for each modeled subpopulation. Primary HIV transmissions among the simulated risk groups are included. Secondary transmissions arising from these primary transmissions were not considered. Abbreviation: HIV, Human immunodeficiency virus.
Table 2.
Model-projected Clinical Outcomes for Current and Reallocation Strategies Over 10 y
| Outcomes | Populations | ||||||
|---|---|---|---|---|---|---|---|
| Total (N = 11 223)a |
MSM (N = 8106)a |
TGW (N = 1999)a |
HSBW (N = 631)a |
First Responders (N = 41)a |
Pregnant People (N = 10)a |
Survivors of Sex Trafficking (N = 436)a |
|
| Total transmissionsb | |||||||
| Current | 6178 | 4745 | 530 | 427 | 30 | 10 | 436 |
| Reallocation | 6344 | 4890 | 547 | 431 | 30 | 10 | 436 |
| Additional transmissions | 166c | 145 | 17 | 4 | 0 | 0 | 0 |
| Cumulative deaths | |||||||
| Current | 1633 | 1138 | 357 | 85 | 5 | 1 | 47 |
| Reallocation | 1823 | 1286 | 402 | 96 | 5 | 1 | 33 |
| Additional deaths | 190c | 148 | 46 | 12 | 0 | 0 | −14d |
| Life-years | |||||||
| Current | 75 792 | 53 619 | 15 657 | 3979 | 252 | 51 | 2234 |
| Reallocation | 74 949 | 52 988 | 15 438 | 3926 | 254 | 53 | 2291 |
| Life-years lost | 843c | 631 | 220 | 53b | −2d | −2d | −57d |
Abbreviations: HIV, human immunodeficiency virus; HSBW, heterosexual Black women; MSM, men who have sex with men; TGW, transgender women.
aN indicates the total combined population size of the undiagnosed cohort and incident cohort over 10 y under Current. The population size at model initiation was equivalent between Current and Reallocation, and was 4926 total, 3216 for MSM, 1452 for TGW 200 for HSBW, 11 for first responders, 1 for pregnant people, and 46 for survivors of sex trafficking.
bIncident cohorts were not simulated for pregnant people or survivors of sex trafficking. For pregnant people, only those whose HIV was not diagnosed during their pregnancy under current allocation was included; for survivors of sex trafficking, only the yearly number of people with HIV freed from sex trafficking were included.
cAll displayed results are rounded to the nearest one.
dNegative numbers indicate deaths averted or life-years gained.
Deaths and Life-years Lost
Over 10 years, Current would result in 1633 total deaths, with 1138 in MSM, 357 in TGW, 85 in HSBW, 5 in first responders, 1 in pregnant people, and 47 in SST.
Reallocation would result in 190 additional deaths compared with Current, an increase of 12% (1823 total deaths; Table 2). MSM would make up the greatest number of projected deaths among PWH with 148 additional deaths over 10 years under Reallocation. Reallocation would result in 843 life-years lost across all subpopulations compared with Current (Table 2).
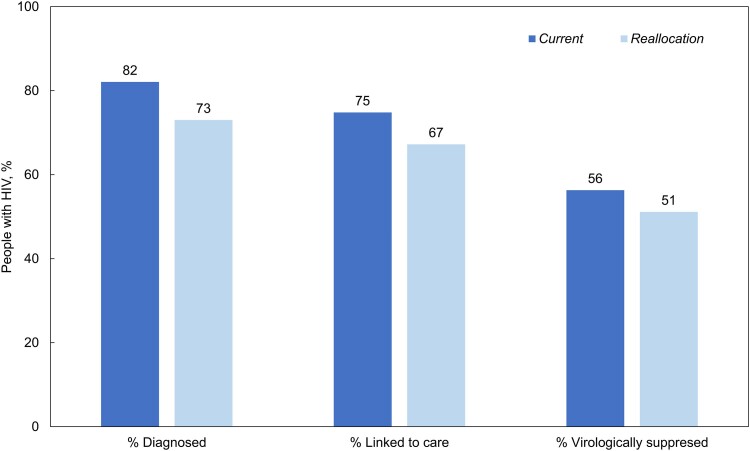
HIV Care Continuum
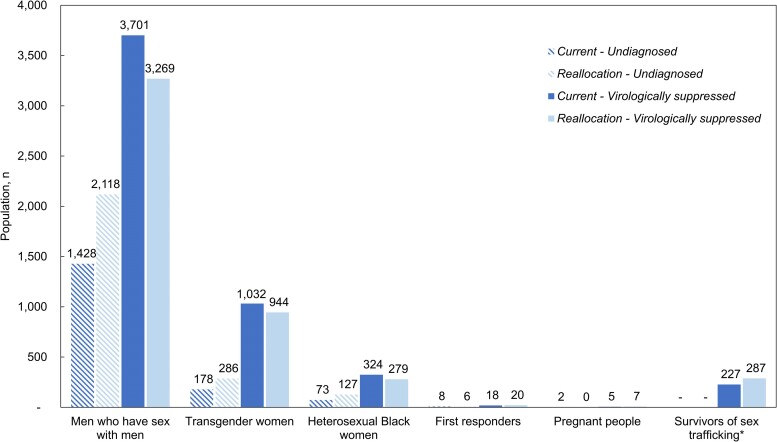
Comparing Current with Reallocation, HIV care outcomes would be worse under Reallocation at 10 years: 82% versus 73% diagnosed, 75% versus 67% linked to HIV care and on ART, and 56% versus 51% virologically suppressed (Figure 2). Reallocation would result in 891 fewer PWH diagnosed among the CDC priority populations to gain only 20 additional PWH diagnosed among the Tennessee-determined priority populations. The average projected time to HIV diagnosis in Current was 2.8 years and in Reallocation was 3.3 years. Reallocation would also result in 731 fewer people linked to care and on ART compared with Current. Last, Reallocation would lead to 565 more PWH with viremia among CDC priority populations to gain 64 more virologically suppressed among Tennessee-determined priority populations (Figure 3).
Figure 2.
HIV care continuum outcomes among modeled people with HIV for the Current and Reallocation strategies at 10 y. Figure shows the proportion of simulated people with HIV who are diagnosed, linked to care, and virologically suppressed at year 10 in the Current (dark shade) and Reallocation (light shade) strategies. Of note, these results are for populations simulated in the present study, defined in the Methods, and include only people in Tennessee with undiagnosed or unlinked HIV at model initiation, or incident HIV over the 10-y time horizon of the simulation. Under Current, there are fewer people with HIV overall because of decreased HIV transmissions; there are also greater proportions of people with HIV who are diagnosed, linked to care, and virologically suppressed. Abbreviation: HIV, Human immunodeficiency virus.
Figure 3.
Number of simulated people with HIV in Tennessee who have undiagnosed HIV, compared to people who have diagnosed HIV and are virologically suppressed at 10 y. Figure presents the projected number of people with HIV across each risk group who are undiagnosed (patterned) compared to those who are diagnosed and virologically suppressed (solid) at 10 y under the Current (dark shade) and Reallocation (light shade) scenarios. Under Current, there are fewer total people with undiagnosed HIV and more people with diagnosed HIV at year 10 compared with Reallocation. Reallocation does increase the number of people with HIV who are virologically suppressed among first responders, pregnant people, and survivors of sex trafficking; however, the increase is relatively small compared to the decrease in virologic suppression among men who have sex with men, transgender women, and heterosexual Black women. *Survivors of sex trafficking are assumed to be diagnosed when freed from sex trafficking in Current and Reallocation, but in Reallocation the linkage to HIV care is assumed to increase from 56% to 90%. Abbreviation: HIV, human immunodeficiency virus.
Economic Outcomes
We assessed the clinical benefits achieved through the prioritization of the $6.2 million/year in HIV prevention funding over 10 years. In Current, this amount of HIV prevention funding would prevent 166 HIV transmissions in the CDC priority groups compared with Reallocation, resulting in an estimated $373 490 spent to avert each transmission (Table 3). Reallocation would not prevent any HIV transmissions in the newly prioritized groups (first responders, pregnant women, and SST) because it would focus on HIV testing and linkage. Under Current, payers would spend $302 440/death averted; under Reallocation, $4 133 330/death averted. Under Current, payers would spend $68 660/life-year saved; under Reallocation, $1 016 390/life-year saved.
Table 3.
Model-projected Economic Outcomes in Tennessee for Current and Reallocation Strategies Over 10 y
| Current | Reallocation | |
|---|---|---|
| HIV prevention funding over 10 ya | $62 000 000 | |
| HIV transmissions averted across modeled populationsb | 166 | 0 |
| Cost per HIV transmission averted, $c | 373 490 | NA |
| HIV diagnoses across 10 y among modeled populationsb | 7734 | 6863 |
| Cost per HIV diagnosis, $ | 8020 | 9030 |
| Deaths averted among modeled populationsb | 205 | 15 |
| Cost per death averted, $ | 302 440 | 4 133 330 |
| Life-years saved among modeled populationsb | 903 | 61 |
| Cost per life-year saved, $ | 68 660 | 1 016 390 |
Abbreviations: HIV, human immunodeficiency virus; NA, not available.
a$6.2 M/year for 10 y.
bModeled populations include men who have sex with men, transgender women, heterosexual Black women, first responders, pregnant people, and survivors of sex trafficking.
cAll costs are in 2022 USD and rounded to the nearest 10.
Sensitivity and Scenario Analyses
We varied the expected reductions in condom use and PrEP use, retention in care at 2 years, reductions in HIV testing under Reallocation, probability of linkage to care under Reallocation, the budgetary reallocation amount, HIV incidence in TGW, and HIV prevalence in SST. Variations in PrEP and condom use reductions under Reallocation were most influential on HIV transmissions over 10 years (Figure 4). Retention in care at 2 years under Reallocation was most influential on deaths and life-years lived when compared with Current. Reducing retention in care to 62% at 2 years (compared with the base case value of 78%) would increase deaths by 143% compared with base case Reallocation projections. Similarly, the reduction in HIV testing under Reallocation had a significant impact on deaths and life-years lived when compared with Current; the number of deaths under Reallocation remained higher compared with Current across assessed ranges. We projected that reducing linkage to care probability by 10% and 25% in Reallocation would lead to 13% and 34% more deaths in Reallocation compared to Current, respectively. Including PWID in our modeled populations would lead to 29% more HIV transmissions and 15% more deaths. Further, a smaller reallocation of $1.2 million instead of the entire $6.2 million would still result in 35 additional HIV transmissions and 22 additional deaths (Figure 4). Under the $1.2 million Reallocation scenario, condom usage would decrease by 0.78%, PrEP provision 5.1%, and HIV testing by 9.1%. Last, a pessimistic case scenario analysis (combining 6 one-way scenario analyses for worst Reallocation outcomes) resulted in projected 1359 additional HIV transmissions, 712 additional deaths, and 2778 life-years lost over 10 years, compared to Current (719%, 275%, and 230% increases over the base case Reallocation).
DISCUSSION
Using a simulation model of HIV disease, we projected the impact of the reallocation of planned HIV prevention funding announced in Tennessee. We found that rejecting $6.2 million of annual CDC prevention funding and using the same amount of state funds to prioritize different populations would lead to 166 additional HIV transmissions, 190 additional deaths, and 843 life-years lost over 10 years in Tennessee. Although Reallocation would improve outcomes for the newly prioritized populations, the scale of improvement was dramatically lower: 15 deaths would be averted in the new priority populations under Reallocation, but an additional 205 deaths would occur among MSM, TGW, and HSBW. At 10 years, there would be more PWH in total, more undiagnosed PWH, and fewer virally suppressed PWH under Reallocation. In terms of economic outcomes and the value of these prevention efforts, we found that under Current, payers are spending $68 660/life-year saved; under Reallocation, this would increase dramatically—requiring more than $1 million/life-year saved. The results are most sensitive to variations in the condom, PrEP, and HIV testing reductions expected under a Reallocation strategy. Additional HIV transmission in Reallocation could vary by approximately ±50% depending on the extent of reductions in condom and PrEP use, and additional deaths in Reallocation could decrease by up to 54% with a lesser reduction in HIV testing or increase by up to 64% with a greater reduction in HIV testing.
These findings offer clinical, epidemiological, and economic support for the current allocation of resources to CDC-identified populations at greatest risk for HIV and highlight how moving away from such evidence-based policies can do harm [34]. As policymakers in other states consider the ramifications of rejecting CDC HIV prevention funding, these results can inform evidence-based policy.
Rejecting CDC HIV prevention funding and shifting priority populations in Tennessee would push Tennessee further from National HIV/AIDS Strategic Plan treatment goals [35]. At present, Tennessee has HIV incidence and mortality higher than the US average [2]. To address these disparities, Tennessee community-based organizations have built a robust HIV prevention infrastructure over several decades to serve communities at highest risk for HIV and its complications [36]. This analysis marshals the available evidence to provide quantitative confirmation of the widely expressed concern that reallocation will set the clock back on efforts to expand HIV prevention and treatment in the state [37, 38].
Although Tennessee policymakers replaced CDC funding with state-provided funding of unclear source and the CDC has additionally pledged $4 million to continue to support community-based organizations through United Way of Greater Nashville, these funds may still not make up for losses because of reallocation. Indeed, we found that even rejecting only $1.2 million of the CDC prevention funds would result in increased HIV transmission and deaths. Moreover, it is anticipated that only 6 organizations will receive United Way of Greater Nashville funding, leaving many smaller community-based organizations across Tennessee, and especially in rural areas, without resources [39, 40]. Often, these smaller organizations are deeply embedded within their communities and serve a diverse client base; an abrupt reduction or removal of funds would have disproportionate impact on the health of Tennesseans [41].
In terms of health equity, Reallocation would worsen existing health disparities in Tennessee among sexual/gender minoritized populations and people of color [42, 43]. Black MSM are at the highest risk for HIV acquisition in Tennessee [44]. People of color, particularly Black men, TGW, and cisgender women, would bear a disproportionate burden of the additional HIV transmissions, deaths, and life-years lost under Reallocation. Furthermore, reallocating HIV prevention funds away from minoritized people at-risk for HIV perpetuates the legacy of systemic racism driving worse health outcomes among people of color in Tennessee and across the United States [45, 46]. A state-sanctioned reprioritization of HIV prevention resources may also discourage minoritized people from using PrEP, obtaining HIV testing, or seeking HIV care [47].
The Current strategy is likely a cost-effective use of resources; prioritizing populations at increased risk of HIV acquisition for HIV prevention and care has been shown to be cost-effective [6, 48]. The current CDC HIV prevention funding averts HIV transmissions at a cost of $348 310/case averted (exclusive of PrEP drug cost), which can be compared to a mean lifetime cost of adult HIV treatment of $420 285 [49]. Under Current, the true overall cost from the state perspective may be substantially lower (ie, approaching $0) because the funding is provided entirely by the CDC.
This analysis has several limitations. First, data about CDC HIV prevention funds distribution across condom provision, PrEP provision, HIV testing, as well as future trends in uptake and discontinuation rates are uncertain, and not all $6.2 M may be spent on HIV testing in the Tennessee-determined priority populations. Although varying these assumptions did not change our policy conclusions, CDC funding distribution data and incorporating yearly changes in the uptake and discontinuation rates would lead to more refined estimates of the impact of redistribution. Second, the simulation assumed mutually exclusive population subgroups, which may underestimate the joint impacts of subgroup risk factors on HIV transmissions and outcomes. Nonetheless, when considering the outcomes for subgroups, the impacts are substantial. Third, we did not conduct probabilistic sensitivity analyses in this study. Assuming the lives of those in subpopulations are valued equally, qualitative conclusions from deterministic sensitivity analyses are robust when we assume the best or worst possible correlation between parameters. Probabilistic sensitivity analyses assuming independent, uniform parameter distributions—because decision uncertainty is near zero—would be unlikely to change policy prescriptions [50, 51]. Last, we simulated the HIV epidemic in Tennessee as a whole, given data limitations on county-level clinical and epidemiologic outcomes, as well as the proportion of reallocation funding in each county. The simulation of county-level outcomes of Reallocation may uncover increased disparities among populations most affected by the HIV epidemic that the state-level analysis cannot show.
In conclusion, we find that the proposed reallocation of HIV prevention funding in Tennessee at a minimum would result in additional HIV transmissions, deaths, and years of life lost over 10 years and increase costs per death averted by 15-fold. Reallocation would greatly harm CDC priority populations, while conferring minimal benefits to the new priority populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Ethan D Borre, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Division of General Internal Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA.
Aima A Ahonkhai, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Vanderbilt Institute for Global Health, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kyu-young Kevin Chi, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
Amna Osman, Nashville CARES, Nashville, Tennessee, USA.
Krista Thayer, Friends For All, Memphis, Tennessee, USA.
Anna K Person, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Andrea Weddle, HIV Medicine Association of the Infectious Diseases Society of America, Arlington, Virginia, USA.
Clare F Flanagan, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA.
April C Pettit, Division of Infectious Diseases, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
David Closs, Friends For All, Memphis, Tennessee, USA.
Mia Cotton, Friends For All, Memphis, Tennessee, USA.
Allison L Agwu, Division of Pediatric Infectious Diseases, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michelle S Cespedes, Division of Infectious Diseases, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Andrea L Ciaranello, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard University Center for AIDS Research, Cambridge, Massachusetts, USA.
Gregg Gonsalves, Public Health Modeling Unit and Department of Health Policy and Management, Yale School of Public Health, New Haven, Connecticut, USA.
Emily P Hyle, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard University Center for AIDS Research, Cambridge, Massachusetts, USA.
A David Paltiel, Public Health Modeling Unit and Department of Health Policy and Management, Yale School of Public Health, New Haven, Connecticut, USA.
Kenneth A Freedberg, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Division of General Internal Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard University Center for AIDS Research, Cambridge, Massachusetts, USA.
Anne M Neilan, Medical Practice Evaluation Center, Massachusetts General Hospital, Boston, Massachusetts, USA; Division of General Internal Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Harvard Medical School, Boston, Massachusetts, USA; Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts, USA; Division of General Academic Pediatrics, Department of Pediatrics, Massachusetts General Hospital, Boston, Massachusetts, USA.
Notes
Author Contributions. All authors contributed substantively to this manuscript in the following ways: study design (E. D. B., A. A. A., K. K. C., A. L. C., K. A. F., A. M. N.), data analysis (E. D. B., K. K. C.), interpretation of results (all authors), drafting the manuscript (E. D. B., K. K. C., K. A. F., A. M. N.), critical revision of the manuscript (all authors), and final approval of submitted version (all authors).
Acknowledgments. The authors thank the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) research team in the Medical Practice Evaluation Center at Massachusetts General Hospital for providing feedback on study design and interpretation.
Meeting presentation disclosure. An earlier version of this analysis was presented at IDWeek 2023 and the Society for Medical Decision Making 45th Annual North American Meeting.
Financial support. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [K08 HD094638-05S1 to A. M. N., UM2 HD111102 to A. M. N. and A. L. C.], the National Institute of Allergy and Infectious Diseases [R01 AI042006 to K. A. F.; R01 MD011770 to A. L. A.; P30 AI094189 to A. L. A.; P30 AI110527 to A. C. P.], the National Institute on Drug Abuse [R37 DA015612 to A. D. P. and G. G.; DP2 DA049282 to G. G.], and the National Institute of Mental Health [R01 MH134724 to A. L. A.] of the National Institutes of Health, the MGH Department of Medicine Transformative Scholar Award (A. M. N.), the MGH Jerome and Celia Reich Endowed Scholar Award (E. P. H.), and the MGH James and Audrey Foster Research Scholar Award (A. L. C.) of the MGH Executive Committee on Research, and the Ryan White Part A and the End the HIV Epidemic to D.C. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders.
REFERENCES
- 1. Tennessee Department of Health . Notification of changes in the HIV program. 2023. Available at: https://wpln.org/wp-content/uploads/sites/7/2023/01/Notification-HIV-Funding-Changes.pdf. Accessed 17 July 2023.
- 2. Centers for Disease Control and Prevention (CDC) . HIV surveillance report 2020. Center for disease control and prevention. Report No.: Volume 33. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-33/index.html. Accessed 17 July 2023.
- 3. Ahonkhai A, Person A, Pettit A. Where do we go from here: HIV prevention in Tennessee and beyond. IDSA Infectious Diseases Society of America. ; Available at: https://www.idsociety.org/science-speaks-blog/2023/where-do-we-go-from-here-hiv-prevention-in-tennessee-and-beyond/#/+/0/publishedDate_na_dt/desc/www.idsociety.org/science-speaks-blog/2023/where-do-we-go-from-here-hiv-prevention-in-tennessee-and-beyond/. Accessed 17 July 2023.
- 4.amfAR. Statement from amfAR on Tennessee's rejection of federal funds for HIV services. amfAR, The Foundation for AIDS Research. Available at: https://www.amfar.org/news/statement-from-amfar-on-tennessees-rejection-of-federal-funds-for-hiv-services/. Accessed 17 July 2023.
- 5. Zuniga JM. IAPAC calls on US congress to reject efforts to gut US HIV response. International Association of Providers of AIDS Care. Available at: https://www.iapac.org/2023/07/14/iapac-calls-on-us-congress-to-reject-efforts-to-gut-us-hiv-response/. Accessed 17 July 2023.
- 6. Borre ED, Hyle EP, Paltiel AD, et al. The clinical and economic impact of attaining national HIV/AIDS strategy treatment targets in the United States. J Infect Dis 2017; 216:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neilan AM, Landovitz RJ, Le MH, et al. Cost-effectiveness of long-acting injectable HIV pre-exposure prophylaxis in the United States. Ann Intern Med 2022; 175:479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herman JL, Flores AR, O’Neill KK. How many adults and youth identify as transgender in the United States?. Available at: https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/. Accessed 17 July 2023.
- 9. United States Bureau of Labor Statistics . Tennessee—May 2022 OEWS state occupational employment and wage estimates. Available at: https://www.bls.gov/oes/current/oes_tn.htm. Accessed 17 July 2023.
- 10. Tennessee Department of Health . General health data. Sections on birth statistics and population. Available at: https://www.tn.gov/health/health-program-areas/statistics/health-data/. Accessed 17 July 2023.
- 11. Centers for Disease Control and Prevention (CDC) . HIV surveillance report 2019. Report No.: Volume 32. Available at: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-32/index.html. Accessed 17 July 2023.
- 12. Tennessee Department of Health . Tennessee HIV epidemiological profile, 2020. Available at: https://www.tn.gov/health/health-program-areas/statistics/health-data/hiv-data.html. Accessed 17 July 2023.
- 13. Wirtz AL, Humes E, Althoff KN, et al. HIV incidence and mortality in transgender women in the Eastern and Southern USA: a multisite cohort study. Lancet HIV 2023; 10:e308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebeiro PF, Gange SJ, Horberg MA, et al. Geographic variations in retention in care among HIV-infected adults in the United States. PLoS One 2016; 11:e0146119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus Abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 16. Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis 2014; 14:572–80. [DOI] [PubMed] [Google Scholar]
- 17. Weller SC, Davis-Beaty K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst Rev 2002:CD003255. [DOI] [PubMed] [Google Scholar]
- 18. Smith DK, Herbst JH, Zhang X, et al. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2015; 68:337. [DOI] [PubMed] [Google Scholar]
- 19. Johnson WD, O’Leary A, Flores SA. Per-partner condom effectiveness against HIV for men who have sex with men. AIDS 2018; 32:1499. [DOI] [PubMed] [Google Scholar]
- 20. Crepaz N, Song R, Lyss SB, et al. Estimated time from HIV infection to diagnosis and diagnosis to first viral suppression during 2014–2018. AIDS 2021; 35:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention (CDC) . HIV infection, risk, prevention, and testing behaviors among heterosexually active adults at increased risk for HIV infection—national HIV behavioral surveillance: 23 U.S. cities, 2019. Report No.: 26. Available at: https://www.cdc.gov/hiv/statistics/systems/nhbs/populations-projects/het.html. Accessed 17 July 2023.
- 22. Local Data: Tennessee [Internet] . AIDSVu. Available at: https://aidsvu.org/local-data/united-states/south/tennessee/. Accessed 17 July 2023.
- 23. Patel D, Johnson CH, Krueger A, et al. Trends in HIV testing among US adults, aged 18–64 years, 2011–2017. AIDS Behav 2020; 24:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spensley CB, Plegue M, Seda R, et al. Annual HIV screening rates for HIV-negative men who have sex with men in primary care. PLoS One 2022; 17:e0266747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Human Trafficking Hotline. Tennessee National human trafficking hotline. Available at: https://humantraffickinghotline.org/en/statistics/tennessee. Accessed 17 July 2023.
- 26. Paltiel AD, Ahmed AR, Jin EY, et al. Increased HIV transmissions with reduced insurance coverage for HIV preexposure prophylaxis: potential consequences of Braidwood Management v. Becerra. Open Forum Infect Dis 2023; 10:ofad139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nashville CARES. Homepage. Available at: https://www.nashvillecares.org/. Accessed 17 July 2023.
- 28.Friends For All. Homepage. Available at: https://www.friendsforall.org. Accessed 17 July 2023.
- 29. Centers for Disease Control and Prevention (CDC) . PS18-1802. Announcements. Funding. HIV/AIDS. CDC. Available at: https://www.cdc.gov/hiv/funding/announcements/ps18-1802/index.html. Accessed 17 July 2023.
- 30. Centers for Disease Control and Prevention (CDC) . PS20-2010. Announcements. Funding. HIV/AIDS. CDC. Available at: https://www.cdc.gov/hiv/funding/announcements/ps20-2010/index.html. Accessed 17 July 2023.
- 31. Centers for Disease Control and Prevention (CDC) . HIV and occupational exposure. 2019. Available at: https://www.cdc.gov/hiv/workplace/healthcareworkers.html. Accessed 17 July 2023.
- 32. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed 17 July 2023.
- 33. Helleberg M, Engsig FN, Kronborg G, et al. Retention in a public healthcare system with free access to treatment: a Danish nationwide HIV cohort study. AIDS 2012; 26:741–8. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention (CDC) . Effectiveness of prevention strategies to reduce the risk of acquiring or transmitting HIV. Available at: https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html. Accessed 17 July 2023.
- 35. White House Office of National AIDS Policy . National HIV/AIDS strategy for the United States 2022–2025. Available at: https://www.hiv.gov/federal-response/national-hiv-aids-strategy/national-hiv-aids-strategy-2022-2025/. Accessed 17 July 2023.
- 36.End HIV 901. Health and Harmony in the 901—official plan for ending the HIV epidemic in Shelby County. Available at: https://endhiv901.org/. Accessed 17 July 2023.
- 37. Ahonkhai AA, Rebeiro PF, Jenkins CA, et al. Individual, community, and structural factors associated with linkage to HIV care among people diagnosed with HIV in Tennessee. PLoS One 2022; 17:e0264508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wester C, Rebeiro PF, Shavor TJ, et al. The 2013 HIV continuum of care in Tennessee. Public Health Rep 2016; 131:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Straube T. In a twist, Tennessee OKs $9M in HIV funding. But is there a catch? POZ. 21 April 2023; Available at: https://www.poz.com/article/tennessee-hiv-groups-get-4m-federal-funds-despite-gop-roadblocks. Accessed 17 July 2023.
- 40. Rajabiun S, Lennon-Dearing R, Hirschi M, et al. Ending the HIV epidemic: one southern community speaks. Soc Work Public Health 2021; 36:647–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denney MR, Pichon LC, Brantley ML. Violence, discrimination, psychological distress, and HIV vulnerability among men who have sex with men in Memphis, Tennessee. Am J Mens Health 2023; 17:15579883231163727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaiser Family Foundation . Tennessee: disparities. Available at: https://www.kff.org/state-category/disparities/. Accessed 17 July 2023.
- 43. Metro Public Health Department . Health equity in Nashville 2021. Metropolitan Government of Nashville & Davidson County; 2021. Available at: https://www.nashville.gov/departments/finance/diversity-equity-and-inclusion/resources. Accessed 17 July 2023.
- 44. Centers for Disease Control and Prevention (CDC) . HIV and African American gay and bisexual men. Available at: https://www.cdc.gov/hiv/group/msm/bmsm.html. Accessed 17 July 2023.
- 45. Wejnert C, Hess KL, Rose CE, et al. Age-specific race and ethnicity disparities in HIV infection and awareness among men who have sex with men—20 US cities, 2008–2014. J Infect Dis 2016; 213:776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nwangwu-Ike N, Frazier EL, Crepaz N, et al. Racial and ethnic differences in viral suppression among HIV-positive women in care. J Acquir Immune Defic Syndr 2018; 79:e56. [DOI] [PubMed] [Google Scholar]
- 47. Adimora AA, Ramirez C, Schoenbach VJ, et al. Policies and politics that promote HIV infection in the Southern United States. AIDS 2014; 28:1393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neilan AM, Bulteel AJB, Hosek SG, et al. Cost-effectiveness of frequent HIV screening among high-risk young men who have sex with men in the United States. Clin Infect Dis 2020; 73:e1927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bingham A, Shrestha RK, Khurana N, et al. Estimated lifetime HIV–related medical costs in the United States. Sex Transm Dis 2021; 48:299. [DOI] [PubMed] [Google Scholar]
- 50. Pitman R, Fisman D, Zaric GS, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force-5. Value Health 2012; 15:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Briggs AH, Weinstein MC, Fenwick EAL, et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group–6. Med Decis Making 2012; 32:722–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.