Abstract
Alkhumra fever is a viral disease caused by the Alkhumra hemorrhagic fever virus (AHFV). It belongs to family Flaviviridae, genus Flavivirus. AHFV is primarily transmitted to humans through the bite of infected ticks, for example, Hyalomma. This disease was first identified in the Kingdom of Saudi Arabia (KSA) in 1995 and then reported in other countries of the Arabian Peninsula and the Middle East. The AHFV genome consists of a positive-sense, single-stranded RNA molecule of approximately 10.2 kilobases (kb) in length. The Open Reading Frame (ORF) encodes a polyprotein precursor that is processed by viral and host proteases to yield individual viral proteins. The polyprotein precursor is cleaved by viral proteases and host signal peptidases into three structural and seven non-structural proteins. AHFV can cause a range of clinical manifestations, from mild flu-like symptoms to severe hemorrhagic fever. In this review, we focus on insightful understanding of molecular biology, pathogenesis, and their potential therapeutic targets for AHFV.
Keywords: Alkhumra hemorrhagic fever virus, Flaviviridae, Kyasanur Forest disease virus, Ornithodoros Savignyi
Introduction
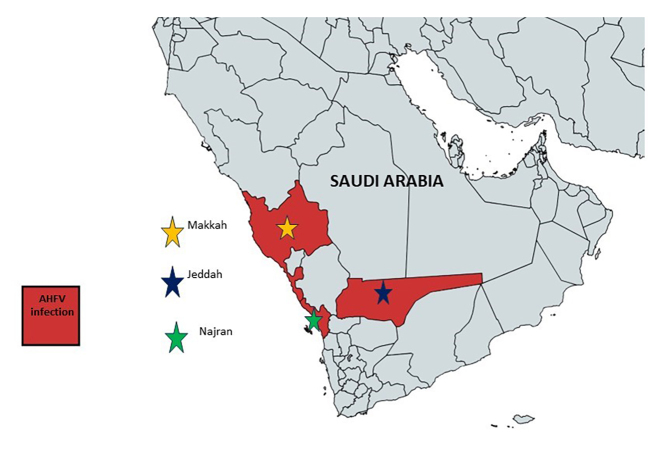
Alkhumra fever is a viral disease caused by the Alkhumra hemorrhagic fever virus (AHFV). It was first reported as hemorrhagic fever in the Kingdom of Saudi Arabia (KSA) in 1995 [1-3]. The virus was recognized as AHFV and belongs to the family Flaviviridae [4,5]. The term “Alkhumra” was derived from the name of the place where the first case was reported, ie, Alkhumra, a district in Jeddah, Saudi Arabia; therefore, it was called “Alkhumra Hemorrhagic Fever Virus” [5-7]. Almost 20 people with AHFV infection were found in Mekkah and later the Najran area of southern Saudi Arabia between 2001 and 2003. Later on, around 2003 and 2007, eight new instances of AHFV infection were recorded from the Najran area (See Figure 1) [8-10]. Thereafter, a rapid surge in infection from this region was observed, with 70 cases in 2008-09 [9,11]. Places like Jeddah, Makkah, and Najran became the most vulnerable regions for AHFV infection. This viral infection further reached to southwestern areas, including new locations like Taif, Alqunfuda, and Jazan, they also showed sporadic cases in subsequent years [10]. The AHFV infection kept spreading and reached Italy when two Italian travelers were found infected on their return journey from southern Egypt [12-14]. Following that, two additional Italian visitors were determined to be afflicted with AHFV. These patients’ travel records revealed that they had recently returned from Egypt. In 2011, another sero-surveillance study revealed the presence of antibodies against AHFV in a female child of 13 years residing near a slaughterhouse in Djibouti; it was the first study about the existence of AHFV antibodies [15]. Later on, in Djibouti, cattle were found infected with AHFV along with ticks in 2010-11 [16].
Figure 1.
Map showing major cities of Saudi Arabia with AHFV infection.
Severe AHFV infection causes hazardous consequences such as severe hemorrhagic disorders, which can lead to death [17-19]. AHFV genome study indicated that it is a distant form of Kyasanur Forest Disease Virus (KFDV), which is widespread in Karnataka, India [20,21]. From 2004-05, samples of sand tampan ticks (Ornithodoros savignyi) isolated from camel shelters and camels were determined to be positive for AHFV using the reverse transcription-PCR technique [22,23]. Ultimately, AHFV was recognized as a zoonotic disease and their hosts were recognized as livestock animals of arid regions like camels, sheep, etc. The most common ways to spread this virus is via contact with skin or exposure of a wound to the blood of an infected animal. It can also be transferred via a tick bite, contaminated raw milk, or directly from infected items [24-30].
Epidemiology and its Geographical Distribution
Summer and wet seasons are the most common times for AHFV infection [29]. The breeding, growth, and development of ticks and mosquitoes (arthropod vectors) during summer and rainy seasons are the optimal conditions. There is a pattern of resemblance between AHFV peak eruption and climatic circumstances (summer and rainy seasons) for other arboviral illnesses and AHFV infections, which enhances vector-host interactions. The most susceptible cohort for AHFV infection is between the ages of 10 and 40, accounting for around 80% of cases [10,24,26,29]. Younger children under the age of 10 are less likely to be infected with AHFV [8-31]. The clinical statistics of AHFV infections from Najran and Makkah were nearly identical [32]. The primary difference between the Najran and Makkah infections were as follows: first, a significant proportion of females had this infection (38% versus 10%), second, an increased number of children who contracted AHFV infection were almost 10 years old (five patients versus none), third, patients with infected family members having AHFV-like illness (25% versus 5%), and finally, lower mortality (1.3% versus 25%) was observed in Najran. These were the primary variations seen as a result of different lifestyle modifications in these places.
In the extended families of Najran (rural), people live together in big houses with backyard gardens used as shelters for livestock animals. The majority of affected family members, including women and children, work in livestock feeding, milking, slaughtering, and butchering. The exceptionally low mortality from AHFV in the Najran outbreak appears to be attributable to AHFV epidemicity establishing in such areas. This might be the result of repetitive viral exposure, which leads to the establishment of herd immunity.
AHFV Classification
The AHFV is a tick (an arthropod) borne hemorrhagic disease. The causative agent of AHFV is from family Flaviviridae, and the genus Flavivirus [1,3,33-35]. AHFV is very similar to KFDV in several ways, like genetics, mode of transmission, etc. The name of this virus was originally a typographical mistake. Several publications incorrectly wrote “ALKHUMRA” virus as “ALKHURMA” which was a typographical error [33-35]. In 2011, the International Committee on Taxonomy of Viruses (ICTV) approved and corrected its name to “ALKHUMRA” [36]. However, still, some authors are using the incorrect name that is “ALKHURMA” in their publications [37-39]. A comparative characteristic of AHFV and KFDV is summarized in Table 1.
Table 1. Comparison of Characteristics of AHFV and KFDV.
| Characteristic | AHFV | KFDV |
| Discovered in year | 1995 | 1957 |
| Genus | Flavivirus | Flavivirus |
| Reservoir | Unknown | Monkey |
| Vector | Unknown | Ticks |
| Transmission | Direct contact of contaminated blood and any products of infected livestock animals or tick bites or may be mosquito bites | Haemaphysalis tick bites that have contact with monkeys |
| Ecosystem | Arid regions (urban and rural) | Forest |
| Geographic distribution | South of Saudi Arabia, south of Egypt, and some African countries | Endemic to Southern part of India (first of reported from the Kyasanur Forest of Karnataka) |
| Incubation period (Days) | Unknown | 2-8 |
| Fatality rate | Less than 1-25% | 3-10% |
| Diagnosis | PCR and Virus isolate sequencing | PCR and Virus isolate sequencing |
| Serological identification | ELISA and other test like serum neutralization | ELISA and other test like serum neutralization |
| Treatment | Treatment unknown and supportive care | Treatment unknown and supportive care |
| Vaccine | Not available | Vaccine used in endemic areas of India |
| Way to prevent and avoid infection and its transmission | • Ignore direct contact of infected livestock animals and their
products. • Wearing personal protective accessories like gloves, apron etc. whenever coming in contact with infected animal or their products having this virus infection. • Apply tick and mosquito repellant cream or spray. •Use of mosquito net and avoid living under trees. • In endemic situations, take high precaution to avoid of contact with ticks and mosquitoes. |
• Control of ticks. • Wearing personal protective accessories like gloves, apron etc. whenever coming in contact with infected animal or their products having this virus infection. • Avoid contact with mammals like monkeys. |
Molecular Characteristics of AHFV
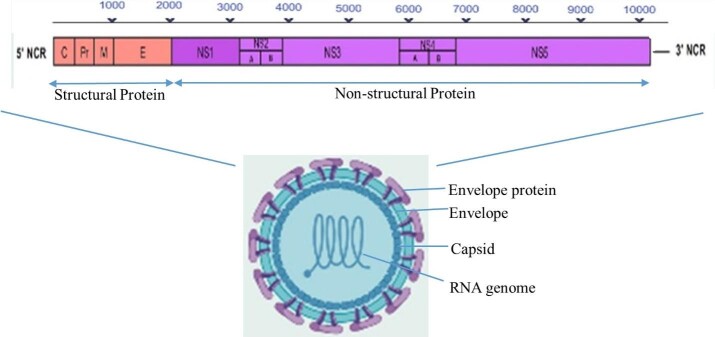
The complete ORF (open reading frame) was isolated from the blood of an AHFV-infected patient in 1995 [40-43] and is comprised of a single positive-sense RNA. The whole viral genome sequence is 10 248 nucleotides (nt) in length that has a single ORF meant for coding a polyprotein, having 3416 amino-acids [44]. AHFV genome encodes a polyprotein for structural and non-structural genes (structural proteins: coat, pre-membrane, and envelope proteins, and non-structural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5; see Figure 2). The viral genome contains a coding sequence that is responsible for viral pathogenicity in the 5’ and 3’ untranslated regions (UTRs). The NS3 region contains protease and helicase domains, whereas the NS5 area contains methyl transferase and RNA-dependent RNA polymerase domains. The NS2B domain acts as a cofactor having protease activity of NS3 [44,45].
Figure 2.
The AHFV genomes open reading frames, structural, and none-structural viral proteins.
Molecular studies of envelope protein and structural genes, evolutionary relationships, and phylogenetic analysis of these polypeptides revealed that AHFV was related to a tick-borne Flavivirus group. Additionally, an independent study showed that there was a close relation between AHFV and KFDV [46]. It was reported that both AHFV and KFDV showed the same lineage with two genetic subtypes. Between 1994-99, studies on the hospitalized patient’s sample of AHFV in Makkah and Jeddah (KSA) showed low diversity and slow microevolution of partial envelope, NS3, and NS5 gene sequences for each isolate. The ancient lineages of AHFV and KFDV have shown that both diverged from each other about 66-177 years ago, and with evolution, the AHFV strains became highly diversified. In 2007, from Jeddah, KSA, a strain of AHFV was found from an O. Savignyi tick viz., tick JE7.
When the sequencing of this strain was compared to that of the strain discovered in 1995, it was discovered that there is 99.7% nt sequence similarity in the envelope gene at its homologous region. Madani et al. in 2014, carried out a broad comparative analysis in which they compared the AHFV strain (from Najran) with 18 other AHFV strains (from Makkah and Jeddah), Dengue Virus (DENV), Langat Virus, KFDV, Tick-Borne Encephalitis Virus (TBEV), and Omsk Hemorrhagic Fever Virus (OHFV) [10,16,21,46]. In 2014, Madani et al. sequenced an AHFV strain isolated from Najran, having 10 546 nt long genome and encoding a single 5410 amino acid long polyprotein. The Narjan sequence of the AHFV strain was relatively short compared to previously isolated AHFV strains with 10 685 to 10 749 nt [47-49]. A comparative phylogenetic study of various strains of AHFV revealed that the Najran strain was very close. It shares about 99% homology with distinct 18 AHFV strains while other Flaviviridae family viruses like KFDV, OHFV, TBEV, and Langat virus isolates showed different clusters due to high variability in their sequence [48,49]. It was found that there is a variation in the sequence of the core protein and NS4a gene of the two different AHFV strains. The factor responsible for varying phylogenetic position and genome lengths of various strains from Najran and other region, was the recombination in virus strains [48,49].
Pathogenesis and Pathology
AHFV was first identified as a KFDV variant in 1995 [50,51]. It was clear since it had 89% nt sequence homology [48,49,51]. The pathogenic mechanism of KFDV and AHFV involves the standard process seen in Flaviviruses, where the envelope protein (E) attaches to the cell surface with glycosaminoglycans. The virus then enters the cell through receptor-mediated, clathrin-dependent endocytosis. The endocytic vesicles with the virus then move to endosomes, where the acidic conditions cause the separation of the E protein’s dimeric form into monomers, which then combine to form trimers. This change in shape results in the breaking apart of particles, merging of the viral and endocytic membrane, and the viral genome being released into the cytoplasm. The positive-stranded RNA is translated into one precursor polypeptide by ribosomes attached to the endoplasmic reticulum (ER), which is later separated into individual proteins by viral and host proteases. Genome replication takes place on host cell membranes derived from the ER, which are triggered by the virus to serve as a site for the viral replication complexes and host cell factors essential for replication. Ultimately, the viral RNA binds with the C protein and is enclosed within a lipid bilayer derived from the ER, which includes hetero-dimers of the prM and E proteins. The C protein helps virus budding by attaching to the ER membrane and containing the viral genome. The virions are then moved to the Golgi complex for the ultimate maturation phase, where prM is converted into M through furin-mediated cleavage. This procedure guarantees that the premature virus particle does not merge with the cell membrane while being exported. Mature infectious particles are discharged into the extracellular environment through exocytosis originating from the Golgi apparatus.
Dodd et al. (2014) conducted the initial animal-model study on the pathogenicity of AHFV. Even though both AHFV and KFDV were shown to infect various organs in mice, AHFV had lower lethality and viral levels compared to KFDV [52,53]. Histopathological analysis of AHFV-infected rat brains revealed the presence of meningoencephalitis, but with sporadic inflammatory infiltration and no necrosis detected. Additionally, the research demonstrated that the brain abnormalities in AHFV-infected rats were comparable to those detailed by Holbrook et al. (2005) [54] in their examination of the brains of Powassan virus-infected mice. Nevertheless, some regions of the cerebrum of mice infected with the Powassan virus showed necrosis in the form of acute neuronal injury, edema, and karyorrhexis. Conversely, OHFV-infected mice showed signs of patchy meningoencephalitis with perivascular cuffing and no necrosis in their brains [54]. In contrast, a previous experiment using a BALB/c mouse model showed that AHFV did not result in observable illness, being present in various internal organs and causing only slight histopathological changes, as well as triggering a proinflammatory response in the spleen and kidney [55]. In a recent study using mice without the type I interferon receptor, Bhatia et al. showed that AHFV-induced damage was primarily seen in the spleen, liver, heart, and lymph nodes with no observed changes in the lungs, kidney, or brain [17]. Because of sporadic and unforeseen occurrences of VHF resulting from specific viral infections like AHFV and KFDV a limited clinical and epidemiological information is available hence incomplete data on pathogenesis is available [56].
Modes of Transmission
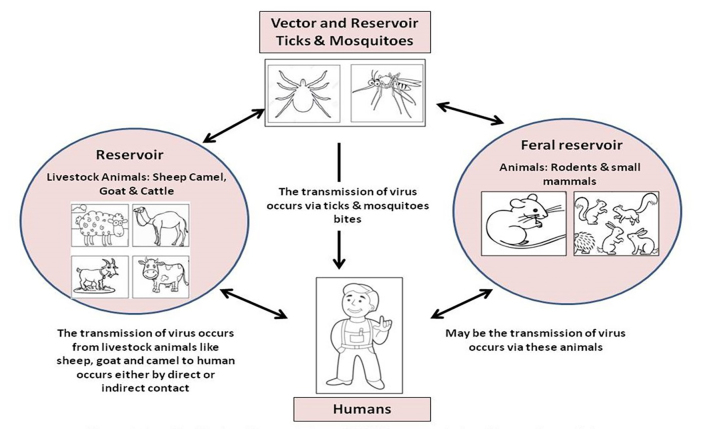
The mode of transmission of AHFV is influenced by several factors like age, sex/gender of patients, weather conditions, etc. Other important factors that affect the transmission are direct and indirect contact with the livestock and their products like meat, raw milk, blood, and body secretions, etc. Contamination can be due to improper housing conditions of livestock animals, especially camels and sheep, which have been exposed to ticks and mosquitoes that are later on responsible for infection (Figure 3). Most of the AHFV infections were reported from such areas with regular contact between livestock animals like localities nearby to the slaughterhouses and butcher areas. It was evident from the study of by Qattan et al. where first eight cases of AHFV infection resulted due to contact with sheep; out of these eight patients, six were butchers [57]. It became clear that the transmission of AHFV infection is either due to direct contact of humans with livestock animals or their different products; there is also a possibility of transmission through mosquito bites, which indicated that ticks might either act as vectors or as reservoirs for transmission of AHFV [1,9,18,58-60].
Figure 3.
Possible modes of transmission.
It was also discovered that patients who had no interaction with infected animals or their products, as well as no tick bites, contracted the virus. It was later discovered that these individuals had been bitten by mosquitos, however this does not establish that mosquitos operate as vectors or reservoirs for AHFV. These people either lived or worked in areas near livestock animals or animal markets or near to slaughterhouses. These findings revealed that mosquitoes were most likely to be the vector of these viruses infecting animals first and later humans. Another study reported that the handling of raw milk or animal meat products was insignificant compared to direct contact with livestock animals, tick bites, and neighboring animal farms. The transmission of AHFV infection from one human to another remains unclear. Despite the fact that there have been multiple incidences of human-to-human transmission of AHFV infection within the same family.
The transfer of infection through the air-droplets, close contact with infected individuals, or transmission by their body fluids is yet to be studied. It is also evident that transmission of AHFV in a group may be due to predisposition to a common source of infection or vectors viz mosquitoes or ticks. It is still unclear that which is/are vectors of this AHFV infection; one of the important suspects is mosquito. Additionally, several reports show that AHFV was transmitted from animals and their products to an individual is either by direct contact or by ticks, this scenario becomes clear in agricultural areas from where diseases were reported. Under such circumstances, from 2007 to 2017 there were seven reports published that shows that the transmission of this virus occurred from ticks discovered AHFV RNA in the camel tick O. savignyi from Jeddah, KSA [16,22,45]. Following that, Mahdi et al. isolated AHFV from H. dromedarii and sand tampan ticks (O. savignyi) in Najran, KSA. Carletti et al. [12] reported that two Italian tourists returning from Egypt had clinical AHFV infection and that one of them had been bitten on the foot by an arthropod described as tick-shaped but not formally recognized. Ravanini et al. [14] and Musso et al. [13] both described AHFV cases involving Italian tourists returning from Egypt who were exposed to an unidentified tick-shaped bug. Horton et al. [50] discovered AHFV RNA in Amblyomma lepidum ticks from imported cattle in Djibouti. In April 2017, AHFV RNA was detected in three patients in Algunfuda, KSA, who had also been bitten by unidentified ticks (personal communication, Ministry of Health, Jeddah, Saudi Arabia). As a result of the above observations, ticks may play an essential role in the epidemiology of AHFV, perhaps as viral vectors or reservoirs. More study in this area is needed to understand the relationship between ticks, mosquitoes, and AHFV transmission due to lack of research.
Clinical Manifestations
Clinically, AHFV infection varies from subclinical to severe or even fatal. Clinical symptoms include retro-orbital pain, fever, headache, leucopenia, vomiting, myalgia, thrombocytopenia, arthralgia, and anorexia. Severe infection can cause hemorrhagic symptoms such as hematemesis, epistaxis, and, in rare cases, encephalitis. Individuals suffering from encephalitis complications die, and such cases have a 25% mortality rate [1,10,14,41,62]. Laboratory manifestations or symptoms involve an increased level of lactate dehydrogenase, creatine kinase, and other liver enzymes. During outbreaks of AHFV in places like Jeddah, Makkah, Najran, and other areas in KSA there were numerous reports focusing on clinical and laboratory manifestation that was seen in these areas. Some examples includes fever, headache, malaise, arthralgia, anorexia, myalgia, backache, nausea and vomiting, chills, retro-orbital pain, diarrhea, abdominal pain, hemorrhagic manifestations, CNS manifestations, leucopenia, high level of liver enzymes, prolonged partial thromboplastin time, thrombocytopenia, increased level of creatine kinase level, and of lactate dehydrogenase. In addition to these common symptoms, a peculiar case of AHFV was noticed, in which the patient had “Rhabdomyolysis and severe muscle weakness” [1,3,10,14].
As for the question of its treatment, no antiviral drug/s have been invented yet. The treatment of AHFV includes symptomatic treatment, for example giving intravenous fluids and ionotropic supplements, in severe conditions. Other treatments can be adopted, like transfusion of fluids, like plasma and blood, giving oxygen by mechanical means, and in case of secondary infections antimicrobial mode of therapy is needed. There are several challenges in AHFV treatment. The first is to develop specific treatment and vaccines that will be available in the future to combat AHFV infections, and the second is to have an animal model for AHFV for testing and determining potent drug targets that will best and suitably control AHFV.
Diagnostic Methods
Detecting early diagnosis of AHFV involves several molecular and immunological techniques [15,60]. Immune-fluorescence assay (IFA) was used to detect Flavivirus specific monoclonal antibodies 4G2. A Polymerase chain reaction (PCR) based 220-base pairs long gene having 89% homology with KFDV NS5 gene was used for the diagnosis of AHFV. Culturing viruses are usually time-consuming and more complicated as compared to Real-time PCR (RT-PCR) [23]. The suspected infection of AHFV can be detected by RT-PCR of body fluid for examples plasma and serum of patients. The initial 7 days of suspected infection is very crucial for its detection and sampling for these tests must be done in the first 7 days to give better detection results. Another point that to be mentioned here is that using buffy coats of individuals having suspected infection gives enhanced sensitivity and also good specificity as compared to using other body fluids, like blood and serum. Various other alternative diagnostics methods are in the pipeline to develop diagnostics assays like other infectious diseases [63-69] and for vaccinations to approach screening of vaccine candidates [70,71].
AHFV Antibodies and its Surveillance in Human Serum
There are very limited sero-surveillance data available for human AHFV antibodies studies from KSA or elsewhere. These data on sero-surveillance from KSA showed the lack of AHFV IgM antibodies. Previously, it was assumed that AHFV-IgG of KSA is not prevalent and found in narrow dimensions, but sero-studies from different parts of KSA have unmasked this and showed that AHFV-IgG positive sera are present in 1.3% out of 1024 soldiers. It suggests broader geographic distribution [72]. To support such studies, it was found that local geographical distribution of AHFV-IgG positive sera has been reported in Tabouk (Northern region) 61.5%, Jazan (South Western region) 7.7%, and Eastern region 23.1% Asir (South Western region) 7.7%, respectively. A strange and intriguing phenomenon was reported in Djibouti, where no AHFV infection was identified, although there were AHFV-IgG positive people [73]. A sero-surveillance investigation in Djibouti found that among 893 people, only a 13-year-old girl who resided near an abattoir had AHFV-neutralizing antibodies [15,60,72]. The reality behind this may be either they have been exposed to AHFV or subclinical infection with AHFV.
Prevention and Control
The limited epidemiological data of AHFV suggests that the control and prevention of infection can be achieved by restricting direct contact with livestock animals and their products like raw milk, meat, etc., and avoiding tick and mosquito bites. The authorities like municipalities, sanitary, health, and veterinary departments near slaughterhouses, animal marketplaces, or infected places should be called immediately to evaluate the risk factors, take action, and propagate awareness among the population regarding its spread and its safe handling measures while treating an infected individual. There should be the establishment of a national-level prevention and control program in this regard. It is observed that countries having such well-equipped national-level prevention and control programs reported a drastic decrease in infection. Avoiding the infection is the only way to decrease the spread of AHFV infection along with interdisciplinary teamwork in the present scenario. Also, currently in progress is the development of human or animal vaccines to prevent this infection. An in silico analysis has recently been done for development of an epitope-based peptide vaccine against AHFV where the authors have analyzed the envelope glycoprotein of ALKV for developing B- and T-cells epitope-based peptide vaccine candidates [72,73]. Data from such studies suggest that these proposed epitopes can be used in the development of a vaccine for ALKV, which may induce both humoral and cell-mediated immunity.
Mortality Due to AHFV Infection
The epidemiological data of AHFV in KSA showed that the mortality rate in Jeddah and Makkah was 20% and 25%, respectively [3]. Subsequently, due to early diagnosis, awareness, improved healthcare, and sanitary facilities as well as the involvement of various authorities have been proven successful in decreasing mortality. For example, the mortality rate was 1% in Najran from 2003 to 2009. A very limited outbreak was observed in Taif and Jazan between 2010 and 2015, and it reached <0.5% in Algunfuda in 2017. Other various data have shown its range of illnesses from asymptomatic infections to severe and fatal infections. The decreasing death rate points out that the AHFV has reached endemicity which in turn leads to the development of herd immunity in the population.
Summary
To summarize, AHFV is a novel hemorrhagic fever virus of Flaviviridae family that has mostly been reported in KSA. Because it originated in the Alkhumra neighborhood of Jeddah, it is known as the Alkhumra hemorrhagic fever virus (AHFV). Its clinical symptoms include flu-like disease, fever, hemorrhagic signs, and, in rare cases, encephalitis. The virus is transmitted from animals (such as sheep, goats, and camels) to people by direct contact or through tick and mosquito bites, and they may operate as a vector or a reservoir. More research is needed to understand the involvement of AHFV vectors. The engagement of authorities such as towns, sanitary, health, and veterinary departments near slaughterhouses, animal marketplaces, or diseased areas to identify risk factors is one of the preventative methods. Different measures should be taken to raise public awareness about AHFV and its spread. People should be educated on how to safely handle diseased people, animals, or their products. There is an urgent need for extensive study on the role of arthropods (mosquitoes and ticks) as vectors, the role of animals in AHFV transmission in humans and cattle, and the seroprevalence of AHFV antibodies.
Acknowledgments
We gratefully acknowledge the facilities used from Multidisciplinary Research Unit, Maulana, Azad Medical College and Associated Hospital, New Delhi, India.
Glossary
- AHFW
Alkhumra Hemorrhagic Fever Virus
- Kb
kilobases
- KSA
Kingdom of Saudi Arabia
- KFDV
Kyasanur Forest Disease Virus
- ICTV
International Committee on Taxonomy of Viruses
- ORF
Open Reading Frame
- Nt
nucleotide
- UTRs
untranslated regions
- TBEV
Tick-Borne Encephalitis Virus
- IFA
Immune-fluorescence assay
- RT-PCR
Real-time PCR
Author Contributions
AS conceptualized the literature search, made the figures, wrote the original draft, edited, and reviewed the final version of the manuscript. SM, CPU, PKM, RRM, BCK edited and reviewed the final version of the manuscript. SCS supervised, edited, and reviewed the final version of the manuscript.
References
- Abdulhaq AA, Hershan AA, Karunamoorthi K, Al-Mekhlafi HM. Human Alkhumra hemorrhagic Fever: Emergence, history and epidemiological and clinical profiles. Saudi J Biol Sci. 2022. Mar;29(3):1900–10. 10.1016/j.sjbs.2021.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Memish ZA. Alkhurma hemorrhagic fever virus. Microbes Infect. 2017. Jun;19(6):305–10. 10.1016/j.micinf.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Alzahrani AG, Al Shaiban HM, Al Mazroa MA, Al-Hayani O, Macneil A, Rollin PE, et al. Alkhurma hemorrhagic fever in humans, Najran, Saudi Arabia. Emerg Infect Dis. 2010. Dec;16(12):1882–8. 10.3201/eid1612.100417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray M, Schaechter M. Academic Press; Oxford: 2009. Hemorrhagic Fever Viruses, in Encyclopedia of Microbiology (Third Edition) pp. 339–353. [Google Scholar]
- Zakham F, Al-Habal M, Taher R, Alaoui A, El Mzibri M. Viral hemorrhagic fevers in the Tihamah region of the western Arabian Peninsula. PLoS Negl Trop Dis. 2017. Apr;11(4):e0005322. 10.1371/journal.pntd.0005322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar LE. Alkhumra Hemorrhagic Fever Virus (AHFV): Current Status and Future Prospects. Advancements of Microbiology. Sciendo. 2023;62(2):83–6. [Google Scholar]
- Madani TA, Azhar EI, Abuelzein TM, Kao M, Al-Bar HM, Niedrig M, et al. Alkhumra, not Alkhurma, is the correct name of the new hemorrhagic fever flavivirus identified in Saudi Arabia. Intervirology. 2012;55(4):259–60. 10.1159/000337238 [DOI] [PubMed] [Google Scholar]
- Madani TA, Azhar EI, Abuelzein TM, Kao M, Al-Bar HM, Abu-Araki H, et al. Alkhumra (Alkhurma) virus outbreak in Najran, Saudi Arabia: epidemiological, clinical, and laboratory characteristics. J Infect. 2011. Jan;62(1):67–76. 10.1016/j.jinf.2010.09.032 [DOI] [PubMed] [Google Scholar]
- Madani TA, Abuelzein EM. Alkhumra hemorrhagic fever virus infection. Arch Virol. 2021. Sep;166(9):2357–67. 10.1007/s00705-021-05083-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri AA, Irekeola AA. Global prevalence of alkhumra hemorrhagic fever virus infection: the first meta-analysis and systematic review. J Infect Public Health. 2024. Jun;17(6):986–93. 10.1016/j.jiph.2024.04.001 [DOI] [PubMed] [Google Scholar]
- Abdulhaq AA, Hershan AA, Karunamoorthi K, Al-Mekhlafi HM. Human Alkhumra hemorrhagic Fever: Emergence, history and epidemiological and clinical profiles. Saudi J Biol Sci. 2022. Mar;29(3):1900–10. 10.1016/j.sjbs.2021.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti F, Castilletti C, Di Caro A, Capobianchi MR, Nisii C, Suter F, et al. Alkhurma hemorrhagic fever in travelers returning from Egypt, 2010. Emerg Infect Dis. 2010. Dec;16(12):1979–82. 10.3201/eid1612.101092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Galati V, Stella MC, Capone A. A case of Alkhumra virus infection. J Clin Virol. 2015. May;66:12–4. 10.1016/j.jcv.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Ravanini P, Hasu E, Huhtamo E, Crobu MG, Ilaria V, Brustia D, et al. Rhabdomyolysis and severe muscular weakness in a traveler diagnosed with Alkhurma hemorrhagic fever virus infection. J Clin Virol. 2011. Nov;52(3):254–6. 10.1016/j.jcv.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, Leparc-Goffart I, et al. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PLoS Negl Trop Dis. 2014. Dec;8(12):e3299. 10.1371/journal.pntd.0003299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Zimmerman D, Deem SL. A Review of Zoonotic Pathogens of Dromedary Camels. EcoHealth. 2019. Jun;16(2):356–77. 10.1007/s10393-019-01413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia B, Haddock E, Shaia C, Rosenke R, Meade-White K, Griffin AJ, et al. Alkhurma haemorrhagic fever virus causes lethal disease in IFNAR-/- mice. Emerg Microbes Infect. 2021. Dec;10(1):1077–87. 10.1080/22221751.2021.1932609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Fagbo SF, Osman Ali A, AlHakeem R, Elnagi FM, Bamgboye EA. Is the epidemiology of alkhurma hemorrhagic fever changing?: A three-year overview in Saudi Arabia. PLoS One. 2014. Feb;9(2):e85564. 10.1371/journal.pone.0085564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumosani TA, Al-Malki AL, Razvi SS, Balgoon MJ, Kaleem M, Huwait EA, et al. Hemorrhagic fever in Saudi Arabia: challenge to public health, effective management and future considerations. Afr Health Sci. 2020. Sep;20(3):1153–63. 10.4314/ahs.v20i3.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PD, Patil S, Jadhav SM, Nyayanit DA, Kumar V, Jain S, et al. Phylogeography of Kyasanur Forest Disease virus in India (1957-2017) reveals evolution and spread in the Western Ghats region. Sci Rep. 2020. Feb;10(1):1966. 10.1038/s41598-020-58242-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia B, Feldmann H, Marzi A. Kyasanur Forest Disease and Alkhurma Hemorrhagic Fever Virus-Two Neglected Zoonotic Pathogens. Microorganisms. 2020. Sep;8(9):1406. 10.3390/microorganisms8091406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohran KA, Farag EA, Reusken CB, Raj VS, Lamers MM, Pas SD, et al. The sample of choice for detecting Middle East respiratory syndrome coronavirus in asymptomatic dromedary camels using real-time reversetranscription polymerase chain reaction. Rev Sci Tech. 2016. Dec;35(3):905–11. 10.20506/rst.35.3.2578 [DOI] [PubMed] [Google Scholar]
- Fakoorziba MR, Golmohammadi P, Moradzadeh R, Moemenbellah-Fard MD, Azizi K, Davari B, et al. Reverse transcription PCR-based detection of Crimean-Congo hemorrhagic fever virus isolated from ticks of domestic ruminants in Kurdistan province of Iran. Vector Borne Zoonotic Dis. 2012. Sep;12(9):794–9. 10.1089/vbz.2011.0743 [DOI] [PubMed] [Google Scholar]
- Alzahrani AG, Al Shaiban HM, Al Mazroa MA, Al-Hayani O, Macneil A, Rollin PE, et al. Alkhurma hemorrhagic fever in humans, Najran, Saudi Arabia. Emerg Infect Dis. 2010. Dec;16(12):1882–8. 10.3201/eid1612.100417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andayi F, Crepey P, Kieffer A, Salez N, Abdo AA, Carrat F, et al. Determinants of individuals’ risks to 2009 pandemic influenza virus infection at household level amongst Djibouti city residents—a CoPanFlu cross-sectional study. Virol J. 2014. Jan;11(1):13. 10.1186/1743-422X-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki AM. Isolation of a flavivirus related to the tick-borne encephalitis complex from human cases in Saudi Arabia. Trans R Soc Trop Med Hyg. 1997;91(2):179–81. 10.1016/S0035-9203(97)90215-7 [DOI] [PubMed] [Google Scholar]
- El-Kamary SS, Strickland GT, Hepatitis V. International Encyclopedia of Public Health. 2nd ed. Academic Press; 2017. pp. 611–20. 10.1016/B978-0-12-803678-5.00209-5 [DOI] [Google Scholar]
- Mehla R, Kumar SR, Yadav P, Barde PV, Yergolkar PN, Erickson BR, et al. Recent ancestry of Kyasanur Forest disease virus. Emerg Infect Dis. 2009. Sep;15(9):1431–7. 10.3201/eid1509.080759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares A. Factors influencing the seasonal patterns of infectious diseases. Int J Prev Med. 2013. Feb;4(2):128–32. [PMC free article] [PubMed] [Google Scholar]
- Shibl A, Senok A, Memish Z. Infectious diseases in the Arabian Peninsula and Egypt. Clin Microbiol Infect. 2012. Nov;18(11):1068–80. 10.1111/1469-0691.12010 [DOI] [PubMed] [Google Scholar]
- Memish ZA, Fagbo SF, Osman Ali A, AlHakeem R, Elnagi FM, Bamgboye EA. Is the epidemiology of alkhurma hemorrhagic fever changing?: A three-year overview in Saudi Arabia. PLoS One. 2014. Feb;9(2):e85564. 10.1371/journal.pone.0085564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, Zaki AM, Fakeeh M, Yousef AI, de Chesse R, Attoui H, et al. Low diversity of Alkhurma hemorrhagic fever virus, Saudi Arabia, 1994-1999. Emerg Infect Dis. 2005. May;11(5):683–8. 10.3201/eid1105.041298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott DC. Hemorrhagic fever viruses. Crit Care Clin. 2005. Oct;21(4):765-83, vii. https://doi.org/ 10.1016/j.ccc.2005.06.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Azhar EI, Abuelzein TM, Kao M, Al-Bar HM, Niedrig M, et al. Alkhumra, not Alkhurma, is the correct name of the new hemorrhagic fever flavivirus identified in Saudi Arabia. Intervirology. 2012;55(4):259–60. 10.1159/000337238 [DOI] [PubMed] [Google Scholar]
- Shayan S, Bokaean M, Shahrivar MR, Chinikar S. Crimean-Congo Hemorrhagic Fever. Lab Med. 2015;46(3):180–9. 10.1309/LMN1P2FRZ7BKZSCO [DOI] [PubMed] [Google Scholar]
- Taxonomy V. The Classification and Nomenclature of Viruses The 9th Report of the ICTV (2011), https://ictv.global/report_9th
- Dolan TT. Ticks and Tick-borne Disease Control: Proceedings of a Joint OAU, FAO, and ILRAD Workshop Held in Kampala, Uganda, 12-14 September 1991. 1993: ILRI (aka ILCA and ILRAD). chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://pdf.usaid.gov/pdf_docs/PNABS630.pdf
- Charrel RN, de Lamballerie X. Le virus Alkhurma (famille Flaviviridae, genre Flavivirus): un pathogène émergent responsable de fièvres hémorragiques au Moyen-Orient [The Alkhurma virus (family Flaviviridae, genus Flavivirus): an emerging pathogen responsible for hemorrhage fever in the Middle East]. Méd Trop (Mars). 2003;63(3):296–9. [PubMed] [Google Scholar]
- Liebert UG. Controversy on virus designation: alkhumra sive Alkhurma hemorrhagic fever flavivirus. Intervirology. 2012;55(4):257–8. 10.1159/000337237 [DOI] [PubMed] [Google Scholar]
- Charrel RN, Zaki AM, Fagbo S, de Lamballerie X. Alkhurma hemorrhagic fever virus is an emerging tick-borne flavivirus. J Infect. 2006. Jun;52(6):463–4. 10.1016/j.jinf.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Al-Tayib OA. An Overview of the Most Significant Zoonotic Viral Pathogens Transmitted from Animal to Human in Saudi Arabia. Pathogens. 2019. Feb;8(1):25. 10.3390/pathogens8010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said S, Kang M. Viral Encephalitis [Updated 2023 Aug 8] StatPearls. [Internet] Treasure Island (FL): StatPearls Publishing; 2024. Jan., Available from https://www.ncbi.nlm.nih.gov/books/NBK470162/
- Charrel RN, Zaki AM, Attoui H, Fakeeh M, Billoir F, Yousef AI, et al. Complete coding sequence of the Alkhurma virus, a tick-borne flavivirus causing severe hemorrhagic fever in humans in Saudi Arabia. Biochem Biophys Res Commun. 2001. Sep;287(2):455–61. 10.1006/bbrc.2001.5610 [DOI] [PubMed] [Google Scholar]
- Luo D, Wei N, Doan DN, Paradkar PN, Chong Y, Davidson AD, et al. Flexibility between the protease and helicase domains of the dengue virus NS3 protein conferred by the linker region and its functional implications. J Biol Chem. 2010. Jun;285(24):18817–27. 10.1074/jbc.M109.090936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakham F, Albalawi AE, Alanazi AD, Truong Nguyen P, Alouffi AS, Alaoui A, et al. Viral RNA metagenomics of Hyalomma ticks collected from dromedary camels in Makkah province, Saudi Arabia. Viruses. 2021. Jul;13(7):1396. 10.3390/v13071396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanisamy N, Akaberi D, Lennerstrand J, Lundkvist Å. Comparative genome analysis of Alkhumra hemorrhagic fever virus with Kyasanur forest disease and tick-borne encephalitis viruses by the in silico approach. Pathog Glob Health. 2018. Jun;112(4):210–26. 10.1080/20477724.2018.1471187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H. Argasid and nuttalliellid ticks as parasites and vectors. Adv Parasitol. 1985;24:135–238. 10.1016/S0065-308X(08)60563-1 [DOI] [PubMed] [Google Scholar]
- Madani TA, Abuelzein TM, Al-Bar HM, Azhar EI, Kao M, Alshoeb HO, et al. Outbreak of viral hemorrhagic fever caused by dengue virus type 3 in Al-Mukalla, Yemen. BMC Infect Dis. 2013. Mar;13(1):136. 10.1186/1471-2334-13-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Azhar EI, Abuelzein TM, Kao M, Al-Bar HM, Farraj SA, et al. Complete genome sequencing and genetic characterization of Alkhumra hemorrhagic fever virus isolated from Najran, Saudi Arabia. Intervirology. 2014;57(5):300–10. 10.1159/000362334 [DOI] [PubMed] [Google Scholar]
- Horton KC, Fahmy NT, Watany N, Zayed A, Mohamed A, Ahmed AA, et al. Crimean Congo Hemorrhagic Fever Virus and Alkhurma (Alkhumra) Virus in Ticks in Djibouti. Vector Borne Zoonotic Dis. 2016. Oct;16(10):680–2. 10.1089/vbz.2016.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, Fagbo S, Moureau G, Alqahtani MH, Temmam S, de Lamballerie X. Alkhurma hemorrhagic fever virus in Ornithodoros savignyi ticks. Emerg Infect Dis. 2007. Jan;13(1):153–5. 10.3201/eid1301.061094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd KA, Bird BH, Jones ME, Nichol ST, Spiropoulou CF. Kyasanur Forest disease virus infection in mice is associated with higher morbidity and mortality than infection with the closely related Alkhurma hemorrhagic fever virus. PLoS One. 2014. Jun;9(6):e100301. 10.1371/journal.pone.0100301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd KA, Bird BH, Khristova ML, Albariño CG, Carroll SA, Comer JA, et al. Ancient ancestry of KFDV and AHFV revealed by complete genome analyses of viruses isolated from ticks and mammalian hosts. PLoS Negl Trop Dis. 2011. Oct;5(10):e1352. 10.1371/journal.pntd.0001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook MR, Aronson JF, Campbell GA, Jones S, Feldmann H, Barrett AD. An animal model for the tickborne flavivirus—omsk hemorrhagic fever virus. J Infect Dis. 2005. Jan;191(1):100–8. 10.1086/426397 [DOI] [PubMed] [Google Scholar]
- Sawatsky B, McAuley AJ, Holbrook MR, Bente DA. Comparative pathogenesis of Alkhumra hemorrhagic fever and Kyasanur forest disease viruses in a mouse model. PLoS Negl Trop Dis. 2014. Jun;8(6):e2934. 10.1371/journal.pntd.0002934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani TA, Kao M, Abuelzein TM, Azhar EI, Al-Bar HM, Abu-Araki H, et al. Propagation and titration of Alkhumra hemorrhagic fever virus in the brains of newborn Wistar rats. J Virol Methods. 2014. Apr;199:39–45. 10.1016/j.jviromet.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Qattan I, Akbar N, Afif H, Abu Azmah S, Al-Khateeb T, Zaki A, et al. A novel flavivirus: makkah Region 1994–1996. Saudi Epidemiol Bull. 1996;3:1–3. [Google Scholar]
- Nepveu-Traversy ME, Fausther-Bovendo H, Babuadze GG. Human Tick-Borne Diseases and Advances in Anti-Tick Vaccine Approaches: A Comprehensive Review. Vaccines (Basel). 2024. Jan;12(2):141. 10.3390/vaccines12020141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, Zaki AM, Fakeeh M, Yousef AI, de Chesse R, Attoui H, et al. Low diversity of Alkhurma hemorrhagic fever virus, Saudi Arabia, 1994-1999. Emerg Infect Dis. 2005. May;11(5):683–8. 10.3201/eid1105.041298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Albarrak A, Almazroa MA, Al-Omar I, Alhakeem R, Assiri A, et al. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg Infect Dis. 2011. Dec;17(12):2316–8. 10.3201/eid1712.110658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger P, Kolodziejek J, Khafaga T, Loney T, Howarth B, Sher Shah M, et al. Potentially Zoonotic Viruses in Wild Rodents, United Arab Emirates, 2019-A Pilot Study. Viruses. 2023. Mar;15(3):695. 10.3390/v15030695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev D, Patel AL, Sonkar SC, Kumari I, Saluja D. Diagnosis of Neisseria gonorrhoeae using molecular beacon. BioMed Res Int. 2015;2015:597432. 10.1155/2015/597432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkar SC, Sachdev D, Mishra PK, Kumar A, Mittal P, Saluja D. A molecular-beacon-based asymmetric PCR assay for easy visualization of amplicons in the diagnosis of trichomoniasis. Biosens Bioelectron. 2016. Dec;86:41–7. 10.1016/j.bios.2016.06.025 [DOI] [PubMed] [Google Scholar]
- Sachdev D, Wasnik K, Patel AL, Sonkar SC, Desai P, Mania-Pramanik J, et al. Multi-centric validation of an in-house-developed beacon-based PCR diagnostic assay kit for Chlamydia and Neisseria and portable fluorescence detector. J Med Microbiol. 2018. Sep;67(9):1287–93. 10.1099/jmm.0.000803 [DOI] [PubMed] [Google Scholar]
- Sonkar SC, Yadav S, Malla N, Dhanda RS, Khurana S, Bagga R, et al. Evaluation of DNA based techniques for the diagnosis of human vaginal trichomoniasis in North Indian population. Br Microbiol Res J. 2016;17(6):1–2. 10.9734/BMRJ/2016/29557 [DOI] [Google Scholar]
- Garg S, Singh VK, Sonkar SC, Kelkar H, Singh S, Garg S, et al. Pattern of serum protein capillary electrophoretogram in SARS- CoV-2 infection. Clin Chim Acta. 2022. Feb;527:11–6. 10.1016/j.cca.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev D, Wasnik K, Patel AL, Sonkar SC, Desai P, Mania-Pramanik J, et al. Multi-centric validation of an in-house-developed beacon-based PCR diagnostic assay kit for Chlamydia and Neisseria and portable fluorescence detector. J Med Microbiol. 2018. Sep;67(9):1287–93. 10.1099/jmm.0.000803 [DOI] [PubMed] [Google Scholar]
- Irungbam M, Chitkara A, Singh VK, Sonkar SC, Dubey A, Bansal A, et al. Evaluation of performance of detection of immunoglobulin G and immunoglobulin M antibody against spike protein of SARS-coV-2 by a rapid kit in a real-life hospital setting. Front Microbiol. 2022. Apr;13:802292. 10.3389/fmicb.2022.802292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkar SC, Arora G, Wasnik K, Ali M, Mittal P, Saluja D. Improved management can be achieved by introducing additional parameters in the syndromic diagnosis of nonviral sexually transmitted infections at low-resource settings. AJOG Glob Rep. 2021. Dec;2(1):100037. 10.1016/j.xagr.2021.100037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Sonkar SC, Raj SR, Chaudhry U, Saluja D. Functional analysis of hypothetical proteins of Chlamydia trachomatis: a bioinformatics based approach for prioritizing the targets. J Comput Sci Syst Biol. 2013;7(1):010-4. [Google Scholar]
- Sharma V, Sonkar SC, Singhal P, Kumar A, Singh RK, Ramachandran VG, et al. Functional impact of allelic variations/haplotypes of TNF-α on reproductive tract infections in Indian women. Sci Rep. 2021. Jan;11(1):627. 10.1038/s41598-020-79963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar LE, et al. Alkhumra hemorrhagic fever virus (ahfv): current status and future prospects. Adv Microbiol. 2023;62(2):83–6. [Google Scholar]
- Ul-Rahman A, Shabbir MA. In silico analysis for development of epitopes-based peptide vaccine against Alkhurma hemorrhagic fever virus. J Biomol Struct Dyn. 2020. Jul;38(10):3110–22. 10.1080/07391102.2019.1651673 [DOI] [PubMed] [Google Scholar]