Abstract
Chikungunya fever (CHIKF) is an acute viral disease caused by the chikungunya virus (CHIKV) transmitted by Aedes mosquitoes. The acute phase presents with limited symptoms and low mortality, but approximately half of cases progress to more chronic illness with persistent and disabling joint symptoms. To better characterize the burden of chronic disease, we analyzed the relationship between pain intensity, the Disease Activity Index by DAS28-ESR, rheumatoid factor (RF) positivity, sex, and age in a retrospective cohort of 133 patients with chikungunya arthritis (CHIKA). We assessed all subjects by clinical evaluations, and laboratory testing, including the Pain Visual Analog Scale (VAS), the Disease Activity Score (DAS28-ESR), and RF measurement. We observed that pain intensity increased significantly with disease activity (ρ = 0.416 and p-value < 0.05) and with age (ρ = 0.259 and p-value = 0.003). Despite a predominance of women in our cohort, sex/gender was not associated with increased pain risk. Our study demonstrated a strong correlation between pain and disease activity, but assessment of these variables in a larger, prospective cohort should be undertaken to further characterize risk variables and improve therapy for patients with CHIKA.
Keywords: Chikungunya fever, chikungunya virus, rheumatoid arthritis, rheumatoid factor
Introduction
Chikungunya (CHIK) is caused by a single-stranded RNA (ribonucleic acid) virus of the genus Alphavirus of the Togaviridae family, called chikungunya virus (CHIKV). CHIK is often a biphasic illness. The acute phase, “chikungunya fever” (CHIKF) begins 3-7 days after CHIKV infection by an Aedes mosquito [1]. CHIKF is characterized by the abrupt onset of high fever, disabling polyarthralgia/polyarthritis, maculopapular skin rash, headache, myalgia, nausea, and diarrhea. This acute phase is generally limited to 10-14 days and has a low mortality rate [2]. However, it is estimated that 51% of infected individuals progress to a chronic phase characterized by persistent arthralgia, disabling chronic arthritis, and myalgia [3].
Since it was first isolated in Tanzania in 1952, CHIKV has caused recurrent epidemics during the second half of the 20th century throughout Asia and sub-Saharan Africa [4]. Beginning in 2004, CHIKV spread to several islands in the Indian Ocean, Southeast Asia, and India. In 2013 CHIK was introduced to the Americas, where more than 3.6 million cases have been reported in 50 countries [5].
During the chronic phase of CHIK, painful musculoskeletal symptoms predominate, varying in intensity [2]. Among patients with chikungunya arthritis (CHIKA), many have nonspecific arthralgia and myalgias, but some develop frank inflammatory arthritis [6,7]. These individuals may meet the American College of Rheumatology (ACR) 2010 criteria for rheumatoid arthritis (RA), with increased inflammatory markers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), in addition to joint effusions, bone erosions, and synovial thickening on imaging studies [6].
Due to the similarities between CHIKA and RA, CHIKA patients have been assessed with outcome measures developed for RA [7-10], such as the Pain Visual Analog Scale (VAS) [11], the Disease Activity Score (DAS28-ESR) [12] and rheumatoid factor (RF) measurement. Using these measurement tools, there is increasing evidence that CHIKA follows a variable clinical course. In some individuals, the illness is self-limited and non-destructive, but others develop a chronic inflammatory course that mimics RA, with irreversible joint damage. For these more chronic patients, a treatment approach similar to RA, including Disease Modifying Antirheumatic Drugs (DMARDs) may be necessary [13].
CHIKA causes a spectrum of pain, disease severity, and patient outcomes. Our study contributes to the existing literature by exploring the correlation between pain intensity, disease activity, and RF positivity in a cohort of Brazilian patients with CHIKA, using Spearman’s correlation coefficient and hypothesis tests (Student’s t-test, Wilcoxon test) [14]. This study also assesses the influence of variables such as age and gender on these outcomes, expanding the understanding of factors that may influence disease progression and severity.
Materials and Methods
Among CHIK cases treated at a rheumatology outpatient clinic in Brazil in 2022, we retrospectively reviewed the medical records of a cohort of 133 individuals with CHIKA, based on data from 196 consecutive patients seen and who provided the necessary information. Cases were confirmed by CHIKV-specific IgM serology by immunochromatography or enzyme-linked immunosorbent assay (ELISA) (VIRCLIA® IgM MONOTEST VCM063).
Data for this study were collected using a standardized clinical and laboratory assessment form, which captured key patient details, including demographic information, laboratory results, and clinical evaluations pertinent to the study’s objectives. The clinical assessments included the Visual VAS for pain and the count of tender and swollen joints, both of which were used to calculate the DAS28-ESR score. In addition, laboratory measures such as complete blood count, CRP, RF, and ESR were obtained to assess the patients’ inflammatory status. All participants met the inclusion criteria, which required confirmed laboratory evidence of CHIKV infection and the presence of new-onset arthritis, as outlined in the methodology. These methods ensured consistent and accurate data collection, enabling a comprehensive analysis of disease activity and pain intensity.
Pain intensity was classified based on patients’ responses to the VAS as follows: 0 = “no pain,” >0 and ≤ 30 = “mild pain,” >30 and ≤ 60 = “moderate pain,” >60 and ≤100 = “severe pain” [11]. Disease activity was classified according to the European League Against Rheumatism: with DAS28-ESR < 2.6 = “remission,” DAS28-ESR ≥ 2.6 and ≤ 3.2 = “low disease activity,” DAS28-ESR > 3.2 and ≤ 5.1 = “moderate disease activity,” DAS28-ESR > 5.1 = “high disease activity.” The RF test was performed using the latex agglutination method and recorded as reactive (positive) when the result was ≥ 8 IU/ml.
This study was approved by the Human Research Ethics Committee of the Universidade Regional do Cariri - Ceará, Brazil (Opinion nº 5,562,805).
Statistical Analysis
Data were analyzed using descriptive and inferential statistics. To describe the demographic and clinical characteristics, means and standard deviations (SD) were calculated for continuous variables, and absolute and relative frequencies for categorical variables. The correlations between continuous variables were evaluated using Spearman’s correlation coefficient, as for all combinations of variables the distribution was not normal in the Shapiro-Wilk normality test [13]. The comparison between sex in relation to the means of the variables “pain” and “DAS28-ESR” was performed using the Student’s t-test for independent samples, and the Wilcoxon test was used for RF positivity [14]. All statistical tests were considered significant for p values < 0.05. Data were analyzed using RStudio (version R-4.3.3 Windows, RStudio Team, Boston, MA) [15].
Results
Of the 133 patients in the cohort, 119 were women (89%). RF was positive for 22 patients (16.5%). Age ranged from 26 to 82 years (mean = 58.6 ± 13 years). The median age of the participants was 58 years (IQR: 49–70). The means ± SD for VAS and DAS28-ESR were 81.8 ± 19.2 and 5.9 ± 1.1, respectively. Most patients (83%) reported pain as severe, with only 2% classified as mild. Only one patient was classified as having low disease activity by DAS28-ESR, while 80% had high disease activity (Table 1).
Table 1. Demographic Profile and Clinical Characteristics of Patients with Chikungunya Arthritis.
| Characteristics | Mean ± SD | n | % |
| Sex/Gender | |||
| Female | 119 | 89.5 | |
| Male | 14 | 10.5 | |
| Age | 58.6 ± 13 | ||
| Tender joint count | 17.1 ± 7.3 | ||
| Swollen joint count | 5.0 ± 6.5 | ||
| Patient global assessment | 81.5 ± 19.2 | ||
| Erythrocyte sedimentation rate | 27.1 ± 17.2 | ||
| Rheumatoid factor | |||
| <8 | 111 | 83.5 | |
| ≥8 | 22 | 16.5 | |
| Disease Activity (DAS28-ESR) | |||
| Remission | 0 | 0 | |
| Low | 1 | 0.7 | |
| Moderate | 26 | 19 | |
| High | 106 | 80 | |
| Pain intensity (VAS) | |||
| Mild | 3 | 2 | |
| Moderate | 19 | 14 | |
| Severe | 111 | 83.5 |
Table 2 presents the comparison of the means of the variables analyzed by sex. Men were younger (55.9 ± 12 years) and none had positive RF.
Table 2. Comparison of Means of Variables Analyzed by Sex in Patients with Chikungunya Arthritis.
| Characteristics | Mean ± SD | p-value | |
| Men | Women | ||
| Age (years) | 55.9 ± 12 | 58.9 ± 14 | 0.363 |
| Time between first symptoms and first visit (days) | 35.4 ± 18 | 43 ± 21 | 0.160 |
| Rheumatoid factor | 0 | 10 ± 33.5 | 0.081 |
| Disease activity (DAS28-ESR) | 5.6 ± 1.1 | 6.0 ± 1.2 | 0.161 |
| Pain intensity (VAS) | 74.2 ± 16.7 | 82.6 ± 19.2 | 0.109 |
Figure 1 presents the Spearman correlation coefficients for the variables analyzed in a heat diagram. The darker the color, the stronger the correlation between the variables. The results suggest a moderate positive correlation between pain intensity and disease activity by DAS28-ESR (ρ = 0.416 and p-value < 0.05). We also observed that this correlation increased significantly with increasing age (ρ = 0.259 and p-value < 0.05). However, there was no correlation between DAS28-ESR and RF (ρ = 0.142 and p-value = 0.102) nor between pain intensity and RF (ρ = 0.167 and p-value = 0.054). Age did not show a significant correlation with these last two variables.
Figure 1.
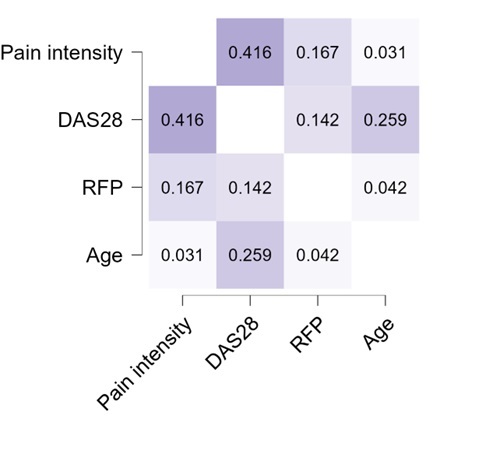
Spearman correlation coefficients of the relationship between the variables pain intensity, DAS-28, Rheumatoid factor, and age in 133 patients with Chikungunya arthritis.
The t-test and Wilcoxon tests did not indicate statistically significant differences in the means stratified by sex/gender, as observed: VAS (t = 1.68 and p-value 0.109); DAS28-ESR (t = 1.46 and p-value = 0.161); RF (Wilcoxon test = 987 and p-value = 0.081).
Discussion
In this study, we assessed whether there is correlation between the variables of pain, disease activity, age, sex/gender, and RF positivity in patients with CHIKA. We chose these variables to evaluate patients with CHIKA since they are established in RA, and assessment tools such as DAS28-ESR, VAS and RF test have also been used in CHIKA [16-21]. However, no previous study has verified the correlation between these variables in patients with CHIKA.
The average age in our cohort was similar to other CHIKA reports that range in age from 48 to 58 years [16,18,21-25]. Although RA can begin at any age, the age range in our CHIKA patients is similar to the age of onset of RA, typically around 40-50 years [26].
As in previous CHIKA reports, our CHIKA patients had high pain scores with a few weeks of illness similar to those persisting up to 40 months [24]. Most of our patients were classified as having moderate or severe pain.
Similarly, as in previous studies, our patients reported high rates of moderate and severe disease activity with high DAS28-ESR scores [16,18,21,25]. These high disease activity scores developed rapidly. In our study, the mean time between symptom onset and first office visit was only 35.4 ± 18 days. Our patients had similar disease activity to reported CHIKA patients with longer-standing illness [17].
Among our cohort of 133 CHIKA patients, RF positivity was found in 16.5%. In previous CHIKA studies, there is wide variation in RF positivity, ranging 3% to 100% [16,21,23].
We did not determine a correlation between RF positivity and pain intensity.
In our cohort, no men tested positive for RF, while 16.5% of the female participants were RF positive. This difference likely reflects the higher prevalence of RF positivity in women, as reported in various rheumatic diseases, including RA. Hormonal and immunological factors are thought to contribute to this increased predisposition in women, particularly in chronic inflammatory conditions such as CHIKA [27]. The relatively small percentage of male participants in this study (10.5%) may have also limited our ability to detect RF positivity in men.
It is possible that with a larger and more gender-balanced sample, RF positivity in men would become evident, or the observed sex/gender differences could be further clarified. Future studies with larger male cohorts are needed to investigate the relationship between gender and RF positivity in CHIKA patients.
In addition, studies have shown that women tend to experience more intense pain and higher disease activity across a range of chronic pain conditions, including RA and osteoarthritis [28,29]. This heightened sensitivity is believed to be influenced by biological factors, such as hormonal effects on nociceptor sensitization, with hormones like prolactin selectively sensitizing nociceptors in females [28,29].
Although our study did not assess seasonal variations, research has demonstrated that seasonal factors can influence disease activity in chronic inflammatory conditions such as RA. For example, studies like those by Mori et al. have shown that RA activity tends to peak in spring and winter, with higher remission rates observed in the fall [30]. Environmental factors, including changes in atmospheric pressure and colder temperatures, are believed to exacerbate musculoskeletal symptoms.
Our study was based on data collected from a single referral center, which limited our ability to include a broader population or conduct multicenter comparisons. While the findings are valid for the CHIK patients evaluated at this center, collaborations with other institutions, particularly in regions with a high prevalence of CHIK, could provide a more diverse and representative sample, improving the generalizability of the results.
Another limitation of this study is the lack of anti-citrullinated peptide antibody (ACPA) testing, primarily due to the cost, as many patients would need to pay for this test out of pocket, making it financially prohibitive for some. Additionally, the retrospective nature of the study and the relatively small cohort size are further limitations. Prospective studies with larger patient samples are needed to better understand the relationships between these variables and improve treatment for CHIKA.
Conclusion
Our study provides insights into the relationship between pain intensity, disease activity, age, and RF positivity in patients with CHIKA. We determined that pain intensity increases significantly with disease activity and with age.
Our results are consistent with previous studies on CHIKA, which have assessed pain intensity and disease activity using VAS and DAS28-VHS, respectively. However, RF positivity has varied widely between studies.
From a public health perspective, better understanding of the determinants of CHIKA severity can help to mitigate the impact of this disease within the community by identifying appropriate testing and treatment algorithms and optimize healthcare resources.
Glossary
- CHIK
Chikungunya
- CHIKA
Chikungunya arthritis
- CHIKF
Chikungunya fever
- CHIKV
Chikungunya virus
- CRP
C-reactive protein
- DAS28-ESR
Disease Activity Score in 28 joints using erythrocyte sedimentation rate
- DMARDs
Disease Modifying Antirheumatic Drugs
- ELISA
Enzyme-Linked Immunosorbent Assay
- ESR
erythrocyte sedimentation rate
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- SD
standard deviation
- VAS
Visual Analog Pain Scale
Financial support or Funding
None.
References
- de Lima Cavalcanti TY, Pereira MR, de Paula SO, Franca RF. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses. 2022. May;14(5):969. 10.3390/v14050969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Ahmed S, Parray HA, Das S. Chikungunya and arthritis: an overview. Travel Med Infect Dis. 2021;44:102168. 10.1016/j.tmaid.2021.102168 [DOI] [PubMed] [Google Scholar]
- Kang H, Auzenbergs M, Clapham H, Maure C, Kim JH, Salje H, et al. Chikungunya seroprevalence, force of infection, and prevalence of chronic disability after infection in endemic and epidemic settings: a systematic review, meta-analysis, and modelling study. Lancet Infect Dis. 2024. May;24(5):488–503. 10.1016/S1473-3099(23)00810-1 [DOI] [PubMed] [Google Scholar]
- Amaral JK, Taylor PC, Schoen RT. Brazil at the Center of Chikungunya Outbreaks. J Glob Infect Dis. 2023. Jul;15(3):131–2. 10.4103/jgid.jgid_21_23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza WM, Ribeiro GS, de Lima ST, de Jesus R, Moreira FR, Whittaker C, et al. Chikungunya: a decade of burden in the Americas. Lancet Reg Health Am. 2024. Jan;30:100673. 10.1016/j.lana.2023.100673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JK, Taylor PC, Schoen RT. Bone erosions and joint damage caused by chikungunya virus: a systematic review. Rev Soc Bras Med Trop. 2024. Apr;57:e00404–02024. 10.1590/0037-8682-0433-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelle E, Ribera A, Degasne I, Marimoutou C, Simon F. Clinical spectrum of post-chikungunya rheumatic musculoskeletal disorders and use of disease-modifying antirheumatic drugs to treat the chronic inflammatory entities: 6-year experience from Reunion Island. BMC Infect Dis. 2014;14(S2 Suppl 2):O20. [Google Scholar]
- Sissoko D, Malvy D, Ezzedine K, Renault P, Moscetti F, Ledrans M, et al. Post-epidemic Chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl Trop Dis. 2009;3(3):e389. 10.1371/journal.pntd.0000389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essackjee K, Goorah S, Ramchurn SK, Cheeneebash J, Walker-Bone K. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad Med J. 2013. Aug;89(1054):440–7. 10.1136/postgradmedj-2012-131477 [DOI] [PubMed] [Google Scholar]
- Watson H, Nogueira-Hayd RL, Rodrigues-Moreno M, Naveca F, Calusi G, Suchowiecki K, et al. Tender and swollen joint counts are poorly associated with disability in chikungunya arthritis compared to rheumatoid arthritis. Sci Rep. 2021. Sep;11(1):18578. 10.1038/s41598-021-98164-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. European Palliative Care Research Collaborative (EPCRC) . Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011. Jun;41(6):1073–93. 10.1016/j.jpainsymman.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009. Jun;68(6):954–60. 10.1136/ard.2007.084459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E, Michelen M, Rigby I, Dagens A, Dahmash D, Cheng V, et al. An evaluation of global Chikungunya clinical management guidelines: A systematic review. EClinicalMedicine. 2022. Sep;54:101672. 10.1016/j.eclinm.2022.101672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J, Li W. Applied Linear Statistical Models. 5th ed. McGraw-Hill; 2005. [Google Scholar]
- Ripley BD, Murdoch DJ, Kalibera T. RStudio Version for R-4.3.3. [software]. 2024.
- Sepúlveda-Delgado J, Vera-Lastra OL, Trujillo-Murillo K, Canseco-Ávila LM, Sánchez-González RA, Gómez-Cruz O, et al. Inflammatory biomarkers, disease activity index, and self-reported disability may be predictors of chronic arthritis after chikungunya infection: brief report. Clin Rheumatol. 2017. Mar;36(3):695–9. 10.1007/s10067-016-3419-2 [DOI] [PubMed] [Google Scholar]
- Chang AY, Martins KA, Encinales L, Reid SP, Acuña M, Encinales C, et al. Chikungunya Arthritis Mechanisms in the Americas: A Cross-Sectional Analysis of Chikungunya Arthritis Patients Twenty-Two Months After Infection Demonstrating No Detectable Viral Persistence in Synovial Fluid. Arthritis Rheumatol. 2018. Apr;70(4):585–93. 10.1002/art.40383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayd RL, Moreno MR, Naveca F, Amdur R, Suchowiecki K, Watson H, et al. Persistent chikungunya arthritis in Roraima, Brazil. Clin Rheumatol. 2020. Sep;39(9):2781–7. 10.1007/s10067-020-05011-9 [DOI] [PubMed] [Google Scholar]
- Yodtaweepornanan P, Pongsittisak W, Satpanich P. Incidence and factors associated with chronic chikungunya arthritis following chikungunya virus infection. Trop Med Int Health. 2023. Aug;28(8):653–9. 10.1111/tmi.13906 [DOI] [PubMed] [Google Scholar]
- Manimunda SP, Vijayachari P, Uppoor R, Sugunan AP, Singh SS, Rai SK, et al. Clinical progression of chikungunya fever during acute and chronic arthritic stages and the changes in joint morphology as revealed by imaging. Trans R Soc Trop Med Hyg. 2010. Jun;104(6):392–9. 10.1016/j.trstmh.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Anna Genaro MS, Marchi MS, Perin MY, Cossô IS, Dezengrini Slhessarenko R. Ferritin, Erythrocyte Sedimentation Rate, and C-Reactive Protein Level in Patients with Chikungunya-Induced Chronic Polyarthritis. Am J Trop Med Hyg. 2020. Nov;103(5):2077–82. 10.4269/ajtmh.20-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquillard E, Combe B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Joint Bone Spine. 2009. Dec;76(6):654–7. 10.1016/j.jbspin.2009.08.005 [DOI] [PubMed] [Google Scholar]
- Ganu MA, Ganu AS. Post-chikungunya chronic arthritis—our experience with DMARDs over two year follow up. J Assoc Physicians India. 2011. Feb;59:83–6. [PubMed] [Google Scholar]
- Watson H, Tritsch SR, Encinales L, Cadena A, Cure C, Ramirez AP, et al. Stiffness, pain, and joint counts in chronic chikungunya disease: relevance to disability and quality of life. Clin Rheumatol. 2020. May;39(5):1679–86. 10.1007/s10067-019-04919-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JK, Taylor PC, Weinblatt ME, Bandeira Í, Schoen RT. Quality of Life and Disability in Chikungunya Arthritis. Curr Rheumatol Rev. 2024;20(1):65–71. 10.2174/1573397119666230726113647 [DOI] [PubMed] [Google Scholar]
- Frazzei G, Musters A, de Vries N, Tas SW, van Vollenhoven RF. Prevention of rheumatoid arthritis: A systematic literature review of preventive strategies in at-risk individuals. Autoimmun Rev. 2023. Jan;22(1):103217. 10.1016/j.autrev.2022.103217 [DOI] [PubMed] [Google Scholar]
- Karlson EW, Deane K. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: a prospective cohort study with 223 526 participants. RMD Open. 2023;9(1): 10.1136/rmdopen-2023-003338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Berkley KJ, O’Connor MI, Nicolella DP, Enoka RM, Boyan BD, et al. Neural and psychosocial contributions to sex differences in knee osteoarthritic pain. Biol Sex Differ. 2012. Dec;3(1):26. 10.1186/2042-6410-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton H, Lee G, Dolatyari M, Ghetti A, Cotta T, Mitchell S, et al. Nociceptors are functionally male or female: from mouse to monkey to man. Brain. 2024. Jun;147(12):4280–91. 10.1093/brain/awae179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Sawada T, Nishiyama S, Shimada K, Tahara K, Hayashi H, et al. Influence of seasonal changes on disease activity and distribution of affected joints in rheumatoid arthritis. BMC Musculoskelet Disord. 2019. Jan;20(1):30. 10.1186/s12891-019-2418-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


