Abstract
Purpose: To report a case of cystoid macular edema (CME) secondary to immune recovery uveitis (IRU) in a patient with previous history of cytomegalovirus (CMV) retinitis and leukemia, which was successfully treated with tocilizumab (TCZ), an interleukin-6 (IL-6) receptor antagonist. Method: The clinical records of the case were reviewed, focusing on demographics, image findings, and clinical course. Results: A 17-year-old female with a past medical history of T-cell acute lymphoblastic leukemia (T-ALL) undergoing chemotherapy for two years presented with active CMV retinitis. She was successfully treated with intravitreal foscarnet injections and systemic ganciclovir. After 5 months of systemic valganciclovir maintenance and following cessation of chemotherapy, the patient developed bilateral CME and vasculitis, and was diagnosed with IRU. CME management was challenging due to a history of bilateral avascular necrosis of the femoral head resulting from prolonged systemic corticosteroid use. Two cycles of monthly TCZ infusions were administered at the dosage of 8mg/kg. Subsequently, the CME and retinal vasculitis resolved significantly without any evidence of inflammation in the anterior chamber and vitreous. Conclusion: The index case report demonstrated the safety and efficacy of the IL-6 receptor antagonist TCZ in treating CME associated with IRU in a non-HIV CMV retinitis patient.
Keywords: Immune recovery uveitis, Cytomegalovirus retinitis, Immune reconstitution inflammatory syndrome, Tocilizumab, IL-6, Cystoid macular edema
Introduction
Immune recovery uveitis (IRU) is the ocular manifestation of immune reconstitution inflammatory syndrome (IRIS), which typically occurs in HIV patients who have an underlying opportunistic infection and experience a significant improvement in immune function after successful treatment with highly active antiretroviral therapy (HAART) [1-3]. Multiple pathogens associated with IRIS have been reported including cytomegalovirus (CMV), varicella-zoster virus, mycobacterium tuberculosis, and atypical mycobacterium [3,4]. While predominantly identified in HIV patients, IRU can also present in non-HIV patients with an immunocompromised status, including organ-transplant recipients and individuals receiving chemotherapy for malignancies [5,6]. In HIV patients, several weeks after starting HAART, recovery of the immune system is characterized by a rise in CD4+ T levels of at least 50 cells/µL to a level of 100 cells/µL [2,3]. Such recovery phase is associated with the onset of IRU, characterized by potentially sight-threatening complications such as cataracts, epiretinal membranes, cystoid macular edema (CME), neovascularization of the retina or optic disc, and retinal detachment [1-3]. CME is the most common complication associated with IRU, and eyes with IRU had a 20-fold higher risk of CME, leading to significant risk of visual loss [1,7,8].
Conventional treatment for CME secondary to IRU often involves the administration of systemic or periocular corticosteroids [2,9]. However, the existing literature is limited, and the reported efficacy is variable [10-13]. Furthermore, treatment strategies for patients with contraindications to systemic steroids have not been firmly established.
An interleukin-6 (IL-6) receptor antagonist, tocilizumab (TCZ), is a fully humanized monoclonal antibody with dual affinity for both soluble and membrane-bound IL-6 receptors. TCZ has been approved for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis by the US Food and Drug Administration (FDA) since 2011 [14]. Despite its off-label application, TCZ has been reported as a successful treatment for refractory non-infectious uveitis-related CME in several case series [15-17]. Furthermore, findings from a multicenter, randomized trial, STOP-UVEITIS showed that TCZ infusion at two different doses (4 or 8 mg/kg, administered every 4 weeks) led to improvements in CME when it was employed in the management of active uveitis [18].
Herein, we report a case of CME secondary to IRU in a 17-year-old female with a history of CMV retinitis and T-cell acute lymphoblastic leukemia (T-ALL), who had contraindications for long-term systemic corticosteroid therapy and was successfully managed by intravenous TCZ.
Case Report
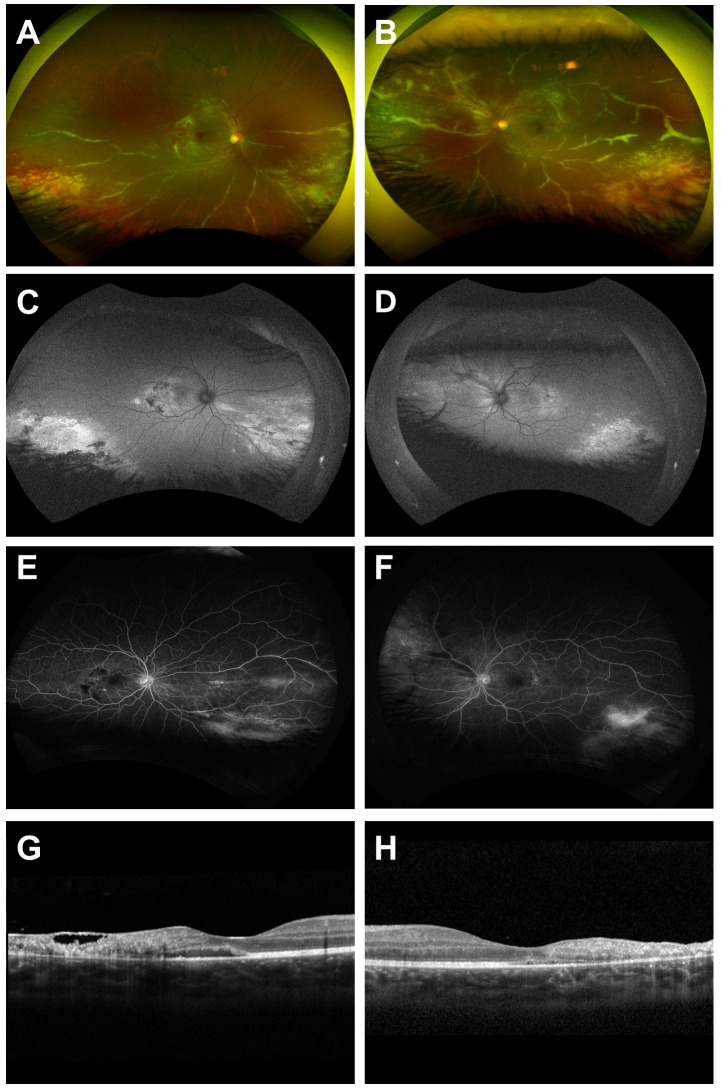
A 17-year-old female was diagnosed with T-ALL since May 2020, and she had been treated with leukapheresis followed by chemotherapy and oral corticosteroids. During the treatment, she developed bilateral avascular necrosis of the femoral head induced by long-term usage of oral corticosteroids and was switched to methotrexate in April 2021. She presented to the Uveitis Service at Byers Eye Institute at Stanford University, Palo Alto, CA in November 2022 with complaints of decreased vision and flashes in both eyes for 2 weeks Upon examination, her best-corrected visual acuity (BCVA) was 20/30 in both eyes. Intraocular pressure was within normal limits. Slit-lamp examination was unremarkable except for 1+ cell in the anterior vitreous of the left eye. Fundus examination of both eyes showed areas of peripheral necrotizing retinitis with hemorrhages consistent with active CMV retinitis in retinal zone 1 and 2. There was bilateral widespread perivascular sheathing (frosted branch angiitis) affecting mainly the inferior arcade in the right eye and all four quadrants in the left eye. Wide-angle fundus autofluorescence (FAF) showed hypo-autofluorescence with a surrounding halo of hyper-autofluorescence in the central, hyper-autofluorescence areas in the peripheral retina in both eyes. Fluorescein angiography (FA) revealed bilateral macular staining associated with hypo-fluorescein blocking effects in the posterior pole, and diffuse areas of leakage in the peripheral retina. On spectral domain optical coherence tomography (SD-OCT), there was subretinal fluid in the right eye; both eyes showed full-thickness retinal hyperreflectivity, disorganization, and thinning (Figure 1).
Figure 1.
Multimodal retinal imaging of active CMV retinitis of the right and left eye. Wide-angle fundus photography shows active necrotizing retinitis with hemorrhage and vascular sheathing of the right eye (A) and the left eye (B). Wide-angle fundus autofluorescence (FAF) shows hypo-autofluorescence with a surrounding halo of hyper-autofluorescence in the central, hyper-autofluorescence areas in the peripheral retina of the right eye (C) and left eye (D). Late-phase fluorescein angiography (FA) shows leakage on the peripheral retina of the right (E) and left eye (F). Spectral-domain optical coherence tomography (SD-OCT) of the right eye shows intra- and sub-retinal fluid, temporal retinal thinning, hyperreflectivity, and disorganization (G) and SD-OCT of the left eye shows temporal retinal thinning, hyperreflectivity, and disorganization (H).
Laboratory test results showed significant leukopenia, with white blood cell count and lymphocyte absolute count at 2400 cells/µL and 240 cells/µL, respectively. CD4 count was 21 cells/µL. The patient was HIV seronegative, indicating the presence of a non-HIV immunodeficiency condition likely secondary to her T-ALL/chemotherapy. Her uveitis workup was unrevealing except for CMV IgG and IgM positivity. Given the high index of suspicion for CMV retinitis, the patient was admitted. Due to the sight threatening nature of retina zone 1 CMV retinitis disease [19], intravitreal foscarnet injections (2.4 mg/0.1 ml) were administered empirically to both eyes. Systemic antiviral therapy was commenced with intravenous ganciclovir 5mg/kg every twelve hours for three consecutive days while the patient was admitted as an inpatient. The intravitreal foscarnet injections were repeated three days after the first for both eyes, and valganciclovir was initiated upon discharged. The polymerase chain reaction from the aqueous fluid obtained at admission was positive for CMV. Two weeks after, the fundus exam showed resolved retinitis and significant reduction in vascular sheathing, with improvement of BCVA to 20/20 in both eyes. Subretinal fluid resolved completely on SD-OCT with partial reconstitution of the normal retinal architecture. The patient was maintained on oral valganciclovir at the dose of 900 mg twice a day for the next 5 months.
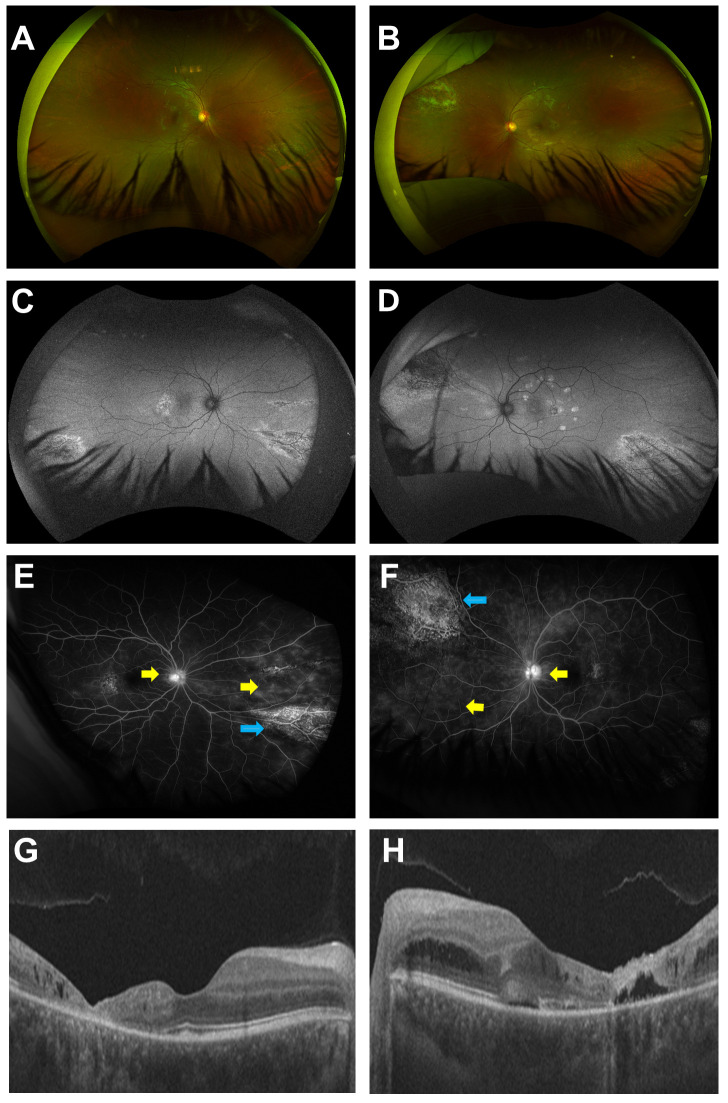
In May 2023, 5 months after successful treatment of CMV retinitis, the patient noticed decreased vision in her left eye. Upon examination, BCVA remained 20/20 in the right eye, but decreased to 20/30 in her left eye. SD-OCT revealed intra-retinal fluid in the right eye and significant CME in the left eye. Repeated FA showed optic disc and peripheral perivascular leakage during the late phase in both eyes. Both CMV retinitis reactivation and IRU were considered as potential diagnoses. But the diagnosis of IRU was solidified as it was preceded by the patient having completed chemotherapy 2 months previously and she had a normalized lymphocyte and CD4 count (1780 cells/µL and 353 cells/µL respectively). In addition, serum CMV DNA plasma was undetectable, and there were no clinical signs of CMV retinitis on dilated fundus examination, wide-angle fundus photography and autofluorescence imaging (Figure 2).
Figure 2.
Multimodal retinal imaging of immune recovery uveitis (IRU) of the right and left eye. Wide-angle fundus photography of the right eye (A) and the left eye (B) are unremarkable except for a residual scar in the superior nasal site of the left eye. Wide angle FAF of the right eye (C) and left eye (D) show hypo-autofluorescence in the central and hypo-autofluorescence areas associated with stippled hyper-autofluorescence areas in the peripheral retina corresponding with the scar from the previous lesions. Late-phase FA of the right (E) and left eye (F) show optic disc and peripheral peri-vascular leakage (yellow arrows) and staining of the retinal scars (blue arrows). SD-OCT of the right eye (G) shows temporal atrophy and intra-retinal fluid in the macula. SD-OCT of the left eye (H) shows cystoid macular edema.
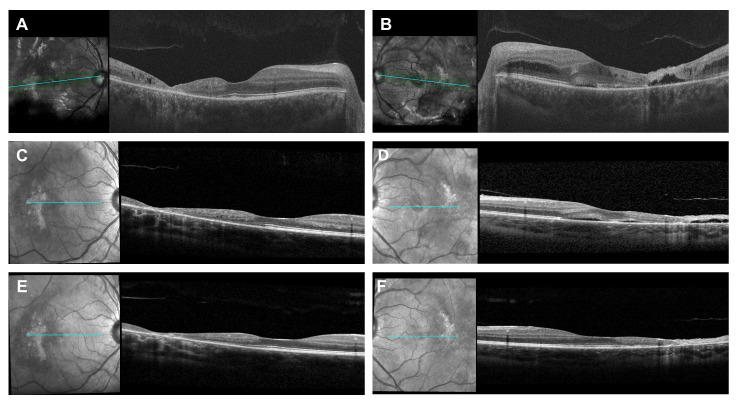
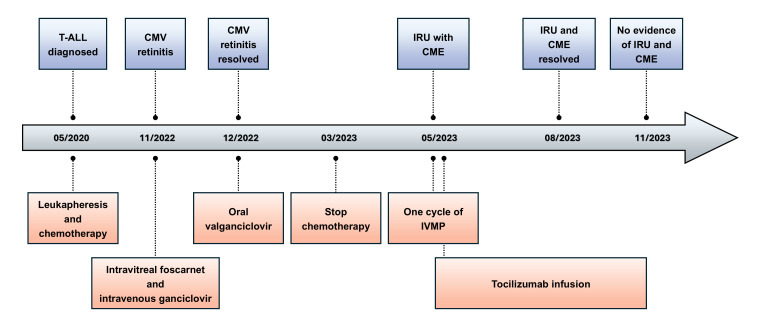
The treatment of IRU for the patient was complicated because long-term corticosteroid was contraindicated due to avascular necrosis of the femoral head. Sub-tenon or intravitreal corticosteroids injection held risks of cataract and ocular hypertension with possible subsequent glaucoma in this young patient with bilateral diseases and clear lens. Given the significant CME secondary to IRU, especially in the left eye, emergent therapy with intravenous methylprednisolone (IVMP) infusion (750 mg daily) for 3 consecutive days was started as a temporalizing measure. After one cycle of IVMP, the patient developed side effects including joint pain, swollen glands, and back pain; she declined further IVMP citing intolerability. After discussions with her pediatric oncologist and infectious control specialist, intravenous TCZ infusion was started at the dose of 8 mg/kg monthly. After two cycles of TCZ treatment, CME resolved completely in both eyes, and both foveal contours were restored (Figure 3). FA showed optic disc and perivascular leakage improved significantly in both eyes. The TCZ infusion therapy was discontinued after two monthly cycles due to patient’s wishes, but at the last follow-up, 3 months after the last infusion, there was no evidence of IRU or CME, and BCVA was 20/20 in both eyes. The patient’s treatment course timeline is illustrated in Figure 4 to summarize the chronological progression.
Figure 3.
SD-OCT of the right and left eye. Before immune recovery uveitis (IRU) treatment, SD-OCT shows intraretinal fluid in the right eye (A) and CME in the left eye (B). After one cycle of IVMP, SD-OCT shows intraretinal resolved in the right eye (C) and decreased CME in the left eye (D). After two cycles of tocilizumab (TCZ) infusion, SD-OCT shows completely resolved CME in right eye (E) and left eye (F).
Figure 4.
Timeline of the patient’s treatment course. Abbreviations: CME, cystoid macular edema; CMV, cytomegalovirus; IRU, immune recovery uveitis; IVMP, intravenous pulse methylprednisolone; T-ALL, T-cell acute lymphoblastic leukemia.
Discussion
The present case reports a difficult scenario involving a young non-HIV immunocompromised patient diagnosed with CMV retinitis and bilateral IRU. IRU poses a diagnosis and therapeutic challenges because of ambiguous clinical features, limited treatment options with suboptimal outcomes, and potential complications [2]. Treatment strategy was further complicated by multiple factors including bilateral diseases, clear lens, and a history of bilateral avascular necrosis of the femoral head induced by long-term use of oral corticosteroids for T-ALL chemotherapy. The index report highlights the first reported case of CME/IRU successfully managed with TCZ, an IL-6 receptor antagonist.
Our case is a patient with non-HIV immunodeficiency due to chemotherapy for T-ALL, which is uncommon in recent literature reports [6,20]. Five months after successful treatment for CMV retinitis, the patient presented with inflammatory signs including sub-retinal fluid in the right eye, significant CME and decreased visual acuity in the left eye, and bilateral optic disc and perivascular leakage.
The typical treatment for CME secondary to IRU often involves the administration of corticosteroids through various routes. The use of oral corticosteroids has demonstrated efficacy in resolving CME in patients with IRU without complications [2,9]. However, the number of case reported is limited [11,13]. While sub-tenon corticosteroid injection could avoid potential problems associated with systemic corticosteroids in immunosuppressed patients, only 40% of eyes showed improvement in visual acuity [10-12]. In severe cases, intravitreal corticosteroid injections have been used successfully [21,22], but care must be taken due to the risk of increased intraocular pressure, cataracts, and more pertinently as with this case, CMV retinitis reactivation [10,12,23,24].
Although the pathogenesis of IRU is still uncertain, it appears to represent an inflammatory reaction to either CMV antigen in the eye or low or subclinical levels of CMV replication and this inflammatory reaction occurs as the immune system recovers competency [3]. Immunohistological study suggested that IRU may be due to T cell-mediated reaction to CMV antigen present in inactive CMV retinitis [13]. IL-6 dysregulation has been confirmed in chronic inflammatory states [25] and aqueous of patients with non-infectious uveitic macular edema [16,26]. Enzyme-linked immunosorbent assay demonstrated that IL-6 level is higher in the aqueous and vitreous of IRU patients with healed CMV retinitis when compared to controls, suggesting that IL-6 plays a role in the pathogenesis of IRU [27].
In our case, the prolonged use of oral corticosteroids initially resulted in bilateral avascular necrosis of the femoral head, a condition that may necessitate additional total hip replacement – a particularly debilitating outcome, especially for young patients. Considering the patient’s clear lens, excellent BCVA and bilateral diseases, the administration of sub-tenon or intravitreal corticosteroid injections poses a potential risk of cataract development and ocular hypertension. At the outset of CME secondary to IRU, especially in the left eye, high-dose pulse IVMP was initially used because of the faster response with the relative more acceptable safety profile than high-dose oral corticosteroids in pediatric uveitis [28]. The intention was to employ IVMP as a temporizing measure to preserve vision while exploring for an effective corticosteroid sparing agent in a patient with a complex past medical history. TCZ, a IL-6 receptor antagonist originally approved for the treatment of rheumatoid arthritis and juvenile idiopathic arthritis but emerging as a promising therapeutic option for immune-mediated conditions beyond its initial indications [14,16] was eventually decided after discussion with her medical team.
In this case, two monthly cycles of TCZ treatment demonstrated remarkable efficacy in reducing macular edema, controlling the inflammatory response associated with IRU. By targeting the IL-6 pathway, TCZ effectively modulated the exaggerated immune response without exacerbating the avascular necrosis of the femoral head. To the best of our knowledge, the successful outcome of this case is the first to suggest that IL-6 receptor antagonist could be considered as a treatment for IRU, especially in patients with contraindications to corticosteroids. Three months after the last dose, the patient continues to have a quiescent disease suggesting that TCZ may have a particular lasting effect on CME secondary to IRU. Possible adverse events of TCZ include infections, mostly cutaneous or soft tissue infection; dyslipidemia, transient decreases in neutrophil count and abnormal transaminase, especially in combination with methotrexate [29]. During the short period of follow-up, our patient did not experience any of the aforementioned.
The major limitation of this case report is its retrospective nature, short follow-up period and the absence of a control group. A future study of greater scale, incorporating a standard-of-care control arm, will be essential to study and confirm the efficacy and investigate the long-term safety profile of TCZ in CME secondary to IRU.
In conclusion, the present case reports a young non-HIV immunocompromised patient diagnosed with CMV retinitis and subsequent bilateral CME secondary to IRU and was successfully treated by TCZ, an IL-6 receptor antagonist. This report expands the treatment spectrum of TCZ beyond typical non-infectious uveitis. The index case provides a foundation for future investigations and expands the armamentarium of clinicians facing similar therapeutic dilemmas.
Glossary
- BCVA
best-corrected visual acuity
- CME
cystoid macular edema
- CMV
cytomegalovirus
- FA
fluorescein angiography
- FAF
fundus autofluorescence
- HAART
highly active antiretroviral therapy
- IL-6
interleukin-6
- IRU
immune recovery uveitis
- IVMP
intravenous methylprednisolone
- SD-OCT
spectral domain optical coherence tomography
- T-ALL
T-cell acute lymphoblastic leukemia
- TCZ
tocilizumab
Author Contributions
BTN, JHH, ZXT, DEF, AM, AOS, XZ, FAA conceived the study concept and design. BTN, JHH, ZXT, DEF, AM, AOS, XZ, FAA, SSM, NTTT, AK, NY, BG, CY, OE, AA, WSY, ASG, QDN commented on the manuscript, and share final responsibility for the decision to submit for publication. BTN and QDN are the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Research to Prevent Blindness Department Challenge Award, National Eye Institute of the National Institutes of Health P30 Award (EY026877), and funding support from the Global Ophthalmic Research Center have been awarded to the Byers Eye Institute at Stanford University.
Conflicts of Interest
Dr. Quan Dong Nguyen has chaired the Steering Committees for the SAKURA, VISUAL, STOP-Uveitis, LINNAEA Studies. Dr. Nguyen also serves on Scientific Advisory Boards for Affibody, Acelyrin, Genentech, Priovant, and Regeneron, among others. No conflict of interests exists for any of the other authors.
References
- Kempen JH, Min YI, Freeman WR, Holland GN, Friedberg DN, Dieterich DT, et al. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113(4):684–94. 10.1016/j.ophtha.2005.10.067 [DOI] [PubMed] [Google Scholar]
- Nguyen QD, Kempen JH, Bolton SG, Dunn JP, Jabs DA. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol. 2000;129(5):634–9. 10.1016/S0002-9394(00)00356-1 [DOI] [PubMed] [Google Scholar]
- Urban B, Bakunowicz-Łazarczyk A, Michalczuk M. Immune recovery uveitis: pathogenesis, clinical symptoms, and treatment. Mediators of inflammation. 2014;2014. 10.1155/2014/971417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens GM, Meyer D, Stoll M, Schmidt RE. Immune reconstitution syndromes in human immunodeficiency virus infection following effective antiretroviral therapy. Immunobiology. 2000;202(2):186–93. 10.1016/S0171-2985(00)80065-0 [DOI] [PubMed] [Google Scholar]
- Baker ML, Allen P, Shortt J, Lewin SR, Spencer A. Immune recovery uveitis in an HIV‐negative individual. Clin Exp Ophthalmol. 2007;35(2):189–90. 10.1111/j.1442-9071.2006.01439.x [DOI] [PubMed] [Google Scholar]
- Rodrigues Alves N, Barão C, Mota C, Costa L, Proença RP. Immune recovery uveitis: a focus review. Graefes Arch Clin Exp Ophthalmol. 2024:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzak R, Rodman J, Pizzimenti J. Cystoid macular edema as a result of immune-recovery uveitis. Optom Vis Sci. 2011;88(2):E344–51. 10.1097/OPX.0b013e3182058fd6 [DOI] [PubMed] [Google Scholar]
- Yoganathan K. Cystoid macular edema secondary to immune recovery uveitis in a man with cytomegalovirus retinitis and AIDS. Clin Ophthalmol. 2010:1065–7. 10.2147/OPTH.S12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavellas MP, Lowder CY, Macdonald JC, Avila CP, Freeman WR. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998;116(2):169–75. 10.1001/archopht.116.2.169 [DOI] [PubMed] [Google Scholar]
- El-Bradey MH, Cheng L, Song MK, Torriani FJ, Freeman WR. Long-term results of treatment of macular complications in eyes with immune recovery uveitis using a graded treatment approach. Retina. 2004;24(3):376–82. 10.1097/00006982-200406000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland GN. Immune recovery uveitis. Ocul Immunol Inflamm. 1999;7(3-4):215–21. 10.1076/ocii.7.3.215.4010 [DOI] [PubMed] [Google Scholar]
- Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145(3):397-408. e1. 10.1016/j.ajo.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Karavellas MP, Azen SP, MacDonald JC, Shufelt CL, Lowder CY, Plummer DJ, et al. Immune recovery vitritis and uveitis in AIDS: clinical predictors, sequelae, and treatment outcomes. Retina. 2001;21(1):1–9. 10.1097/00006982-200102000-00001 [DOI] [PubMed] [Google Scholar]
- Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014:349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adán A, Mesquida M, Llorenç V, Espinosa G, Molins B, Hernández MV, et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 2013;251(11):2627–32. 10.1007/s00417-013-2436-y [DOI] [PubMed] [Google Scholar]
- Karkhur S, Hasanreisoglu M, Vigil E, Halim MS, Hassan M, Plaza C, et al. Interleukin-6 inhibition in the management of non-infectious uveitis and beyond. J Ophthalmic Inflamm Infect. 2019;9(1):1–14. 10.1186/s12348-019-0182-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas-Revenga N, Calvo-Río V, Mesquida M, Adán A, Hernández MV, Beltrán E, et al. Anti-IL6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol. 2019;200:85–94. 10.1016/j.ajo.2018.12.019 [DOI] [PubMed] [Google Scholar]
- Sepah YJ, Sadiq MA, Chu DS, Dacey M, Gallemore R, Dayani P, et al. Primary (month-6) outcomes of the STOP-uveitis study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71–80. 10.1016/j.ajo.2017.08.019 [DOI] [PubMed] [Google Scholar]
- Ude IN, Yeh S, Shantha JG. Cytomegalovirus retinitis in the highly active anti-retroviral therapy era. Ann Eye Sci. 2022;7:7. 10.21037/aes-21-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saricay LY, Baldwin G, Leake K, Johnston A, Shah AS, Patel NA, et al. Cytomegalovirus retinitis and immune recovery uveitis in a pediatric patient with leukemia. J AAPOS. 2023;27(1):52–5. 10.1016/j.jaapos.2022.10.004 [DOI] [PubMed] [Google Scholar]
- Morrison VL, Kozak I, LaBree LD, Azen SP, Kayicioglu OO, Freeman WR. Intravitreal triamcinolone acetonide for the treatment of immune recovery uveitis macular edema. Ophthalmology. 2007;114(2):334–9. 10.1016/j.ophtha.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Hu J, Coassin M, Stewart JM. Fluocinolone acetonide implant (Retisert) for chronic cystoid macular edema in two patients with AIDS and a history of cytomegalovirus retinitis. Ocul Immunol Inflamm. 2011;19(3):206–9. 10.3109/09273948.2010.538120 [DOI] [PubMed] [Google Scholar]
- D’Alessandro L, Bottaro E. Reactivation of CMV retinitis after treatment with subtenon corticosteroids for immune recovery uveitis in a patient with AIDS. Scand J Infect Dis. 2002;34(10):780–2. 10.1080/00365540260348644 [DOI] [PubMed] [Google Scholar]
- Ufret-Vincenty RL, Singh RP, Lowder CY, Kaiser PK. Cytomegalovirus retinitis after fluocinolone acetonide (Retisert™) implant. Am J Ophthalmol. 2007;143(2):334–5. 10.1016/j.ajo.2006.09.020 [DOI] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8 Suppl 2:1–6. 10.1186/ar1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas J, Llorenç V, Adán A, Molins B. Systemic regulatory T cells and IL-6 as prognostic factors for anatomical improvement of uveitic macular edema. Front Immunol. 2020;11:579005. 10.3389/fimmu.2020.579005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RD, Song MK, Smith IL, Karavellas MP, Bartsch DU, Torriani FJ, et al. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina. 2006;26(2):165–9. 10.1097/00006982-200602000-00007 [DOI] [PubMed] [Google Scholar]
- Ghoraba HH, Matsumiya W, Khojasteh H, Akhavanrezayat A, Karaca I, Or C, et al. Safety of Intravenous Methylprednisolone in Refractory and Severe Pediatric Uveitis. Clin Ophthalmol. 2022;16:1697–706. 10.2147/OPTH.S366370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Ding C. Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clinical Medicine Insights: Arthritis and Musculoskeletal Disorders. 2010;3:CMAMD. S4864. 10.4137/CMAMD.S4864 [DOI] [PMC free article] [PubMed] [Google Scholar]