Abstract
Chikungunya virus infection (CHIKV) increases the risk of persistent arthralgia; however, there is no consistent evidence regarding prognostic biomarkers of progression to chronic arthropathy. This systematic review provides an overview of currently available literature about the potential role of the acute immunologic response in predicting long-term joint pain in patients with a diagnosis of CHIKV. We searched for observational studies using the terms “chikungunya,” “cytokines,” “biomarkers,” and “joint pain” in PubMed/MEDLINE, LILACS, Cochrane Library Plus, and SCOPUS databases, restricting to articles published in English and up to April 2024. PROSPERO registration number: CRD42021279400. Thirty-eight studies were selected for qualitative synthesis with a maximum duration from diagnosis to clinical evaluation of 60 months. The sample sizes ranged from 8 to 346 participants (age range: 0-90 years). We identified an immunologic profile during the acute phase of CHIKV that includes increased levels of proinflammatory cytokines (IFN-α, IFN-γ, IL-2R, IL-6, IL-7, and IL-8), anti-inflammatory cytokines (IL-1Ra and IL-4), chemokines (MCP-1, MIG, and IP-10) and growth factors (VEGF and G-CSF). Only one out of two studies reported differences in cytokine levels during the acute phase, predicting persistent joint pain at 20 months of follow-up. Also, persistence of anti-CHIKV IgG seemed to be a potential prognostic marker. The evidence suggests the existence of an inflammatory response in the acute phase of CHIKV that persists during its chronic phase; however, there is no unequivocal candidate set of biomarkers of progression toward long-term articular sequelae.
Keywords: Chikungunya fever, biomarkers, arthralgia, chronic pain, cytokines
Introduction
Chikungunya, an arthropod-borne disease caused by the Chikungunya virus (CHIKV), is an acute infection associated to the development of rheumatic clinical manifestations that can persist for years [1]. The main long-term consequence of CHIKV infection, the post-CHIK chronic inflammatory rheumatism (pCHIK-CIR), is defined by the persistence of joint and extra-articular symptoms for more than 3 months after the onset of CHIKV disease or the development of specific immune-mediated inflammatory pathology during follow-up [2]. The frequency of persistent rheumatic manifestations ranges from 17% to 53%, a wide variation partially explained by the heterogenicity of clinical definitions and follow-up times at which they were implemented [3-6].
Although the first epidemic of CHIKV occurred in Tanzania in 1952 [7], the emergence of this infection in Europe and America is recent. Also, understanding the pathogenesis of persistent rheumatic manifestations is still limited, consistent with Chikungunya being classified as a neglected disease by the World Health Organization (WHO) [8]. It has been suggested that CHIKV may persist in immune-privileged niches such as the synovial tissue, contributing directly to its damage and the progression toward the chronic phase of the disease; however, there is no consistency in the finding of viral RNA or its proteins in macrophages from synovial fluid obtained from patients afflicted by the infection [9,10].
Alternatively, it has been postulated that the immune response, ie, the production of cytokines, chemokines, and growth factors induced by CHIKV, could be associated with chronic articular disease persistence [11-13]. Lee et al. were the first to demonstrate elevated levels of interleukin-8 (IL-8), IFN-γ-induced protein 10 (IP-10), monokine-induced by IFN-γ (MIG), and monocyte chemoattractant protein (MCP-1) in one patient with CHIKV infection [14] a finding inconsistently replicated by other authors [10,15-24] owned in part to the limited availability of longitudinal studies in clinical settings. This systematic review aims to establish an immunological profile of acute CHIKV disease based on the available evidence; also, to identify and critically appraise the evidence on prognostic biomarkers of the immune response in relation to persistent arthralgia, one of the most commonly and consistently reported symptoms of chronic articular compromise, post-CHIKV infection.
Materials and Methods
This systematic review was conducted following the AMSTAR (A Measurement Tool to Assess Systematic Reviews) instrument [25] and the reporting of results following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines [26]. Review protocol was registered in the PROSPERO international register of systematic reviews (CRD42021279400). The AMSTAR and PRISMA checklists of this review can be found in Appendix A: Supplementary material I and II.
Data Sources and Search Strategy
PubMed/MEDLINE, LILACS, Cochrane Library Plus, and SCOPUS databases were searched for observational studies published in any language, since their inception to April 2024. We used the search terms “chikungunya,” “cytokines,” “biomarkers,” “arthralgia,” and “joint pain.” We also checked the abstract books of the meetings of the American Society of Tropical Medicine and Hygiene (ASTMH) from 2011 to 2023 and the reference lists of the identified articles to supplement electronic searching.
Eligibility Criteria
We included observational studies, which evaluated the relationship between markers of immunological response and acute phase of CHIKV disease or arthralgia in patients with a diagnosis of CHIKV infection. Studies published in a language different from English were excluded as well as those in which markers were measured in a biological matrix different from serum or plasma, or after an in vitro stimulation of human cells.
Study Selection
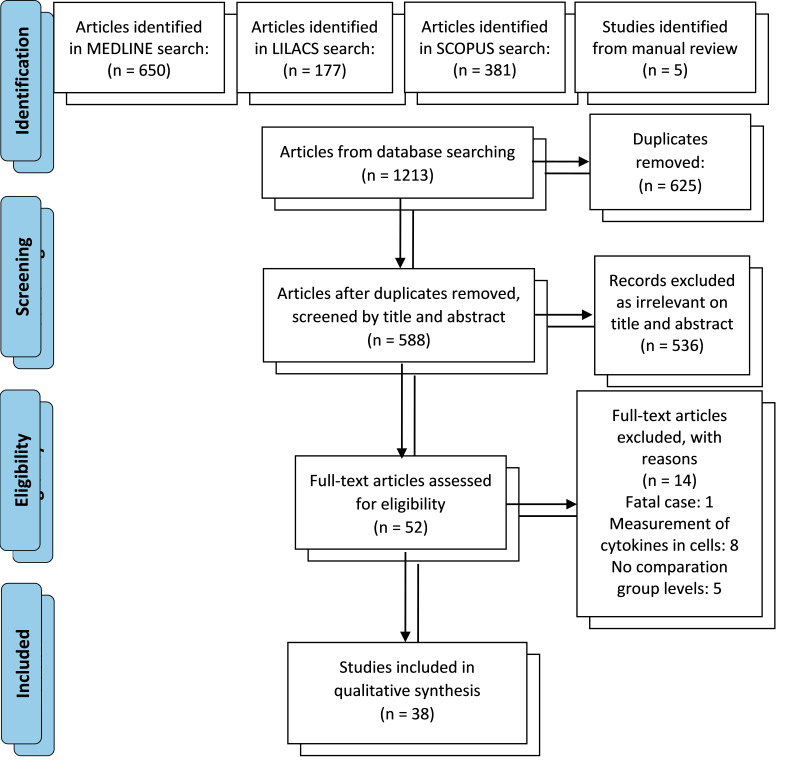
Studies identified through the search strategy were recorded in an Excel spreadsheet and the duplicated records were removed. We screened all the titles and abstracts based on the eligibility criteria (authors: AL-P, RMGR). Discrepancies were resolved by consensus and if necessary, a third reviewer was consulted to reach a final decision. After retrieval of full-text articles, one author again checked eligibility. Figure 1 shows the flow chart of the study.
Figure 1.
Search flow chart.
Data Extraction
The following information was retrieved by one author, for each study: author name, study design, country, publication year, patient age, sample size (prevalent or incident cases and controls, if applicable), maximum disease duration, the test used for CHIKV diagnosis and type of biological matrix used for the measurement of markers’ concentrations.
Bias Risk Assessment
Two authors independently assessed the methodological quality of the eligible observational studies using the Newcastle-Ottawa Scale (NOS). According to the NOS criteria for selection (four points at most), comparability (two points at most), and the adequacy of outcome measures (three points at most), a maximum of nine points could be awarded. The risk of bias was categorized based on the obtained score as follows: high-risk (0-3), intermediate-risk (4-6), and low-risk (7-9).
Results
Study Selection
The search strategy identified 1213 articles; however, after removing 625 duplicates and excluding 536 non-eligible articles based on the screening of their titles and abstracts, we retrieved and reviewed 52 full-text articles of which 38 studies were selected for qualitative synthesis (Figure 1).
Studies’ Characteristics and Risk of Bias
Among the included studies, 52.6% were conducted in Asia, 31.6% in America, 13.2% in Europe, and 2.6% in Africa with a predominance of case-control and cohort designs (55.3% and 34.2%, respectively; Table 1). The sample sizes ranged from 8 to 346 participants (age range: 0 to 90 years old), and the maximum duration from diagnosis to clinical evaluation was 60 months. CHIKV infection was defined as a positive result of a reverse transcription-polymerase chain reaction (RT-PCR) test to detect the CHIKV genome (n=8), an enzyme-linked immunosorbent assay (ELISA) to detect IgM or IgG anti-CHIKV (n=13) or both (n=17). The most often evaluated biomarkers were IL-6 (n=21), tumor necrosis factor-alpha (TNF-α; n=19), interferon-gamma (IFN-γ; n=17), IL-8 (n=17), and IL-10 (n=17), with most studies (71.1%) using serum as a biological matrix for biomarker quantification. In relation to risk of bias, most of the studies (73.5% and 23.5%) were classified as at intermediate- and low-risk according to the NOS (Table 2).
Table 1. Characteristics of the Included Studies.
| Author’s name, pub year | Study design | Country | Patient age (years) | Sample size (n) | Maximum disease duration | CHIKV diagnosis | Sample | |
| Cases | Controls | |||||||
|
| ||||||||
| Hoarau, 2010 [10] | Cohort | France | 19-90 | 32 | 8 | 12 months | PCR & serology | Serum |
| Chaaithanya, 2011 [36] | Cohort | India | 25-55 | 22 | 6 | 10 months | Serology | Serum |
| Kelvin, 2011 [18] | Cohort | Italy | No data | 50 | 10 | 12 months | PCR & serology | Serum |
| Chow, 2011 [29] | Cohort | Singapore | 23-67 | 30 | 8 | 2-3 months | PCR | Plasma |
| Chopra, 2012 [28] | Cohort | India | 15-24 | 225 | 49 | 24 months | Serology | Serum |
| Lohachanakul, 2012 [20] | Cohort | Thailand | 20-54 | 35 | 27 | 1 month | PCR & serology | Plasma |
| Moro, 2012 [41] | Cohort | Italy | 0-60 | 250 | 0 | 12 months | Serology | Serum |
| Kam, 2012 [34] | Cohort | Singapore | 23-67 | 30 | 0 | 2-3 months | Serology | Plasma |
| Gérardin, 2013 [40] | Cohort | France | 15-65 | 346 | 0 | 24 months | Serology | Serum |
| Venugopalan, 2014 [30] | Cohort | India | Adults | 110 | 80 | 1 month | Serology | Serum |
| Chang, 2018 [39] | Cohort | Colombia | Adults | 242 | 0 | 20 months | Serology | Serum |
| Nayak, 2020 [42] | Cohort | India | 15-77 | 72 | 0 | 20 months | PCR & serology | Plasma |
| Jacob-Nascimento, 2021 [63] | Cohort | Brazil | 14-50 | 253 | 81 | >3 months | PCR & serology | Serum |
| Alves de Souza, 2022 [15] | Cohort | Brazil | 28-66 | 78 | 10 | 3 months | PCR & serology | Plasma |
| Chirathaworn, 2010 [64] | Case-Control | Thailand | No data | 28 | 20 | 13 days | PCR & serology | Serum |
| Wauquier, 2011 [24] | Case-Control | Gabon | Adults | 69 | 30 | 1 week | PCR | Plasma |
| Chirathaworn, 2013 [16] | Case-Control | Thailand | 2-84 | 46 | 20 | 13 days | PCR & serology | Serum |
| Schilte, 2013 [38] | Case-Control | France | Adults | 20 | 22 | 36 months | PCR | Serum |
| Kashyap, 2014 [17] | Case-Control | India | 35-85 | 8 | 5 | 11 days | PCR & serology | Serum |
| Reddy, 2014 [65] | Case-Control | India | 21-80 | 48 | 37 | 3 months | PCR & serology | Plasma |
| Rojas, 2015 [43] | Case-Control | Colombia | Adults | 73 | 0 | 1 month | Serology | Serum |
| Dutta, 2017 [66] | Case-Control | India | 9-76 | 173 | 157 | >8 days | PCR & serology | Serum |
| Chattopadhya, 2017 [27] | Case-Control | India | 5-65 | 30 | 30 | No data | Serology | Serum |
| Banerjee, 2018 [67] | Case-Control | India | Adults | 40 | 25 | 2 weeks | PCR & serology | Plasma |
| Tanabe, 2019 [68] | Case-Control | Brazil | 7-82 | 29 | 21 | 5 days | PCR & serology | Serum |
| Cavalcanti, 2019 [31] | Case-Control | Brazil | 41-69 | 45 | 49 | 5 months | Serology | Serum |
| Ninla-Aesong, 2019 [35] | Case-Control | Thailand | No data | 93 | 30 | 60 months | Serology | Serum |
| Sánchez-Arcila, 2020 [23] | Case-Control | Brazil | Adults | 33 | 37 | <8 days | PCR | Serum |
| Cavalcanti, 2020 [32] | Case-Control | Brazil | 41-69 | 44 | 49 | 5 months | Serology | Serum |
| Krishnan, 2021 [19] | Case-Control | India | 22-65 | 16 | 10 | 14 days | PCR & serology | Plasma |
| Rocha, 2022 [33] | Case-Control | Brazil | 15-89 | 80 | 32 | <19 days | PCR & serology | Serum |
| Liu., 2022 [69] | Case-Control | Brazil | 18-66 | 40 | 13 | 6 months | PCR | Serum |
| Babu, 2023 [70] | Case-Control | India | 12-70 | 196 | 24 | 1 month | PCR & serology | Serum |
| Restrepo, 2022 [22] | Case-Control | Colombia | 18-15 | 83 | 10 | 3 months | PCR & serology | Serum |
| Dhenni, 2021 [71] | Cross-sectional | Indonesia | 1-78 | 32 | 4 | <9 days | PCR | Serum |
| Ng, 2009 [21] | Case series | Singapore | 22-65 | 10 | 9 | <10 days | PCR | Plasma |
| Chopra, 2014 [72] | Case series | India | No data | 70 | 80 | >6 weeks | Serology | Serum |
| Sepúlveda-Delgado, 2017 [37] | Case series | Mexico | 27-64 | 10 | 0 | 12 months | PCR & serology | No data |
Table 2. Newcastle-Ottawa Risk of Bias Assessment.
| Author’s name | Study design | Selection | Comparability | Exposure | Score | Risk of bias |
| Hoarau, 2010 [10] | Cohort | 3 | 0 | 1 | 4 | Intermediate |
| Chaaithanya, 2011 [36] | Cohort | 3 | 0 | 2 | 5 | Intermediate |
| Kelvin, 2011 [18] | Cohort | 3 | 0 | 2 | 5 | Intermediate |
| Chow, 2011 [29] | Cohort | 2 | 0 | 2 | 4 | Intermediate |
| Chopra, 2012 [28] | Cohort | 3 | 0 | 2 | 5 | Intermediate |
| Lohachanakul, 2012 [20] | Cohort | 4 | 0 | 0 | 4 | Intermediate |
| Moro, 2012 [41] | Cohort | 4 | 1 | 3 | 8 | Low |
| Kam, 2012 [34] | Cohort | 2 | 0 | 0 | 2 | High |
| Gérardin, 2013 [40] | Cohort | 4 | 1 | 2 | 7 | Low |
| Venugopalan, 2014 [30] | Cohort | 3 | 0 | 1 | 4 | Intermediate |
| Chang, 2018 [39] | Cohort | 4 | 1 | 2 | 7 | Low |
| Nayak, 2020 [42] | Cohort | 4 | 0 | 2 | 6 | Intermediate |
| Jacob-Nascimento, 2021 [63] | Cohort | 4 | 2 | 1 | 7 | Low |
| Alves de Souza, 2022 [15] | Cohort | 3 | 0 | 1 | 4 | Intermediate |
| Chirathaworn, 2010 [64] | Case-Control | 2 | 0 | 3 | 5 | Intermediate |
| Wauquier, 2011 [24] | Case-Control | 3 | 0 | 3 | 6 | Intermediate |
| Chirathaworn, 2013 [16] | Case-Control | 1 | 0 | 3 | 4 | Intermediate |
| Schilte, 2013 [38] | Case-Control | 4 | 1 | 3 | 8 | Low |
| Kashyap, 2014 [17] | Case-Control | 1 | 0 | 3 | 4 | Intermediate |
| Reddy, 2014 [65] | Case-Control | 1 | 1 | 3 | 5 | Intermediate |
| Rojas, 2015 [43] | Case-Control | 4 | 1 | 3 | 8 | Low |
| Dutta, 2017 [66] | Case-Control | 3 | 2 | 3 | 8 | Low |
| Chattopadhya, 2017 [27] | Case-Control | 2 | 1 | 3 | 6 | Intermediate |
| Banerjee, 2018 [67] | Case-Control | 2 | 1 | 3 | 6 | Intermediate |
| Tanabe, 2019 [68] | Case-Control | 1 | 0 | 3 | 4 | Intermediate |
| Cavalcanti, 2019 [31] | Case-Control | 3 | 1 | 2 | 6 | Intermediate |
| Ninla-Aesong, 2019 [35] | Case-Control | 4 | 1 | 3 | 8 | Low |
| Sánchez-Arcila, 2020 [23] | Case-Control | 2 | 0 | 3 | 5 | Intermediate |
| Cavalcanti, 2020 [32] | Case-Control | 2 | 1 | 2 | 5 | Intermediate |
| Krishnan, 2021 [19] | Case-Control | 1 | 1 | 2 | 4 | Intermediate |
| Rocha, 2022 [33] | Case-Control | 3 | 0 | 3 | 6 | Intermediate |
| Liu, 2022 [69] | Case-Control | 3 | 0 | 3 | 6 | Intermediate |
| Restrepo, 2022 [22] | Case-Control | 1 | 0 | 3 | 4 | Intermediate |
| Babu, 2023 [70] | Case-Control | 3 | 0 | 2 | 5 | Intermediate |
Profile of Immune Response in the Acute Disease
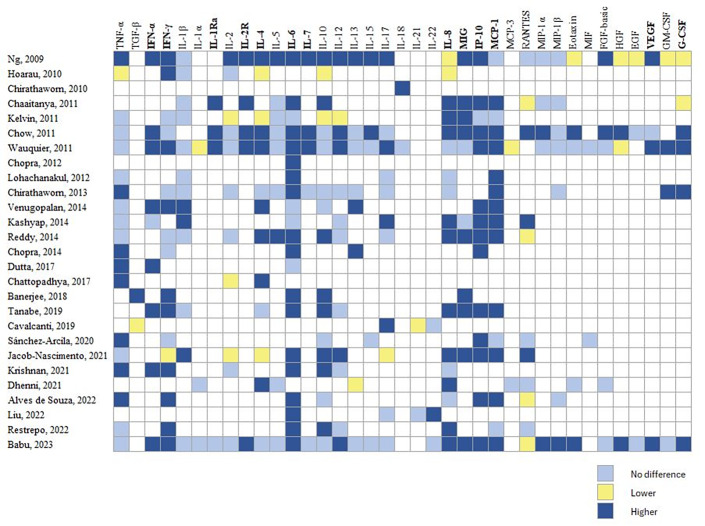
Twenty-seven studies (71.1%) evaluated immune response markers during the acute phase of CHIKV infection. Figure 2 shows a heat map of 37 immune mediators from at least two studies, indicating comparisons among CHIKV cases and healthy controls (HC). We observed higher levels of proinflammatory cytokines (IFN-α, IFN-γ, interleukin 2 receptor (IL-2R), IL-6, IL-7, and IL-8) and anti-inflammatory cytokines (IL-4 and the IL-1 antagonist receptor (IL-1Ra)) as well as chemokines (MCP-1, MIG, and IP-10) and growth factors (vascular endothelial growth factor (VEGF), and granulocyte colony-stimulating factor (G-CSF)) in the acute phase of CHIKV infection compared with HC. This pattern persisted regardless of the precedence of cases (Asia or America), except by IL-4, and IFN-α, which were not included in the pattern of studies from America. The clinical laboratory parameter, C-reactive protein (CRP) was reported to increase in the acute phase of CHIKV infection compared with HC [10,21,22,27-30]. Moreover, recently three molecules were reported as potential markers of acute CHIKV infection: IL-27 [31], galectin 9 (GAL-9) [32], and high mobility group box 1 protein (HMGB1) [33].
Figure 2.
Heat map of immune profile reported in the acute phase of chikungunya infection.
Biomarkers of Persistent Joint Pain
The duration of arthropathy symptoms among CHIKV cases ranged from 3 months [15,29,34] to 60 months [35]. Concurrent evaluation of biomarkers between 6 and 12 months post-CHIKV infection showed higher IL-6 levels and lower eotaxin, HGF, IL-5, and Regulated on Activation, Normal T-Expressed and Secreted (RANTES) levels in cases with persistent joint pain as compared to recovered cases [29,36,37] (Table 3). Moreover, studies with longer disease duration (36 and 60 months) found higher concentrations of IL-1α and matrix metalloproteinases 1 and 3 (MMP-1 and MMP-3) among cases with persistent joint pain as compared to recovered cases [35,38].
Table 3. Concurrent Markers of Persistent Joint Pain in Chikungunya Infection.
| Study | Outcome | Sample size (n) | Evaluation time of markers and outcome (months) |
Contrast of markers' concentrations (Recovered patients as
reference group) |
|||
| Chronic | Recovered | Higher | Lower | Non difference | |||
|
| |||||||
| Chow, 2011 [29] | Joint pain | 4 | 26 | 3 | IL-6, GM-CSF | Eotaxin, HGF | |
| Restrepo, 2022 [22] | Musculoskeletal disorder | 28 | 55 | 3 | IL-10, MIP-1 | IFN-γ, TNF-α, IL-6, IL-12, IL-8, MCP-1, RANTES | |
| Sepúlveda-Delgado, 2017 [37] | Joint pain | 6 | 4 | 3 and 12 | IL-6, RF | CRP | |
| Chaaithanya, 2011 [36] | Joint pain | 10 | 6 | 10 | IL-1β, IL-1Ra, MCP-1, IL-6, IL-8, G-CSF, MIP-1α, MIP-1β | IL-5, RANTES | IL-2R, IL-10, IP-10, MIG |
| Schilte, 2013 [38] | Joint pain | 20 | 22 | 36 | IL-1α | GM-CSF, IFN-γ, IL-1β, IL-10, IL-12, IL-15, IL-17, IL-18, IL-1RA, IL-2, IL-23, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, MCP-1, MIP-1 α, MIP-1β, RANTES, TNF-α, TNF- β, IP-10 | |
| Ninla-Aesong, 2019 [35] | Joint pain | 63 | 30 | 60 | MMP-1, MMP-3 | TNF-α | IL-4, IL-10, IL-12, IL-6, IL-1β, IL-8, IL-17A, RANTES, IFN-γ, TGF- β, MCP-1 |
About prognosis, only one out of two studies [10,39] reported cytokines levels measured during the acute phase predicting persistent joint arthropathy at 20 months of follow-up (Table 4) [39]. Although Hoarau et al. also observed lower IL-4 and IL-13 levels in the early days of CHIKV infection in patients with persistent arthralgia at 12 months of follow-up, such differences were not statistically significant [10]. Also, CRP levels were higher in the first 5 days in chronic cases compared to the recovered case (60.2±59.7 vs 11.3±10.1 mg/l) [10].
Table 4. Predictor Markers of Persistent Joint Pain in Chikungunya Infection.
| Study | Follow-up (months) | Outcome | Sample size (n) | Time of markers evaluation |
Contrast of markers' concentrations (Recovered patients as
reference group) |
|||
| Chronic | Recovered | Higher | Lower | Non difference | ||||
|
| ||||||||
| Hoarau, 2010 [10] | 12 | Joint pain > 1 joint, > 3 months | 9 | 6 | 5 days | CRP | TNF-α, IL-8, IL-6, IFN-γ, IFN-α, IL-12, IL-4, IL-13 | |
| Chang, 2018 [39] | 20 | Joint pain | 121 | 121 | Acute phase | IL-1β, IL-6, TNF-α, IL-10, IL-12, IL-13, IL-17, IL-2, IL-4, IL-5 | ||
Several studies evaluated anti-CHIKV IgG levels as biomarkers of chronic arthropathy [34,40-43]. The TELECHIK study reported an association between higher IgG levels and increased risk of persistent rheumatic pain at 24 months of follow-up (OR=6.2; 95 CI%: 2.8-13.2) [40]. Rojas et al. observed associated higher IgG levels with a positive squeeze test in the sub-acute phase (OR=1.1; 95% CI: 1.01-1.12) [43]. An early antibody response, indicated by increased IgG3 levels, could clear the virus faster and protect against persistent arthralgia [34]. Notably, the early appearance of neutralizing antibodies (irrespective of the isotype) during the febrile phase of CHIKV infection increased the risk of developing chronic arthritis [42].
Discussion
In this systematic review we identified an immunologic profile characterized by a set of increased biomarkers during the acute phase of the infection by CHIKV that includes proinflammatory cytokines (IFN-α, IFN-γ, IL-2R, IL-6, IL-7, and IL-8), anti-inflammatory cytokines (IL-1Ra and IL-4), chemokines (MCP-1, MIG, and IP-10) and growth factors (VEGF and G-CSF). IL-27, GAL-9, and HMGB1 also could contributed to such profiles, while IL-6, IL-4, IgG, and CRP emerged as potential prognostic biomarkers of chronic complications.
Previous systematic reviews provide context for our findings. Teng et al. [44], reported a similar acute immune response profile in CHIKV infection but found no changes in VEGF and IL-8 levels. They observed increased concentration of IL-2, IL-10, IL-12, IL-15, IL-17, IL-18, monocyte chemoattractant protein 1α and β (MIP-1α and MIP-1β) and the basic fibroblast growth factor (FGF-β). These differences may be attributed to the genetic background of the populations in the studies reviewed by Teng et al. which mostly originated in Asia and Europe [45,46]. Ferreira et al. revealed higher biomarker concentrations in severe acute infection or chronic cases; however, their quantitative synthesis focused on IL-6, CRP and TNF-α showing no significant differences [47]. Our results partially agree with those from Ferreira et al. in relation to their qualitative finding of increased levels of IL-6, IL-8, MCP-1, IL-1α, GM-CSF, IL-1Ra, MIP-1α, and MIP-1β; however, these results cannot be directly contrasted because Ferreira et al. did not report separate data for patients evaluated during acute infection from those with chronic disease and considered as control group a mix of healthy individuals and recovered patients.
As the first line of defense against viral infections, IFN-α induces an antiviral state to restrict the infection and promotes the expression of class I major histocompatibility complex (MHC I) on infected cells. This favors the recognition and destruction of infected cells by CD8+ T cells [48]. Our results are in accordance with previous reports showing high levels of IFN-α and a predominance of CD8+ T cells during the early stages of CHIKV infection [24]. We also found increased concentrations of IFN-γ, IP-10 and MIG among CHIKV infection cases compared to healthy controls. IFN-γ is crucial for maintaining the Th1/Th2 balance and induces these chemokines, which recruit macrophages, monocytes, NK cells, and T cells to the affected joints [14,36].
Elevated MCP-1 levels during the acute stage of CHIKV infection could attract monocytes to infection sites, correlating with increased CD14+ and CD14+CD16+ monocyte subpopulations [49]. In addition, MCP-1 stimulates monocytes differentiation into osteoclasts, which could partially explain early joint pain in CHIKV patients [50]. Likewise, IL-6 and IL-8 may contribute to the differentiation of monocyte into osteoclasts [50], and IL-7 may stimulate T cells to secrete osteoclastogenic cytokines [51]. Furthermore, IL-8 and VEGF may be relevant to CHIKV pathogenesis. IL-8 contributes to joint inflammation by attracting neutrophils and T cells to the inflammatory site [52,53], while VEFG, a proinflammatory growth factor, has been reported at high levels during the acute and chronic phase of the Mayaro virus infection and detected in synovial fibroblasts cultures from rheumatoid arthritis patients [54,55].
Other molecules, whose production was elevated during the acute phase of CHIKV infection but for which no replication studies are available, could also contribute to the pathogenesis of long-term disease’s complications. One of these is IL-27 [31] which might play both, proinflammatory and anti-inflammatory roles. In the first case, IL-27 downregulates the regulatory T cells but stimulates the Th1 and Th17 response, whereas in its anti-inflammatory role IL-27 stimulates the production of IL-10 by T cells and decreases the Th17 response [31]. IL-27 also induces a strong IFN-independent antiviral response to CHIKV in monocyte-derived macrophages by activating JAK-STAT signaling pathway and inducing IFN-stimulated genes in the antiviral state [56,57]. GAL-9 is another relevant molecule to CHIKV infection pathogenesis, with higher levels among CHIKV infection cases compared to healthy controls and is associated with the duration of morning stiffness [31]. Increased levels of GAL-9 have also been observed in rheumatoid arthritis patients, which suggests that the Galectin family is implicated in the processes of osteoclast genesis and inflammatory bone destruction [58]. On the other hand, HMGB1, which is secreted by infected or active immune cells to potentiate the proinflammatory response, has been associated with viremia in CHIKV infection as proposed as a disease biomarker [59]. Furthermore, HMGB1 has been observed in the synovial fluid of rheumatoid arthritis cases, contributing to cartilage and bone destruction [59]. While increased CRP levels have been found in CHIKV cases, CRP does not seem to have a prognostic role for the persistence of articular manifestations of CHIKV [60]. Interestingly, Hoarau et al. reported that early CRP levels discriminated between patients with persistent joint pain and those who recovered at 12 months of follow-up [10], suggesting that CRP might be a simple and affordable prognostic marker of disease progression, a finding that requires replication.
When contrasted against patients who recovered from CHIKV infection, those with persistent clinical findings showed higher levels of IL-6 at 3, 10, and 12 months post-onset of infection. Similarly, MCP-1 remains elevated after 10 months of follow-up in patients with arthralgia compared to those without [36]. As mentioned before, MCP-1 and IL-6 are related to bone degradation [50], with IL-6 inducing its own production and upregulating MCP-1 expression [48]. On the other hand, matrix metalloproteinases (MMP-1, MMP-3, MMP-9, and MMP-13) which degraded the extracellular matrix in cartilage may also contribute to chronic arthralgia in CHIKV infection [61]. Ninla-Aesong et al. proposed that the activation of MMP-1 and MMP-3, secondary to the increase of Th1 markers (IL-6, IL-8, IL-1β, MCP-1, and TNF-α) [35], might partially contribute to the pathogenesis of chronic arthralgia in Chikungunya.
Although the body of evidence suggests an important contribution of immune mechanisms in the pathogenesis of the long-term articular complications in CHIKV infection, its prognostic potential remains poorly developed. In fact, only two prospective studies have evaluated the association between acute-phase biomarkers and the development of relevant clinical outcomes [10,39]. However, they reported conflicting results for TNF-α, IL-6, and IL-12 due to the differences in study design. Hoarau et al. found no association between cytokine levels and persistent joint pain, possibly due to their smaller sample size and shorter follow-up [10]. In contrast, Chang et al. conducted a nested case-control study, with 242 age- and gender-matched CHIKV infection cases, assessing joint pain by telephone survey at 20 months [39].
The titers of anti-CHIKV IgG antibodies also have been related to persistent joint pain [40,41,43]. Experimental and epidemiological studies suggest that pre-existing neutralizing antibodies are associated with a lower risk of both symptomatic and asymptomatic CHIKV infections [62]. Consistently, a Colombian cohort study observed that a positive test for anti-CHIKV IgG antibodies doubled the likelihood of having a symptomatic infection compared to a negative result (preliminary data). In relation to IgG subtypes, Kam et al. suggest that a strong and rapid response of the IgG3 subtype could clear the virus faster and potentially protect against the development of persistent joint pain after CHIKV infection [34].
The available evidence is consistent with the existence of an inflammatory response from the acute phase to the chronic phase of CHIKV infection; however, there is no clear profile of prognostic biomarkers for long-term sequelae, specifically arthralgia. This might be partially explained by the high heterogeneity among studies regarding eligibility criteria for cases and controls (sociodemographic composition, geographical origin, comorbidities, etc.), candidate biomarkers, timing of measurements, definition of outcomes, and follow-up durations. Most of the studies in this review had a high risk of bias, mainly attributable to the lack of adjustment for relevant covariates. Moreover, some studies in this review are reported as cohorts, only two prospectively evaluated the relationships between exposure(s) and outcomes. Finally, our search strategy might be considered as highly sensitive (ie, selected terms, databases, and checking reference lists) and we do not expect that publication bias due to the omission of publications in languages other than English has significantly impacted our conclusions.
Conclusions
The evidence suggests the existence of an inflammatory response in the acute phase of CHIKV that persists during its chronic phase; however, there is no unequivocal candidate set of biomarkers of progression toward long-term articular sequelae. This may be due to the heterogeneity of the studied populations, the definition of outcomes, and the timing for quantification of biomarkers during disease.
Given the current gaps in understanding CHIKV pathogenesis, further research should focus on key areas such as synovial membrane biopsies and predisposing genetic factors could provide insights associated with chronic disease. Since alphavirus target synovial fibroblast and cause arthritis, new studies could investigate the expression of biomarker discussed in this review on these cells. Additionally, evaluating polymorphisms in genes encoding JAK-STAT pathway components, IFNs, or toll-like receptors like TLR3/8, IL-27 receptor, should be informative. These approaches could enhance our understanding of disease mechanisms and inform more effective diagnostic and therapeutic approaches.
Glossary
- CHIKV
Chikungunya virus infection
- pCHIK-CIR
post-CHIK chronic inflammatory rheumatism
Appendix A.
Author Contributions
AL-P (ORCID: 0000-0002-6770-258X): Conceptualization; Methodology; Writing - original draft. VH (ORCID: 0000-0002-6295-1640): Conceptualization, Methodology; Writing - review & editing. SU-I (ORCID: 0000-0002-2865-3041): Writing - review & editing. RMGM (ORCID: 0000-0002-4851-1753): Articles selection; Writing – review. LAVC (ORCID: 0000-0002-3873-0901): Conceptualization; Funding acquisition; Methodology; Writing - review & editing.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding
The work was partially supported by European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement No. 825746), the Canadian Institutes of Health Research, Institute of Genetics - CIHR-IG (Grant Agreement N.01886-000), Universidad Industrial de Santander, and Centro de Atención y Diagnóstico de Enfermedades Infecciosas (CDI).
References
- Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, et al. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013. Sep;99(3):345–70. 10.1016/j.antiviral.2013.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, et al. Société de pathologie infectieuse de langue francaise . French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015. Jul;45(7):243–63. 10.1016/j.medmal.2015.05.007 [DOI] [PubMed] [Google Scholar]
- López Rodríguez MA. Evaluación de las manifestaciones reumatológicas y las alteraciones paraclínicas luego de dos años de la presentación de la infección por virus de chikungunya en un brote en el municipio de Capitanejo, Santander. [Tesis de Especialización en Medicina Interna]. Universidad Industrial de Santander; 2018.
- Edington F, Varjão D, Melo P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: A systematic review of the literature and meta-analysis. Joint Bone Spine. 2018. Dec;85(6):669–78. 10.1016/j.jbspin.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales AJ, Gil-Restrepo AF, Ramírez-Jaramillo V, Montoya-Arias CP, Acevedo-Mendoza WF, Bedoya-Arias JE, et al. Post-chikungunya chronic inflammatory rheumatism: results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000 Res. 2016. Mar;5(360):360. 10.12688/f1000research.8235.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama K, de Roo AM, Louwsma T, Hofstra HS, Gurgel do Amaral GS, Vondeling GT, et al. Clinical outcomes of chikungunya: A systematic literature review and meta-analysis. PLoS Negl Trop Dis. 2024. Jun;18(6):e0012254. 10.1371/journal.pntd.0012254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharz EJ, Cebula-Byrska I. Chikungunya fever. Eur J Intern Med. 2012. Jun;23(4):325–9. 10.1016/j.ejim.2012.01.009 [DOI] [PubMed] [Google Scholar]
- WHO . Neglected tropical diseases [Internet]. 2019. [cited 2024 Jul 30]. Available from: https://www.who.int/neglected_diseases/diseases/en/
- Chang AY, Martins KA, Encinales L, Reid SP, Acuña M, Encinales C, et al. Chikungunya arthritis mechanisms in the Americas: A cross-sectional analysis of chikungunya arthritis patients twenty-two months after infection demonstrating no detectable viral persistence in synovial fluid. Arthritis Rheumatol. 2018. Apr;70(4):585–93. 10.1002/art.40383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010. May;184(10):5914–27. 10.4049/jimmunol.0900255 [DOI] [PubMed] [Google Scholar]
- Chen W, Foo SS, Sims NA, Herrero LJ, Walsh NC, Mahalingam S. Arthritogenic alphaviruses: new insights into arthritis and bone pathology. Trends Microbiol. 2015. Jan;23(1):35–43. 10.1016/j.tim.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Avila-Trejo AM, Rodríguez-Páez LI, Alcántara-Farfán V, Aguilar-Faisal JL. Multiple factors involved in bone damage caused by chikungunya virus infection. Int J Mol Sci. 2023. Aug;24(17):13087. 10.3390/ijms241713087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira-Freitas JE, Campagnolo ML, Dos Santos Cortez M, de Melo FF, Zarpelon-Schutz AC, Teixeira KN. Long chikungunya? An overview to immunopathology of persistent arthralgia. World J Virol. 2024. Jun;13(2):89985. 10.5501/wjv.v13.i2.89985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Wong CK, Lam WY, Wong A, Lim W, Lam CW, et al. Chikungunya fever, Hong Kong. Emerg Infect Dis. 2006. Nov;12(11):1790–2. 10.3201/eid1211.060574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Souza TM, Fernandes-Santos C, Araújo da Paixão de Oliveira J, Tomé LC, Fiestas-Solórzano VE, Nunes PC, et al. Increased Indoleamine 2,3-Dioxygenase 1 (IDO-1) activity and inflammatory responses during chikungunya virus infection. Pathogens. 2022. Apr;11(4):444. 10.3390/pathogens11040444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirathaworn C, Poovorawan Y, Lertmaharit S, Wuttirattanakowit N. Cytokine levels in patients with chikungunya virus infection. Asian Pac J Trop Med. 2013. Aug;6(8):631–4. 10.1016/S1995-7645(13)60108-X [DOI] [PubMed] [Google Scholar]
- Kashyap RS, Morey S, Bhullar S, Baheti N, Chandak N, Purohit H, et al. Determination of Toll-like receptor-induced cytokine profiles in the blood and cerebrospinal fluid of Chikungunya patients. Neuroimmunomodulation. 2014;21(6):338–46. 10.1159/000358240 [DOI] [PubMed] [Google Scholar]
- Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, et al. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl Trop Dis. 2011. Aug;5(8):e1279. 10.1371/journal.pntd.0001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SM, Mahalingam J, Sabarimurugan S, Muthu T, Venkidasamy B, Krishnasamy K, et al. Comparison of cytokine expression profile in chikungunya and dengue co-infected and mono-infected patients’ samples. Pathogens. 2021. Feb;10(2):166. 10.3390/pathogens10020166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohachanakul J, Phuklia W, Thannagith M, Thonsakulprasert T, Ubol S. High concentrations of circulating interleukin-6 and monocyte chemotactic protein-1 with low concentrations of interleukin-8 were associated with severe chikungunya fever during the 2009-2010 outbreak in Thailand. Microbiol Immunol. 2012. Feb;56(2):134–8. 10.1111/j.1348-0421.2011.00417.x [DOI] [PubMed] [Google Scholar]
- Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, et al. IL-1β, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4(1):e4261. 10.1371/journal.pone.0004261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo BN, Marín K, Romero P, Arboleda M, Muñoz AL, Bosch I, et al. Role of cytokines, chemokines, C3a, and mannose-binding lectin in the evolution of the chikungunya infection. Am J Clin Exp Immunol. 2022. Jun;11(3):51–63. [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Arcila JC, Badolato-Correa J, de Souza TM, Paiva IA, Barbosa LS, Nunes PC, et al. Clinical, virological, and immunological profiles of DENV, ZIKV, and/or CHIKV-infected Brazilian patients. Intervirology. 2020;63(1-6):33–45. 10.1159/000510223 [DOI] [PubMed] [Google Scholar]
- Wauquier N, Becquart P, Nkoghe D, Padilla C, Ndjoyi-Mbiguino A, Leroy EM. The acute phase of Chikungunya virus infection in humans is associated with strong innate immunity and T CD8 cell activation. J Infect Dis. 2011. Jul;204(1):115–23. 10.1093/infdis/jiq006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017. Sep;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (Chinese edition). Journal of Chinese Integrative Medicine. 2009. [PMC free article] [PubMed] [Google Scholar]
- Chattopadhya D, Verghese A, Broor S. The pro-inflammatory markers and cytokine profile in acute chikungunya virus infections in a rural community from north India. J Commun Dis. 2017;49(3):67–72. 10.24321/0019.5138.201725 [DOI] [Google Scholar]
- Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute Chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect. 2012. May;140(5):842–50. 10.1017/S0950268811001300 [DOI] [PubMed] [Google Scholar]
- Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, et al. Persistent arthralgia induced by Chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011. Jan;203(2):149–57. 10.1093/infdis/jiq042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopalan A, Ghorpade RP, Chopra A. Cytokines in acute chikungunya. PLoS One. 2014. Oct;9(10):e111305. 10.1371/journal.pone.0111305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualberto Cavalcanti N, MeloVilar K, Branco Pinto Duarte AL, Jesus Barreto de Melo Rêgo M, Cristiny Pereira M, da Rocha Pitta I, et al. IL-27 in patients with Chikungunya fever: A possible chronicity biomarker? Acta Trop. 2019. Aug;196:48–51. 10.1016/j.actatropica.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Gualberto Cavalcanti N, Melo Vilar K, Branco Pinto Duarte AL, Barreto de Melo Rêgo MJ, Pereira MC, da Rocha Pitta I, et al. Increased serum levels of galectin-9 in patients with chikungunya fever. Virus Res. 2020. Sep;286:198062. 10.1016/j.virusres.2020.198062 [DOI] [PubMed] [Google Scholar]
- Rocha DC, Souza TM, Nunes PC, Mohana-Borges R, Paes MV, Guimarães GM, et al. Increased circulating levels of High Mobility Group Box 1 (HMGB1) in acute-phase Chikungunya virus infection: potential disease biomarker. J Clin Virol. 2022. Jan;146:105054. 10.1016/j.jcv.2021.105054 [DOI] [PubMed] [Google Scholar]
- Kam YW, Simarmata D, Chow A, Her Z, Teng TS, Ong EK, et al. Early appearance of neutralizing immunoglobulin G3 antibodies is associated with chikungunya virus clearance and long-term clinical protection. J Infect Dis. 2012. Apr;205(7):1147–54. 10.1093/infdis/jis033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninla-Aesong P, Mitarnun W, Noipha K. Proinflammatory cytokines and chemokines as biomarkers of persistent arthralgia and severe disease after chikungunya virus infection: A 5-year follow-up study in southern Thailand. Viral Immunol. 2019. Dec;32(10):442–52. 10.1089/vim.2019.0064 [DOI] [PubMed] [Google Scholar]
- Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, et al. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011. Aug;24(4):265–71. 10.1089/vim.2010.0123 [DOI] [PubMed] [Google Scholar]
- Sepúlveda-Delgado J, Vera-Lastra OL, Trujillo-Murillo K, Canseco-Ávila LM, Sánchez-González RA, Gómez-Cruz O, et al. Inflammatory biomarkers, disease activity index, and self-reported disability may be predictors of chronic arthritis after chikungunya infection: brief report. Clin Rheumatol. 2017. Mar;36(3):695–9. 10.1007/s10067-016-3419-2 [DOI] [PubMed] [Google Scholar]
- Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137. 10.1371/journal.pntd.0002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AY, Tritsch S, Reid SP, Martins K, Encinales L, Pacheco N, et al. The cytokine profile in acute chikungunya infection is predictive of chronic arthritis 20 months post infection. Diseases. 2018. Oct;6(4):95. 10.3390/diseases6040095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérardin P, Fianu A, Michault A, Mussard C, Boussaïd K, Rollot O, et al. Predictors of Chikungunya rheumatism: a prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res Ther. 2013. Jan;15(1):R9. 10.1186/ar4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro ML, Grilli E, Corvetta A, Silvi G, Angelini R, Mascella F, et al. Study Group “Infezioni da Chikungunya in Emilia-Romagna” . Long-term chikungunya infection clinical manifestations after an outbreak in Italy: a prognostic cohort study. J Infect. 2012. Aug;65(2):165–72. 10.1016/j.jinf.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Nayak K, Jain V, Kaur M, Khan N, Gottimukkala K, Aggarwal C, et al. Antibody response patterns in chikungunya febrile phase predict protection versus progression to chronic arthritis. JCI Insight. 2020. Apr;5(7):e130509. 10.1172/jci.insight.130509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas EM, et al. Chikungunya rheumatism during the subacute phase of the disease and its correlation with Ig G antibodies: Findings from an outbreak in Colombia. In: Abstract Book 64th Annual Meeting ASTMH [Internet]. 2015. p. 520. Available from: https://www.astmh.org/ASTMH/media/Documents/ASTMH-2015-Abstract-Book-Final.pdf
- Teng TS, Kam YW, Lee B, Hapuarachchi HC, Wimal A, Ng LC, et al. A systematic meta-analysis of immune signatures in patients with acute chikungunya virus infection. J Infect Dis. 2015. Jun;211(12):1925–35. 10.1093/infdis/jiv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J, Zabaleta J, Garai J, Ji P, Imtiaz S, Spagnardi M, et al. Immune-related gene expression and cytokine secretion is reduced among African American colon cancer patients. Front Oncol. 2020. Sep;10:1498. 10.3389/fonc.2020.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Hong CC, Ruiz-Narváez EA, Evans SS, Zhu Q, Schaefer BA, et al. Genetic ancestry and population differences in levels of inflammatory cytokines in women: role for evolutionary selection and environmental factors. PLoS Genet. 2018. Jun;14(6):e1007368. 10.1371/journal.pgen.1007368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira AS, Baldoni NR, Cardoso CS, Oliveira CD. Biomarkers of severity and chronification in chikungunya fever: a systematic review and meta-analysis. Rev Inst Med Trop São Paulo. 2021. Mar;63:e16. 10.1590/s1678-9946202163016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum FM, Ng LF. Cellular and molecular mechanisms of chikungunya pathogenesis. Antiviral Res. 2015. Aug;120:165–74. 10.1016/j.antiviral.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Michlmayr D, Pak TR, Rahman AH, Amir ED, Kim EY, Kim-Schulze S, et al. Comprehensive innate immune profiling of chikungunya virus infection in pediatric cases. Mol Syst Biol. 2018. Aug;14(8):e7862. 10.15252/msb.20177862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuklia W, Kasisith J, Modhiran N, Rodpai E, Thannagith M, Thongsakulprasert T, et al. Osteoclastogenesis induced by CHIKV-infected fibroblast-like synoviocytes: a possible interplay between synoviocytes and monocytes/macrophages in CHIKV-induced arthralgia/arthritis. Virus Res. 2013. Nov;177(2):179–88. 10.1016/j.virusres.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology (Oxford). 2008. Jun;47(6):753–9. 10.1093/rheumatology/ken053 [DOI] [PubMed] [Google Scholar]
- Constant LE, Rajsfus BF, Carneiro PH, Sisnande T, Mohana-Borges R, Allonso D. Overview on chikungunya virus infection: from epidemiology to state-of-the-art experimental models. Front Microbiol. 2021. Oct;12:744164. 10.3389/fmicb.2021.744164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RC, Garcia CC, Teixeira MM, Amaral FA. The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol. 2014. May;10(5):593–619. 10.1586/1744666X.2014.894886 [DOI] [PubMed] [Google Scholar]
- Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis [Internet]. Arthritis Rheum. 2003. Jun;48(6):1521–9. [cited 2023 Apr 23] Available from: https://pubmed.ncbi.nlm.nih.gov/12794819/ 10.1002/art.11143 [DOI] [PubMed] [Google Scholar]
- Santiago FW, Halsey ES, Siles C, Vilcarromero S, Guevara C, Silvas JA, et al. Long-term arthralgia after mayaro virus infection correlates with sustained pro-inflammatory cytokine response. PLoS Negl Trop Dis. 2015. Oct;9(10):e0004104. 10.1371/journal.pntd.0004104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-López JF, Fernandez GJ, Urcuqui-Inchima S. Interleukin 27 as an inducer of antiviral response against chikungunya virus infection in human macrophages. Cell Immunol. 2021. Sep;367:104411. 10.1016/j.cellimm.2021.104411 [DOI] [PubMed] [Google Scholar]
- Valdés-López JF, Hernández-Sarmiento LJ, Tamayo-Molina YS, Velilla-Hernández PA, Rodenhuis-Zybert IA, Urcuqui-Inchima S. Interleukin 27, like interferons, activates JAK-STAT signaling and promotes pro-inflammatory and antiviral states that interfere with dengue and chikungunya viruses replication in human macrophages [Internet]. Front Immunol. 2024. Apr;15:1385473. [cited 2024 Aug 1] Available from: https://pubmed.ncbi.nlm.nih.gov/38720890/ 10.3389/fimmu.2024.1385473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Asano T, Matsuoka N, Temmoku J, Sato S, Matsumoto H, et al. Differential regulation and correlation between galectin-9 and anti-CCP antibody (ACPA) in rheumatoid arthritis patients. Arthritis Res Ther. 2020. Apr;22(1):80. 10.1186/s13075-020-02158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Li S, Zhu L. Potential effects of HMGB1 on viral replication and virus infection-induced inflammatory responses: A promising therapeutic target for virus infection-induced inflammatory diseases. Cytokine Growth Factor Rev. 2021. Dec;62:54–61. 10.1016/j.cytogfr.2021.08.003 [DOI] [PubMed] [Google Scholar]
- Anna Genaro MS, Marchi MS, Perin MY, Cossô IS, Dezengrini Slhessarenko R. Ferritin, erythrocyte sedimentation rate, and C-reactive protein level in chikungunya-induced chronic polyarthritis. Am J Trop Med Hyg. 2020. Nov;103(5):2077–82. 10.4269/ajtmh.20-0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LT, Sharma AR, Chakraborty C, Saibaba B, Ahn ME, Lee SS. Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci. 2017. Mar;18(3):e601. 10.3390/ijms18030601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon IK, Srikiatkhachorn A, Alera MT, Fernandez S, Cummings DA, Salje H. Pre-existing chikungunya virus neutralizing antibodies correlate with risk of symptomatic infection and subclinical seroconversion in a Philippine cohort. Int J Infect Dis. 2020. Jun;95:167–73. 10.1016/j.ijid.2020.03.073 [DOI] [PubMed] [Google Scholar]
- Jacob-Nascimento LC, Carvalho CX, Silva MM, Kikuti M, Anjos RO, Fradico JR, et al. Acute-phase levels of CXCL8 as risk factor for chronic arthralgia following chikungunya virus infection. Front Immunol. 2021. Oct;12:744183. 10.3389/fimmu.2021.744183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirathaworn C, Rianthavorn P, Wuttirattanakowit N, Poovorawan Y. Serum IL-18 and IL-18BP levels in patients with Chikungunya virus infection. Viral Immunol. 2010. Feb;23(1):113–7. 10.1089/vim.2009.0077 [DOI] [PubMed] [Google Scholar]
- Reddy V, Mani RS, Desai A, Ravi V. Correlation of plasma viral loads and presence of Chikungunya IgM antibodies with cytokine/chemokine levels during acute Chikungunya virus infection. J Med Virol. 2014. Aug;86(8):1393–401. 10.1002/jmv.23875 [DOI] [PubMed] [Google Scholar]
- Dutta SK, Tripathi A. Association of toll-like receptor polymorphisms with susceptibility to chikungunya virus infection. Virology. 2017. Nov;511:207–13. 10.1016/j.virol.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Banerjee N, Mukhopadhyay S. Oxidative damage markers and inflammatory cytokines are altered in patients suffering with post-chikungunya persisting polyarthralgia. Free Radic Res. 2018. Aug;52(8):887–95. 10.1080/10715762.2018.1489131 [DOI] [PubMed] [Google Scholar]
- Tanabe IS, Santos EC, Tanabe EL, Souza SJ, Santos FE, Taniele-Silva J, et al. Cytokines and chemokines triggered by Chikungunya virus infection in human patients during the very early acute phase. Trans R Soc Trop Med Hyg. 2019. Nov;113(11):730–3. 10.1093/trstmh/trz065 [DOI] [PubMed] [Google Scholar]
- Liu X, Poo YS, Alves JC, Almeida RP, Mostafavi H, Tang PC, et al. Interleukin-17 contributes to chikungunya virus-induced disease. MBio. 2022. Apr;13(2):e0028922. 10.1128/mbio.00289-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu N, Mahilkar S, Jayaram A, Ibemgbo SA, Mathur G, Shetty U, et al. Cytokine profile, neutralisation potential and viral replication dynamics in sera of chikungunya patients in India: a cross-sectional study. Lancet Reg Health Southeast Asia. 2023. Sep;19:100269. 10.1016/j.lansea.2023.100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhenni R, Yohan B, Alisjahbana B, Lucanus A, Riswari SF, Megawati D, et al. Comparative cytokine profiling identifies common and unique serum cytokine responses in acute chikungunya and dengue virus infection. BMC Infect Dis. 2021. Jul;21(1):639. 10.1186/s12879-021-06339-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, Saluja M, Venugopalan A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014. Feb;66(2):319–26. 10.1002/art.38221 [DOI] [PubMed] [Google Scholar]