Summary
A cluster of 129 patients with coronavirus disease 2019 (COVID-19) nosocomial infections was analysed during the Omicron strain epidemic. The incubation period for nosocomial Omicron strain infections was found to be 3 days. The transmission route of the first patient with COVID-19 (FCP) in each room is a critical factor within these clusters. There have been few cases of healthcare-worker-to-patient transmission, and most FCPs maintained high levels of activity in daily living. The primary routes of nosocomial infection among FCPs likely involved patient visits or direct conversations between patients. Therefore, hospital clusters can potentially be mitigated by educating patients on infection control measures, such as proper mask-waring and hand hygiene.
Keywords: Nosocomial infections, COVID-19, Clusters, Omicron, Infection control, Incubation period
Introduction
On 5th May 2023, the World Health Organization declared an end to the public health emergency of international concern related to coronavirus disease 2019 (COVID-19). [1] Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, was initially reported in 2019 in Wuhan, China. To date, SARS-CoV-2 has undergone multiple mutations, changing its pathogenicity and immune evasion capabilities. [2] The virulence of SARS-CoV-2 has gradually decreased, and as of April 2024, the Omicron strain is, now predominant, and has shown lower mortality rates among hospitalised patients than the wild-type, alpha, and delta strains. [3] Despite reduced virulence, nosocomial COVID-19 infections are associated with increased mortality, procedural delays, and extended hospital stays [4] as well as higher mortality rates and long-term multi-organ dysfunction than influenza. [5] Omicron strains, particularly, exhibit high immune evasion and transmissibility. [6] Family infection rates were 36.4%, 29.7 %, and 42.7% for alpha, delta, and omicron strains, respectively. [6] Given these characteristics, stringent nosocomial infection control measures remain crucial. We observed a COVID-19 cluster of 129 patients with nosocomial infections between 15th November and 26th December 2022. This study aimed to examine the challenges associated with these clusters and propose effective countermeasures.
Methods
Analysis period
St. Marianna University Hospital is a tertiary-level teaching hospital with 1,175 beds.
In 2022, it admitted an average of 300 new patients daily, with an average inpatient stay of 11 days. This study enrolled newly diagnosed patients with COVID-19 in multibed hospital rooms (eight patients per room) between 15th November and 26th December 2022.
Definition of hospital-acquired COVID-19 and analysis method
During this period, all hospitalised patients underwent reverse transcription polymerase chain reaction testing to COVID-19 infection. All hospitalised patients were monitored three times a day (morning, noon, and evening or bedtime) for COVID-19 symptoms such as fever, upper respiratory symptoms, and joint pain. Vital signs were measured at least once daily to facilitate early detection of first patients with COVID-19 (FCPs) and prevent the spread of infection. Patients with suspected COVID-19 were promptly tested. SARS-CoV-2 testing was performed whenever symptoms or vital signs indicated a potential infection. To rule out the possibility that the patient was infected outside the hospital and developed the disease following admission, COVID-19 for nosocomial infections was diagnosed if: 1) the SARS-CoV-2 reverse transcription polymerase chain reaction test results were negative at admission, and 2) COVID-19 infection developed ≥6 days after hospitalisation. The FCP in each room was defined as the initial patient diagnosed with symptomatic COVID-19. Patients who spent more than one night in the same room as an FCP were considered close contacts. Those who subsequently developed COVID-19 were categorised as patients with post-exposure COVID-19 (PCP). The day of the diagnosis was considered day 0 of onset, and the day of close contact was considered day 0 of exposure. To test for COVID-19 infection, SARS-CoV-2 antigen quantification assays were performed on days 0 and 5 for close contacts. Close contacts were assessed for symptoms. Quantitative antigen testing was performed in the presence of symptoms suggestive of COVID-19, such as fever, upper respiratory symptoms, or joint pain. If the test result from the close contact was negative on the fifth day, the possibility of infection was ruled out, and the patient could return to the general ward.
Once the FCP was identified, all patients in the same room were tested for SARS-CoV-2 antigen quantification (day 0 of exposure test). We classified the patients into two groups based on the results of the day 0 exposure test. On the 0 exposure test, if there were asymptomatic patients with COVID-19 in the same room other than the FCP, the cluster was categorised into the WA group, whereas cases where there were no other patients with COVID-19 in the same room other than the FCP on day 0 of the exposure test were categorised into the WoA group. In the WA group, PCPs who tested positive on day 0 of the exposure test were defined as D0-PCPs. Additionally, at the time of the day 0 exposure test, none of the patients other than the FCP showed symptoms suggestive of COVID-19. Those diagnosed with COVID-19 were isolated in dedicated rooms for patients with COVID-19. Close contacts remained in their original room until 5 days had passed since close contact and their antigen test was negative. We investigated the COVID-19 incubation period among the WoA and WA groups of close contacts. In the WoA group, the differences in activity between the FCP and PCP groups were compared. Patients who could walk independently or with a cane were included in the highly active group, whereas those who needed assistance from a healthcare worker to walk or use a wheelchair, and those who were bedridden comprised the low-activity group [7].
Ethics
The St. Marianna University School of Medicine Ethics Committee approved the study protocol (approval number: 6069).
Results and discussion
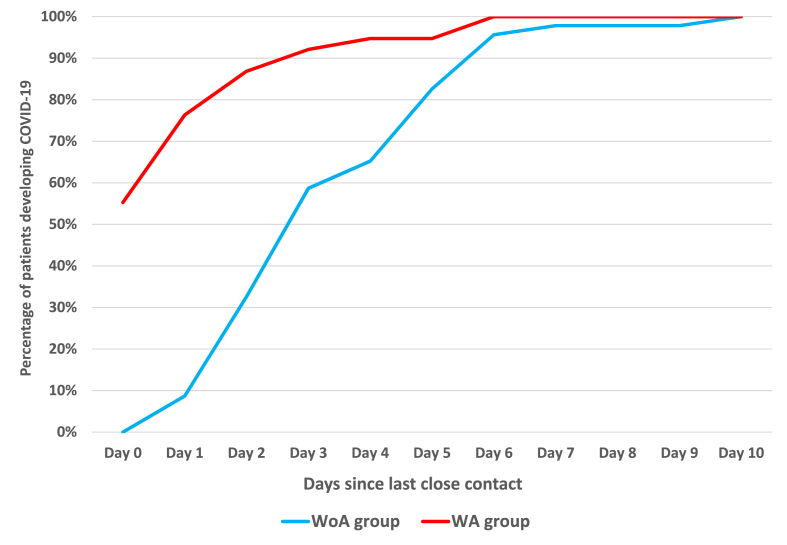
There were 25 FCPs in the WoA group and 20 in the WA group. Additionally, there were 46 PCPs in the WoA group and 38 in the WA group, encompassing 21 D0-PCPs. In the WoA group, 59% developed COVID-19 on day 3 after close contact, whereas in the WA group, 55% of close contacts had already developed COVID-19 by day 0, and 92% had developed COVID-19 by day 3 (Figure 1). In the WA group, the incubation period was analysed for 17 PCPs, excluding D0-PCPs. Of these, 47% of those in close contact developed COVID-19 on the first day, and 71% developed it by the second day. The difference in activity between FCPs and PCPs was notable: 76% of FCPs were active, whereas none were bedridden. In contrast, among PCPs, 48% were active and 52% had low activity levels (Table I).
Figure 1.
Difference in incubation period between patients in the WoA and WA groups, including patients with post-exposure COVID-19. The percentage of patients developing COVID-19 was calculated by dividing the number of patients who developed COVID-19 after close contact by the total number of patients who had close contact up to the respective date.
Abbreviations: COVID-19, Coronavirus disease 2019.
Table I.
IADL classification of patients with COVID-19
| High activity |
Low activity |
||||
|---|---|---|---|---|---|
| ADL | Walk independently | Walk with a cane | Walk with the assistance of a healthcare worker | Use a wheelchair with the assistance of a healthcare worker | Bedridden |
| FCP | 56.0% | 20.0% | 4.0% | 20.0% | 0.0% |
| PCP | 37.0% | 10.9% | 0.0% | 26.1% | 26.1% |
Abbreviations: ADL, Activities of daily living; FCP, the first patient diagnosed with symptomatic COVID-19; PCP, patients with post-exposure COVID-19.
During the outbreak, >90% of the prevalent strains were BA.5 (Omicron). [8] Although nosocomial COVID-19 mortality was reportedly reduced during the Omicron strain epidemic, some studies suggest that this reduction is attributable to vaccination or prior infection, with strain differences having minimal impact on mortality. [3,4] In addition, Omicron exhibited higher transmissibility than the pre-Omicron strains, highlighting the ongoing importance of monitoring nosocomial infections. [6].
The incubation period of the Omicron strain was reduced to approximately 3 days, whereas that of the Alpha strain was approximately 5 days. [3] There are no data on the incubation period for nosocomial infections with Omicron strains. Therefore, the definition of nosocomial COVID-19 infection was established with an incubation period of 5 days. More than half of the PCPs in the WoA group were antigen-positive on day 3, and the incubation period for nosocomial infections did not differ significantly from that for community-acquired infections (Figure 1). In contrast, in the WA group, D0-PCPs who tested positive on day 0 accounted for 55% of PCPs, with 71% of the remaining PCPs developing COVID-19 by the second day. The short incubation period among close contacts in the WA group suggests that they may have been infected not only by the FCP but also by DO-PCPs. Therefore, the WA group was not suitable for estimating the route of infection.
In the WoA group, the incubation period for PCP was approximately 3 days, indicating potential effectiveness of post-exposure prophylaxis in the future. Even during the Omicron strain outbreak, >95% of cases among close contacts tested antigen test-positive until the 6th day of the illness, supporting the rationale for transferring them to a non-COVID-19 ward on the 6th day after confirming negative antigen test results on the 5th day.
In the WoA group, the FCPs were primarily active patients. None of the bedridden patients, who had minimal contact with anyone other than healthcare workers, became FCPs. This finding suggests that patients with nosocomial infections are unlikely to acquire SARS-CoV-2 from healthcare workers. Data from the wild-type and Alpha strains indicated a low risk of SARS-CoV-2 transmission from healthcare workers to patients. [9] Even with the highly transmissible Omicron strain, the risk of transmission from healthcare workers to patients remains low. Since no molecular epidemiologic analysis has been performed, the transmission route of SARS-CoV-2 in the WoA group FCP was not fully understood in this study. In the BA.5 variant, upper respiratory symptoms such as cough, nasal discharge, and sore throat may take up to three days to appear after COVID-19 onset, and exposure to asymptomatic patients with COVID-19 during hospitalisation is possible. [10] Given that healthcare worker-to-patient transmission is unlikely to occur and that most FCPs are highly active, it is possible that FCPs may have been exposed outside their rooms during conversations with other patients or during visits. Therefore, in addition to implementing infection control measures by healthcare workers, it is crucial to ensure patients cooperation with infection control practices, such as wearing masks and practicing hand hygiene.
Limitations
SARS-CoV-2 testing for close contacts was performed only at symptom onset, except for testing on days 0 and 5. Therefore, the onset of asymptomatic cases with COVID-19 may be estimated later than it actually occurred.
Conclusions
An analysis of COVID-19 nosocomial clusters during the BA.5 epidemic revealed that transmission from healthcare workers to patients was rare. The FCP may have contracted the virus through visits or conversations between patients. Therefore, cooperation from both patients and medical staff in adhering to infection control measures, such as wearing masks and practicing hand hygiene, is crucial in preventing hospital clusters.
Author contributions
Tomonori Takano: Conceptualization, Methodology, Software, Validation, Investigation, Writing - Original Draft. Yoshiko Nakatani: Conceptualization, Methodology. Investigation. Akihiro Nagai: Visualization, Methodology, Investigation. Natsuki Izumoto: Visualization, Methodology, Investigation. Y uta Ono: Conceptualization, Visualization, Methodology, Investigation. Atsushi Inoue: Conceptualization, Methodology, Software. Hiromu Takemura: Supervision, Project administration, Validation. Hiroyuki Kunishima: Supervision, Project administration, Validation. All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version submitted.
Funding
This research was supported by AMED under Grant Number 24fk0108685h0002.
Conflict of interest statement
None.
Acknowledgements
We would like to extend our gratitude to all the staff members who worked with the patients with COVID-19.
References
- 1.WHO, director, General. Opening remarks at the media briefing. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing---5-may-2023, (accessed 13 May 2024).
- 2.Carabelli A.M., Peacock T.P., Thorne L.G., Harvey W.T., Hughes J., COVID-19 Genomics UK Consortium. Peacock S.J., et al. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21:162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedberg P., Parczewski M., Serwin K., Marchetti G., Bai F., Ole Jensen B.E., et al. In-hospital mortality during the wild-type, alpha, delta, and omicron SARS-CoV-2 waves: a multinational cohort study in the EuCARE project. Lancet Reg Health Eur. 2024;38 doi: 10.1016/j.lanepe.2024.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dave N., Sjöholm D., Hedberg P., Ternhag A., Granath F., Verberk J.D.M., et al. Nosocomial SARS-CoV-2 infections and mortality during unique COVID-19 epidemic waves. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.41936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Y., Choi T., Al-Aly Z. Long-term outcomes following hospital admission for COVID-19 versus seasonal influenza: a cohort study. Lancet Infect Dis. 2024;24:239–255. doi: 10.1016/S1473-3099(23)00684-9. [DOI] [PubMed] [Google Scholar]
- 6.COVID Past SARS-CoV-2 infection protection against reinfection: a systematic review and meta-analysis. Lancet. 2023;401:833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edemekong P.F., Bomgaars D.L., Sukumaran S., Schoo C. In: Encyclopedia of the neurological sciences. Aminoff M.J., Daroff R.B., editors. 2023. Activities of daily living; pp. 47–48. Published Online. [Google Scholar]
- 8.Summary on SARS-CoV-2 variants of voncern for increased infectivity/transmissibility and antigenic changes. https://www.niid.go.jp/niid/images/cepr/covid-19/230216_SARSCoV-2_No24en.pdf
- 9.Evans S., Stimson J., Pople D., Bhattacharya A., Hope R., White P.J., et al. Quantifying the contribution of pathways of nosocomial acquisition of COVID-19 in English Hospitals. Int J Epidemiol. 2022;51:393–403. doi: 10.1093/ije/dyab241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakakubo S., Kishida N., Okuda K., Kamada K., Iwama M., Suzuki M., et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect Dis. 2023;23:1244–1256. doi: 10.1016/S1473-3099(23)00271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]