Abstract
Introduction
Most studies have shown a declining incidence of upper gastrointestinal bleeding (UGIB) in recent years. Data regarding mortality were controversial; in non-variceal bleeding, the increasing age of the population, increased use of anti-thrombotic and anticoagulant therapy in patients with cardiovascular diseases, and the use of non-steroidal anti-inflammatory drugs are counterbalanced by the progress in endoscopic therapy with stable mortality.
Material and Method
We performed a retrospective, cross-sectional study that included patients admitted with UGIB in Clinical Emergency Hospital Craiova during 2013–2020.
Results
3571 patients with UGIB were selected; a trend toward increased admission for UGIB from 2013 to 2019 was noted, with a significant decrease in 2020. Non-variceal bleeding remains the most frequent form, with a slight increase in variceal bleeding, of Mallory-Weiss syndrome and angiodysplasia, and a 3-fold decrease for unknown etiology bleeding (with no endoscopy performed) during the 2017–2020 period as compared to 2013–2016. There was a trend toward decreased mortality, with lower mortality in 2017–2020 (12.83%) compared to 2013–2016 (17.41%). The mortality for variceal bleeding and peptic ulcer bleeding has declined, but mortality for non-variceal bleeding has slightly increased during 2013–2020. Mortality has decreased in admissions during regular hours/after hours and weekdays/weekends, but the difference (off-hours and weekend effects) had increased. The percentage of endoscopies performed in the first 24 hours after admission and the rate of therapeutic endoscopy increased during 2017–2020; the median time between admission and endoscopy was 17.0 hours during 2017–2020 and 59.1 hours during 2013–2016. The proportion of patients who needed emergency surgery for uncontrolled bleeding has significantly declined since 2013–2015, with an average value of 1% in the last 5 years of the study.
Conclusion
Increased admissions for UGIB, with lower mortality, especially for peptic ulcer bleeding and variceal bleeding were noted; higher percentages of therapeutic endoscopies and endoscopies performed during the first 24 hours after admission were also recorded.
Keywords: upper gastrointestinal bleeding, peptic ulcer bleeding, endoscopy, emergency surgery
Introduction
Acute upper gastrointestinal bleeding (UGIB) represents a potentially severe complication associated with significant hospitalization, morbidity, and mortality. The etiology is dominated by peptic ulcer disease, erosive gastritis, varices (esophageal and rarely gastric), esophagitis, Mallory-Weiss syndrome, and neoplasms.1,2 In 3 to 19% of cases, no apparent source of bleeding is found (obscure bleeding);2–4 the use of enteroscopy (capsule endoscopy, spiral, or balloon-aided endoscopy) has decreased the frequency of obscure bleeding diagnosis.5,6
Non-variceal bleeding is the most frequent form of UGIB; the aging population and increased use of anti-thrombotic and non-steroidal anti-inflammatory drugs are factors that adversely affect the incidence and mortality.7 The progress in medical care and endoscopic therapy has counterbalanced the former factors and contributed to a stable mortality of 5 to 10% in non-variceal UGIB,1,8–14 although values as high as 14–15% have been estimated in some studies.1–19 In variceal bleeding (most frequently caused by the rupture of esophageal varices) the 6-week mortality rate is 10–20%.18,20 Early endoscopy may contribute to a reduction in mortality in both forms;21 the general recommendation is to perform endoscopy within 24 hours of admission2 but in severe cases of bleeding or in variceal bleeding a very early (<12 hours of admission) or immediately after stabilization is recommended.
The selection of severe cases represents an important factor for prognostic stratification; the most used scores were the Rockall score and the Glasgow-Blatchford score8,18,22–24-Tables 1 and 2, but other scores such as Baylor score (Table 3), AIM65, Cedar Sinai score, PNED, T-score, ANN score, and Cambridge score were proposed.10,16,23,25,26
Table 1.
Rockall Score10
| 0 | 1 | 2 | 3 | |
| Age | <60 | 60–79 | ≥80 | – |
| Shock | P<100 sBP≥100 |
P≥100 ≥100 |
sBP<100 | – |
| Comorbidities | NO major | – | Cardiac failure, coronary ischemia | Renal/liver failure Disseminated malignancy |
| Diagnosis | MW No lesion No stigmata |
Other exc. malignancy | Malignancy | |
| Bleeding stigmata | No/dark spot | – | Blood, adherent clot Visible/spurting vessel |
– |
Abbreviations: P, pulse; sBP, systolic Blood Pressure; MW, Mallory-Weiss syndrome.
Table 2.
Glasgow-Blatchford Score10
| Urea (mg/dl) | 39–47 48-60 60–149 ≥150 |
2 3 4 6 |
| Hb (g/dl) | Men 12–12.99 Men ≥10 Woman ≥10 Both sexes <10 |
1 3 1 6 |
| sBP (mm Hg) | 100–109 90-99 <90 |
1 2 3 |
| Pulse (>100/min) | 1 | |
| Melena | 1 | |
| Syncope | 2 | |
| Liver disease | 2 | |
| Cardiac failure | 2 |
Abbreviation: sBP, systolic blood pressure.
Table 3.
Baylor Score15
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| Age | 30–49 | 50–59 | 60–69 | ≥70 | |
| Number of diseases | 1–2 | 3–4 | >5 | ||
| Severity of diseases | Chronic | Acute | |||
| Endoscopy score | |||||
| Site of bleeding | Posterior DU | ||||
| Bleeding stigmata | IIb | IIa | I |
Abbreviation: DU, duodenal ulcer.
Several studies have shown a declining trend of incidence, hospitalization, and mortality of UGIB, and especially of peptic ulcer bleeding in recent years.2,4,23,27 The effect can be related to several factors: improved access to endoscopy and endoscopic treatment, reduced time to endoscopy, progress of endoscopic hemostasis procedures, improved general care of patients with UGIB, increased use of proton pump inhibitors and vasoactive drugs in variceal bleeding.2,9,18 Significant therapeutic endoscopy advances have led to a decline in rebleeding rates and emergency surgical interventions.2,9
The purpose of our study was to evaluate the temporal trend for admission rates, mortality, and etiology changes in patients with acute UGIB over a significant timeframe in a tertiary care unit.
Materials and Method
We performed a retrospective, cross-sectional study to analyze the admissions, etiology, and mortality in cases with acute UGIB admitted in Clinical Emergency County Hospital Craiova for eight years (2013–2020). In the first four years, patients with UGIB were admitted to the Surgery Departments; endoscopy was regularly available only Monday to Friday on working days from 8 to 15 and emergency endoscopy was performed outside this schedule when necessary. By 2017, the Gastroenterology Department took responsibility for UGIB management, and the endoscopy regular schedule was expanded to 8–20 during regular working days and 8–15 during weekends and holidays.
The trend for admissions, etiology, total mortality, case-fatality (nonvariceal, peptic ulcer bleeding, variceal bleeding, UGIB with no endoscopy performed), the timing of endoscopy, the need for emergency surgery, and the influence of admission time were assessed. We quantified admissions as PROGRAMME IN/OUT (IN=admissions during regular hours, OUT=admissions after regular hours schedule) and also as WEEK IN/OFF (IN=admissions during working days, OFF=admissions during weekends and holidays) and stratified patients regarding risk factors for mortality (age, pre-endoscopic and after endoscopy Baylor bleeding score, Forrest score, Charlson comorbidity index) in non-variceal and variceal bleeding (Table 4 and Table 5). Charlson comorbidity index (CCI) was developed as a method for the estimation of mortality risk by weighing associated diseases with 1.2 and 6 points and also by adding supplemental points in patients at advanced age;28 however, the accuracy in the prognosis of peptic ulcer bleeding is inferior to specific bleeding scores. The intervals between the onset of bleeding and endoscopy and between admission and endoscopy were estimated.
Table 4.
Forrest Classification10
| Prevalence (%) | Rebleeding Rate (%) | ||
|---|---|---|---|
| Active bleeding | IA (spurting bleeding) | 10 | 90 |
| IB (oozing bleeding) | 10 | 10–20 | |
| Bleeding stigmata, no active bleeding | IIA (visible vessel) | 25 | 50 |
| IIB (adherent clot) | 10 | 25–50 | |
| IIC (hematin spot) | 10 | 7–10 | |
| No bleeding stigmata | III (clean base) | 35 | 3–5 |
Table 5.
Charlson Comorbidity Index28
| Points | |
|---|---|
| Age >40 | 1 |
| >50 | 2 |
| >60 | 3 |
| >70 | 4 |
| Chronic pulmonary disease | 1 |
| Myocardial infarction | 1 |
| Peripheral vascular disease | 1 |
| Chronic Heart Failure | 1 |
| Peptic ulcer disease | 1 |
| Mild Liver Disease | 1 |
| Rheumatological disease | 1 |
| Dementia | 1 |
| Diabetes without chronic complications | 1 |
| Diabetes with chronic complications | 2 |
| Hemiplegia or paraplegia | 2 |
| Renal disease | 2 |
| Solid tumors | 2 |
| Leukemia/lymphoma | 2 |
| Moderate/severe liver disease | 3 |
| HIV/AIDS | 6 |
| Metastatic solid tumors | 6 |
Inclusion and Exclusion Criteria
All patients admitted with UGIB in the Clinical Emergency County Hospital Craiova were included. The diagnosis was based on hematemesis, melena, or hematochezia and confirmed by endoscopy; in patients with melena or hematochezia and no endoscopy performed, the nazo-gastric tube has confirmed upper digestive source of bleeding. The study was conducted following the Declaration of Helsinki. Informed consent was obtained from all admitted patients and approval by the Local Ethics Committee of the Emergency Clinical County Hospital of Craiova was also obtained. Patients under 18 years of age, those who denied consent for data usage, and those with missing data were excluded.
In non-variceal bleeding, proton pump inhibitor therapy (80 mg iv bolus followed by 8 mg/hour 72 hours) was initiated before endoscopy; blood transfusions were recommended if the hemoglobin value was below 8 g/dl. Vitamin K or plasma concentrate was used in cases with over-dosage of vitamin K antagonists, and thrombocyte concentrate was used in cases with thrombocyte counts below 50.000/dl. In case of possible variceal bleeding (patients with known cirrhosis or previous variceal bleeding), the treatment with Terlipressin was initiated before endoscopy and was continued 3–5 days after endoscopy, antibiotics for prevention of infections, and corrective measures for coagulation disturbances were used; Sengstaken-Blakemore tube was used in unstable cases before endoscopy to help stabilize the patients.
Endoscopic therapy was performed in cases with active bleeding (Forrest Ia, Ib) and cases with a high risk of rebleeding (Forrest IIa, IIb); adrenaline injection combined with either clip placement or electrocoagulation was used for non-variceal bleeding, while in variceal bleeding EVL (endoscopic variceal ligation) procedure was used for esophageal varices or type I or II of GOV (gastro-esophageal varices) with active bleeding, with stigmata of recent bleeding or in case of high-risk varices (large, red signs present). Surgery was imposed in life-threatening non-variceal bleeding cases and cases with repeated endoscopic hemostatic failure with continued bleeding, whereas Sengstaken-Blakemore was used in variceal bleeding with persistent bleeding.
Statistical data were analyzed and provided using MedCalc version 22.009. Continuous variables were compared using the Student test; the Chi-square or the Fisher test were used for categorical variables. The received operating area under the curve (AUROC) was used to assess the predictive value of the Baylor bleeding score and the Charlson comorbidity index for mortality prediction.
Results
3571 patients with UGIB were selected during the analyzed period. The median age was 62.6 years in 2013–2016 and 62.9 years in 2017–2020. 19.1% were variceal bleeding (681 cases) and 58.3% were non-variceal bleeding (2081 cases); 226 cases had no known cause (obscure bleeding) and in 583 cases endoscopy was not performed during the bleeding episode (patient refusal, dementia, immediate death, alcohol withdrawal, other contraindications). Characteristics of the patients are illustrated in Table 6.
Table 6.
Characteristics of Patients with UGIB
| Characteristics | 2013–2016 -1585 Patients- |
2017–2020 -1986 Patients- |
P-value |
|---|---|---|---|
| Age yrs±STD (Minimum-maximum) | 62.6±13.9 (18–94) | 62.9±13.9 (16–99) | 0.5724 |
| <60/60-79/>80 (%) | 39.6/50.8/9.6 | 37.5/50.5/13 | 0.0593 |
| M/F (%M) | 64.9 | 63.3 | 0.8099 |
| Etiology (endoscopy performed) | |||
| Ulcer | 39.1 | 36.9 | 0.6373 |
| Gastric/duodenal/esophageal erosions | 12.9 | 15.4 | 0.0049 |
| MW/Boerhaave syndrome | 3.3 | 6.8 | <0.0001 |
| Esophageal/gastric/jejunal varices | 21.8 | 23.6 | 0.0189 |
| Angiodysplasia/Dieulafoy/GAVE | 0.7 | 3.5 | 0.0006 |
| Tumors | 5.8 | 5.8 | 0.6106 |
| Anticoagulants/antithrombotic | 5.1 | 3.5 | 0.0995 |
| Other | 0.3 | 0.2 | 0.9119 |
| Obscure | 11.1 | 5.3 | <0.0001 |
| Unknown (endoscopy not done) | 26.9 | 8.4 | <0.0001 |
| Mortality (%) -all patients | 12.8 | 7.4 | 0.0001 |
| - Patients with endoscopy (%) | 6.5 | 7.9 | 0.1743 |
| - Patients without endoscopy (%) | 36.8 | 35.9 | 0.8467 |
| - Variceal bleeding | 23.4 | 22.1 | 0.7025 |
| - Non-variceal bleeding | 6.6 | 7.0 | 0.7166 |
| - Cirrhosis with non-variceal bleeding | 12.5 | 10.8 | 0.7218 |
| Cirrhosis (%) | 27.6 | 32.0 | 0.0056 |
| Endoscopy <6h/<12h/<24h | 6.5/14.5/38.5 | 40.3/57.8/83.1 | <0.0001 |
| Endoscopic therapy (%) | 7.3 | 23.7 | <0.0001 |
| Emergency surgery (%) | 3.7 | 0.8 | <0.0001 |
| Mean hospital stay (days) | 7.9 | 7.4 | 0.0401 |
Note: Statistically significant P-values are marked with italicized fonts.
Abbreviations: STD, standard deviation; GAVE, Gastric antral vascular ectasia.
Admission Rate and Etiology of Bleeding
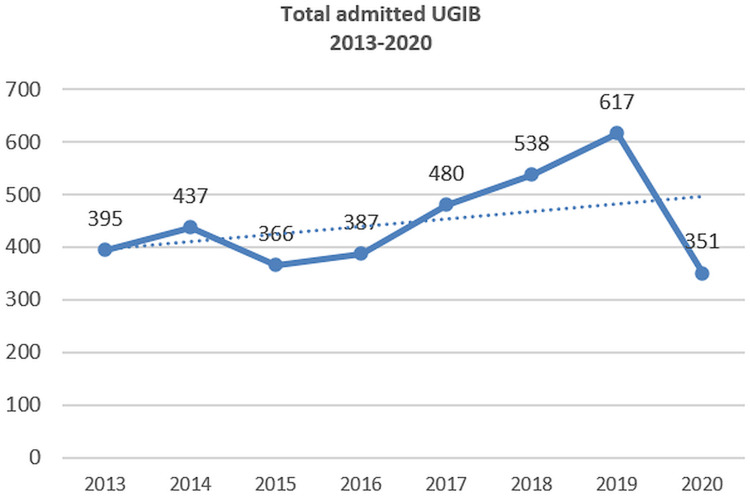
During the 2013–2020 interval, we noted a trend toward increased admission for UGIB from 2013 to 2019, with a decrease in 2020 (the first pandemic year) - Figure 1. The etiology was similar, with the ratio between non-variceal and variceal bleeding being 3.04 during 2013–2016 and 3.08 in 2017–2020; an increased frequency of Mallory-Weiss bleeding and a decreased frequency of tumoral bleeding was seen. A 3-fold reduction for unknown etiology bleeding (with no endoscopy performed) and a 2-fold reduction of obscure bleeding were observed during 2017–2020 period, as compared with 2013–2016 period (8.4 versus 26.9%, OR=0.2490, 95% CI 0.2052–0.3021, P<0.0001, and 5.3 versus 11.1%, OR=0.4465, 95% CI 0.3393 to 0.5875, P<0.0001). The reduction of bleeding with no endoscopy was related to the improvement of the permanent endoscopy schedule, whereas the lower rate of obscure bleeding can be related to a more accurate endoscopy.
Figure 1.
Admissions for UGIB (2013–2020).
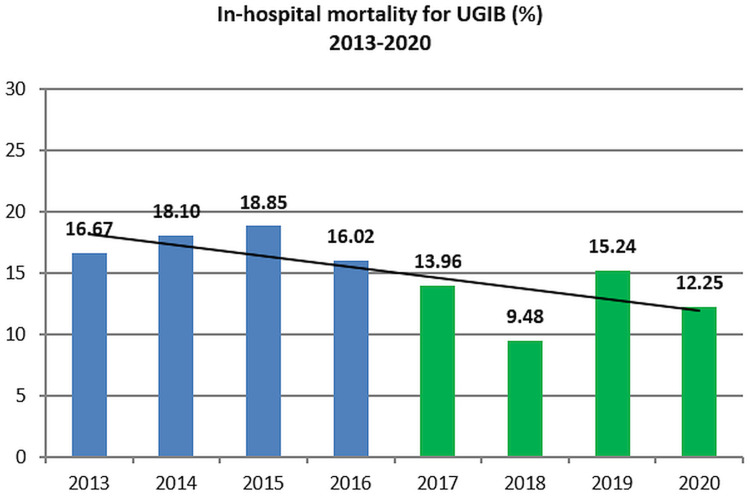
Mortality
During the analyzed period of 8 years, there was a trend toward decreased mortality (Figure 2) with lower mortality in the 2017–2020 period (12.8%) as compared with the 2013–2016 period (17.4%, OR=0.6988, 95% CI 0.5806 to 0.8410, P=0.0001). The mortality in 2019 (15.2%) was higher than in 2017–2018 (11.6%, OR=1.3708, 95% CI 1.0241 to 1.8350, P=0.0340). The appearance of the COVID-19 pandemic may have altered admission and possibly mortality in UGIB,28,29 although our study has included only the first year of the pandemic.
Figure 2.
In-hospital mortality for UGIB (2013–2020).
Associated Comorbidities
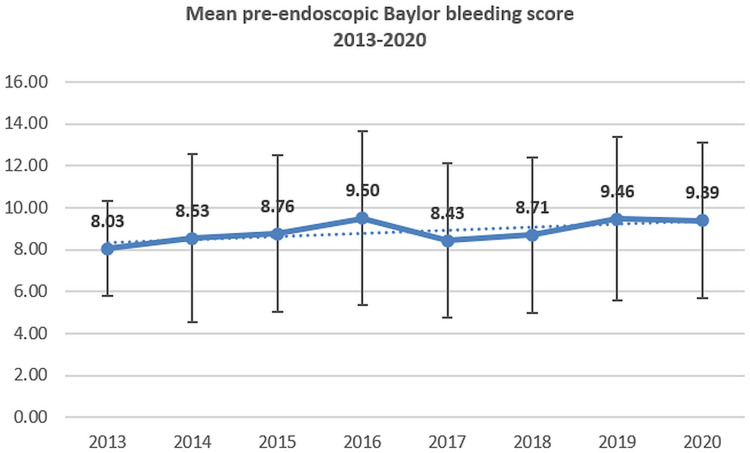
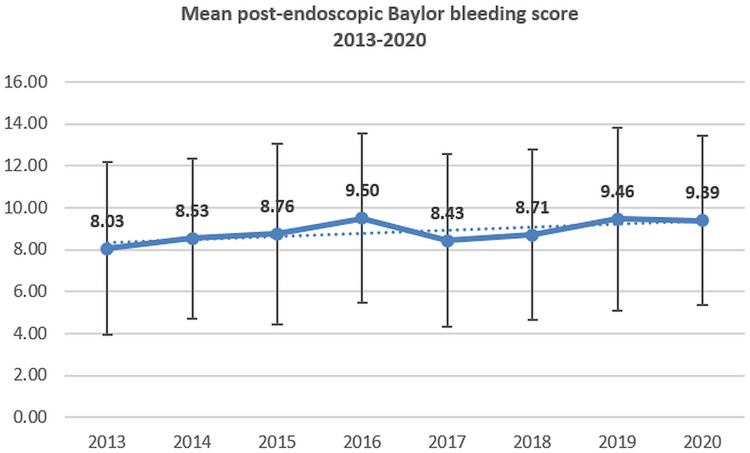
The percentage of patients with cirrhosis and the mean values of CCI have no significant variations during the analyzed period; however, a slight increase for pre- and after-endoscopy BBS was recorded (Figures 3–4).
Figure 3.
The mean value of pre-endoscopic Baylor bleeding score in UGIB (2013–2020).
Figure 4.
The mean value of after-endoscopic Baylor bleeding score in UGIB (2013–2020).
Case Fatality Rate Related to the Type of Bleeding
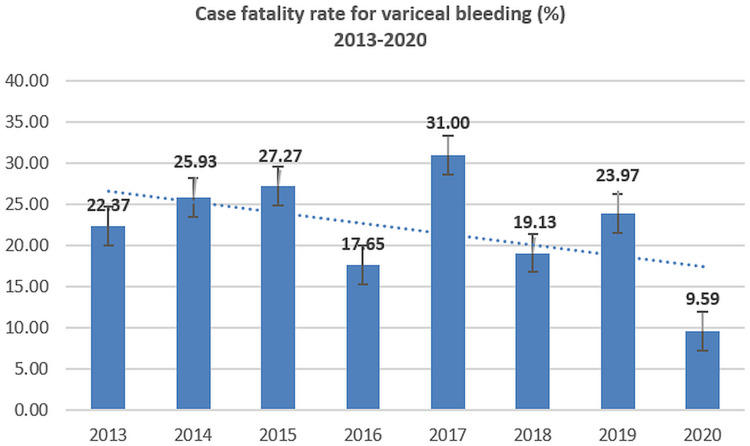
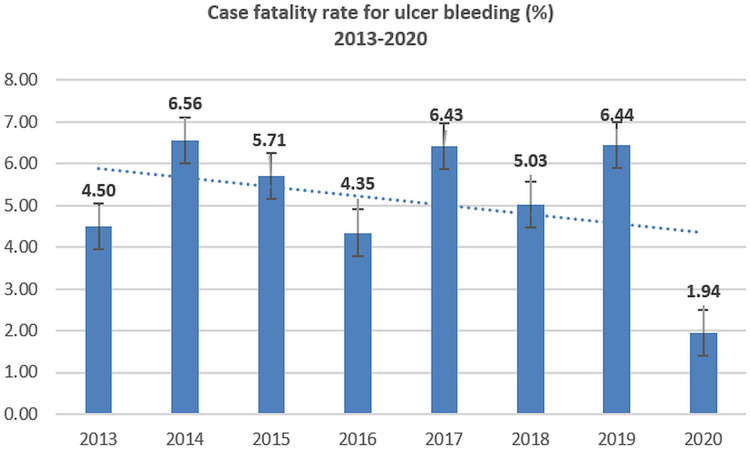
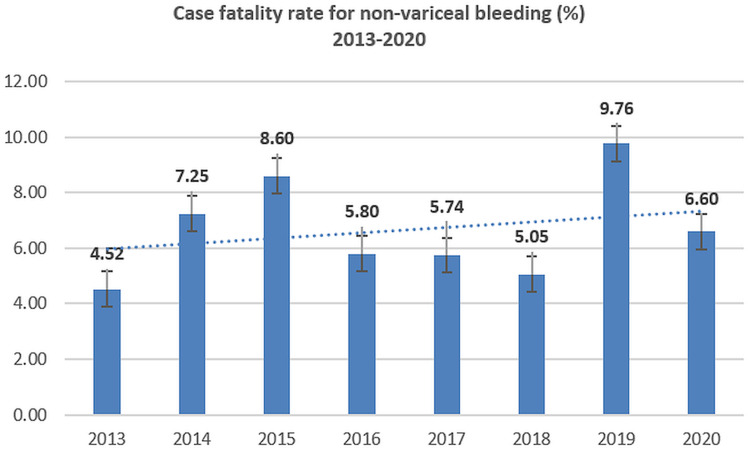
Case fatality ratios for variceal bleeding and peptic ulcer bleeding have declined; however, the mortality for non-variceal bleeding has slightly increased during 2013–2020 (Figures 5–8).
Figure 5.
Case-fatality rate for variceal bleeding (2013–2020).
Figure 6.
Case-fatality rate for peptic ulcer bleeding (2013–2020).
Figure 7.
Case-fatality rate for non-variceal UGIB (2013–2020).
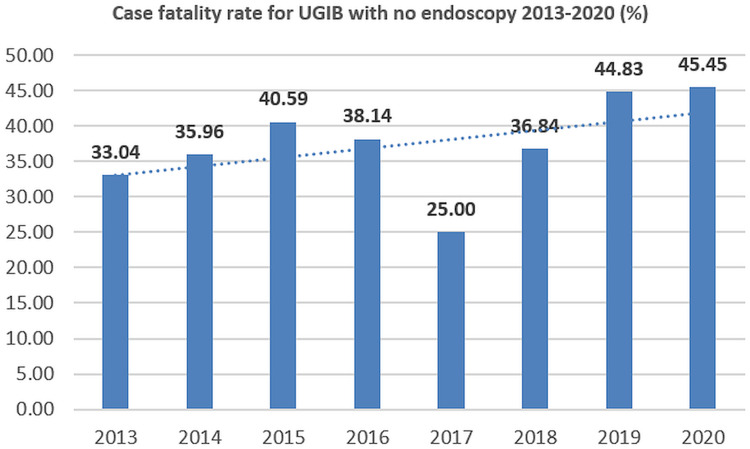
Figure 8.
Case-fatality rate for UGIB with no endoscopy (2013–2020).
The Effect of Admission Time
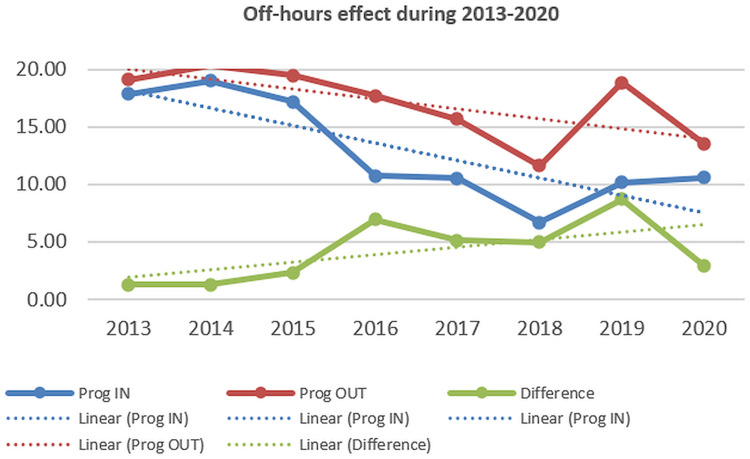
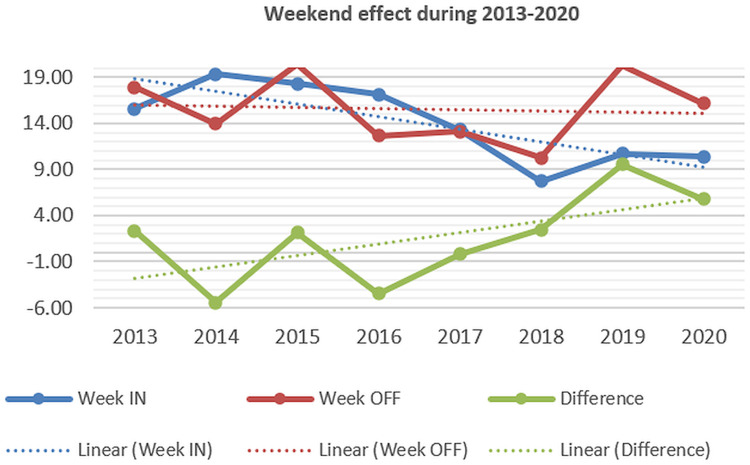
Several studies in the literature have analyzed the potential increased mortality in patients admitted after-hours (off-hours or after-hours effect) and during weekends; the possible explanations were a lower rate of endoscopy after-hours or during weekends because of schedule, decreased quality of medical care because less staff or less experienced staff involved during after-hours and weekends, or more severe cases admitted.28 In our study, we noted decreasing mortality in admissions during both regular hours/after-hours and weekdays/weekends, nevertheless, the differences (off-hours and weekend effects) have increased from 2013 to 2020 (Figures 9–10).
Figure 9.
Mortality in UGIB adjusted by admission during regular hours/after hours (2013–2020).
Figure 10.
Mortality in UGIB adjusted by admission during weekdays/weekends (2013–2020).
Endoscopy-Timing, Percentage, Therapy
41.6% of patients admitted from 2013 to 2016 have performed endoscopy during the first 24 hours compared with 84.7% for those during the 2017–2020 period. The median time between admission and endoscopy was 17.0 hours during 2017–2020 compared to 59.1 hours during 2013–2016 (P<0.0001). The percentage of therapeutic endoscopy has also increased during the 2017–2020 period (23.7% versus 7.3% during the 2013–2016 period, P<0.0001).
Surgery
The proportion of patients who needed emergency surgery for uncontrolled bleeding has declined since 2016, with an average value of 1% in the last 5 years of the study. The extension of endoscopy availability to 8–20 during weekdays and 8–15 during weekends, the possibility of emergency endoscopy until midnight, together with admission of UGIB to Gastroenterology instead of the Surgery Department may have contributed to this decline.
Discussions
Admissions for UGIB have increased from 2013 to 2019, mostly related to the increased availability of emergency endoscopy in our center. In 2020 a decreased admission for UGIB was attributed to the beginning of the COVID-19 pandemic, with multiple causes (lockdown imposed during March-May 2020, “fear effect” related to the hospital visits and admissions, the need for triage and dedicated spaces).29–32
The etiology of UGIB has only limited changes during the 2013–2020 period, mainly related to an increased proportion of angiodysplasia and Mallory-Weiss syndrome. Non-variceal bleeding was the most frequent form, and peptic ulcer represented the first cause of non-variceal bleeding during the analyzed period, with no significant variation during 2013–2020. Although the prevalence of peptic ulcer has decreased during the last decades as a result of proton pump inhibitor treatment and H. pylori eradication; the prevalence of peptic ulcer bleeding was stable because of the aging population, frequent use of NSAID and also antithrombotic therapy.29,31,33 Erosive diseases (esophagitis, gastritis, and duodenitis) accounted for 12.9% of UGIB during 2013–2016 and 15.4% during 2017–2020; tumors represent the third cause of non-variceal bleeding, with 5.8% of UGIB during both the 2013–2016 and 2017–2020 period; most cases were gastric adenocarcinoma, but esophageal carcinoma, GIST, or gastric polyps can also be associated with a risk of UGIB.34 Mallory-Weiss syndrome has increased during 2017–2020, while antithrombotic therapy and angiodysplasia/Dieulafoy lesions have represented the fifth and sixth cause of UGIB. We noted a 3-fold reduction in unknown etiology bleeding and a 2-fold reduction in obscure bleeding; the main explanation was related to the increased accessibility to endoscopy during after-hours and weekends and better investigation of obscure cases of UGIB including colonoscopy, capsule or balloon-aided enteroscopy. UGIB with no endoscopy performed has a higher mortality rate compared to both variceal and non-variceal bleeding with endoscopy performed,30 because of the lack of endoscopic therapy.
Mortality rates progressively decreased during 2013–2020, with lower mortality in the second half. The reduction was mainly induced by a reduction in case fatality rate for variceal bleeding and PUB, while for patients with no endoscopy performed a slightly increased mortality trend was counterbalanced by a superior decrease in the number of cases (because of higher accessibility at emergency endoscopy). Studies in the literature are contradictory; a study in Canada from 1993–2003 found a stable mortality of 3–3.5% during the period,35 while a decrease was noted in several studies in the UK,36–39 and also in Scotland.40 A study in Turkey found that mortality for nonvariceal UGIB doubled in 2015–2016 as compared with 1993–1995 (6% versus 3% during 1993–1995, P=0.06).41 Some longitudinal studies have shown that the mortality rate has decreased,42,43 with an increased proportion of neoplasms, angiodysplasia, Dieulafoy lesions, and esophagitis as the causes of UGIB.42 In the USA, a longitudinal study from 2012 to 2021 has shown a slightly increased mortality over time, partially explained by the Covid-19 pandemic;44 another study in Finland has shown a declining trend for mortality in men (which remains between 5–10%) and a stable trend of fatality in women.45
Despite significant progress in the endoscopic and pharmacological management of UGIB during the last decades, the downward trend for mortality in UGIB was very slow. Age and comorbidities represent the main factors for mortality, and increasing the use of anti-thrombotic therapy can be a significant factor in increasing the prevalence of UGIB. Aging population together with increased consumption of NSAID and anti-thrombotic drugs can slow down or even stop the decline of mortality in UGIB. In our study, the mean age was similar during 2017–2020 compared to 2013–2016 (62.6±13.9 versus 62.9±13.9 years, P-value=0.5724). Data for NSAID and AT use was available only for the 2017–2020 period and was published in one of our previously published papers;29 no differences were seen between NSAID use but a significant increase in AT drugs was noted, which may be an explanation for increased admissions during the 2017–2020 period.
The assessment of severity variations during 2013–2020 was contradictory; the Charlson comorbidity index was not significantly changed during the whole analyzed periods, but a slightly increasing trend for both pre-endoscopic and after-endoscopy Baylor scores was recorded, which may suggest a potential trend toward more severe UGIB admitted cases.
The “weekend effect” (increased mortality in patients admitted during weekends) was noted in patients with UGIB in several studies,39,40,46–49 although other studies have shown no difference.50–52 Some meta-analyses were available; higher mortality was noted for weekend admissions,53–56 but only non-variceal UGIB had a significant weekend effect in some meta-analyses.53–55 Some studies have shown a more pronounced effect in European hospitals.56 For off-hours admissions, higher mortality was also noted in a systematic review and meta-analysis in non-variceal UGIB.57 In our study both off-hours and weekend effects were observed; although the mortality has decreased in both after-hours/regular hours and weekdays/weekend admitted patients, the intensity of off-hour and weekend effects have increased, which suggests that medical care and endoscopic therapy improvements were more significant during regular hours and weekdays.
A significant improvement in endoscopy and endoscopic therapy was noted during the 2017–2020 period, with an increasing percentage of total and therapeutic endoscopies, procedures performed during the first 24 hours, and the median time between admission and endoscopy. The improvement in endoscopy may represent the main reason for improved mortality, although improved care of patients with UGIB may also have an important role. A significantly lower percentage of patients needed surgery for uncontrollable UGIB during 2017–2020 compared to 2013–2016 (OR=5.0803, 95% CI 2.8711 to 8.9896, P<0.0001). This finding was consistent with international data35,49–61 and studies from Romania.59 Mortality rates of 10–30% in patients with emergency surgery for UGIB have been noted;62–64 improvements in mortality for surgical cases were however observed.63,65
Conclusions
The temporal trend for UGIB was marked by increased admissions between 2013 and 2019, with a decrease in 2020. The etiology of UGIB was stable, but the mortality rates progressively decreased, with a decreasing case fatality for variceal bleeding and PUB. Significant improvements in endoscopy and endoscopic therapy were noted during 2017–2020. Less than 1% of patients needed surgery for uncontrollable UGIB during 2017–2020.
Funding Statement
There is no funding to report.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the EMERGENCY HOSPITAL OF CRAIOVA (protocol code 11977/24.03.2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
References
- 1.Kim BS, Li BT, Engel A, et al. Diagnosis of gastrointestinal bleeding: a practical guide for clinicians. World J Gastrointest Pathophysiol. 2014;5(4):467–478. doi: 10.4291/wjgp.v5.i4.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18(11):1202–1207. doi: 10.3748/wjg.v18.i11.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masoodi M, Saberifiroozi M. Etiology and outcome of acute gastrointestinal bleeding in Iran: a review article. Middle East J Dig Dis. 2012;4(4):193–198. [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Encinosa W.Hospitalizations for gastrointestinal bleeding in 1998 and 2006: statistical brief #65.In:Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet].Rockville (MD):Agency for Healthcare Research and Quality (US);2008.Dec.Available from.http://www.ncbi.nlm.nih.gov/books/NBK54562/. [PubMed] [Google Scholar]
- 5.Estevinho MM, Pinho R, Fernandes C, et al. Diagnostic and therapeutic yields of early capsule endoscopy and device-assisted enteroscopy in the setting of overt GI bleeding: a systematic review with meta-analysis. Gastrointest Endosc. 2022;95(4):610–625.e9. doi: 10.1016/j.gie.2021.12.009 Epub 2021 Dec 21. PMID: 34952093. [DOI] [PubMed] [Google Scholar]
- 6.Vere CC, Foarfă C, Streba CT, Cazacu S, Pârvu D, Ciurea T. Videocapsule endoscopy and single balloon enteroscopy: novel diagnostic techniques in small bowel pathology. Rom J Morphol Embryol. 2009;50(3):467–474. PMID: 19690776. [PubMed] [Google Scholar]
- 7.Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterol. 2009;9:41. doi: 10.1186/1471-230X-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheasgreen C, Leontiadis GI. Recent advances on the management of patients with non-variceal upper gastrointestinal bleeding. Ann Gastroenterol. 2013;26(3):191–197. [PMC free article] [PubMed] [Google Scholar]
- 9.Jairath V, Martel M, Logan RF, Barkun AN. Why do mortality rates for nonvariceal upper gastrointestinal bleeding differ around the world? A systematic review of cohort studies. Can J Gastroenterol. 2012;26(8):537–543. doi: 10.1155/2012/862905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley AJ. Update on risk scoring systems for patients with upper gastrointestinal haemorrhage. World J Gastroenterol. 2012;18(22):2739–2744. doi: 10.3748/wjg.v18.i22.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thandassery RB, Sharma M, John AK, et al. Clinical application of AIMS65 scores to predict outcomes in patients with upper gastrointestinal hemorrhage. Clin Endosc. 2015;48(5):380–384. doi: 10.5946/ce.2015.48.5.380 Epub 2015 Sep 30. PMID: 26473120; PMCID: PMC4604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youn YH, Park YJ, Kim JH, Jeon TJ, Cho JH, Park H. Weekend and nighttime effect on the prognosis of peptic ulcer bleeding. World J Gastroenterol. 2012;18(27):3578–3584. doi: 10.3748/wjg.v18.i27.3578 PMID: 22826623; PMCID: PMC3400860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn DW, Park YS, Lee SH, et al. Clinical outcome of acute nonvariceal upper gastrointestinal bleeding after hours: the role of urgent endoscopy. Korean J Intern Med. 2016;31(3):470–478. doi: 10.3904/kjim.2014.099 Epub 2016 Apr 6. PMID: 27048253; PMCID: PMC4855084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nahon S, Hagège H, Latrive JP, et al. (Groupe des Hémorragies Digestives Hautes de l’ANGH). Epidemiological and prognostic factors involved in upper gastrointestinal bleeding: results of a French prospective multicenter study. Endoscopy. 2012;44(11):998–1008. doi: 10.1055/s-0032-1310006 Epub 2012 Oct 29. PMID: 23108771. [DOI] [PubMed] [Google Scholar]
- 15.Biecker E. Diagnosis and therapy of non-variceal upper gastrointestinal bleeding. World J Gastrointest Pharmacol Ther. 2015;6(4):172–182. doi: 10.4292/wjgpt.v6.i4.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trawick EP, Yachimski PS. Management of non-variceal upper gastrointestinal tract hemorrhage: controversies and areas of uncertainty. World J Gastroenterol. 2012;18(11):1159–1165. doi: 10.3748/wjg.v18.11.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota Y, Yamauchi H, Nakatani K, et al. Factors for unsuccessful endoscopic hemostasis in patients with severe peptic ulcer bleeding. Scand J Gastroenterol. 2021;56(12):1396–1405. doi: 10.1080/00365521.2021.1969593 Epub 2021 Aug 29. PMID: 34455892. [DOI] [PubMed] [Google Scholar]
- 18.Jafar W, Jafar AJN, Sharma A. Upper gastrointestinal haemorrhage: an update. Frontline Gastroenterol. 2016;7(1):32–40. doi: 10.1136/flgastro-2014-100492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta R, Nageshwar Reddy D. Upper GI bleeding - has mortality changed with advancements in therapy? Trop Gastroenterol. 2013;34(1):5–6. doi: 10.7869/tg.2012.83 PMID: 23923367. [DOI] [PubMed] [Google Scholar]
- 20.Voicu MN, Popescu F, Florescu DN, et al. Clostridioides difficile infection among cirrhotic patients with variceal bleeding. Antibiotics. 2021;10(6):731. doi: 10.3390/antibiotics10060731 PMID: 34204307; PMCID: PMC8233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim LG, Ho KY, Chan YH, et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy. 2011;43(4):300–306. doi: 10.1055/s-0030-1256110 [DOI] [PubMed] [Google Scholar]
- 22.Gralnek IM, Stanley AJ, Morris AJ, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European society of gastrointestinal endoscopy (ESGE) guideline - update 2021. Endoscopy. 2021;53(3):300–332. doi: 10.1055/a-1369-5274 Epub 2021 Feb 10. PMID: 33567467. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y, Loffroy R, Lau JY, Barkun A. Multidisciplinary management strategies for acute non-variceal upper gastrointestinal bleeding. Br J Surg. 2014;101(1):e34–50. doi: 10.1002/bjs.9351 [DOI] [PubMed] [Google Scholar]
- 24.Sung JJ, Chiu PW, Chan FKL, et al. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67(10):1757–1768. doi: 10.1136/gutjnl-2018-316276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimi Bakhtavar H, Morteza Bagi HR, Rahmani F, Shahsavari Nia K, Ettehadi A. Clinical scoring systems in predicting the outcome of acute upper gastrointestinal bleeding; a narrative review. Emerg. 2017;5(1):e36. [PMC free article] [PubMed] [Google Scholar]
- 26.Monteiro S, Gonçalves TC, Magalhães J, Cotter J. Upper gastrointestinal bleeding risk scores: who, when and why? World J Gastrointest Pathophysiol. 2016;7(1):86–96. doi: 10.4291/wjgp.v7.i1.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cazacu SM, Burtea DE, Iovănescu VF, et al. Outcomes in patients admitted for upper gastrointestinal bleeding and COVID-19 infection: a study of two years of the pandemic. Life. 2023;13(4):890. doi: 10.3390/life13040890 PMID: 37109419; PMCID: PMC10146262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433 Epub 2011 Feb 17. PMID: 21330339. [DOI] [PubMed] [Google Scholar]
- 29.Popa P, Iordache S, Florescu DN, et al. Mortality rate in upper gastrointestinal bleeding associated with anti-thrombotic therapy before and during Covid-19 pandemic. J Multidiscip Healthc. 2022;15:2679–2692. PMID: 36425876; PMCID: PMC9680964. doi: 10.2147/JMDH.S380500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazacu SM, Alexandru DO, Statie RC, et al. The accuracy of pre-endoscopic scores for mortality prediction in patients with upper GI bleeding and no endoscopy performed. Diagnostics. 2023;13(6):1188. doi: 10.3390/diagnostics13061188 PMID: 36980496; PMCID: PMC10047350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cazacu SM, Turcu-Stiolica A, Florescu DN, et al. The reduction of after-hours and weekend effects in upper gastro-intestinal bleeding mortality during the COVID-19 pandemic compared to the pre-pandemic period. J Multidiscip Healthc. 2023;16:3151–3165. PMID: 37908341; PMCID: PMC10615097. doi: 10.2147/JMDH.S427449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marginean CM, Popescu M, Vasile CM, et al. Challenges in the differential diagnosis of COVID-19 pneumonia: a pictorial review. Diagnostics. 2022;12(11):2823. doi: 10.3390/diagnostics12112823 PMID: 36428883; PMCID: PMC9689132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cazacu SM, Surlin VM, Rogoveanu I, et al. Trends for admission and mortality in peptic ulcers at a tertiary referral hospital during the 2017-2021 period. Chirurgia. 2024;119(4):404–416. doi: 10.21614/chirurgia.2923 PMID: 39250610. [DOI] [PubMed] [Google Scholar]
- 34.Barbu LA, Mărgăritescu ND, Ghiluşi MC, et al. Severe upper gastrointestinal bleeding from gastrointestinal stromal tumor of the stomach. Rom J Morphol Embryol. 2016;57(4):1397–1401. PMID: 28174810. [PubMed] [Google Scholar]
- 35.Targownik LE, Nabalamba A. Trends in management and outcomes of acute nonvariceal upper gastrointestinal bleeding: 1993-2003. Clin Gastroenterol Hepatol. 2006;(12):1459–1466. doi: 10.1016/j.cgh.2006.08.018 [DOI] [PubMed] [Google Scholar]
- 36.Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59(8):1022–1029. doi: 10.1136/gut.2008.174599 Epub 2010 Mar 31. PMID: 20357318. [DOI] [PubMed] [Google Scholar]
- 37.Nedjat-Shokouhi B, Glynn M, Denton ERE, Greenfield SM. Provision of out-of-hours services for acute upper gastrointestinal bleeding in England: results of the 2014-2015 BSG/NHS England national survey. Frontline Gastroenterol. 2017;8(1):8–12. doi: 10.1136/flgastro-2016-100706 Epub 2016 Jul 21. PMID: 28839878; PMCID: PMC5369431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shokouhi BN, Khan M, Carter MJ, et al. The setting up and running of a cross-county out-of-hours gastrointestinal bleed service: a possible blueprint for the future. Frontline Gastroenterol. 2013;4(3):227–231. doi: 10.1136/flgastro-2012-100243 Epub 2012 Nov 12. PMID: 28839729; PMCID: PMC5369799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crooks C, Card T, West J. Reductions in 28-day mortality following hospital admission for upper gastrointestinal hemorrhage. Gastroenterology. 2011;141(1):62–70. doi: 10.1053/j.gastro.2011.03.048 Epub 2011 Mar 27. PMID: 21447331; PMCID: PMC3194090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed A, Armstrong M, Robertson I, Morris AJ, Blatchford O, Stanley AJ. Upper gastrointestinal bleeding in Scotland 2000-2010: improved outcomes but a significant weekend effect. World J Gastroenterol. 2015;21(38):10890–10897. doi: 10.3748/wjg.v21.i38.10890 PMID: 26478680; PMCID: PMC4600590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danış N, Tekin F, Akarca US, et al. Changing patterns of upper gastrointestinal bleeding over 23 years in Turkey. Turk J Gastroenterol. 2019;30(10):877–882. doi: 10.5152/tjg.2019.19239 PMID: 31258140; PMCID: PMC6812949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci. 2018;63(5):1286–1293. doi: 10.1007/s10620-017-4882-6 Epub 2017 Dec 27. PMID: 29282637. [DOI] [PubMed] [Google Scholar]
- 43.Taefi A, Cho WK, Nouraie M. Decreasing trend of upper gastrointestinal bleeding mortality risk over three decades. Dig Dis Sci. 2013;58(10):2940–2948. doi: 10.1007/s10620-013-2765-z Epub 2013 Jul 5. PMID: 23828142. [DOI] [PubMed] [Google Scholar]
- 44.Merza N, Masoud AT, Ahmed Z, Dahiya DS, Nawras A, Kobeissy A. Trends of upper gastrointestinal bleeding mortality in the United States before and during the COVID-19 era: estimates from the centers for disease control WONDER database. Gastroenterol Res. 2023;16(3):165–170. doi: 10.14740/gr1626 Epub 2023 Jun 11. PMID: 37351079; PMCID: PMC10284642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vora P, Pietila A, Peltonen M, Brobert G, Salomaa V. Thirty-year incidence and mortality trends in upper and lower gastrointestinal bleeding in Finland. JAMA Network Open. 2020;3(10):e2020172. doi: 10.1001/jamanetworkopen.2020.20172 PMID: 33034641; PMCID: PMC7547368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dorn SD, Shah ND, Berg BP, Naessens JM. Effect of weekend hospital admission on gastrointestinal hemorrhage outcomes. Dig Dis Sci. 2010;55(6):1658–1666. doi: 10.1007/s10620-009-0914-1 Epub 2009 Aug 12. PMID: 19672711. [DOI] [PubMed] [Google Scholar]
- 47.Shaheen AA, Kaplan GG, Myers RP. Weekend versus weekday admission and mortality from gastrointestinal hemorrhage caused by peptic ulcer disease. Clin Gastroenterol Hepatol. 2009;7(3):303–310. doi: 10.1016/j.cgh.2008.08.033 Epub 2008 Sep 3. PMID: 18849015. [DOI] [PubMed] [Google Scholar]
- 48.Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296–302e1. doi: 10.1016/j.cgh.2008.08.013 Epub 2008 Aug 19. PMID: 19084483. [DOI] [PubMed] [Google Scholar]
- 49.Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663–668. doi: 10.1056/NEJMsa003376 PMID: 11547721. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Han B, Li H, et al. Effect of admission time on the outcomes of liver cirrhosis with acute upper gastrointestinal bleeding: regular hours versus off-hours admission. Can J Gastroenterol Hepatol. 2018;2018:3541365. PMID: 30631756; PMCID: PMC6304553. doi: 10.1155/2018/3541365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jairath V, Kahan BC, Logan RF, et al. Mortality from acute upper gastrointestinal bleeding in the United Kingdom: does it display a”weekend effect”? Am J Gastroenterol. 2011;106(9):1621–1628. doi: 10.1038/ajg.2011.172 Epub 2011 May 24. PMID: 21606977. [DOI] [PubMed] [Google Scholar]
- 52.Abougergi MS, Travis AC, Saltzman JR. Impact of day of admission on mortality and other outcomes in upper GI hemorrhage: a nationwide analysis. Gastrointest Endosc. 2014;80(2):228–235. doi: 10.1016/j.gie.2014.01.043 Epub 2014 Mar 25. PMID: 24674354. [DOI] [PubMed] [Google Scholar]
- 53.Shih PC, Liu SJ, Li ST, Chiu AC, Wang PC, Liu LY. Weekend effect in upper gastrointestinal bleeding: a systematic review and meta-analysis. PeerJ. 2018;6:e4248. doi: 10.7717/peerj.4248 eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A, Agarwal R, Ananthakrishnan AN. ”Weekend effect” in patients with upper gastrointestinal hemorrhage: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113(1):13–21. doi: 10.1038/ajg.2017.430 Epub 2017 Nov 14. PMID: 29134968. [DOI] [PubMed] [Google Scholar]
- 55.Weeda ER, Nicoll BS, Coleman CI, Sharovetskaya A, Baker WL. Association between weekend admission and mortality for upper gastrointestinal hemorrhage: an observational study and meta-analysis. Intern Emerg Med. 2017;12(2):163–169. doi: 10.1007/s11739-016-1522-7 Epub 2016 Aug 17. PMID: 27534406. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Hao D, Liu W, Wang L, Wang X. Does weekend hospital admission affect upper gastrointestinal hemorrhage outcomes?: a systematic review and network meta-analysis. J Clin Gastroenterol. 2020;54(1):55–62. doi: 10.1097/MCG.0000000000001116 PMID: 30119093. [DOI] [PubMed] [Google Scholar]
- 57.Xia XF, Chiu PWY, Tsoi KKF, Chan FKL, Sung JJY, Lau JYW. The effect of off-hours hospital admission on mortality and clinical outcomes for patients with upper gastrointestinal hemorrhage: a systematic review and meta-analysis of 20 cohorts. United Eur Gastroenterol J. 2018;6(3):367–381. doi: 10.1177/2050640617732651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haas JM, Gundrum JD, Rathgaber SW. Comparison of time to endoscopy and outcome between weekend/weekday hospital admissions in patients with upper GI hemorrhage. WMJ. 2012;111(4):161–165. PMID: 22970530. [PubMed] [Google Scholar]
- 59.Botianu A, Matei D, Tantau M, Acalovschi M. Mortality and need of surgical treatment in acute upper gastrointestinal bleeding: a one year study in a tertiary center with a 24 hours / day-7 days/week endoscopy call. Has anything changed? Chirurgia. 2013;108(3):312–318. PMID: 23790778. [PubMed] [Google Scholar]
- 60.Tarasconi A, Coccolini F, Biffl WL, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. 2020;15:3. PMID: 31921329; PMCID: PMC6947898. doi: 10.1186/s13017-019-0283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morales Uribe CH, Sierra Sierra S, Hernández Hernández AM, Arango Durango AF, López GA. Upper gastrointestinal bleeding: risk factors for mortality in two urban centers in Latin America. Rev Esp Enferm Dig. 2011;103(1):20–24. doi: 10.4321/s1130-01082011000100004 PMID: 21341933. [DOI] [PubMed] [Google Scholar]
- 62.Hsu E, Law SD. Refractory nonvariceal upper gastrointestinal bleeding requiring surgical intervention. Cureus. 2019;11(2):e4135. doi: 10.7759/cureus.4135 PMID: 31058018; PMCID: PMC6485538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke MG, Bunting D, Smart NJ, Lowes J, Mitchell SJ. The surgical management of acute upper gastrointestinal bleeding: a 12-year experience. Int J Surg. 2010;8(5):377–380. doi: 10.1016/j.ijsu.2010.05.008 Epub 2010 Jun 9. PMID: 20538082. [DOI] [PubMed] [Google Scholar]
- 64.Langner I, Langner S, Partecke LI, et al. Acute upper gastrointestinal hemorrhage: is a radiological interventional approach an alternative to emergency surgery? Emerg Radiol. 2008;15(6):413–419. doi: 10.1007/s10140-008-0736-z Epub 2008 May 30. PMID: 18512090. [DOI] [PubMed] [Google Scholar]
- 65.Laohathai S, Jaroensuk J, Laohathai S, Laohavinij W. Impact of acute care surgery model in aspects of patients with upper gastrointestinal hemorrhage: result from a single tertiary care center in Thailand. Trauma Surg Acute Care Open. 2021;6(1):e000570. doi: 10.1136/tsaco-2020-000570 PMID: 33748427; PMCID: PMC7934772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.