Abstract
Purpose
Glioblastoma (GBM) is associated with metabolic disturbances, yet the relationships between metabolites with GBM have not been comprehensively explored. This study aims to fill this gap by integrating Mendelian randomization (MR) analysis with clinical validation.
Patients and Methods
Summary data from genome-wide association study (GWAS) of cerebrospinal fluid (CSF) metabolites, plasma metabolites, and GBM were obtained separately. A total of 338 CSF metabolites and 1400 plasma metabolites were utilized as exposures. Concurrently, GBM was designated as the outcome. A two-sample bidirectional MR study was conducted to investigate the potential association. The inverse variance weighted (IVW) analyses were conducted as causal estimates, accompanied by a series of sensitivity analyses to evaluate the robustness of the results. Additionally, metabolite levels in clinical plasma and CSF samples were quantified using liquid chromatography-mass spectrometry to validate the findings.
Results
MR analysis identified eight CSF metabolites and six plasma metabolites that were closely associated with GBM. Among these, elevated levels of 7-alpha-hydroxy-3-oxo-4-cholestenoate (7-HOCA) in both CSF and plasma were found to promote GBM. In terms of clinical validation, compared to the control group, 7-HOCA levels were significantly higher in both the CSF and plasma of GBM group.
Conclusion
This study provides a comprehensive analysis of the metabolic factors contributing to GBM. The identification of specific metabolites, particularly 7-HOCA, that have vital roles in GBM pathogenesis suggests new biomarkers and therapeutic targets, offering potential pathways for improved diagnosis and treatment of GBM.
Keywords: 7-alpha-hydroxy-3-oxo-4-cholestenoate, metabolite, glioblastoma, cerebrospinal fluid, Mendelian randomization, plasma
Introduction
Gliomas, particularly glioblastoma (GBM), represent the most common and aggressive form of malignant primary brain tumors within the central nervous system.1 Despite advancements in therapeutic strategies, the median survival time for GBM patients remains under 15 months, with a 5-year survival rate below 10%, primarily due to the tumor’s heterogeneity and resistance to existing treatment modalities.2 Therefore, the exploration of etiological factors and biomarkers are crucial for early diagnosis and targeted therapy of GBM patients. For instance, the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) promoter, as well as the isocitrate dehydrogenase (IDH) mutations have been demonstrated to correlate with the prognosis of glioma patients.3,4
With the rapid advancement of metabolomics, an increasing number of studies have confirmed that metabolic dysregulation is a significant cause of neurological diseases. For example, studies in both rats and humans have found that the accumulation of methylmalonic acidemia can induce epileptic seizures.5,6 β-amyloid (Aβ) and Tau are considered classic biomarkers for the diagnosis of Alzheimer’s disease (AD).7 Yan et al found that cerebrospinal fluid (CSF) metabolites in the tryptophan-kynurenine pathway are effective biomarkers for acute neuroinflammation.8 Sun et al discovered that levels of homocysteine can predict cognitive dysfunction following ischemic stroke.9 Regarding gliomas, research has revealed that the levels of D-2-hydroxyglutarate in the CSF of patients with IDH-mutant gliomas are significantly elevated compared to those with IDH wild-type gliomas. This finding aids in the pathological grading and prognostic assessment of gliomas.10 Additionally, glycolysis, glutamine metabolism, and lipid metabolism have been demonstrated to facilitate the proliferation of gliomas and can serve as therapeutic targets for glioma treatment.11
Plasma metabolites, as the end products of cellular activities, mirror the evolving pathophysiological condition of GBM, thus offering potential insights into novel aspects of its biology.12 Concurrently, the CSF provides a unique perspective on the internal milieu of the brain, potentially unlocking vital information on metabolic alterations specifically relevant to neurologic diseases. Due to its close connection with the extracellular space of the brain, analysis of CSF metabolomics may provide a more immediate assessment of the biochemical milieu influencing the advancement of GBM.13 Studies have found that undergoing a biopsy before surgical resection is associated with worse prognosis in patients with GBM. In this context, liquid biopsies, plasma or CSF studies may offer a potential solution.14,15 The relationship between plasma and CSF metabolites is complex and influenced by the blood-brain barrier (BBB). Some metabolites can cross the BBB, while others cannot, leading to distinct but sometimes correlated profiles between plasma and CSF.
To capitalize on the revelations provided by metabolomics, incorporating genomic data from Genome Wide Association Studies (GWAS) is crucial. GWAS enables the identification of genetic variations that affect susceptibility and progression of diseases, including GBM.16,17 By amalgamating GWAS outcomes with the metabolic alterations observed in both plasma and CSF, a more comprehensive understanding of GBM’s pathogenesis can be achieved. Employing this combined methodology, Mendelian Randomization (MR) analysis emerges as a powerful mechanism for inferring causality from observational data.18 MR leverages genetic variants as instrumental variables (IVs) to delineate the causative pathways between metabolites in plasma or CSF and GBM, thus circumventing the confounding factors frequently encountered in observational research.
This study is dedicated to investigating plasma metabolites and CSF metabolites closely associated with GBM. By employing the MR analysis to identify metabolites significantly related to GBM, the aim is to comprehend their biological significance and their potential as biomarkers. Furthermore, this study conducts external validation of the MR analysis by measuring the levels of metabolites in the plasma and CSF of patients with GBM.
Materials and Methods
Study Design
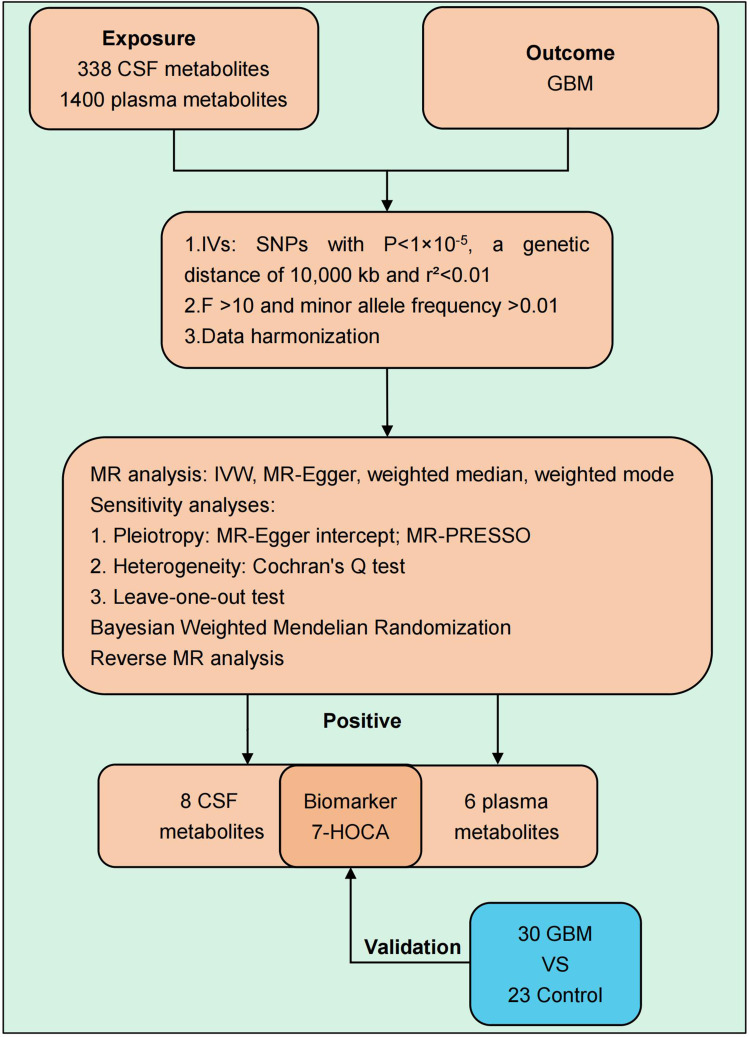
Our investigation employed a two-sample MR approach to separately evaluate the potential associations of 338 CSF metabolites and 1400 plasma metabolites with GBM. The research framework is depicted in Figure 1.
Figure 1.
The flow chart of this study.
We utilized GWAS summary data for CSF metabolites, plasma metabolites, and GBM sourced from extant literature. The GWAS Catalog provides a repository for extensive GWAS summary statistics (https://www.ebi.ac.uk/gwas/home). All study subjects in GWAS were of European ancestry. To ensure the reliability of the results, it is imperative that three critical criteria are satisfied: (1) The IVs must exhibit a strong association with the exposure. (2) The IVs should not be associated with any confounders that could influence both the exposure and the outcome. (3) The IVs should influence the outcome solely through their effect on the exposure.19
GWAS Data on Exposures
The primary dataset on CSF metabolites was procured from two distinct longitudinal studies focused on AD: the Wisconsin AD Research Center and the Wisconsin Registry for AD Prevention. Both studies underwent rigorous quality control processes for the CSF metabolite and genotype information. The refined dataset encompasses 338 CSF metabolites derived from 291 normal individuals of European descent. Out of the 338 metabolites analyzed, 39 remain unidentified due to unresolved chemical properties. The remaining 299 metabolites have been chemically characterized and categorized into eight principal metabolic clusters: amino acids, carbohydrates, cofactors and vitamins, energy substrates, lipids, nucleotides, peptides, and xenobiotic metabolism,20(Table S1). The primary plasma metabolite dataset was obtained from a large-scale GWAS of 8299 unrelated European participants in the Canadian Longitudinal Study of Aging. These individuals underwent genome-wide genotyping and plasma metabolite measurement, resulting in 1091 metabolite concentrations and 309 concentration ratios21 (Table S2).
GWAS Data on Outcome
The GBM dataset was sourced from the FinnGen database (https://www.finngen.fi). This dataset, derived from a comprehensive GWAS, involved 253 individuals with GBM and 314,193 controls of European descent, analyzing approximately 19.34 million genetic variants.
Identification of IVs
To identify appropriate IVs, we screened single nucleotide polymorphisms (SNPs) that exhibited a strong association with the respective metabolite exposures, adhering to a significance threshold of p < 1×10.−522 To ensure the independence of the IVs, we performed linkage disequilibrium (LD) clumping with parameters set to r2 = 0.01 and a clumping distance of 10,000 kb. SNPs showing a significant link to the outcome at a threshold of p < 5×10−5 were excluded from consideration as IVs.
Additionally, Phenoscanner was used to eliminate confounding factors (http://www.phenoscanner.medschl.cam.ac.uk/). If a particular SNP exhibits a close association with a confounding factor, it was excluded from the analysis. Steiger filtering was applied to reduce the risk of reverse causation. Harmonization of SNPs was also carried out to standardize the effect alleles, ensuring that they were consistent across both exposure and outcome datasets. Finally, to ensure that the IVs were robust, only SNPs with an F-statistic >10 were selected.23,24
Bidirectional MR Analysis
To conduct the bidirectional MR analysis, our study incorporated four methodologies: inverse variance weighted (IVW), weighted median, weighted mode, and MR-Egger. The foundational MR outcomes were ascertained utilizing the IVW method. To evaluate the consistency of the MR result, we executed an extensive array of sensitivity examinations. These included Cochran’s Q test to detect heterogeneity, the Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) test for outlier detection and adjustment, the MR-Egger intercept for pleiotropy assessment, and the leave-one-out approach for individual variant influence analysis. After applying the MR-PRESSO Global test, SNPs classified as outliers were systematically excluded until the global test’s p-value exceeded the threshold of 0.05.25 Furthermore, a reverse MR analysis was conducted using the same methodology to examine potential bidirectional causal relationships.
Bayesian Weighted MR (BWMR) Analysis
BWMR analysis serves as a complement to two-sample MR analysis, employing statistical methods to evaluate potential causal relationships within observational data, notably in epidemiological studies. By integrating Bayesian techniques and the principles of MR, BWMR addresses uncertainties and biases more effectively, thereby enhancing precision and robustness in estimating causal effects compared to conventional approaches.26 In this study, when performing BWMR analysis on previously identified results, causal relationships with p < 0.05 were considered more robust and reliable.
External Validation
Patient specimens were obtained from the Fourth Hospital of Hebei Medical University, collected from June 2022 to March 2024. Plasma and CSF metabolites were analyzed in 30 patients with GBM (IDH wild-type). A control group of 23 patients who presented with headaches but had negative examination results was included. The control group underwent brain CT or MRI scans, CSF analysis, and plasma parameter assessments, all of which showed no abnormalities. After removal from the body, the specimens were centrifuged, and the supernatant was immediately stored at −80°C. Metabolite quantification was conducted using liquid chromatography-mass spectrometry (LC-MS).
Statistical Analysis
MR analyses were performed using R (version 4.3.0), packages “Two Sample MR” (version 0.5.6), “MR-PRESSO” (version 1.0) and “ggplot2” (version 3.3.6). To compare the levels of metabolite between the experimental group and the control group, we employed the Independent Samples t-test. A p-value of less than 0.05 was considered statistically significant. The statistical analyses were performed using SPSS software (version 26.0).
Results
Associations Between CSF Metabolites and GBM
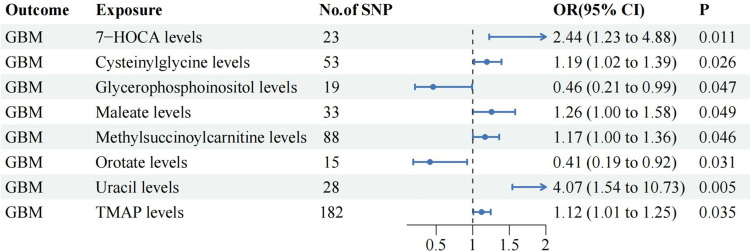
The IVW analysis identified six CSF metabolites that were potentially associated with an increased risk of GBM, namely, 7-alpha-hydroxy-3-oxo-4-cholestenoate (7-HOCA) (OR: 2.44, 95% CI 1.23–4.88, P=0.011), Cysteinylglycine (OR: 1.19, 95% CI 1.02–1.39, P=0.026), Maleate (OR: 1.26, 95% CI 1.00–1.58, P=0.049), Methylsuccinoylcarnitine (OR: 1.17, 95% CI 1.00–1.36, P=0.046), Uracil (OR: 4.07, 95% CI 1.54–10.73, P=0.005), N,n,n-trimethyl-alanylproline betaine (TMAP) (OR: 1.12, 95% CI 1.01–1.25, P=0.035). Meanwhile, three CSF metabolites were potentially associated with a decreased risk of GBM, including Phenyllactate (OR: 0.84, 95% CI 0.71–0.99, P=0.038), Orotate (OR: 0.41, 95% CI 0.19–0.92, P=0.031) and Glycerophosphoinositol (OR: 0.46, 95% CI 0.21–0.99, P=0.047). Additionally, except for Phenyllactate (PBWMR=0.100), all the above relationships were validated by BWMR analysis (PBWMR <0.05). (Figure 2, Table S3)
Figure 2.
The significant relationships between CSF metabolites and GBM.
Associations Between Plasma Metabolites and GBM
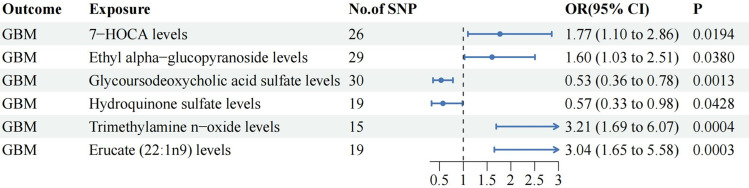
The IVW analysis revealed that five plasma metabolites promote the occurrence of GBM, namely 7-HOCA (OR: 1.77, 95% CI 1.10–2.86, P=0.019), Ethyl alpha-glucopyranoside (OR: 1.60, 95% CI 1.03–2.51, P=0.038), Erucate (22:1n9) (OR: 3.04, 95% CI 1.65–5.58, P=0.0003), Guaiacol sulfate (OR: 1.69, 95% CI 1.00–2.83, P=0.049), and Trimethylamine n-oxide (OR: 3.21, 95% CI 1.69–6.07, P=0.0004). Conversely, three plasma metabolites were found to reduce the risk of GBM, namely Hydroquinone sulfate (OR: 0.57, 95% CI 0.33–0.98, P=0.043), Glycoursodeoxycholic acid sulfate (OR: 0.53, 95% CI 0.36–0.78, P=0.001), and Phenylalanine to phosphate ratio (OR: 0.62, 95% CI 0.38–0.99, P=0.047). The BWMR analysis indicated that, except for Guaiacol sulfate (PBWMR=0.062) and Phenylalanine to phosphate ratio (PBWMR=0.057), the aforementioned results were robust and reliable (PBWMR <0.05). (Figure 3, Table S4)
Figure 3.
The significant relationships between plasma metabolites and GBM.
Sensitive Analysis
Across all metabolic outcomes assessed, MR-Egger analysis did not indicate the presence of horizontal pleiotropy, and Cochran’s Q test found no signs of heterogeneity (P >0.05). MR estimates obtained from the IVW, weighted median, weighted mode, and MR-Egger methods showed a consistent direction of effect, reinforcing the reliability of the potential causal inferences (all beta values > 0 or < 0). Furthermore, the MR-PRESSO test did not identify any significant horizontal directional pleiotropy (Global Test P >0.05) (Table S5). The application of the Steiger test indicated that the potential causal relationships identified in the study are unlikely to be affected by reverse causation (steiger_test_direction=true). The leave-one-out analysis, shown in Figure S1, demonstrated that the overall range of the estimates remained consistent and did not cross 0, indicating the robustness of the findings. Upon manual inspection of the SNPs, it was determined that none of the SNPs were closely linked to the confounding factors.27
Reverse MR Analysis
Using the same threshold, a reverse MR analysis was conducted with eight CSF metabolites and six plasma metabolites as exposures, and GBM as the outcome. The results showed insufficient SNPs for the analysis, suggesting reverse causality is unlikely.
External Validation
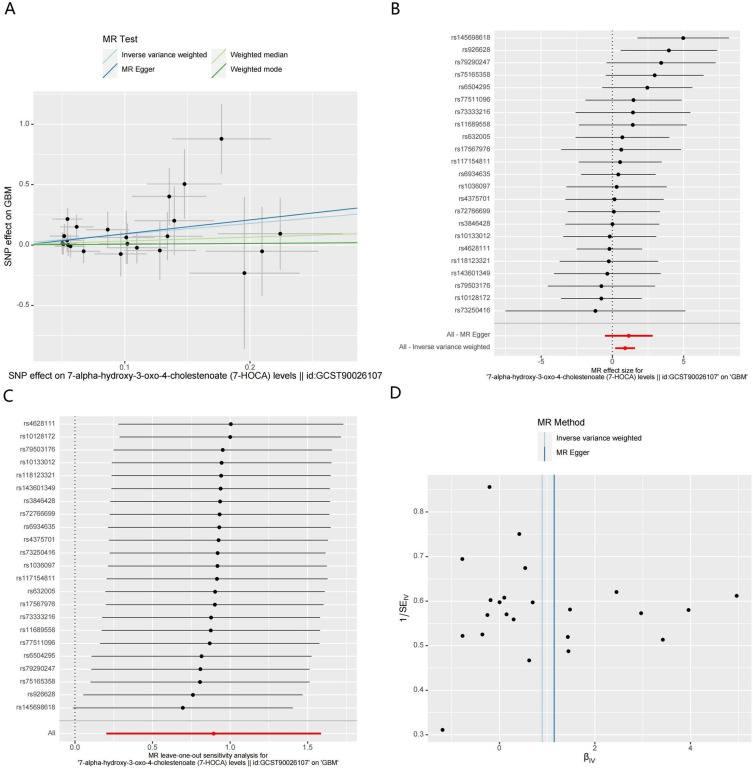
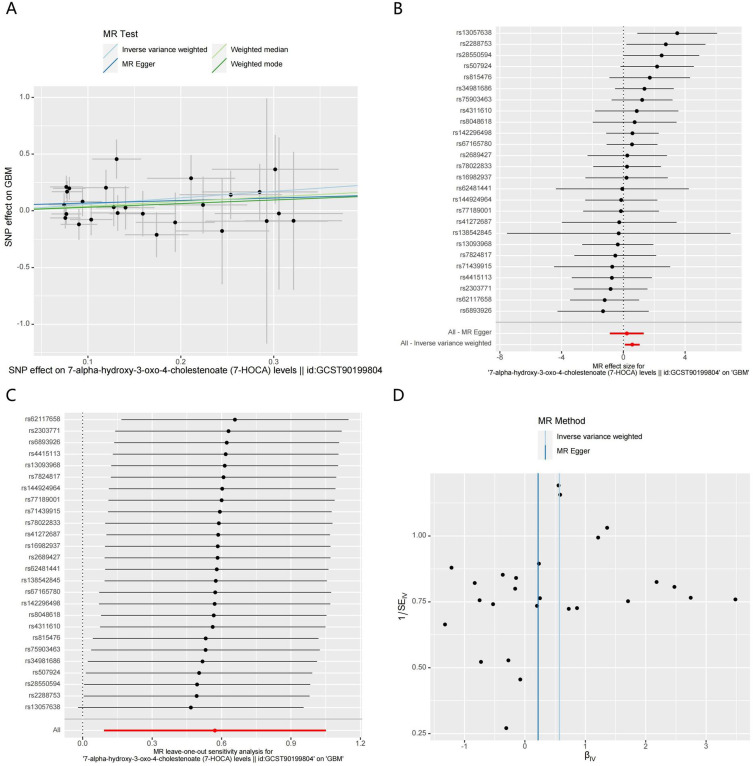
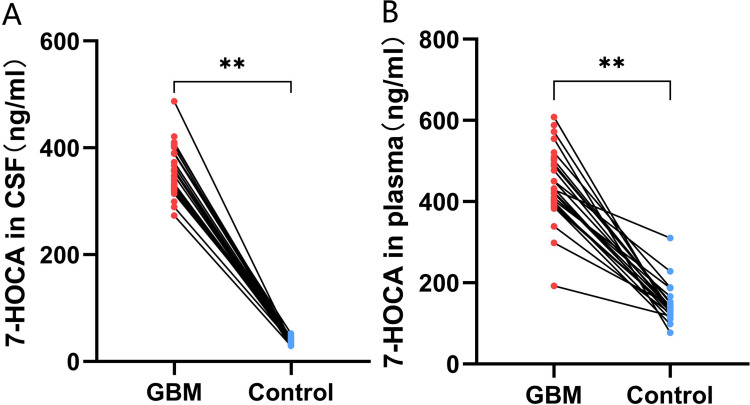
The MR analysis results indicated that higher levels of 7-HOCA in both CSF and plasma could promote the occurrence of GBM (Figures 4A-D and 5A-D). External validation showed that the levels of 7-HOCA in CSF and plasma were significantly higher in the GBM group compared to the control group (Figure 6A and B). Therefore, 7-HOCA may serve as a therapeutic target and predictor for GBM (Table S6).
Figure 4.
MR results of CSF 7-HOCA levels and GBM. (A). Scatter plot. (B) Forest Plot. (C) Leave-one-out analysis. (D) Funnel plots.
Figure 5.
MR results of plasma 7-HOCA levels and GBM. (A) Scatter plot. (B) Forest Plot. (C) Leave-one-out analysis. (D) Funnel plots.
Figure 6.
External validation of the relationship between 7-HOCA and GBM. (A) Difference in CSF 7-HOCA levels between GBM and control. (B) Comparison of plasma 7-HOCA levels between GBM and control. (**p<0.001).
Discussion
Recent studies have suggested that metabolic dysregulation plays a crucial role in the pathogenesis and progression of GBM. This study aims to comprehensively explore the role of metabolites in GBM. Plasma is one of the most easily accessible clinical samples. In this study, we analyzed the potential relationships between 1400 plasma metabolites and GBM, making it the most extensive study of plasma metabolites to date. Historically, human metabolomic research has largely focused on readily obtainable specimens like blood or urine. Yet, for neurological conditions, CSF is particularly pertinent.28 CSF, which bathes the brain and spinal cord and is shielded from the bloodstream by the BBB, may more accurately mirror the physiological events within the nervous system disease (CNS) than other biological fluids. To date, thorough and methodical research into the potential impact of CSF metabolites on GBM is limited. The challenge lies in the procurement of CSF, which is more invasive than collecting blood or urine, necessitating a lumbar puncture and thus rendering CSF a scarce and valuable specimen. To the best of our knowledge, this is the first study to comprehensively explore the associations between 338 CSF metabolites and GBM. Our analysis identified eight CSF metabolites and six plasma metabolites that exhibit a robust relationship with GBM. Notably, we discovered that elevated levels of 7-HOCA in both CSF and plasma are associated with an increased risk of GBM.
7-HOCA is a hydroxycholesterol derivative with a hydroxyl group at the 7-alpha position, a keto group at the 3 position, and a double bond between the 4 and 5 positions of the cholestenoate backbone. As a bile acid intermediate and cholesterol metabolite, 7-HOCA plays a role in the classical (or neutral) pathway of bile acid synthesis, which occurs primarily in the liver. This pathway begins with the conversion of cholesterol to 7-alpha-hydroxycholesterol by the enzyme cholesterol 7-alpha-hydroxylase (CYP7A1), a rate-limiting step in bile acid synthesis. 7-HOCA is subsequently converted to chenodeoxycholic acid and cholic acid through multiple enzymatic steps involving further hydroxylation, oxidation, and conjugation reactions.29,30 Saeed et al found that patients with BBB impairment had significantly elevated levels of 7-HOCA compared to healthy individuals, suggesting that 7-HOCA could reflect the integrity of the BBB.31 To date, our study is the first to explore the relationship between 7-HOCA and GBM. Previous research indicated that bile acid and cholesterol metabolism disorders can lead to various neurological diseases. Targeting the cholesterol-bile acid pathway may aid in the treatment of certain neurological diseases. For example, levels of lithocholic acid were significantly higher in AD patients compared to controls. Additionally, levels of glycodeoxycholic acid and glycolithocholic acid were significantly elevated in AD patients compared to those with mild cognitive impairment. However, the contribution of these bile acids to the pathogenesis of AD remains unknown.32 UDCA and TUDCA are neuroprotective secondary bile acids that can cross the blood-brain barrier. Graham et al found that their levels are significantly decreased in Parkinson’s mice compared to the control group.33 In a Phase II clinical trial evaluating the efficacy of targeting bile acid signaling as a treatment strategy for Amyotrophic Lateral Sclerosis (ALS), TUDCA treatment was found to have potential neuroprotective effects. It was shown to slow functional deterioration in ALS patients, with a 15% increase in the ALS Functional Rating Scale-Revised (ALSFRS-R) scores.34 Studies have shown that 7-HOCA may play a protective role in hemorrhagic stroke by eliminating 27-hydroxycholesterol and promoting the synthesis of bile acids as downstream products, thereby contributing to lipid homeostasis.35
The brain, the organ with the highest cholesterol content, accounts for approximately 20% of the body’s total cholesterol, underscoring the importance of cholesterol homeostasis for its function. Unlike other organs, the brain synthesizes its cholesterol de novo, primarily through the mevalonate pathway alongside the Bloch and Kandutsch-Russell pathways, due to the BBB preventing cholesterol uptake from peripheral circulation and dietary sources. Hydroxylation of brain cholesterol allows it to cross the BBB and contribute to bile acid formation in the liver, facilitating bodily excretion.36 Recent mechanistic studies have revealed that glioma cells augment cholesterol synthesis by increasing oxygen consumption, glycolysis, and activity of the pentose phosphate pathway. Importantly, targeting downstream components of the mevalonate pathway with pharmacological inhibitors has been shown to induce glioma cell death.37 Furthermore, Farnesyl Diphosphate Synthase (FDPS) plays a crucial role in cholesterol homeostasis and cellular functions, with inhibition of FDPS impeding the formation of glioma stem cell secondary spheres, as demonstrated by Kim et al.38 Additionally, research by Lewis et al indicates that under conditions of hypoxia and serum deprivation, SREBP, which regulates fatty acid and cholesterol biosynthesis genes, is upregulated in GBM cells. Inhibition of SREBP activity under these conditions results in GBM cell death, highlighting the potential of targeting cholesterol metabolism as an effective strategy for GBM treatment.39
In addition to 7-HOCA, we have identified seven other CSF metabolites that are strongly correlated with GBM for the first time. Previous literature indicated that these metabolites are closely associated with various neurological diseases. Uracil is part of the pyrimidine metabolism pathway and is involved in nucleic acid metabolism and synthesis. Hanin et al found that uracil has neuroprotective effects, and levels of uracil in the CSF of patients with status epilepticus were significantly lower than those in the control group.40 Orotate is an intermediate in the pyrimidine synthesis pathway, formed from carbamoyl phosphate through an acylation reaction, and is subsequently converted into uridine monophosphate, which further participates in the synthesis of nucleic acids and RNA. Panyard et al found that abnormalities in orotate levels in CSF are closely associated with the occurrence of attention deficit hyperactivity disorder (ADHD).20 TMAP is involved in the metabolism of arginine, a critical amino acid that plays a role in various metabolic processes. Research indicated that TMAP levels are lower in individuals with the APOE ε4 allele, which is associated with an increased risk of AD. This suggests that TMAP may be involved in the metabolic changes observed in AD.41 Methylsuccinoylcarnitine is an ester of carnitine and methylsuccinic acid. It is involved in the metabolism of fatty acids and energy production. Elevated levels of methylsuccinoylcarnitine have been observed in the CSF of patients with Parkinson’s disease, suggesting a disruption in energy metabolism pathways.42 Maleate is the cis-isomer of maleic acid, which is a dicarboxylic acid. Research indicated that levels of maleate in CSF are associated with metabolic disturbances related to neurodevelopmental disorders such as autism and epilepsy.43 Glycerophosphoinositol is derived from phosphatidylinositol, a phospholipid that plays a crucial role in cell signaling and membrane dynamics. Brudvig et al found that levels of glycerophosphoinositol were abnormally elevated in patients with Batten disease.44 Cysteinylglycine is a dipeptide composed of cysteine and glycine. It is an intermediate in the metabolism of glutathione, a critical antioxidant in the body. Altered levels of cysteinylglycine have been observed in Parkinson’s disease patients, indicating potential disruptions in antioxidant defenses and redox balance.45
Disruption of plasma metabolites can have profound effects on the nervous system, contributing to the pathophysiology of various neurological disorders. After excluding variables with insufficient SNPs, unclear functions, and those failing BWMR analysis, we ultimately identified six plasma metabolites closely associated with GBM. Ethyl alpha-glucopyranoside is an ethylated derivative of glucose, primarily associated with carbohydrate and energy metabolism systems. The primary energy source for the brain is glucose, and energy metabolism disorders are a cause of neurological diseases.46 Glycoursodeoxycholic acid sulfate is a sulfated bile acid metabolite derived from glycoursodeoxycholic acid. As previously mentioned, bile acid metabolism disorders, including abnormal secretion of glycoursodeoxycholic acid sulfate, can lead to various neurological dysfunctions.47 Hydroquinone sulfate is a metabolite of hydroquinone, formed through the sulfation process in the liver. Some studies suggest that hydroquinone derivatives may have antioxidant properties, which could potentially protect against oxidative stress-related neurological damage.48 Consistent with this, we found that hydroquinone sulfate has a negative regulatory effect on GBM. Trimethylamine N-oxide is a metabolite derived from the oxidation of trimethylamine, which is produced by gut microbiota from dietary choline, betaine, and carnitine. Research has linked high levels of trimethylamine N-oxide with neurodegenerative diseases like Alzheimer’s and cognitive impairment, suggesting it may affect the CNS through inflammation, oxidative stress, and vascular dysfunction.49 Erucate (22:1n9), also known as erucic acid, is a monounsaturated omega-9 fatty acid. Research indicated that erucic acid is one of the early metabolic markers of cognitive changes in AD.50
More importantly, our clinical validation revealed that 7-HOCA levels were concurrently elevated in both the CSF and plasma of GBM patients, which could have potential clinical implications. High levels of 7-HOCA might correlate with the severity or aggressiveness of GBM. This could help in stratifying patients based on risk and tailoring more personalized treatment approaches. Besides, the concurrent elevation of 7-HOCA in CSF and plasma may serve as a biomarker for the diagnosis of GBM. Its presence in both fluids could help in early detection and monitoring disease progression or response to treatment.
The novelty of this study lies in the comprehensive evaluation of the relationship between CSF metabolites, plasma metabolites, and GBM. To the best of our knowledge, this is the first study to demonstrate that 7-HOCA is closely associated with GBM, identifying it as a potential tumor biomarker. However, this study has some limitations. 1) The FinnGen database does not provide IDH wild-type status confirmation for GBM population. This raises the possibility that some cases could be misclassified as GBM. 2) The study lacks functional experiments to validate the biological mechanisms. 3) Although MR aims to minimize confounding, it is still possible that unaccounted pleiotropic effects or reverse causation are influencing the results. 4) The external validation cohort’s relatively small size may not provide sufficient statistical power to robustly confirm the findings from the MR analysis. 5) The GWAS data for metabolites were derived solely from individuals of European descent, and the results may not generalize to populations of different genetic backgrounds. 6) False-positive associations are a concern in such large-scale analyses. 7) The study design is observational, and the cross-sectional nature of the metabolite measurements means that changes in metabolite levels over time in relation to disease progression or treatment response are not captured.
Conclusion
In conclusion, our study identified eight CSF metabolites and six plasma metabolites that are significantly associated with GBM. Among these, levels of 7-HOCA were notably elevated in both plasma and CSF of GBM patients, highlighting its potential as a biomarker and a therapeutic target. However, further studies are warranted to validate these findings and explore their clinical applications.
Acknowledgments
We are grateful for the data shared by the FinnGen database and the GWAS Catalog.
Funding Statement
This work was supported by the Medical Science Research Project Plan of Hebei Provincial Health Commission (Grant No. 20210974, 20240302, and 20230032). The S&T Program of Hebei, Grant No. 22377717D.
Abbreviations
GBM, Glioblastoma; AD, Alzheimer’s disease; CSF, cerebrospinal fluid; IDH, isocitrate dehydrogenase; BBB, blood-brain barrier; GWAS, genome-wide association study; MR, Mendelian Randomization; IVs, instrumental variables; SNP, single nucleotide polymorphism; LD, linkage disequilibrium; IVW, inverse variance weighted; MR-PRESSO, Mendelian Randomization Pleiotropy Residual Sum and Outlier; BWMR, Bayesian weighted Mendelian randomization; 7-HOCA, 7-alpha-hydroxy-3-oxo-4-cholestenoate; TMAP, N,n,n-trimethyl-alanylproline betaine; CNS, nervous system disease.
Data Sharing Statement
The data sources for this study are all publicly available and have been detailed in the article, further inquiries can be directed to the corresponding author.
Ethics Approval and Consent to Participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The Fourth Hospital of Hebei Medical University (2020KY303).
Informed consent was obtained from all individual participants included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. doi: 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadowski K, Jażdżewska A, Kozłowski J, Zacny A, Lorenc T, Olejarz W. Revolutionizing glioblastoma treatment: a comprehensive overview of modern therapeutic approaches. Int J Mol Sci. 2024;25(11):5774. doi: 10.3390/ijms25115774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toyoda M, Shibahara I, Shigeeda R, et al. Clinical and molecular features of patients with IDH1 wild-type primary glioblastoma presenting unexpected short-term survival after gross total resection. J Neurooncol. 2024;169(1):39–50. doi: 10.1007/s11060-024-04687-2 [DOI] [PubMed] [Google Scholar]
- 4.Estival A, Sanz C, Ramirez JL, et al. Pyrosequencing versus methylation-specific PCR for assessment of MGMT methylation in tumor and blood samples of glioblastoma patients. Sci Rep. 2019;9(1):11125. doi: 10.1038/s41598-019-47642-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YN, Pi YL, Yan X, Li YQ, Qi ZJ, Zhang HF. Methylmalonic acidemia complicated by homocystinuria diseases: a report of three cases. Adv Ther. 2020;37(1):630–636. doi: 10.1007/s12325-019-01149-4 [DOI] [PubMed] [Google Scholar]
- 6.Salvadori MG, Banderó CR, Jesse AC, et al. Prostaglandin E(2) potentiates methylmalonate-induced seizures. Epilepsia. 2012;53(1):189–198. doi: 10.1111/j.1528-1167.2011.03326.x [DOI] [PubMed] [Google Scholar]
- 7.Guha D, Misra V, Chettimada S, Yin J, Gabuzda D. CSF extracellular vesicle Aβ42 and Tau/Aβ42 ratio are associated with cognitive impairment in older people with HIV. Viruses. 2023;16(1):72. doi: 10.3390/v16010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan J, Kuzhiumparambil U, Bandodkar A, Bandodkar S, Dale RC, Fu S. Cerebrospinal fluid metabolites in tryptophan-kynurenine and nitric oxide pathways: biomarkers for acute neuroinflammation. Dev Med Child Neurol. 2021;63(5):552–559. doi: 10.1111/dmcn.14774 [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Chen Z, Li G, Weng Y, Hou Y. Correlation between risk factors of cognitive dysfunction and blood pressure variability after acute ischemic stroke in northwest Shanghai. Int J Neurosci. 2024;1–11. [DOI] [PubMed] [Google Scholar]
- 10.Ballester LY, Lu G, Zorofchian S, et al. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol Commun. 2018;6(1):85. doi: 10.1186/s40478-018-0588-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi J, Chowdhry S, Wu S, Zhang W, Masui K, Mischel PS. Altered cellular metabolism in gliomas - an emerging landscape of actionable co-dependency targets. Nat Rev Cancer. 2020;20(1):57–70. doi: 10.1038/s41568-019-0226-5 [DOI] [PubMed] [Google Scholar]
- 12.Hsia T, Yekula A, Batool SM, et al. Glioblastoma-derived extracellular vesicle subpopulations following 5-aminolevulinic acid treatment bear diagnostic implications. J Extracell Vesicles. 2022;11(11):e12278. doi: 10.1002/jev2.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stringer BW, De Silva MI, Greenberg Z, et al. Human cerebrospinal fluid affects chemoradiotherapy sensitivities in tumor cells from patients with glioblastoma. Sci Adv. 2023;9(43):eadf1332. doi: 10.1126/sciadv.adf1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu P, Pichardo-Rojas PS, Dono A, et al. The detrimental effect of biopsy preceding resection in surgically accessible glioblastoma: results from the national cancer database. J Neurooncol. 2024;168(1):77–89. doi: 10.1007/s11060-024-04644-z [DOI] [PubMed] [Google Scholar]
- 15.Sheehan JP, Trifiletti DM, Lehrer EJ. Tissue biopsy before resection in glioblastoma: is there an opportunity to improve outcomes with liquid biopsies and pre-operative stereotactic radiosurgery. J Neurooncol. 2024;168(2):379–380. doi: 10.1007/s11060-024-04678-3 [DOI] [PubMed] [Google Scholar]
- 16.Mountjoy E, Schmidt EM, Carmona M, et al. An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nat Genet. 2021;53(11):1527–1533. doi: 10.1038/s41588-021-00945-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789–794. doi: 10.1038/ng.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Han Y, He C, et al. Association between psychiatric disorders and glioma risk: evidence from Mendelian randomization analysis. BMC Cancer. 2024;24(1):118. doi: 10.1186/s12885-024-11865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao SS, Burgess S. Vitamin D is associated with reduced risk of Sjögren’s syndrome: a Mendelian randomization study. Rheumatology. 2024;63(2):e32–e33. doi: 10.1093/rheumatology/kead356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panyard DJ, Kim KM, Darst BF, et al. Cerebrospinal fluid metabolomics identifies 19 brain-related phenotype associations. Commun Biol. 2021;4(1):63. doi: 10.1038/s42003-020-01583-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Lu T, Pettersson-Kymmer U, et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet. 2023;55(1):44–53. doi: 10.1038/s41588-022-01270-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Yan B, Zhao B, et al. Assessing the causal effects of human serum metabolites on 5 major psychiatric disorders. Schizophr Bull. 2020;46(4):804–813. doi: 10.1093/schbul/sbz138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 2017;13(11):e1007081. doi: 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottigliengo D, Foco L, Seibler P, Klein C, König IR, Del Greco MF. A Mendelian randomization study investigating the causal role of inflammation on Parkinson’s disease. Brain. 2022;145(10):3444–3453. doi: 10.1093/brain/awac193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Ming J, Hu X, Chen G, Liu J, Yang C. Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics. 2020;36(5):1501–1508. doi: 10.1093/bioinformatics/btz749 [DOI] [PubMed] [Google Scholar]
- 27.Cote DJ, Samanic CM, Smith TR, et al. Alcohol intake and risk of glioma: results from three prospective cohort studies. Eur J Epidemiol. 2021;36(9):965–974. doi: 10.1007/s10654-021-00800-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simrén J, Ashton NJ, Blennow K, Zetterberg H. An update on fluid biomarkers for neurodegenerative diseases: recent success and challenges ahead. Curr Opin Neurobiol. 2020;61:29–39. doi: 10.1016/j.conb.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 29.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72(1):137–174. doi: 10.1146/annurev.biochem.72.121801.161712 [DOI] [PubMed] [Google Scholar]
- 30.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50(10):1955–1966. doi: 10.1194/jlr.R900010-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saeed A, Floris F, Andersson U, et al. 7α-hydroxy-3-oxo-4-cholestenoic acid in cerebrospinal fluid reflects the integrity of the blood-brain barrier. J Lipid Res. 2014;55(2):313–318. doi: 10.1194/jlr.P044982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marksteiner J, Blasko I, Kemmler G, Koal T, Humpel C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics. 2018;14(1):1. doi: 10.1007/s11306-017-1297-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham SF, Rey NL, Ugur Z, et al. Metabolomic profiling of bile acids in an experimental model of prodromal Parkinson’s disease. Metabolites. 2018;8(4):71. doi: 10.3390/metabo8040071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elia AE, Lalli S, Monsurrò MR, et al. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2016;23(1):45–52. doi: 10.1111/ene.12664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Shen Y, Li Q, et al. Exploring the causal association between genetically determined circulating metabolome and hemorrhagic stroke. Front Nutr. 2024;11:1376889. doi: 10.3389/fnut.2024.1376889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad F, Sun Q, Patel D, Stommel JM. Cholesterol metabolism: a potential therapeutic target in glioblastoma. Cancers. 2019;11(2):146. doi: 10.3390/cancers11020146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kambach DM, Halim AS, Cauer AG, et al. Disabled cell density sensing leads to dysregulated cholesterol synthesis in glioblastoma. Oncotarget. 2017;8(9):14860–14875. doi: 10.18632/oncotarget.14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HY, Kim DK, Bae SH, et al. Farnesyl diphosphate synthase is important for the maintenance of glioblastoma stemness. Exp Mol Med. 2018;50(10):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CA, Brault C, Peck B, et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene. 2015;34(40):5128–5140. doi: 10.1038/onc.2014.439 [DOI] [PubMed] [Google Scholar]
- 40.Hanin A, Chollet C, Demeret S, Di Meglio L, Castelli F, Navarro V. Metabolomic changes in adults with status epilepticus: a human case-control study. Epilepsia. 2024;65(4):929–943. doi: 10.1111/epi.17899 [DOI] [PubMed] [Google Scholar]
- 41.Hammond TC, Xing X, Yanckello LM, et al. Human gray and white matter metabolomics to differentiate apoe and stage dependent changes in Alzheimer’s disease. J Cell Immunol. 2021;3(6):397–412. doi: 10.33696/immunology.3.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeWitt PA, Li J, Wu KH, Lu M. Diagnostic metabolomic profiling of Parkinson’s disease biospecimens. Neurobiol Dis. 2023;177:105962. doi: 10.1016/j.nbd.2022.105962 [DOI] [PubMed] [Google Scholar]
- 43.Brister D, Werner BA, Gideon G, et al. Central nervous system metabolism in autism, epilepsy and developmental delays: a cerebrospinal fluid analysis. Metabolites. 2022;12(5):371. doi: 10.3390/metabo12050371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brudvig JJ, Swier VJ, Johnson TB, et al. Glycerophosphoinositol is elevated in blood samples from CLN3 (Δex7-8) pigs, Cln3 (Δex7-8) Mice, and CLN3-affected individuals. Biomark Insights. 2022;17:11772719221107765. doi: 10.1177/11772719221107765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baldini F, Hertel J, Sandt E, et al. Parkinson’s disease-associated alterations of the gut microbiome predict disease-relevant changes in metabolic functions. BMC Biol. 2020;18(1):62. doi: 10.1186/s12915-020-00775-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang S, Lachance BB, Mattson MP, Jia X. Glucose metabolic crosstalk and regulation in brain function and diseases. Prog Neurobiol. 2021;204:102089. doi: 10.1016/j.pneurobio.2021.102089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kiriyama Y, Nochi H. The biosynthesis, signaling, and neurological functions of bile acids. Biomolecules. 2019;9(6):232. doi: 10.3390/biom9060232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giner RM, Ríos JL, Máñez S. Antioxidant activity of natural hydroquinones. Antioxidants. 2022;11(2):343. doi: 10.3390/antiox11020343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie L, Pan L, Liu B, Cheng H, Mao X. Research progress on the association between trimethylamine/trimethylamine - N - oxide and neurological disorders. Postgrad Med J. 2024;100(1183):283–288. doi: 10.1093/postmj/qgad133 [DOI] [PubMed] [Google Scholar]
- 50.Darst BF, Huo Z, Jonaitis EM, et al. Metabolites associated with early cognitive changes implicated in Alzheimer’s disease. J Alzheimers Dis. 2021;79(3):1041–1054. doi: 10.3233/JAD-200176 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sources for this study are all publicly available and have been detailed in the article, further inquiries can be directed to the corresponding author.