ABSTRACT
Background
Studies are currently being conducted on rabbits requiring serial glucose monitoring. The FreeStyle Libre 2 (FSL2), a serial glucose monitoring device, has been validated in humans, dogs and cats, but not in rabbits.
Objectives
This study aimed to evaluate the accuracy of the FSL2 in rabbits.
Methods
Six healthy rabbits were used in this study. Interstitial glucose (IG) was measured using the FSL2, and blood glucose (BG) was measured using a portable blood glucose meter (PBGM); their results were compared with those from a clinical chemistry analyser. For the first 3 h, IG and BG were measured at 1‐h intervals. Subsequently, they were measured every 8 h over a 48‐h period. Regular insulin 0.2 U/kg was then administered to the rabbits, and IG and BG were measured every 15 min over a 90‐min period.
Results
Before insulin treatment, no measurements fell within the hypoglycaemic range (BG < 100 mg/dL). In the euglycaemic range (BG ≥ 100 mg/dL), the PBGM and FSL2 showed 85.7% and 23.8% accuracies, respectively. After insulin treatment, the PBGM showed 95.5% and 81.3% accuracies in the hypoglycaemic and euglycaemic ranges, respectively. The FSL2 showed 68.1% and 37.5% accuracies in the hypoglycaemic and euglycaemic ranges, respectively. Parkes consensus error grid analysis showed that the PBGM and FSL2 had 100% agreement for Zones A (no effect on clinical action) and B (altered clinical action unlikely to affect outcome) in rabbits with and without insulin treatment.
Conclusions
There was limited agreement between the FSL2 and reference standard BG measurements. However, the FSL2 allows clinically acceptable identification of hypoglycaemic states in rabbits.
Keywords: FreeStyle Libre 2, FSL2, hypoglycaemia, interstitial glucose, rabbit
The manuscript describes the accuracy of a flash glucose monitoring system, the FreeStyle Libre 2, in rabbits compared to a portable blood glucose meter that has been validated in rabbits. The readings obtained using the portable blood glucose meter and the FreeStyle Libre 2 were strongly correlated with those obtained using the reference method. Furthermore, both the portable blood glucose meter and FreeStyle Libre 2 met the ISO 15197:2013 criteria for analytical and clinical accuracy, although the FreeStyle Libre 2 was not as accurate as the portable blood glucose meter and reference methods.

1. Introduction
The use of rabbits in experimental research has a long historical tradition because they possess desirable characteristics as laboratory animal models, including a long lifespan, ease of handling, and relatively low cost (Wang et al. 2010). In experiments using rabbits, such as diabetic models and insulin‐induced hypoglycaemia, serial blood glucose monitoring is essential (Díez et al. 2013; Wada et al. 2019).
Chemistry analysers employing hexokinase methods are considered standard for evaluating blood glucose (BG) concentrations (Neeley 1972). However, these laboratory methods require relatively large sample volumes and are time‐consuming. Therefore, these methods have limitations in experimental rabbit studies. Portable blood glucose meters (PBGM) offer researchers a means of promptly assessing BG concentrations while requiring only a small sample volume. Some PBGMs have been validated for use in rabbits through comparison with a hexokinase reference method (K. Silva et al. 2020; DeClue et al. 2004). However, they have certain drawbacks, such as the need for repeated venipunctures, which can be stressful and painful for the animals. Rabbits present challenges for vascular access because of their small blood vessels, making repeated blood sampling difficult. PBGM also carries the risk of missing BG peaks or nadirs, if they occur between two sampling times. Thus, the application of the PBGM is limited in experiments that require serial glucose measurements or the detection of hypoglycaemia over time.
The FreeStyle Libre 2 (FSL2), a continuous glucose monitoring system (CGMS), is routinely used in human patients with diabetes, and several studies have confirmed its clinical accuracy in veterinary medicine (DeClue et al. 2004; Deiting and Mischke 2021; Wiedmeyer et al. 2005). The FSL2 measures interstitial glucose (IG) levels with a small catheter inserted under the skin that records measurements, with readings automatically stored at 15‐min intervals. These characteristics of FSL2 make it possible to overcome the shortcomings of the PBGM. Furthermore, they allow for the evaluation of glucose trends over time while decreasing the overall cost of care and blood sampling‐associated patient discomfort (Shea and Hess 2021). The FSL2 is a flash glucose monitoring system that has been validated in various mammals, including humans, dogs and cats. This device can be simply applied to rabbits to ameliorate the overall blood sampling‐associated stress, which potentially affects BG levels. However, the accuracy of the FSL2 has not yet been evaluated in rabbits.
Thus, the aim of this study was to evaluate the analytical and clinical accuracy of the FSL2, including its accuracy in rabbits with insulin‐induced hypoglycaemia, according to ISO 15197:2013 criteria.
2. Materials and Methods
2.1. Animals
Six White New Zealand rabbits (three males, three females) aged 5 months and weighing 3.54–3.94 kg (median, 3.66 kg) were included in the study. The rabbits were given free access to commercial pellet feed and water and were housed individually in suspended wire cages with automatic water dispensers and manual feeders. The rabbits were maintained under a 12‐h light–dark cycle at a temperature (19°C–24°C) and humidity (40%–70%). This study was approved by the Institutional Animal Care and Use Committee (CBNUA‐2038‐22‐01).
2.2. Glucose Measurement
One hour before glucose measurement, an FSL2 sensor (FreeStyle Libre 2, Abbott, IL, USA) was placed on each rabbit. Rabbits were secured by polycarbonate rabbit restrainers. The skin between the scapulae was shaved and cleaned using isopropyl alcohol. The sensor applicator was lifted and separated from the sensor pack. In this manner, the sensor applicator was ready for use. Then, 2–4 drops of tissue adhesive (Vetbond, 3M Animal Care Products, MN, USA) were applied on the skin surface of the sensor. The sensor applicator was placed over the shaved skin between the scapulae and pushed down firmly to apply the sensor. The FSL2 automatically monitors IG with a detection range of 40–500 mg/dL as described in the product manual.
IG measurements were compared to BG concentrations obtained using a PBGM (Accu‐Chek Active, Roche Diabetes Care, Basel, Switzerland), validated for rabbits (K. Silva et al. 2020). PBGM performs measurements using the enzyme glucose dehydrogenase, based on reflectance photometry (Vashist et al. 2011). PBGM requires 0.1–0.2 µL of blood with a BG detection range of 10–600 mg/dL as described in the product manual.
To compare the glucose readings measured using the FSL2 and PBGM to the reference measurements taken using the hexokinase method (Hitachi‐7020; Hitachi High‐Tech, Tokyo, Japan), paired 0.5 mL blood samples were collected from the marginal ear vein via a 24G catheter, with discarding the catheter's dead space. Blood samples were placed in plain tubes and centrifuged for BG analysis.
2.3. Data Collection
One hour after FSL2 application, continuous IG measurements were obtained. The sensor was scanned at the first, second and third hours after placement and then every 8 h for 48 h. Simultaneously, the BG levels were measured using the PBGM and a chemistry analyser. Blood (0.5 mL) was drawn from the marginal ear vein; one drop was used to measure glucose with the PBGM, while the rest was collected in a plain tube, stored at 4°C, and submitted within 3 h for analysis using a chemistry analyser. Thirty minutes before blood collection from the marginal ear vein, lidocaine cream was applied to a 1 cm area around the venipuncture site.
After 48 h, the glucose levels of rabbits with insulin‐induced reduced BG levels were measured. A 24G catheter was placed in the marginal ear vein for the administration of dextrose solution and anaesthetic drugs. Anaesthesia was induced using alfaxalone (Alfaxan multidose, Zoetis, NJ, USA; 1.5 mg/kg, intravenously) and maintained with a continuous alfaxalone infusion (0.2 mg/kg/min, intravenously) throughout glucose measurements to minimize the fluctuation of BG by stress (Harcourt‐Brown and Harcourt‐Brown 2012). Baseline glucose concentrations were measured before anaesthesia. Immediately after anaesthesia, regular human insulin (Humulin R, Eli Lilly, IN, USA) 0.2 U/kg was injected subcutaneously. Blood was collected from the marginal ear vein at 15‐min intervals for the remaining 90 min. The IG and BG measurements were performed using the same methods as those used in the previous set of measurements. Severe hypoglycaemia (BG < 60 mg/dL) was not observed during the experiment; therefore, dextrose administration was not required.
2.4. Statistical Analyses
Glucose concentrations were compared between the two test methods (PBGM and FSL2) and with a reference method (chemistry analyser). Data were assessed for normal distribution using the Shapiro–Wilk test. The correlation between the BG and IG measurements was assessed using Spearman's rank correlation, and the groups were compared using the Wilcoxon signed‐rank test. The data homogeneity of variance was verified using the Brown–Forsythe test. Bland–Altman plots were generated, and the bias and 95% limits of agreement were calculated. All statistical analyses were performed using a commercial statistical program (SPSS version 23.0; IBM, Armonk, NY, USA). The accuracy of the test methods was assessed according to the ISO 15197:2013 criteria (International Organization for Standardization. ISO 15197 2013). Analytical accuracy was evaluated by determining the number of glucose concentration pairs within ±15 mg/dL of the reference BG reading for BG concentrations <100 mg/dL and within ±15% of the reference BG for readings ≥100 mg/dL. To meet analytical accuracy, the ISO 15197:2013 criteria require that at least 95% of the readings fall within the defined limits. Analytical accuracy was also assessed by calculating relative differences for each time point (100 × [IG—BG]/BG) as previously described (Clarke and Kovatchev 2009) and then calculating mean absolute relative difference (MARD), median absolute relative difference (mARD), mean relative difference (MRD) and mean absolute difference (MAD).
Clinical accuracy was assessed using Parkes consensus error grids for Type 1 diabetes mellitus, categorizing glucose reading pairs into five clinical risk categories (Zones A–E): (Zone A) no effect on clinical action, (Zone B) altered clinical action unlikely to affect outcome, (Zone C) altered clinical action likely to affect clinical outcome, (Zone D) altered clinical action could have substantial medical risk and (Zone E) altered clinical action could have dangerous consequences (Parkes et al. 2000). Based on the ISO 15197:2013 criteria, 99% of measured glucose levels should fall within Zones A and B.
3. Results
3.1. Application of the FSL2
The application of the FSL2 appeared to be easy to perform and well tolerated by all rabbits. No significant adverse events were recorded while using it; only one rabbit showed mild erythema at the sensor application site at the end of the wearing period, which spontaneously resolved within the subsequent 24 h. Before insulin treatment, throughout the 48‐h period of glucose measurements, the 2/6 sensor was detached from the rabbit skin at the 32‐h mark.
3.2. Measurements Taken Using the PBGM and FSL2
For the non‐insulin‐treated rabbits, the median BG concentrations measured using the reference method and PBGM and the median IG concentration obtained by the FSL2 were 130.5 mg/dL (range, 67–152 mg/dL), 126 mg/dL (range, 103–161 mg/dL) and 159.5 mg/dL (range, 102–208 mg/dL), respectively. After insulin treatment, the median BG concentration measured using the reference method and PBGM and the median IG concentration obtained using the FSL2 were 120.5 mg/dL (range, 63–155 mg/dL), 106 mg/dL (range, 60–150 mg/dL) and 134.5 mg/dL (range, 72–207 mg/dL), respectively.
The results obtained using the PBGM and the reference method were strongly correlated (r = 0.79, p < 0.01), as were those obtained using the FSL2 and the reference method (r = 0.78, p < 0.01) (Figure 1).
FIGURE 1.
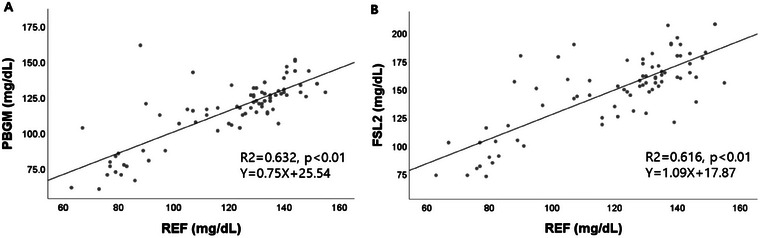
Correlation between readings from (A) the portable blood glucose meter and the reference method, and (B) the FreeStyle Libre 2 and the reference method. The solid line represents the best fit. FSL2, FreeStyle Libre 2; PBGM, portable blood glucose meter; REF, reference method.
3.3. Bland–Altman Analysis
Bland–Altman analysis of glucose measurements obtained using the PBGM and reference method showed a slight negative proportional bias and heteroscedasticity, with more variation for higher glucose concentrations (Figure 2). Constant bias (95% limits of agreement) was estimated to be −8.8 mg/dL (−24.6 to 6.9 mg/dL). Bland–Altman analysis of glucose measurements taken using the FSL2 and reference method showed considerable variation between methods and heteroscedasticity (greater variation for higher glucose concentrations) (Figure 2). The constant bias was estimated to be 17.5 mg/dL and the 95% limits of agreement were −11.532 to 46.637 mg/dL.
FIGURE 2.
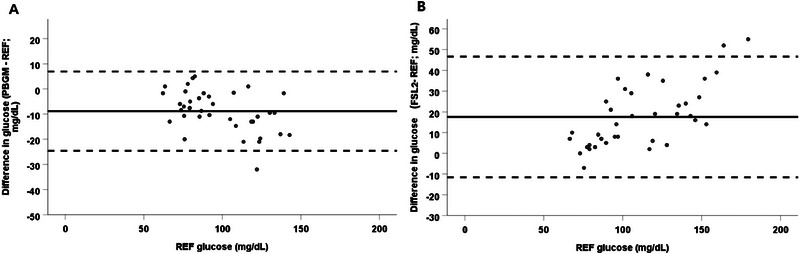
Bland–Altman analysis of glucose measurements between (A) the portable blood glucose meter and (B) the FreeStyle Libre 2 and the reference method. (A) A negative proportional bias and heteroscedasticity with more variation for higher blood glucose concentrations is apparent. Constant bias was estimated to be −8.818 mg/dL and the 95% limits of agreement were −24.610 to 6.974 mg/dL. (B) Considerable variation between methods and heteroscedasticity are the most apparent findings. Constant bias was estimated to be 17.553 mg/dL and the 95% limits of agreement were −11.532–46.637 mg/dL. FSL2, FreeStyle Libre 2; PBGM, portable blood glucose meter; REF, reference method.
3.4. ISO 15197:2013 Criteria
In insulin‐treated rabbits, the proportions of readings in the hypoglycaemic range (reference method BG < 100 mg/dL) for which the test method measurement was within ±15 mg/dL of the reference BG for the PBGM and FSL2 were 95.5% (21/22) and 68.1% (15/22), respectively. The PBGM readings were above the 95% mandated by the ISO 15197:2013 criteria (Table 1). The proportion of readings in the euglycaemic range (reference method BG ≥100 mg/dL) within ±15% of the reference BG for the PBGM and FSL2 were 81.3% (13/16) and 37.5% (6/16), respectively; both were below the 95% mandated by the ISO 15197:2013 criteria.
TABLE 1.
Analytical accuracy of the portable blood glucose meter and the FreeStyle Libre 2.
| Noninsulin‐treated rabbits | ||
|---|---|---|
| PBGM | FSL2 | |
| Hypoglycaemic range (REF BG < 100 mg/dL) | ||
| Percentage of measurements within ±15 mg/dL of the REF BG value | — | — |
| Euglycaemic range (REF BG ≥ 100 mg/dL) | ||
| MARD (%) | 10.40 | 29.07 |
| mARD (%) | 5.70 | 21.88 |
| MRD (%) | 2.80 | 28.09 |
| Percentage of measurements within ±15% of the REF BG value | 85.7 (36/42) | 23.8 (10/42) |
| Insulin‐treated rabbits | ||
|---|---|---|
| PBGM | FSL2 | |
| Hypoglycaemic range (REF BG < 100 mg/dL) | ||
| MAD (mg/dL) | 6.58 | 13.27 |
| Percentage of measurements within ±15 mg/dL of the REF BG value | 95.5 (21/22) | 68.1 (15/22) |
| Euglycaemic range (REF BG ≥ 100 mg/dL) | ||
| MARD (%) | 10.31 | 18.46 |
| mARD (%) | 10.60 | 17.83 |
| MRD (%) | −10.21 | 18.46 |
| Percentage of measurements within ±15% of the REF BG value | 81.3 (13/16) | 37.5 (6/16) |
Note: The two test methods (FSL2 and PBGM) were compared to a reference standard.
Abbreviations: BG, blood glucose; FSL2, FreeStyle Libre 2; MAD, mean absolute difference; MARD, mean absolute relative difference; mARD, median absolute relative difference; MRD, mean relative difference; PBGM, portable blood glucose meter; REF, reference method.
In noninsulin‐treated rabbits, no results were included in the hypoglycaemic range (reference method BG < 100 mg/dL). The proportion of readings in the euglycaemic range (reference method BG ≥ 100 mg/dL) within ±15% of the reference BG for the PBGM and FSL2 were 85.7% (36/42) and 23.8% (10/42), respectively; both were below the 95% mandated by ISO 15197:2013 criteria. The MAD, MARD, mARD and MRD are listed in Table 1.
The Parkes consensus error grids for the PBGM and FSL2 are presented in Figure 3. In insulin‐treated rabbits, for the PBGM and FSL2, 100% (38/38) of the pairs were in Zones A and B of the error grid. In noninsulin‐treated rabbits, for the PBGM and FSL2, 97.6% (41/42) of the pairs were in Zones A and B of the error grid and 1 result in Zone C, which was inadvertently stored at room temperature (19°C–24°C) for 3 h. After the result of a sample corresponding to Zone C was removed from the Parkes consensus error grids for the PBMG and FSL2, 100% (41/41) of the pairs were in Zones A and B of the error grid. Based on the ISO 15197:2013 criteria, ≥99.0% of the measured glucose results should fall within Zones A and B.
FIGURE 3.
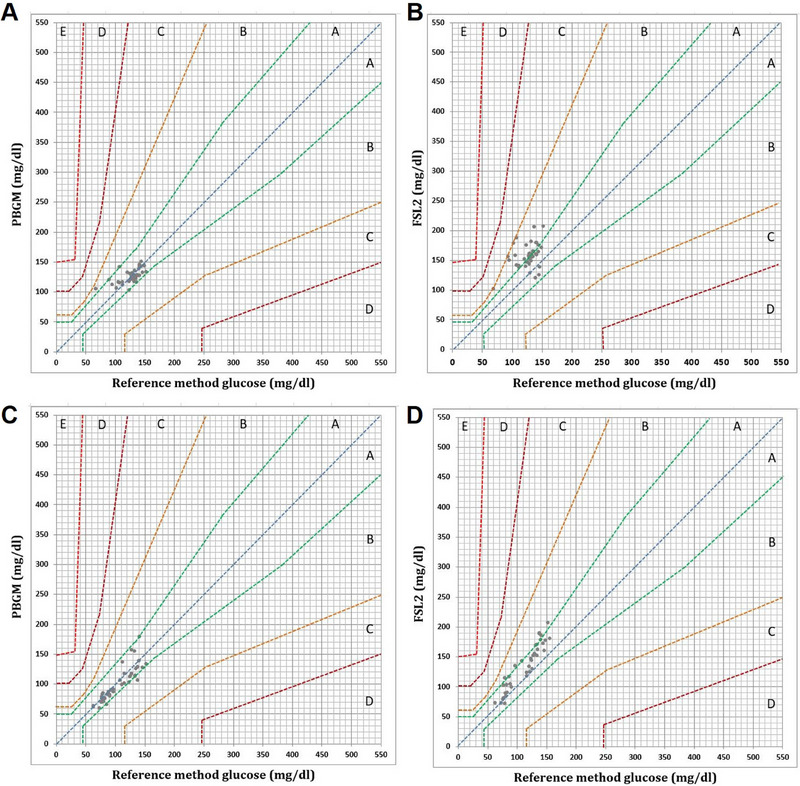
The Parkes consensus error grid (for Type 1 diabetes mellitus) of (A) the PBGM readings for noninsulin‐treated rabbits, (B) the FSL2 readings for noninsulin‐treated rabbits, (C) the PBGM readings for insulin‐treated rabbits and (D) the FSL2 readings for insulin‐treated rabbits. Zones are categorized as follows: (Zone A) no effect on clinical action; (Zone B) altered clinical action unlikely to affect outcome; (Zone C) altered clinical action likely to affect clinical outcome; (Zone D) altered clinical action could have substantial medical risk and (Zone E) altered clinical action could have dangerous consequences. Based on the ISO 15197:2013 criteria, (Zone C) altered clinical action likely to affect clinical outcome; (Zone D)FreeStyle Libre 2; PBGM, portable blood glucose meter.
4. Discussion
This study aimed to evaluate the accuracy of the FSL2 in rabbits. In this experimental model, rabbits showed limited agreement between results obtained using the FSL2 and reference standard glucose measurements. Both the PBGM and FSL2 met the ISO 15197:2013 criteria for analytical and clinical accuracy. In insulin‐treated rabbits, readings taken using the FSL2 and PBGM were clinically acceptable, and the analytical accuracies of the FSL2 and PBGM in the hypoglycaemic range (BG < 100 mg/dL) were higher than those in the euglycaemic range (BG > 100 mg/dL). The PBGM came close to satisfying the analytical accuracy requirements of this study. The FSL2 was not as accurate as the PBGM and reference methods but succeeded in reliably detecting hypoglycaemia in our study.
Bland–Altman analysis of the PBGM and FSL2 showed tendencies in BG; on average, the BG measured using the PBGM was 8.818 mg/dL lower and that measured using the FSL2 was 17.553 mg/dL higher than that measured using the reference method. The 95% limits of agreement were wider for the FSL2 than for the PBGM. Measurements obtained using both PBGM and FSL2 deviated more from reference measurements at higher glucose concentrations, and this heteroscedasticity was greater for FSL2 than for PBGM. Consequently, PBGM measurements were more closely related to the reference measurements than the FSL2 measurements and thus more accurate in detecting BG concentrations.
ISO 15197:2013 criteria are applied in human medicine to evaluate methods of glucose measurement for accuracy and to optimize patient safety (International Organization for Standardization. ISO 15197 2013). Smaller differences are more important in the more dangerous hypoglycaemic range. In contrast, there is a larger gap between glucose concentrations in the euglycaemic range, which affects clinical results. For the non‐insulin‐treated rabbits, only 85.7% of the PBGM and 23.8% of the FSL2 readings were within ±15% of the reference BG measurement for the euglycaemic range. The PBGM was more analytically accurate than the FSL2 in rabbit samples. The clinical relevance of the lack of agreement between the FSL2 and the reference analyser was demonstrated using the Parkes error grid. This error grid was developed to assess the accuracy of BG measurements in self‐monitoring human patients with diabetes mellitus (Parkes et al. 2000). The risk categories were designated by 100 physicians from the American Diabetes Association. For a device to be considered clinically accurate, at least 99% of the values must fall within Zones A and B, indicating no effect on the clinical outcome. Although the patient species and clinical scenarios may differ, these guidelines for evaluating clinical accuracy are also employed as a framework for evaluating the diagnostic performance of glucometers in veterinary medicine (Corradini et al. 2016; Malerba et al. 2020; Del Baldo, Fracassi, et al. 2021).
In the present study, the percentage of PBGM and FSL2 readings for the noninsulin‐treated rabbits in the two acceptable Zones A and B was 100%, which was compatible with a previous study (Selleri, Di Girolamo, and Novari 2014.). The PBGM used in this study, which has previously been validated for clinical accuracy in rabbits. However, one blood sample was corresponding to Zone C because it was inadvertently stored at room temperature for 3 h. Glucose is lost through glycolysis at a rate of 5%–7% per hour at room temperature in human blood (Bruns and Knowler 2009). Therefore, the single measurement from Zone C was removed, and the clinical accuracy increased to 100%.
For insulin‐treated rabbits, the PBGM and FSL2 failed to meet the ISO 15197:2013 criteria for analytical accuracy, with the exception of PBGM in the hypoglycaemic range. In the hypoglycaemic range, the analytical accuracies of PBGM and FSL2 were 95.5% and 68.1%, respectively. In the euglycaemic range, they were 81.3% and 37.5%, respectively. The PBGM was more analytically accurate than the FSL2 in insulin‐treated rabbits. The analytical accuracy of the PBGM and FSL2 was higher in the hypoglycaemic range (BG < 100 mg/dL) than in the euglycaemic range (BG > 100 mg/dL). When evaluating the accuracy of the FSL2 in the hypoglycaemic range in different species, dogs exhibit a decrease in the analytical accuracy of the FSL2, whereas cats and humans show an increase in accuracy (Babaya et al. 2020; Del Baldo et al. 2021; Howard et al. 2021). Interspecies differences in the accuracy of the FSL2 within the hypoglycaemic range (BG < 100 mg/dL) may arise from variations in skin thickness or hydration status (Foxx et al. 2022; D. Silva et al. 2021). To accurately identify the underlying causes of interspecific differences in FSL2 accuracy within the hypoglycaemic range (BG < 100 mg/dL), additional studies evaluating skin thickness and hydration status across species, followed by an assessment of FSL2 accuracy, are required. The percentage of readings that did not affect the clinical outcome was 100% for PBGM and FSL2, indicating acceptable clinical accuracy.
In the insulin‐treated rabbits in this study, the clinical accuracy of the FSL2 was acceptable, and the analytical accuracy was higher in the hypoglycaemic range (BG < 100 mg/dL) than in the euglycaemic range (BG > 100 mg/dL). Therefore, the FSL2 is applicable in experiments requiring the rapid detection of hypoglycaemia and fluctuating BG levels in the hypoglycaemic range, such as in diabetic rabbit models, insulinoma rabbits and insulin‐induced hypoglycaemia models (Del Baldo et al. 2021; Díez et al. 2013; Wada et al. 2019); it is also more convenient than the PBGM, which presents challenges in serial monitoring. The FSL2 provides continuous readings and identifies changes in glucose levels that would be missed by PBGM. Therefore, although the FSL2 may be less accurate than the PBGM, it could be a substitute for the PBGM because it is clinically applicable and facilitates the easy and rapid detection of hypoglycaemia. In addition, it minimizes fluctuations in experimental results owing to reduced stress in rabbits, reduces staff workloads and saves time.
This study had several limitations. Firstly, the study involved only a small number of rabbits, which potentially affected the results owing to the limited number of measurements. Our sample size was limited by the number of devices; therefore, no a priori sample size calculations were performed. However, the number of rabbits was based on a previous study using experimental rabbits for the following validation study (Cutler et al. 2020). Our study provided preliminary results for further validation studies to investigate the utility of FSL2 in rabbits, including pet rabbits. Second, this study focused only on hypoglycaemic and euglycaemic rabbits. Studies involving hyperglycaemic rabbits are required to evaluate the accuracy of the FSL2 across all BG ranges. Third, certain factors such as skin thickness and hydration status might affect FSL2 performance (Del Baldo et al. 2021; Genua et al. 2021; Malerba et al. 2020); however, these factors were not evaluated in our study. Finally, the effect of alfaxalone‐induced anaesthesia on BG levels has not been evaluated in insulin‐treated rabbits. Previous studies have confirmed that alfaxalone anaesthesia in dogs does not affect BG levels (Muñoz, Robertson, and Wilson 2017). However, the lack of effects on BG levels has not been verified in rabbits.
5. Conclusion
Although the ISO 15197:2013 requirements were not fulfilled for rabbits, the FSL2 was clinically acceptable for identifying hypoglycaemic states and rapid changes in hypoglycaemic concentrations. Further studies with larger sample sizes and hyperglycaemia are needed to assess the performance of the FSL2 across all BG ranges in rabbits.
Author Contributions
Minseok Choi: conceptualization, methodology, data curation, formal analysis, validation, investigation, writing–original draft, visualization, software, resources. Yeon Chae: data curation, formal analysis, validation. Jayeon Park: methodology, data curation, investigation, software. Yelim Lee: methodology, software, data curation, investigation. Kyung‐Mee Park: conceptualization, methodology, investigation, supervision. Dong‐Hyuk Jeong: methodology, data curation, investigation, supervision. Byeong‐Teck Kang: writing–review and editing, supervision. Taesik Yun: writing–review and editing, supervision. Hakhyun Kim: conceptualization, writing–review and editing, project administration, funding acquisition, formal analysis, supervision, methodology, resources, investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.70166
Funding: This work was supported by the Basic Research Lab Program (2022R1A4A1025557) through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Babaya, N. , Noso S., Hiromine Y., et al. 2020. “Flash Glucose Monitoring in Type 1 Diabetes: A Comparison With Self‐Monitoring Blood Glucose.” Journal of Diabetes Investigation 11, no. 5: 1222–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns, D. E. , and Knowler W. C.. 2009. “Stabilization of Glucose in Blood Samples: Why It Matters.” Clinical Chemistry 55, no. 5: 850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, W. , and Kovatchev B.. 2009. “Statistical Tools to Analyze Continuous Glucose Monitor Data.” Diabetes Technology & Therapeutics 11, no. S1: S45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini, S. , Pilosio B., Dondi F., et al. 2016. “Accuracy of a Flash Glucose Monitoring System in Diabetic Dogs.” Journal of Veterinary Internal Medicine 30, no. 4: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler, D. C. , Koenig A., Di Girolamo N., and Mayer J.. 2020. “Investigation for Correction Formulas on the Basis of Packed Cell Volume for Blood Glucose Concentration Measurements Obtained With Portable Glucometers When Used in Rabbits.” American Journal of Veterinary Research 81, no. 8: 642–650. [DOI] [PubMed] [Google Scholar]
- DeClue, A. E. , Cohn L. A., Kerl M. E., and Wiedmeyer C. E.. 2004. “Use of Continuous Blood Glucose Monitoring for Animals With Diabetes Mellitus.” Journal of the American Animal Hospital Association 40, no. 3: 171–173. [DOI] [PubMed] [Google Scholar]
- Deiting, V. , and Mischke R.. 2021. “Use of the ‘FreeStyle Libre’ Glucose Monitoring System in Diabetic Cats.” Research in Veterinary Science 135: 253–259. [DOI] [PubMed] [Google Scholar]
- Del Baldo, F. , Diana A., Canton C., Linta N., Chiocchetti R., and Fracassi F.. 2021. “The Influence of Skin Thickness on Flash Glucose Monitoring System Accuracy in Dogs With Diabetes Mellitus.” Animals 11, no. 2: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Baldo, F. , Fracassi F., Pires J., et al. 2021. “Accuracy of a Flash Glucose Monitoring System in Cats and Determination of the Time Lag Between Blood Glucose and Interstitial Glucose Concentrations.” Journal of Veterinary Internal Medicine 35, no. 3: 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez, R. , García J. J., Diez M. J., et al. 2013. “Hypoglycemic and Hypolipidemic Potential of a High Fiber Diet in Healthy Versus Diabetic Rabbits.” BioMed Research International 2013: 960568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxx, J. , Mans C., Strunk A., and Gasper D.. 2022. “Long‐Term Medical Management of Insulinoma in a Rabbit.” Journal of Exotic Pet Medicine 42: 47–49. [Google Scholar]
- Genua, I. , Sánchez‐Hernandez J., Martínez M. J., et al. 2021. “Accuracy of Flash Glucose Monitoring in Patients With Diabetes Mellitus on Hemodialysis and Its Relationship With Hydration Status.” Journal of Diabetes Science and Technology 15, no. 6: 1308–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt‐Brown, F. M. , and Harcourt‐Brown S. F.. 2012. “Clinical Value of Blood Glucose Measurement in Pet Rabbits.” Veterinary Record 170, no. 26: 674. [DOI] [PubMed] [Google Scholar]
- Howard, L. A. , Lidbury J. A., Jeffery N., Washburn S. E., and Patterson C. A.. 2021. “Evaluation of a Flash Glucose Monitoring System in Nondiabetic Dogs With Rapidly Changing Blood Glucose Concentrations.” Journal of Veterinary Internal Medicine 35, no. 6: 2628–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization . 2013. “ISO 15197:2013: In Vitro Diagnostic Test Systems—Requirements for Blood‐Glucose Monitoring Systems for Self‐Testing in Managing Diabetes Mellitus.” https://www.iso.org/standard/54976.html.
- Malerba, E. , Cattani C., Del Baldo F., et al. 2020. “Accuracy of a Flash Glucose Monitoring System in Dogs With Diabetic Ketoacidosis.” Journal of Veterinary Internal Medicine 34, no. 1: 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz, K. A. , Robertson S. A., and Wilson D. V.. 2017. “Alfaxalone Alone or Combined With Midazolam or Ketamine in Dogs: Intubation Dose and Select Physiologic Effects.” Veterinary Anaesthesia and Analgesia 44, no. 4: 766–774. [DOI] [PubMed] [Google Scholar]
- Neeley, W. E. 1972. “Simple Automated Determination of Serum or Plasma Glucose by a Hexokinase‐Glucose‐6‐Phosphate Dehydrogenase Method.” Clinical Chemistry 18: 509–515. [PubMed] [Google Scholar]
- Parkes, J. L. , Slatin S. L., Pardo S., and Ginsberg B. H.. 2000. “A New Consensus Error Grid to Evaluate the Clinical Significance of Inaccuracies in the Measurement of Blood Glucose.” Diabetes Care 23, no. 8: 1143–1148. [DOI] [PubMed] [Google Scholar]
- Selleri, P. , Di Girolamo N., and Novari G.. 2014. “Performance of Two Portable Meters and a Benchtop Analyzer for Blood Glucose Concentration Measurement in Rabbits.” Journal of the American Veterinary Medical Association 245, no. 1: 87–98. [DOI] [PubMed] [Google Scholar]
- Shea, E. K. , and Hess R. S.. 2021. “Validation of a Flash Glucose Monitoring System in Outpatient Diabetic Cats.” Journal of Veterinary Internal Medicine 35, no. 4: 1703–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, D. D. , Cecci G. R. M., Biz G., Chiaro F. N., and Zanutto M. S.. 2021. “Evaluation of a Flash Glucose Monitoring System in Dogs With Diabetic Ketoacidosis.” Domestic Animal Endocrinology 74: 106525. [DOI] [PubMed] [Google Scholar]
- Silva, K. G. , Rotta I., Costa L. B., and Sotomaior C. S.. 2020. “Comparison of 2 Portable Human Glucometers for the Measurement of Blood Glucose Concentration in White New Zealand Rabbits.” Journal of Veterinary Diagnostic Investigation 32, no. 5: 683–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist, S. K. , Zheng D., Al‐Rubeaan K., Luong J. H., and Sheu F. S.. 2011. “Technology Behind Commercial Devices for Blood Glucose Monitoring in Diabetes Management: A Review.” Analytica Chimica Acta 703, no. 2: 124–136. [DOI] [PubMed] [Google Scholar]
- Wada, K. , Masamune T., Ino H., et al. 2019. “Severe Hypoglycemia Reduces the Shivering Threshold in Rabbits.” BMC Anesthesiology 19, no. 1: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wan R., Mo Y., Zhang Q., Sherwood L. C., and Chien S.. 2010. “Creating a Long‐Term Diabetic Rabbit Model.” Experimental Diabetes Research 2010: 289614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmeyer, C. E. , Johnson P. J., Cohn L. A., et al. 2005. “Evaluation of a Continuous Glucose Monitoring System for Use in Veterinary Medicine.” Diabetes Technology & Therapeutics 7, no. 6: 885–895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


