Abstract
Purpose
This study investigated whether abnormal cerebral activity observed in adolescents and adults with ADHD also occurs in children during the early developmental stages of executive function.
Methods
The study included 52 children with ADHD aged 4.0–9.0 years and 34 healthy control children. Changes in oxygenated hemoglobin (HbO) levels were measured while participants completed GO/NOGO tasks to assess brain activation and connectivity.
Results
Children with ADHD demonstrated a stable prefrontal activation deficit during the GO/NOGO tasks (pFDR < 0.05). Additionally, hyperconnectivity was observed between the motor area and the prefrontal lobe in these children (uncorrected p <0.01). The logistic regression model incorporating brain activation and connectivity features achieved an area under the ROC curve of 0.86 (95% CI, [0.78, 0.95]), with a sensitivity of 0.79 and specificity of 0.85.
Conclusion
The findings suggest that prefrontal region abnormalities are present in children with ADHD at early developmental stages. This underscores the importance of targeting the prefrontal cortex in interventions and highlights the role of multi-network coordination in ADHD-related brain abnormalities. Limitations include the cross-sectional design and relatively small sample size, which should be addressed in future longitudinal studies.
Keywords: attention deficit hyperactivity disorder, fNIRS, execution function, brain network
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a neuro developmental disorder characterized by inattention, hyperactivity, and impulsiveness.1,2 ADHD symptoms often manifest in preschool children, with 5.5% - 7.6% estimated prevalence.3,4 Understanding the early characteristics of children with ADHD is of great significance for early identification and intervention.
The period between ages 4 and 9, during which executive functions develop rapidly, is particularly important for understanding ADHD’s neurodevelopmental trajectory. This stage is marked by significant maturation in brain regions related to executive functions, such as the prefrontal cortex. These regions undergo substantial structural and functional changes that enhance complex information processing. Understanding the specific neurodevelopmental changes in this age group is essential for early diagnosis and effective intervention in ADHD. A meta-analysis has revealed developmental delays in executive function among preschool children with ADHD.5 Executive function encompasses several components, including inhibition, working memory, and shifting. The GO/NOGO task and continuous performance task (CPT) are commonly used measures of executive function in both research and clinical settings. In the context of relatively simple response inhibition tasks, children’s executive functions improve rapidly from age 4 to mid-childhood.6 A recent large-scale study mapping brain development trajectories indicates that individual differences in the total volume of cortical gray matter peak at age 4.7 The overall volume of various cortical functional areas reaches its peak before the age of 10.7 Thus, during this period (age 4 to mid-childhood), the brain undergoes significant structural and functional maturation, enhancing the ability to process complex information.6,7 However, the characteristics of brain activity in children with ADHD aged 4–9 during this period of rapid executive function development remain unclear.
Functional near-infrared spectroscopy (fNIRS) offers a unique advantage over other neuroimaging techniques such as fMRI and EEG, especially in young children. Due to its robust resistance to motion artifacts and ease of use in naturalistic settings, fNIRS provides a non-invasive and ecologically valid method to measure brain activation during cognitive tasks.8 This makes it especially useful for studying children who may have difficulty complying with the requirements of fMRI or EEG, especially children with ADHD. Notably, a consistent hypoactivity within the right frontal lobe region during the execution of executive function tasks has been observed in children with ADHD.9 Furthermore, children with ADHD exhibit fewer instances of robust connectivity during a GO/NOGO task within the frontoparietal network and a prevalence of default network connectivity, primarily anchored in bilateral frontoparietal cortices, alongside heightened global brain connectivity.10 Employing a Flanker task, which demands inhibitory control, revealed atypical functional connectivity patterns in children with ADHD compared to their neurotypical counterparts, including the cerebellum, right inferior frontal gyrus, left dorsolateral prefrontal cortex, bilateral precentral gyrus, right supplementary motor area, and left precuneus.11 Moreover, the utilization of fNIRS signals during executive function tasks effectively discriminates between children with ADHD and healthy control group,12,13 underscoring its diagnostic utility in clinical settings.
fMRI studies on children and adults suggest that the neurobiological basis of ADHD stems from dysfunction across multiple brain networks and a reduced degree of segregation between these networks.14–16 Similar findings have emerged from fNRIS task-based studies on elementary school children with ADHD, highlighting imbalances among various brain networks centered in the frontal lobe. However, the clinical diagnosis of ADHD typically begins at age 4,17 yet neuroimaging studies on ADHD commence at age 6.9 Considering that children’s executive functions improve rapidly from age 4 to mid-childhood, there is a notable gap in research examining preschool children. Currently, there is a dearth of studies addressing cerebral activity changes during executive function tasks in this younger, less compliant demographic.
This study aims to investigate the cerebral activity of children with ADHD aged 4–9 when performing an executive function task. This study also explores the clinical applicability of fNIRS in assisting with diagnosing early childhood ADHD. We selected children aged 4.0–9.0 years as our study participants, including both children with ADHD and healthy controls. fNIRS was employed to record changes in oxygenated hemoglobin concentrations (HbO) as children completed the GO/NOGO task. We hypothesize that, behaviorally, children with ADHD will exhibit a higher number of errors. Regarding brain activation, we anticipate insufficient activation in the frontal lobe of children with ADHD. In terms of brain network connectivity, we expect children with ADHD to demonstrate hyperconnectivity in the motor areas.
Materials and Methods
Participants
Participants were recruited from children who were visited the Beijing Children Hospital from July 2022 to November 2023. Children with ADHD were recruited from the developmental behavioral clinic, and the inclusion criteria were as follows: (1) Diagnosis of ADHD according to the DSM-V by a clinician; (2) Intelligence Quotient (IQ) ≥ 80 on the Chinese-Wechsler Intelligence Scale for Children (C-WISC) or Level ≥ 3 on the Raven’s Progressive Matrice (RPM) or Developmental Quotient (DQ) ≥ 80 on the Children Neuropsychological and Behavior Scale(CNBS); (3) Never taken any central stimulants, atomoxetine or other psychiatric drugs; (4) Ages between 4.0 and 9.0 years old. Children in control group were recruited from the medical examination clinic, and the inclusion criteria were as follows: (1) The chief complaint does not include any attention issues; (2) IQ≥ 80 on the C-WISC or Level ≥ 3 on RPM or DQ ≥ 80 on CNBS; (3) Ages between 4.0 and 9.0 years old. To improve diagnostic reliability, all cases were selected following the unanimous agreement of a panel of experts, which included two psychiatrists and a psychologist. Exclusion criteria were: history of tics; neurological disorders, or sensory impairments (seizures or brain injury); mental health conditions including autism spectrum disorder, motor or communication disorders and Tourette’s syndrome.
The sample included 86 children aged between 4.0 and 9.0 years. The ADHD group comprised 52 participants (6.77 ± 1.19 years, 47 males), while the control group consisted of 34 participants (6.32 ± 1.48 years, 27 males). This study was reviewed and approved by the Beijing Children Hospital of Ethics Committee/Institutional Review Board. Informed consent was obtained from all participating children and their parents or legal guardians. All procedures performed in the study were in accordance with the ethical standards of the institutional and national research committee and with the 1964 helsinki declaration and its later amendments or comparable ethical standards.
The Audio-Visual Integration Response Inhibition Task
The test was adapted from the Go/NoGo paradigm and the Continuous Performance Test (CPT) paradigm to assess executive function in children under audio-visual conditions. Stimuli include pictures and sounds of kittens and puppies. To ensure younger children understood the task, a brief training session was conducted before the experiment. During this session, children practiced the task with simplified instructions and visual feedback, completing 8 practice trials. A trained examiner closely monitored each child for signs of confusion or difficulty in understanding, offering clarifications or breaks as needed. If comprehension issues were detected, the practice session was repeated up to two additional times. If the child did not achieve at least 6 correct responses (75%) in both practice sessions, they were not allowed to proceed to the formal testing phase. In the formal testing phase, children first complete the rest condition by quietly looking at the central fixation point on the screen for 30 seconds. They then alternated between two task conditions: “searching for animals” (Go condition) and “searching for puppies” (NoGo condition), each performed twice. In the Go condition, children responded by tapping the screen when they saw or heard any animal, reflecting general executive function ability. In the NoGo condition, they were instructed to respond only to pictures or sounds of puppies, assessing both executive function and inhibitory control. Each trial began with a 1-second fixation point followed by a stimulus, and children had 2 seconds to respond. If a response was made in time, the next trial commenced; otherwise, the stimulus disappeared after 2 seconds. A 12-second fixation interval was inserted between each condition. The entire task, including practice, took approximately 30 minutes. Behavioral data collected during the task included accuracy, mean reaction time, reaction time variability, omission errors, and commission errors.
fNIRS Data Acquisition and Processing
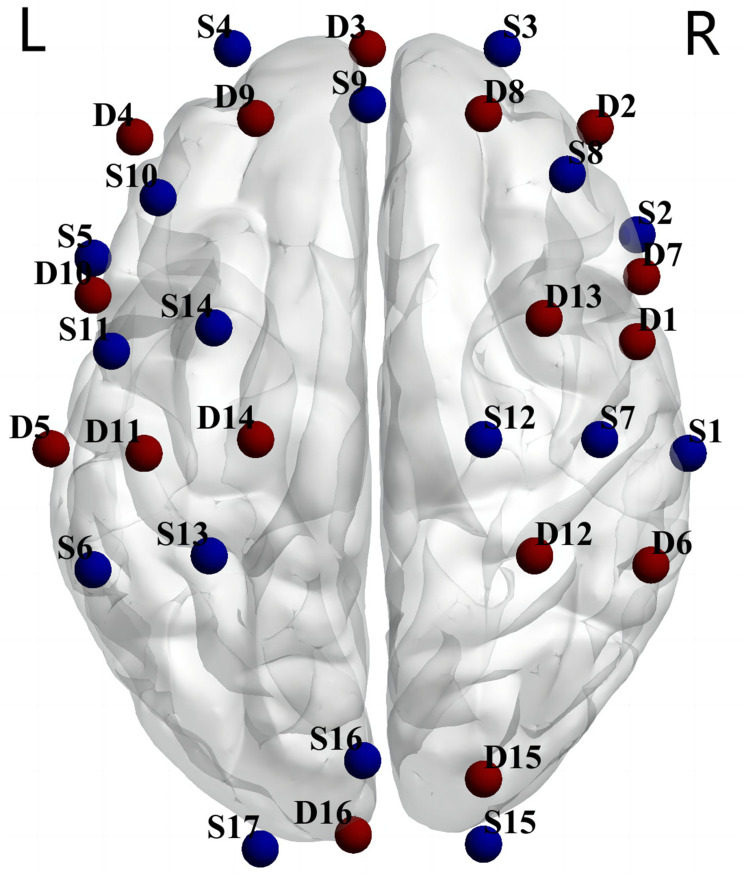
We acquired cortical neural activity by a 40-channel near-infrared optical imaging system (NirSmart-6000B, Danyang Huichuang Medical Equipment Co., Ltd, China) operating at three wavelengths (730/808/850 nm) with a sampling rate of 11 hz. An elastic cap containing 40 channels (15 light source optodes and 16 light detector probes) was arranged (see Figure 1), and placed on the head of each subject. The source-detector distance was 3 cm.
Figure 1.
The probe setting for 40-channel fNIRS.
Notes: S denotes source optode and D denotes detector probe.
The fNIRS data underwent preprocessing and analysis using the NIRS Brain AnalyzIR Toolbox.18 The data processing pipeline proceeded as follows: (1) conversion of raw fNIRS light intensity data to optical density, (2) application of wavelet-based motion correction to eliminate movement artifacts, (3) band-pass filtering (0.01–0.5Hz) to eliminate low-frequency noise (eg, data drift) and high-frequency noise such as (eg, cardiac signal), and (4) conversion to HbO and deoxygenated hemoglobin concentrations (HbR) using the modified Beer-Lambert law with a partial pathlength factor of 0.1. To specifically address movement artifacts in young children prone to excessive movement, a trained examiner closely monitored the participants throughout the task. During transitions between different task conditions, the examiner provided short breaks to help the children regain focus and minimize movement. During data preprocessing, visual inspection was used to detect segments with excessive motion. Participants showing substantial head movement or channel signal deviations exceeding 20% were excluded from further analysis. Given the superior signal-to-noise ratio of HbO compared to HbR, this study exclusively reports changes in HbO data.19–21
Statistical Analysis
We estimated task-evoked hemodynamic responses in each channel for two task phases by NIRS-SPM22 using a general linear model (GLM). The GLM analysis was utilized to assess brain activation in response to the tasks. In NIRS-SPM, we used the canonical hemodynamic response function (HRF) for modeling the HbO signals. To reduce high-frequency noise, we applied wavelet-based high-pass filtering. For prewhitening, we used autoregressive modeling (AR(1)). These settings were chosen to improve the robustness of hemodynamic response estimates and minimize the impact of noise artifacts. The GLM equation is as follows: “beta ~ −1 + group:cond +age+gender + (1|subject)”. Task-related activations were identified using a significance threshold of p < 0.05 (FDR-corrected). Pearson’s correlation coefficients between channels were calculated using preprocessed HbO concentration changes. To improve the normality of the correlation coefficients, we chose the z matrix (ie, Fisher’s r-to-z transformation) for the next calculation step. And the results obtained were used as functional connectivity (FC) values. The outcomes of the FC analysis reflect the functional connectivity of the brain during the tasks. The results from group-level analyses were visualized using the nirs2img function (https://www.alivelearn.net/?p=2230) to convert the t statistic values of significant channels and the corresponding MNI coordinates into *.img files. The converted images were then rendered over the 3D brain model using Brainnet Viewr.23 To comprehensively assess the effectiveness of brain activation and connectivity features as a diagnostic-aid tool, we employed exploratory factor analysis for dimensionality reduction of brain features, followed by logistic regression analysis. The predictive model was evaluated by Receiver Operating Characteristic (ROC) curve analysis.
Results
Behavioral Performance
Table 1 showed the behavioral scores of ADHD group and control group on the GO/NOGO task. Children with ADHD committed more omiss errors (t = 2.57, p = 0.012) and conmiss errors (t = 2.75, p = 0.008) in the NOGO condition compared to the control group.
Table 1.
Behavioral Differences in the GONOGO Task Between ADHD Group and Control Groups
| Index (condition) | ADHD Group | Control Group | T | p | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Accuracy (GO) | 0.97 | 0.04 | 0.98 | 0.04 | −1.46 | 0.149 |
| Accuracy (NOGO) | 0.92 | 0.10 | 0.96 | 0.04 | −2.75 | 0.008 |
| Omission errors (NOGO) | 2.90 | 2.99 | 1.59 | 1.74 | 2.57 | 0.012 |
| Commission errors (NOGO) | 5.33 | 6.18 | 2.71 | 2.43 | 2.75 | 0.008 |
Difference in Brain Activation
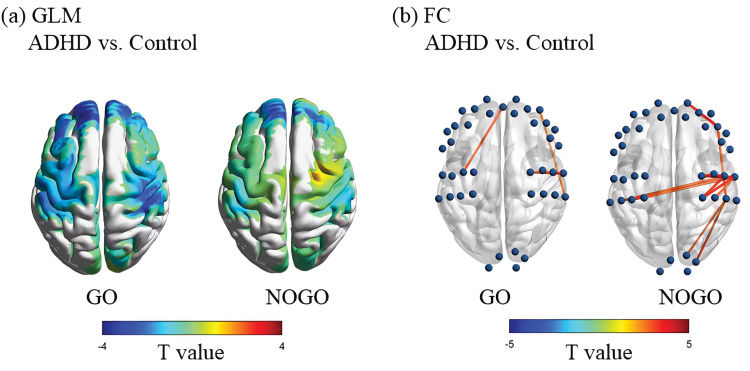
Figure 2(a) shows the difference in activation of different channels in GLM analysis between ADHD group and control children under GO and NOGO conditions, and only pFDR < 0.05 is reported in Table 2. Compared with the control group, children with ADHD showed insufficient activation in the GO condition in bilateral prefrontal regions (CH5, 6, 8, 10, 26, 27, 22, 23, 24, t = [−2.72,-4.84]), right superior marginal gyrus (CH16, t = −3.70), and left posterior central gyrus (CH32, t = −3.31). Compared with the control group, children with ADHD showed insufficient activation of bilateral prefrontal regions (CH21, 22, 23, 24, t= [−2.72,-4.84]) under NOGO conditions.
Figure 2.
Differences in brain activation and functional connectivity of brain areas during GONOGO tasks between ADHD and control groups.
Notes: (a) The colors of the brain regions in the figure represent the t-values from the general linear model (GLM) analysis comparing the ADHD group and the control group during the GO or NOGO conditions. (b) The blue spheres represent different channels, and the lines connecting the spheres indicate significant differences in functional connectivity (FC) between the two groups in these channels. The thickness of the lines reflects the strength of the t-values. Only channels with a p-value < 0.01 are shown.
Table 2.
Differences in Brain Region Activation in GLM Analysis Between ADHD and Control Groups
| Condition | Label of Channel | X | Y | Z | Brian Region (LPBA40) | β | T | p | pfdr |
|---|---|---|---|---|---|---|---|---|---|
| GO | CH5 (S3-D2) | 41 | 61 | 6 | R middle frontal gyrus | −15.56 | −2.8 | 0.006 | 0.026 |
| CH6 (S3-D3) | 16 | 72 | 12 | R middle frontal gyrus | −11.28 | −3.44 | 0.001 | 0.006 | |
| CH8 (S4-D3) | −15 | 72 | 12 | L middle frontal gyrus | −8.34 | −2.67 | 0.008 | 0.030 | |
| CH10 (S4-D9) | −28 | 62 | 26 | L middle frontal gyrus | −14.76 | −4.84 | <0.001 | <0.001 | |
| CH26 (S10-D9) | −35 | 45 | 38 | L middle frontal gyrus | −14.2 | −3.54 | 0.001 | 0.005 | |
| CH27 (S10-D10) | −53 | 27 | 36 | L middle frontal gyrus | −12.74 | −2.72 | 0.007 | 0.029 | |
| CH22 (S9-D3) | 2 | 64 | 27 | R superior frontal gyrus | −8.56 | −3.02 | 0.003 | 0.014 | |
| CH23 (S9-D8) | 14 | 57 | 41 | R middle frontal gyrus | −9.44 | −3.04 | 0.003 | 0.014 | |
| CH24 (S9-D9) | −12 | 57 | 42 | L superior frontal gyrus | −13.52 | −4.61 | <0.001 | <0.001 | |
| CH16 (S7-D6) | 57 | −29 | 56 | R supramarginal gyrus | −16.86 | −3.7 | <0.001 | 0.004 | |
| CH32 (S13-D11) | −43 | −30 | 70 | L postcentral gyrus | −15.8 | −3.31 | 0.001 | 0.008 | |
| NOGO | CH21 (S8-D8) | 35 | 47 | 37 | R middle frontal gyrus | −10.14 | −2.8 | 0.006 | 0.047 |
| CH22 (S9-D3) | 2 | 64 | 27 | R superior frontal gyrus | −8.75 | −3.45 | 0.001 | 0.014 | |
| CH23 (S9-D8) | 14 | 57 | 41 | R middle frontal gyrus | −11.02 | −3.92 | <0.001 | 0.005 | |
| CH24 (S9-D9) | −12 | 57 | 42 | L superior frontal gyrus | −8.45 | −3.17 | 0.002 | 0.024 |
Difference in Brain Functional Connectivity
Figure 2(b) presents the differential activation of various channels in the FC analysis between the ADHD and control groups under GO and NOGO conditions, with both Figure 2(b) and Table 3 reporting uncorrected p <0.01. During the GO condition, children with ADHD exhibited stronger connectivity compared to the control group in several areas: the right prefrontal area to the right superior marginal gyrus (CH5-CH2, t=2.64), the right anterior central gyrus to the right posterior central gyrus (CH31-CH1, t=3.43; CH31-CH15, t=2.89), and the left anterior central gyrus to the right prefrontal area (CH34-CH22, t=3.06). In the NOGO condition, children with ADHD showed significantly stronger internal connections within the right motor area (anterior and posterior central gyrus) (CH17-CH1, CH30-CH1, CH30-CH15, CH31-CH1, CH31-CH15, CH31-CH18, t=[2.76, 4.01]), stronger bilateral motor area connections (left posterior central gyrus to right posterior central gyrus) (CH32-CH1, CH32-CH15, CH33-CH1, t=[2.81, 3.56]), and enhanced connectivity from the right supramarginal gyrus to the right supraoccipital gyrus (CH36-CH16, t=2.87; CH38-CH16, t=2.75). Additionally, they exhibited stronger connections from the right marginal gyrus to the right prefrontal area (CH19-C16, t=2.92), from the left marginal gyrus to the right motor area (right posterior central gyrus) (CH14-CH1, t=3.50; CH15-CH14, t=2.81), and increased internal connectivity within the right prefrontal region (right inferior frontal gyrus to right middle frontal gyrus) (CH19-CH6, t=3.55).
Table 3.
Differences in FC Between ADHD and Control Groups
| Condition | Channel A | Channel B | Brian Region A | Brian Region B | T | p |
|---|---|---|---|---|---|---|
| GO | CH5 (S3-D2) | CH2 (S1-D6) | R middle frontal gyrus | R supramarginal gyrus | 2.64 | 0.010 |
| CH31 (S12-D13) | CH1 (S1-D1) | R precentral gyrus | R postcentral gyrus | 3.43 | 0.001 | |
| CH31 (S12-D13) | CH15 (S7-D1) | R precentral gyrus | R postcentral gyrus | 2.89 | 0.005 | |
| CH34 (S14-D11) | CH22 (S9-D3) | L precentral gyrus | R superior frontal gyrus | 3.06 | 0.003 | |
| NOGO | CH17 (S7-D12) | CH1 (S1-D1) | R postcentral gyrus | R postcentral gyrus | 2.76 | 0.007 |
| CH30 (S12-D12) | CH1 (S1-D1) | R postcentral gyrus | R postcentral gyrus | 3.36 | 0.001 | |
| CH30 (S12-D12) | CH15 (S7-D1) | R postcentral gyrus | R postcentral gyrus | 3.27 | 0.002 | |
| CH31 (S12-D13) | CH1 (S1-D1) | R precentral gyrus | R postcentral gyrus | 3.21 | 0.002 | |
| CH31 (S12-D13) | CH15 (S7-D1) | R precentral gyrus | R postcentral gyrus | 4.01 | <0.001 | |
| CH31 (S12-D13) | CH18 (S7-D13) | R precentral gyrus | R precentral gyrus | 3.04 | 0.003 | |
| CH32 (S13-D11) | CH1 (S1-D1) | L postcentral gyrus | R postcentral gyrus | 3.56 | 0.001 | |
| CH32 (S13-D11) | CH15 (S7-D1) | L postcentral gyrus | R postcentral gyrus | 3.00 | 0.004 | |
| CH33 (S13-D14) | CH1 (S1-D1) | L postcentral gyrus | R postcentral gyrus | 2.81 | 0.006 | |
| CH36 (S15-D15) | CH16 (S7-D6) | R superior occipital gyrus | R supramarginal gyrus | 2.87 | 0.005 | |
| CH38 (S16-D15) | CH16 (S7-D6) | R superior occipital gyrus | R supramarginal gyrus | 2.75 | 0.007 | |
| CH19 (S8-D2) | CH6 (S3-D3) | R inferior frontal gyrus | R middle frontal gyrus | 3.55 | 0.001 | |
| CH14 (S6-D11) | CH1 (S1-D1) | L supramarginal gyrus | R postcentral gyrus | 3.50 | 0.001 | |
| CH15 (S7-D1) | CH14 (S6-D11) | R postcentral gyrus | L supramarginal gyrus | 2.81 | 0.006 | |
| CH19 (S8-D2) | CH16 (S7-D6) | R inferior frontal gyrus | R supramarginal gyrus | 2.92 | 0.005 |
Specificity and Sensitivity of Brain Indicators in Predicting Group Outcomes
The exploratory factor analysis identified 15 brain activation indicators with significant intergroup differences from the GLM analysis (see Table 2), which could be reduced to 5 factors that cumulatively explained 76.18% of the variance. For the functional connectivity analysis, 18 brain activation indicators exhibiting significant intergroup differences (see Table 3) were also reduced to 4 factors, explaining 69.90% of the variance. The 5 factors derived from the brain activation indicators and the 4 factors from the connectivity indicators were used as predictor variables in the logistic regression model, with patient grouping serving as the response variable. The logistic regression model, which integrated brain activation and connectivity features, revealed an area under the ROC curve of 0.86 (95% CI, [0.78, 0.95]). The model demonstrated a sensitivity of 0.79 and a specificity of 0.85.
Discussion
The purpose of this study was to investigate the cerebral activity of children with ADHD aged 4–9 during the period of rapid executive function development when performing an executive function task. In terms of behavioral performance on the GO/NOGO task, consistent with our hypothesis, children with ADHD made more errors of omission and impulsivity in NOGO conditions requiring inhibitory control. In terms of brain activation, consistent with the conclusions of previous studies, children with ADHD showed insufficient activation of the frontal region in both GO and NOGO conditions. In terms of brain network connectivity, ADHD children showed hyperconnectivity, mainly characterized by internal hyperconnectivity in the right motor area, hyperlinks between bilateral motor areas, hyperlinks in the right margin back to the right suprachional gyrus, and internal hyperlinks in the right prefrontal area. The logistic regression model that integrated brain activation and connectivity features produced a strong area under the ROC curve of 0.86 (95% CI, [0.78, 0.95]), with a sensitivity of 0.79 and specificity of 0.85.
In the GO/NOGO task paradigm, the results of this study are consistent with previous studies. A qualitative analysis of fNIRS studies of participants with ADHD aged 6–16 showed a consistent hypoactivity in the prefrontal region in multiple executive function tasks.9 One intervention study found that the prefrontal hemodynamic increased when children with ADHD completed executive function tasks after taking atomoxetine,24 This suggests that the prefrontal functional activity measured by fNIRS can be considered as a target for executive function enhancement and drug intervention in children with ADHD.8 Previous studies have shown that commonly used ADHD medications can have certain side effects. For instance, methylphenidate may increase the risk of arrhythmias in children with ADHD.25 Transcranial direct current stimulation (tDCS) is a popular non-invasive brain stimulation technique used in ADHD interventions. Research indicates that tDCS can help improve effort maintenance and enhance task performance in children with ADHD, especially during activities requiring sustained attention. Stimulation of the right prefrontal cortex has been linked to increased effort maintenance when anticipating delayed rewards.26 Additionally, anodal stimulation of the left dorsolateral prefrontal cortex (DLPFC) has shown effects on executive control functions, such as working memory and interference inhibition.27
Although the prefrontal region is closely related to executive function, attention, and higher-order motor control, the abnormal brain development of children with ADHD is not limited to this region, but is related to the abnormal cooperation and dysfunction between different brain networks.28,29 In this study, while hyperactivation in the motor area was only observed under a more lenient threshold, previous studies using resting state data have shown hyperconnectivity in cortical areas, including prefrontal and motor areas. For instance, ADHD children under 12 years exhibit hyperconnectivity between cortical seeds and the middle frontal gyrus.30 Children with ADHD showed hyperconnectivity within the somatomotor network compared to healthy controls.31 In elementary school-age children, parent-rated inattentive symptoms correlate with increased connectivity between the frontal-motor striatum and regions of the default mode network.32 Unlike typically developing children, those with ADHD show age-related increases in functional connectivity between frontal corticostriatal circuits and the somatomotor network.32 Interhemispheric cortical inhibitory mechanisms are also compromised in children with ADHD.31,33 Abnormally strong interhemispheric somatomotor connectivity during rest is observed in school-age children with ADHD.31 Additionally, patients with ADHD exhibit significantly altered dynamic functional connectivity in the cingulo-opercular and sensorimotor networks.34 The sign of maturity of brain networks is functional specialization, that is, the increased degree of separation of different brain networks. In this study, it was found that there were short-range FC hyperconnections in the brain network of motor and frontal regions when children with ADHD completed executive function tasks, which may indicate abnormal synaptic pruning development in these regions. Long-range FC enhancement, such as superior marginal return to the visual area, motor area, and frontal area, may indicate compensation for abnormal myelination development in these areas in children with ADHD.
The clinical implications of this study can be summarized in three points. First, children in the early stages of ADHD show good acceptance of fNIRS equipment. By designing executive function tasks that align with children’s cognitive levels and operational habits, we can effectively collect fNIRS signals from them. Second, this study confirms that during the rapid development of executive function, the brain function and network connectivity in children with ADHD differ from those in typically developing children. Interestingly, we found significant hyperconnectivity in the motor cortex of children with ADHD, which aligns with clinical observations that hyperactivity symptoms are more pronounced in early childhood ADHD. Further research is needed to explore the relationship between motor cortex hyperconnectivity and behavioral manifestations, as well as developmental trajectories. Lastly, this study confirms that bilateral prefrontal cortex and bilateral motor cortex are promising targets for neurointervention therapy. While previous studies have utilized repetitive transcranial magnetic stimulation (rTMS) to activate the prefrontal cortex as an adjunct treatment for ADHD,35,36 our findings of hyperactivation in the motor cortex suggest a new potential target for neuromodulation.
Limitations and Strengths
The interpretation of the results should be approached with caution due to the limitations of this study. One key limitation is the small sample size, with fewer than 20 participants in each age group. This restricts our ability to capture the dynamic changes in cerebral blood oxygen activity across different ages in children with ADHD. Additionally, the study lacks extensive behavioral assessments, making it challenging to fully explore the relationship between brain activity and behavior. In our functional connectivity (FC) analysis, we used an uncorrected threshold of p < 0.01, based on the exploratory nature of this part of the study. Given the relatively small sample size and the preliminary goals of identifying potential connectivity patterns in ADHD, we opted for an uncorrected threshold to avoid overly conservative corrections that could obscure meaningful exploratory findings. We acknowledge that this choice may increase the risk of Type I errors, and we emphasize that our findings in FC analysis should be interpreted with caution. Future studies with larger samples will aim to validate these preliminary findings using more stringent correction methods.
The advantage of this study is that the sample included ADHD children aged 4–6 who had not been paid attention to in previous studies, and the GO/NOGO paradigm was adopted to investigate the brain activity of children when they completed executive function tasks. Functional connectivity analysis was first used in children with ADHD to interpret the characteristics of cerebral blood oxygen activity in children with ADHD from the perspective of brain network.
Conclusion
This study investigates the cerebral activity in children with ADHD during the early developmental stages of executive function. Our findings reveal that children aged 4 to 9 with ADHD showed increased errors of omission and impulsivity in conditions requiring inhibitory control, which aligns with our initial hypothesis regarding their behavioral performance. These children had extensive hypoactivity in frontal area, hyperconnection within motor area and prefrontal area, and hyperconnection between superior limbal gyrus and visual area, motor area and prefrontal area when completing executive control tasks. The logistic regression model that integrated brain activation and connectivity features produced a strong area under the ROC curve of 0.86 (95% CI, [0.78, 0.95]), with a sensitivity of 0.79 and specificity of 0.85. The results support the prefrontal region as one of the targets for intervention of ADHD brain abnormalities, and support the view that ADHD brain abnormalities are the result of multi-network coordination. Future research, including longitudinal studies, should focus on how these neural patterns change over time. It is also important to explore interventions that can specifically target the identified connectivity issues in ADHD, providing potential for more personalized treatments.
Funding Statement
This work is supported by STI 2030—Major Projects 2021ZD0200508, National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Danielson ML, Bitsko RH, Ghandour RM, Holbrook JR, Kogan MD, Blumberg SJ. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199–212. doi: 10.1080/15374416.2017.1417860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faraone SV, Banaschewski T, Coghill D, et al. The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. 2021;128:789–818. doi: 10.1016/j.neubiorev.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu A, Xu Y, Yan Q, Tong L. The prevalence of attention deficit/hyperactivity disorder among Chinese children and adolescents. Sci Rep. 2018;8(1):11169. doi: 10.1038/s41598-018-29488-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salari N, Ghasemi H, Abdoli N, et al. The global prevalence of ADHD in children and adolescents: a systematic review and meta-analysis. Ital J Pediatr. 2023;49(1):48. doi: 10.1186/s13052-023-01456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shephard E, Zuccolo PF, Idrees I, et al. Systematic review and meta-analysis: the science of early-life precursors and interventions for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2022;61(2):187–226. doi: 10.1016/j.jaac.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 6.Best JR, Miller PH. A developmental perspective on executive function. Child Development. 2010;81(6):1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethlehem RAI, Seidlitz J, White SR, et al. Brain charts for the human lifespan. Nature. 2022;604(7906):525–533. doi: 10.1038/s41586-022-04554-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grazioli S, Mauri M, Crippa A, et al. Light up ADHD: II. Neuropharmacological effects measured by near infrared spectroscopy: is there a biomarker? J Affective Disorders. 2019;244:100–106. doi: 10.1016/j.jad.2018.10.100 [DOI] [PubMed] [Google Scholar]
- 9.Gossé LK, Bell SW, Hosseini SMH. Functional near-infrared spectroscopy in developmental psychiatry: a review of attention deficit hyperactivity disorder. Eur Arch Psychiatry Clin Neurosci. 2022;272(2):273–290. doi: 10.1007/s00406-021-01288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutoko S, Monden Y, Tokuda T, et al. Atypical dynamic-connectivity recruitment in attention-deficit/hyperactivity disorder children: an insight into task-based dynamic connectivity through an fNIRS study. Front Human Neurosci. 2020;14. doi: 10.3389/fnhum.2020.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar U, Arya A, Agarwal V. Altered functional connectivity in children with ADHD while performing cognitive control task. Psychiatry Res-Neuroimag. 2022;326:111531. doi: 10.1016/j.pscychresns.2022.111531 [DOI] [PubMed] [Google Scholar]
- 12.Gu Y, Miao S, Zhang Y, Yang J, Li X. Compressibility analysis of functional near-infrared spectroscopy signals in children with attention-deficit/hyperactivity disorder. IEEE J Biomed Health Inform. 2023;27(11):5449–5458. doi: 10.1109/JBHI.2023.3303470 [DOI] [PubMed] [Google Scholar]
- 13.Yang CM, Shin J, Kim JI, Lim YB, Park SH, Kim BN. Classifying children with ADHD based on prefrontal functional near-infrared spectroscopy using machine learning. Clin Psychopharmacol Neurosci. 2023;21(4):693–700. doi: 10.9758/cpn.22.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duffy KA, Rosch KS, Nebel MB, et al. Increased integration between default mode and task-relevant networks in children with ADHD is associated with impaired response control. Develop Cognitive Neurosci. 2021;50:100980. doi: 10.1016/j.dcn.2021.100980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JS, Monaghan A, Leyland-Craggs A, Astle DE. Testing the triple network model of psychopathology in a transdiagnostic neurodevelopmental cohort. Neuroimage-Clin. 2023;40:103539. doi: 10.1016/j.nicl.2023.103539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman LJ, Sudre G, Price J, Shastri GG, Shaw P. Evidence from “big data” for the default-mode hypothesis of ADHD: a mega-analysis of multiple large samples. Neuropsychopharmacol. 2023;48(2):281–289. doi: 10.1038/s41386-022-01408-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolraich ML, Hagan JF, Allan C, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2019;144(4):e20192528. doi: 10.1542/peds.2019-2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santosa H, Zhai X, Fishburn F, Huppert T. The NIRS brain analyzIR toolbox. Algorithms. 2018;11(5):73. doi: 10.3390/a11050073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng X, Li X, Hu Y. Synchronous brain activity during cooperative exchange depends on gender of partner: a fNIRS‐based hyperscanning study. Human Brain Mapp. 2015;36(6):2039–2048. doi: 10.1002/hbm.22754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49(5):765–775. doi: 10.1016/j.neuron.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 21.Peng XR, Bundil I, Schulreich S, Li SC. Neural correlates of valence-dependent belief and value updating during uncertainty reduction: an fNIRS study. NeuroImage. 2023;279:120327. doi: 10.1016/j.neuroimage.2023.120327 [DOI] [PubMed] [Google Scholar]
- 22.Ye J, Tak S, Jang K, Jung J, Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44(2):428–447. doi: 10.1016/j.neuroimage.2008.08.036 [DOI] [PubMed] [Google Scholar]
- 23.Xia M, Wang J, He Y. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi: 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ota T, Iida J, Nakanishi Y, et al. Increased prefrontal hemodynamic change after atomoxetine administration in pediatric attention-deficit/hyperactivity disorder as measured by near-infrared spectroscopy. Psychiatry Clin Neurosci. 2015;69(3, SI):161–170. doi: 10.1111/pcn.12251 [DOI] [PubMed] [Google Scholar]
- 25.Tanır Y, Erbay MF, Özkan S, Özdemir R, Örengül AC. The effects of methylphenidate on ventricular repolarization parameters in children with attention-deficit hyperactivity disorder. Alpha Psychiatry. 2023;24(5):174–179. doi: 10.5152/alphapsychiatry.2023.231185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vöckel J, Kühnel A, Rossberg R, et al. Transcranial direct current stimulation enhances effort maintenance in ADHD. Brain Stimulation. 2024;17(4):899–906. doi: 10.1016/j.brs.2024.07.018 [DOI] [PubMed] [Google Scholar]
- 27.Nejati V, Salehinejad MA, Nitsche MA, Najian A, Javadi AH. Transcranial direct current stimulation improves executive dysfunctions in ADHD: implications for inhibitory control, interference control, working memory, and cognitive flexibility. J Atten Disord. 2020;24(13):1928–1943. doi: 10.1177/1087054717730611 [DOI] [PubMed] [Google Scholar]
- 28.Cai W, Chen T, Szegletes L, Supekar K, Menon V. Aberrant time-varying cross-network interactions in children with attention-deficit/hyperactivity disorder and the relation to attention deficits. Biol Psych. 2018;3(3):263–273. doi: 10.1016/j.bpsc.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai W, Griffiths K, Korgaonkar MS, Williams LM, Menon V. Inhibition-related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Mol Psychiatry. 2021;26(8):4016–4025. doi: 10.1038/s41380-019-0564-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu N, Liu Q, Yang Z, et al. Different functional alteration in attention‐deficit/hyperactivity disorder across developmental age groups: a meta‐analysis and an independent validation of resting‐state functional connectivity studies. CNS Neurosci Ther. 2023;29(1):60–69. doi: 10.1111/cns.14032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, Lidstone D, Crocetti D, Mostofsky SH, Nebel MB. Increased interhemispheric somatomotor functional connectivity and mirror overflow in ADHD. Neuroimage-Clin. 2021;31:102759. doi: 10.1016/j.nicl.2021.102759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikolaidis A, He X, Pekar J, Rosch K, Mostofsky SH. Frontal corticostriatal functional connectivity reveals task positive and negative network dysregulation in relation to ADHD, sex, and inhibitory control. Develop Cognitive Neurosci. 2022;54:101101. doi: 10.1016/j.dcn.2022.101101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu ZM, Bralten J, Cao QJ, et al. White matter microstructural alterations in children with ADHD: categorical and dimensional perspectives. Neuropsychopharmacol. 2017;42(2):572–580. doi: 10.1038/npp.2016.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Lan Z, Xue SW, et al. Brain state-dependent dynamic functional connectivity patterns in attention-deficit/hyperactivity disorder. J Psychiatr Res. 2021;138:569–575. doi: 10.1016/j.jpsychires.2021.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Liang SC, Sun CK, et al. A meta-analysis on the therapeutic efficacy of repetitive transcranial magnetic stimulation for cognitive functions in attention-deficit/hyperactivity disorders. BMC Psychiatry. 2023;23(1):756. doi: 10.1186/s12888-023-05261-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagy NAS, Amin GR, Khalil SA, Mahmoud DAM, Elkholy H, Shohdy M. The therapeutic role of repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder in Egypt a randomized sham controlled clinical trial. Middle East Curr Psychiatry. 2022;29(1):55. doi: 10.1186/s43045-022-00210-3 [DOI] [Google Scholar]