Abstract
Focal status epilepticus, particularly the motor variant of epilepsia partialis continua (EPC), is a rare condition characterized by near‐continuous, chronic focal motor seizures, and associated with poor outcomes. Medications, including anesthetics, are often unsuccessful. Surgical resection can result in motor deficits. We report a medically complex pediatric case of super‐refractory EPC that was successfully managed with combined focal resection and responsive neuromodulation. This case introduces neuromodulation as a treatment modality for this challenging condition.
Introduction
Status epilepticus is a medical emergency, with a cumulative incidence of 3–42 episodes per 100,000 1 , 2 children. Focal motor status epilepticus involving the primary motor cortex includes epilepsia partialis continua (EPC), characterized by prolonged, focal myoclonic activity that can persist for years. 3 , 4 , 5 In adults, EPC is associated with neurologic decline in 36% of patients. 6 Pediatric EPC is associated with significant neurological consequences in 65% and lethal outcomes in 16%. 3 The most common cause of pediatric EPC is Rasmussen encephalitis or other inflammatory and immune‐mediated disorders, followed by mitochondrial disorders, and malformations of cortical development. 3 , 7 , 8 , 9
Approximately 15% of cases of status epilepticus are super‐refractory to medical management despite escalation to anesthetics for at least 24 h. 10 Due to concerns for permanent neuronal damage, efforts are made to stop the ictal activity but can be difficult to achieve. 11 , 12 , 13 Novel therapies to treat status epilepticus have been reported in adult 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 and pediatric populations, including vagal nerve stimulation (VNS), 14 , 15 cortical stimulation, 14 , 23 transcranial magnetic stimulation (TMS), 14 , 16 , 21 electroconvulsive therapy (ECT), 14 , 17 and thalamic deep brain stimulation (DBS). 14 , 24 , 25
The responsive neurostimulator (RNS) is a closed‐loop brain stimulation device FDA‐approved to treat chronic multifocal epilepsy in adults. 26 , 27 , 28 Typical use involves device implantation followed by months or years of outpatient parameter adjustment for optimal seizure control. In adults, the median reduction in seizures was 44% at 1 year, 53% at 2 years, 60% at 3 years, and 75% at 9 years. 29 , 30 RNS has rarely been used off‐label in adults to treat super‐refractory status epilepticus. A recent case series reported acute RNS implantation and initiation in 10 adult patients with refractory status epilepticus: 70% had resolution of status epilepticus, though two patients died of status epilepticus complications and one had a recurrence. 31
Although not FDA‐approved for use in pediatric populations, the RNS device has been reported to have similar or improved long‐term efficacy and safety when used to treat drug‐refractory epilepsy in children. 32 , 33 Building on this, we present a case of successful treatment of super‐refractory focal motor status epilepticus in a child using combined limited focal resection and acute RNS implantation and treatment. We review the details of the case and the stimulation parameters used that led to seizure control. Importantly, this reversible, non‐destructive treatment targeting eloquent cortex, in combination with resection targeting non‐eloquent cortex, led to sustained seizure control and avoided the morbidities associated with a larger resection. Tailored, multimodal approaches can be considered as an option for children with similar presentations in the future.
Case
Our patient is a 6‐year‐old left‐handed female with a complex history of diffuse left hemispheric polymicrogyria and pachygyria, maternally inherited DYNC1H1 variant (cytoplasmic dynein 1 heavy chain), mild right hemiparesis, mild global developmental delay, near‐continuous epileptiform discharges during sleep localized to the left frontopolar region, and drug‐refractory daily brief focal motor seizures localized to the left centroparietal region. She had no prior history of clinical status epilepticus. Baseline structural and electrophysiologic imaging are shown in Figure 1.
Figure 1.
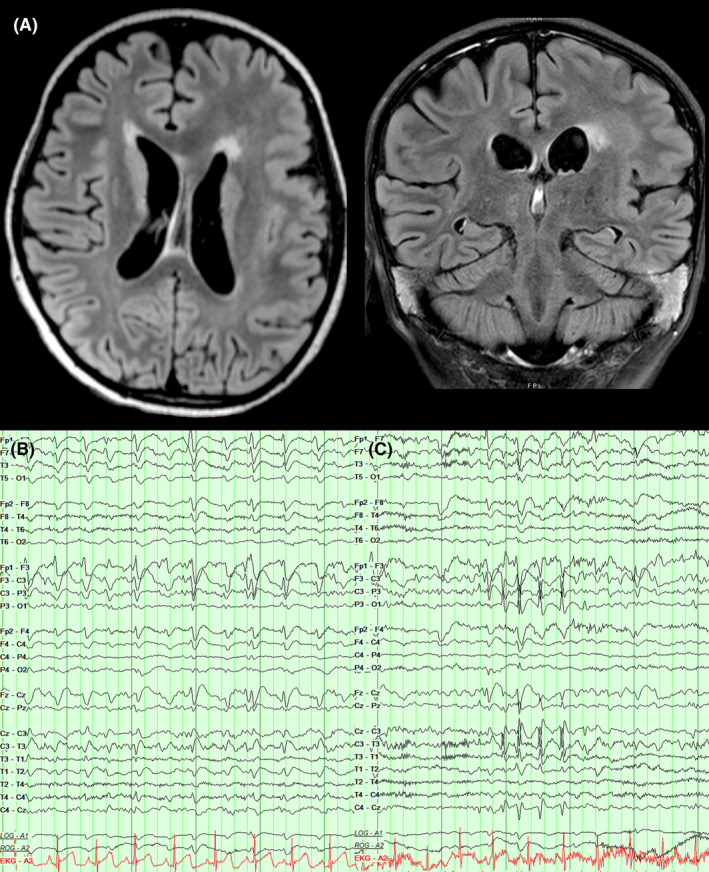
Baseline structural and electrographic findings. (A) Axial and coronal T2 FLAIR images demonstrating diffuse left frontal pachygyria and polymicrogyria. (B) EEG recording 2 months prior to status presentation revealed abundant left frontopolar epileptiform discharges that were continuous at 2 Hz during non‐rapid eye movement sleep. (C) EEG recording 2 months prior to status presentation also revealed bursts of left central fast activity and spikes that coincided with right hand and face myoclonic seizures.
The patient developed continuous right thumb twitching that escalated to include right face and arm jerking. She was evaluated in a local hospital, diagnosed with focal status epilepticus, and treated with escalating medications, including lorazepam, fosphenytoin, levetiracetam, lacosamide, topiramate, midazolam, ketamine, and phenobarbital without response. She was intubated and burst‐suppressed on propofol, which was terminated after 48 h due to the development of fever, metabolic acidosis, rhabdomyolysis, cardiac arrhythmia, and transaminitis consistent with propofol infusion syndrome. Her course was further complicated by COVID‐19 and subsequent multisystem inflammatory syndrome in children, treated with a 8 days of dexamethasone (0.15 mg/kg), IVIG (2 g/kg) and anakinra (15 mg/kg). Her focal status epilepticus persisted and local epilepsy consultation recommended hemispherotomy. On hospital day 19, she was transferred to our institution.
Neurological examination revealed near‐continuous right‐sided blinking, right facial twitching, tongue biting, and right hand, thumb, and finger contraction. At the time of transfer, she was treated with levetiracetam (74.1 mg/kg/day), valproic acid (22.6 mg/kg/day), phenobarbital (5.7 mg/kg/day), diazepam (0.4 mg/kg/day). Midazolam (0.2 mg/kg/h) and lorazepam (0.4 mg/kg/day) were added and diazepam (0.4 mg/kg/day) and clonazepam (0.2 mg/kg/day) were cross‐tapered. She was treated with solumedrol (30 mg/kg) for 5 days without effect. Video EEG demonstrated 2–5 Hz left frontal discharges time‐locked to her myoclonic jerks. Ictal PET demonstrated hypermetabolism diffusely throughout the left hemisphere, but most prominently in the left frontal region. Preoperative functional and electrophysiological imaging is shown in Figure 2.
Figure 2.
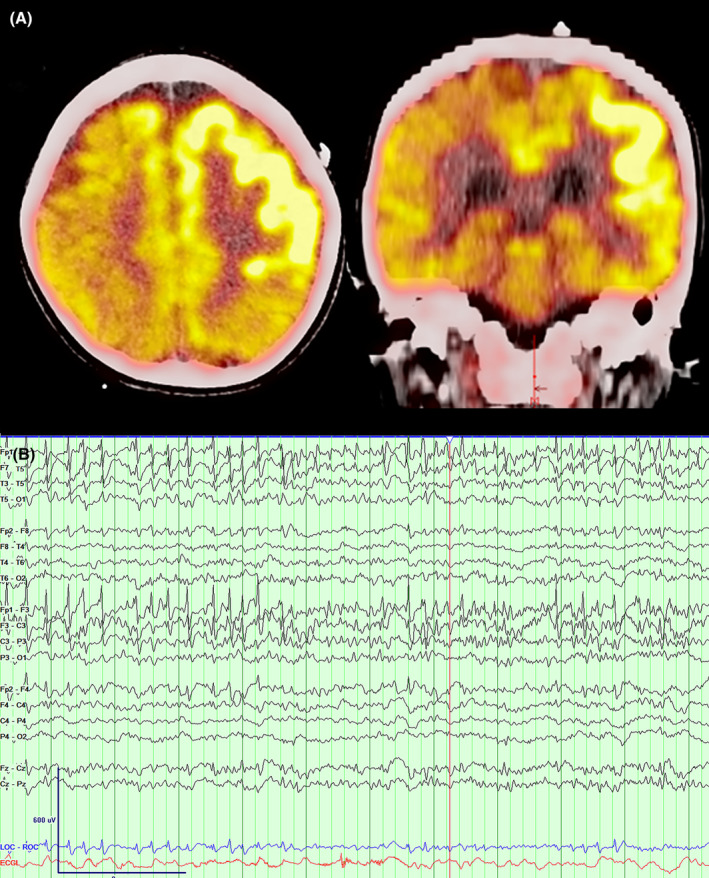
Perioperative functional and electrophysiological imaging. (A) Axial and coronal PET imaging reveal ictal hypermetabolism diffusely over the left frontal region. (B) EEG 1 week prior to surgical intervention reveals focal status over the left hemisphere with a dominant epileptic focus over the left frontopolar region.
After a multidisciplinary epilepsy surgery conference discussion, the patient was suspected to have two independent epileptogenic zones: a frontopolar focus and a focus over the primary motor cortex. She underwent resection of the frontopolar ictal focus guided by intra‐operative electrocorticography, and an RNS was implanted with two depth electrodes targeting presumed superior and inferior motor cortex identified by intraoperative sensorimotor mapping (Fig. 3). Immediately, post‐operatively, she was seizure‐free. However, on post‐operative day (POD) 1, the patient developed frequent right‐hand contractions, right facial jerking and tongue biting. Over the first 9 post‐operative days, antiseizure medication changes included lacosamide initiation (11 mg/kg/day), levetiracetam increase (100 mg/kg/day), and clonazepam decrease (0.09 mg/kg/day); each without a notable change in clinical seizures. After post‐operative day 9, antiseizure medications remained stable or were decreased due to inefficacy. RNS recordings revealed abundant epileptic spikes maximal over presumed primary motor cortex contacts (channels 1 and 3). Lead‐to‐lead neurostimulation at these contacts resulted in worsened myoclonus. Lead‐to‐lead stimulation over the contacts estimated to be immediately anterior to the primary motor cortex (channels 2 and 4) was tolerated and initiated on POD1 with a second treatment using a grouped bipolar stimulation between channels 1 and 2. We used a non‐specific detector at channels 1 and 3 resulting in ~4500 stimulations/day. Current was increased by 0.5 uC/cm2 every 2–3 days and frequency increased from 40 to 333 Hz over the following 24 days. Using the limited observations available, adjustments that increased total energy delivered appeared to correspond best to reduced activity. For each programming session, several test stimulations were delivered and confirmed to be tolerated without after‐discharges or clinical events. Detected events that triggered stimulation increased over time and long episodes estimated to reflect longer periods of epileptic activity decreased over time (Fig. 4). On post‐operative day 24, when treatment reached an estimated charge density of 4.1 μC/cm2 (4 mA, 333.3 Hz, 160 μs pulse width, 200 ms burst duration), the EPC stopped. The patient successfully tapered off multiple antiseizure medications, returned to baseline cognitive and motor function, and remained clinically seizure‐free. Occasional myoclonus was observed when a levetiracetam taper was attempted at 11 months post‐operatively. An adjustment to a full lead‐to‐lead stimulation across all channels (channels 1 and 2 to channels 3 and 4) was now tolerated, and a levetiracetam taper was successfully reattempted. Aside from this breakthrough event, she has remained seizure‐free for over 17 months with only occasional epileptiform activity observed on RNS recordings.
Figure 3.
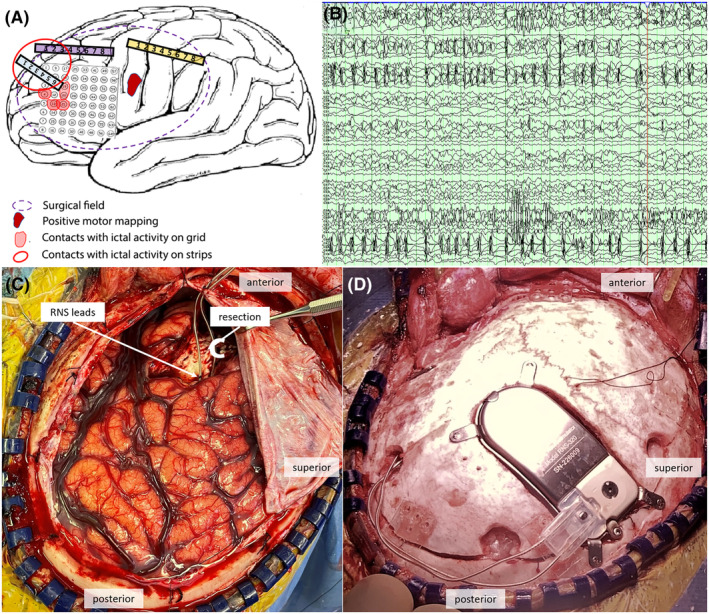
Operative data and treatment. (A) Diagram summarizing the intraoperative recordings and results. (B) Example ictal intraoperative recording localized the ictal focus for resection. (C) Frontopolar resection focus and RNS lead placement targeting motor cortex. (D) Neurostimulator placement.
Figure 4.
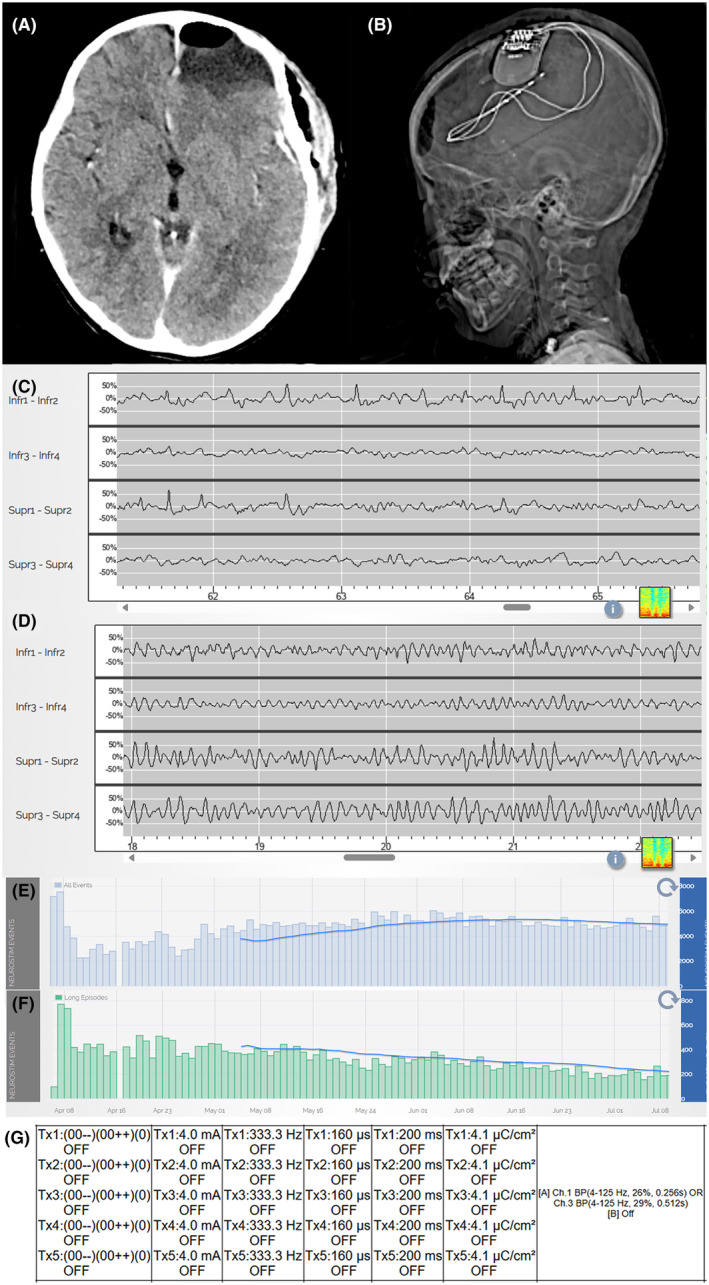
Surgical and neuromodulatory treatment. (A) Post‐operative CT reveals resection of left frontopolar ictal focus. (B) RNS placement with two depth electrodes targeting motor cortex. (C) RNS recording on POD1 shows abundant epileptic spikes maximal in channels 1 and 3, which were often time‐locked to right facial and right‐hand myoclonic activity. (D) RNS recording on POD 30 shows absent epileptiform activity. (E) Using a non‐specific detector, abundant events were detected resulting in ~4500 stimulations per day. (F) Longer bursts of epileptiform activity, recognized as long events, decreased with time. (G) Final stimulation settings that corresponded with resolution of epilepsia partialis continua.
Discussion
We report a pediatric case of super‐refractory focal motor status epilepticus consistent with EPC that responded to a combination of limited resection and acute treatment with RNS. This case demonstrates the potential role of neuromodulation to achieve seizure control in the acute setting. Use of this non‐destructive surgical treatment in eloquent brain targets avoids the functional morbidities that would result from alternate, destructive options. In EPC, faster adjustments and higher neurostimulation settings than used in the outpatient setting may be required.
Although RNS remains an off‐label treatment option in children, the nondestructive and reversible nature of neuromodulation mitigates risks and motivates its use. In our case, although hemispherotomy would likely have resulted in seizure freedom, this neurosurgical cure would have introduced a new permanent hemiplegia and hemifield defect. In contrast, the use of a tailored approach, with a combined limited resection of non‐eloquent cortex and neuromodulation in eloquent cortex, enabled our patient to recover to her preoperative functional baseline.
Although long‐term remodeling likely contributes to the efficacy of neuromodulatory treatment in the outpatient population, 26 , 30 in the setting of status epilepticus, an immediate, abortive response is the goal. The typical outpatient treatment protocol following RNS includes approximately 1 month of recording without stimulation, followed by stimulation adjustments every 3 months. 26 Here, we increased current every 2–3 days and seizure control was obtained in 3.5 weeks, consistent with prior experience in adults. 31 It is possible that even faster uptitration may have achieved faster results. In addition, although lower stimulation currents are typically used adjacent to the motor cortex, with 0.5 μC/Cm2 used in the prior adult case report of EPC, 34 here we escalated treatment to 4.1 μC/Cm2 (4.0 mA) in contacts adjacent to the primary motor cortex. This charge density coincided with resolution of status epilepticus and caused no observed after‐discharges, discomfort, myoclonus, or other evident side effects. Overall, our experience supports the application of acute RNS treatment with settings tailored based on patient tolerance and response. 34 Although optimal parameter settings remain an area of active research, here we used high frequency stimulation (333 Hz) and a relatively non‐specific detector, which resulted in a relatively high stimulation rate and total energy delivered. 26 , 30 , 35 , 36
Despite the positive outcome we observed in this case, there are several limitations. Multiple treatments were offered simultaneously, confounding causal analysis. However, the resolution of status epilepticus by post‐op day 24 concordant with RNS uptitration while antiseizure medication was being weaned is consistent with a response to neurostimulation. It is possible that the patient's focal status epilepticus self‐resolved. However, the presence of persistent, intermittent epileptiform activity on her RNS recordings and the improvement in seizure control even compared to her premorbid baseline suggest that RNS is contributing to obtaining and sustaining seizure freedom. Finally, it remains unclear which stimulation parameters were most effective or whether they would generalize to other cases.
This case suggests that acute, aggressive RNS treatment can be considered in drug‐refractory pediatric status epilepticus. Future work to test detection and stimulation parameters is required to further optimize outcomes and response in pediatric status epilepticus.
Author Contributions
PH and CJC wrote the manuscript. All authors edited and approved the manuscript.
Conflict of Interest
CJC provides consulting to Biogen Inc, Ovid Pharmaceuticals, Novartis, Ionis Pharmaceuticals. RMR provides consulting to Neuropace.
Acknowledgments
We would like to thank our patient and family for agreeing to share their story, Wendy Wan and Mike Nozzilillo for their support during RNS programming; our EEG technologists for their assistance; the many MGH and OSH staff who participated in the care of this patient.
Funding Information
NINDS: R01NS119483
Funding Statement
This work was funded by NINDS grant R01NS119483.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions, since this is a case report about a specific patient. Some of the data that support the findings of this study can be made available on request from the corresponding author and with permission of the patient and their family. Portions of the data, specifically the RNS recordings that support the findings of this study are available from Neuropace. Restrictions apply to the availability of these data per Neuropace.
References
- 1. Leitinger M, Trinka E, Giovannini G, et al. Epidemiology of status epilepticus in adults: a population‐based study on incidence, causes, and outcomes. Epilepsia. 2019;60(1):53‐62. doi: 10.1111/epi.14607. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gurcharran K, Grinspan ZM. The burden of pediatric status epilepticus: epidemiology, morbidity, mortality, and costs. Seizure. 2019;68:3‐8. [DOI] [PubMed] [Google Scholar]
- 3. Kravljanac R, Djuric M, Jovic N, et al. Etiology, clinical features and outcome of epilepsia partialis continua in cohort of 51 children. Epilepsy Res. 2013;104(1–2):112‐117. [DOI] [PubMed] [Google Scholar]
- 4. Mameniškienė R, Wolf P. Epilepsia partialis continua: a review. Seizure. 2017;44:74‐80. [DOI] [PubMed] [Google Scholar]
- 5. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus – report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56(10):1515‐1523. doi: 10.1111/epi.13121. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 6. Cockerell OC, Rothwell J, Thompson PD, et al. Clinical and physiological features of epilepsia partialis continua: cases ascertained in the UK. Brain. 1996;119(2):393‐407. doi: 10.1093/brain/119.2.393 [DOI] [PubMed] [Google Scholar]
- 7. Bien CG, Elger CE. Epilepsia partialis continua: semiology and differential diagnoses. Epileptic Disord. 2008;10(1):3‐7. https://pubmed.ncbi.nlm.nih.gov/18367424/ [DOI] [PubMed] [Google Scholar]
- 8. Bien CG, Granata T, Antozzi C, et al. Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain. 2005;128(3):454‐471. doi: 10.1093/brain/awh415 [DOI] [PubMed] [Google Scholar]
- 9. Sinha S, Satishchandra P. Epilepsia Partialis continua over last 14 years: experience from a tertiary care center from south India. Epilepsy Res. 2007;74(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 10. Shorvon S, Ferlisi M. The treatment of super‐refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(10):2802‐2818. doi: 10.1093/brain/awr215 [DOI] [PubMed] [Google Scholar]
- 11. Fujikawa DG. Programmed mechanisms of status epilepticus‐induced neuronal necrosis. Epilepsia Open. 2023;8(S1):S25‐S34. doi: 10.1002/epi4.12593. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine‐induced status epilepticus. Brain Res. 1996;725(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 13. Wasterlain CG, Fujikawa DG, Penix LR, Sankar R. Pathophysiological mechanisms of brain damage from status epilepticus. Epilepsia. 1993;34:S37‐S53. doi: 10.1111/j.1528-1157.1993.tb05905.x. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 14. Trinka E, Brigo F. Neurostimulation in the treatment of refractory and super‐refractory status epilepticus. Epilepsy Behav. 2019;101:106551. [DOI] [PubMed] [Google Scholar]
- 15. Dibué‐Adjei M, Brigo F, Yamamoto T, et al. Vagus nerve stimulation in refractory and super‐refractory status epilepticus – a systematic review. Brain Stimul. 2019;12(5):1101‐1110. [DOI] [PubMed] [Google Scholar]
- 16. Rotenberg A, Bae EH, Takeoka M, et al. Repetitive transcranial magnetic stimulation in the treatment of epilepsia partialis continua. Epilepsy Behav. 2009;14(1):253‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeiler FA, Matuszczak M, Teitelbaum J, et al. Electroconvulsive therapy for refractory status epilepticus: a systematic review. Seizure. 2016;35:23‐32. [DOI] [PubMed] [Google Scholar]
- 18. Valentin A, Ughratdar I, Cheserem B, Morris R, Selway R, Alarcon G. Epilepsia partialis continua responsive to neocortical electrical stimulation. Epilepsia. 2015;56(8):e104‐e109. doi: 10.1111/epi.13067. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 19. Ahmed J, Metrick M, Gilbert A, et al. Electroconvulsive therapy for super refractory status epilepticus. J ECT. 2018;34(1):5‐9. https://journals.lww.com/ectjournal/fulltext/2018/03000/electroconvulsive_therapy_for_super_refractory.19.aspx [DOI] [PubMed] [Google Scholar]
- 20. Valentín A, Nguyen HQ, Skupenova AM, et al. Centromedian thalamic nuclei deep brain stimulation in refractory status epilepticus. Brain Stimul. 2012;5(4):594‐598. [DOI] [PubMed] [Google Scholar]
- 21. Zeiler FA, Matuszczak M, Teitelbaum J, et al. Transcranial magnetic stimulation for status epilepticus. Epilepsy Res Treat 2015;2015:678074. /pmc/articles/PMC4670661/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhys Potter A, Hallal‐Peche F, Stavropoulos I, et al. Potential predictive value of repetitive transcranial magnetic stimulation before chronic cortical stimulation for epilepsia partialis continua. Brain Stimul. 2023;16(1):71‐74. https://pubmed.ncbi.nlm.nih.gov/36640829/ [DOI] [PubMed] [Google Scholar]
- 23. Child ND, Stead M, Wirrell EC, et al. Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia. 2014;55(3):e18‐e21. doi: 10.1111/epi.12525. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 24. Lehtimäki K, Långsjö JW, Ollikainen J, et al. Successful management of super‐refractory status epilepticus with thalamic deep brain stimulation. Ann Neurol. 2017;81(1):142‐146. doi: 10.1002/ana.24821. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 25. Sa M, Singh R, Pujar S, et al. Centromedian thalamic nuclei deep brain stimulation and Anakinra treatment for FIRES – two different outcomes. Eur J Paediatr Neurol. 2019;23(5):749‐754. [DOI] [PubMed] [Google Scholar]
- 26. Heck CN, King‐Stephens D, Massey AD, et al. Two‐year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432‐441. doi: 10.1111/epi.12534. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jarosiewicz B, Morrell M. The RNS system: brain‐responsive neurostimulation for the treatment of epilepsy. Expert Rev Med Devices. 2021;18(2):129‐138. doi: 10.1080/17434440.2019.1683445. https://www.tandfonline.com/doi/abs/ [DOI] [PubMed] [Google Scholar]
- 28. Sun FT, Morrell MJ, Wharen RE. Responsive cortical stimulation for the treatment of epilepsy. Neurotherapeutics. 2008;5(1):68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergey GK, Morrell MJ, Mizrahi EM, et al. Long‐term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nair DR, Morrell MJ, Skarpaas TL, et al. Nine‐year prospective efficacy and safety of brain‐responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):E1244‐E1256. https://pubmed.ncbi.nlm.nih.gov/32690786/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ernst LD, Raslan AM, Wabulya A, et al. Responsive neurostimulation as a treatment for super‐refractory focal status epilepticus: a systematic review and case series. J Neurosurg. 2023;140(1):201‐209. https://thejns.org/view/journals/j‐neurosurg/140/1/article‐p201.xml [DOI] [PubMed] [Google Scholar]
- 32. Kerezoudis P, Gyftopoulos A, Alexander AY, et al. Safety and efficacy of responsive neurostimulation in the pediatric population: evidence from institutional review and patient‐level meta‐analysis. Epilepsy Behav. 2022;129:108646. [DOI] [PubMed] [Google Scholar]
- 33. Nanda P, Sisterson N, Walton A, et al. Centromedian region thalamic responsive neurostimulation mitigates idiopathic generalized and multifocal epilepsy with focal to bilateral tonic–clonic seizures. Epilepsia. 2024. doi: 10.1111/epi.18070. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PubMed] [Google Scholar]
- 34. Yang JC, Harid NM, Nascimento FA, et al. Responsive neurostimulation for focal motor status epilepticus. Ann Clin Transl Neurol. 2021;8(6):1353‐1361. doi: 10.1002/acn3.51318. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alcala‐Zermeno JL, Starnes K, Gregg NM, Worrell G, Lundstrom BN. Responsive neurostimulation with low‐frequency stimulation. Epilepsia. 2023;64(2):e16‐e22. doi: 10.1111/epi.17467. https://onlinelibrary.wiley.com/doi/full/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Razavi B, Rao VR, Lin C, et al. Real‐world experience with direct brain‐responsive neurostimulation for focal onset seizures. Epilepsia. 2020;61(8):1749‐1757. https://pubmed.ncbi.nlm.nih.gov/32658325/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions, since this is a case report about a specific patient. Some of the data that support the findings of this study can be made available on request from the corresponding author and with permission of the patient and their family. Portions of the data, specifically the RNS recordings that support the findings of this study are available from Neuropace. Restrictions apply to the availability of these data per Neuropace.


