ABSTRACT
This retrospective, multicenter cohort study aimed to determine whether cancer cachexia serves as a biomarker for determining the most effective treatment for patients having non-small-cell lung cancer (NSCLC) with high programmed death ligand 1 (PD-L1) expression treated with immune checkpoint inhibitors (ICIs) alone or combined with chemotherapy (ICI/chemotherapy). We included 411 patients with advanced NSCLC with a PD-L1 tumor proportion score of ≥50%. The patients were treated with pembrolizumab monotherapy or ICI/chemotherapy. Cancer cachexia was defined as a weight loss of >5% of the total body weight or a body mass index of <20 kg/m2 coupled with an additional weight loss of >2% within 6 months before starting treatment. Eighty-five (21%) patients met the cancer cachexia criteria. Overall survival (OS) was significantly shorter in patients with cachexia than in those without cachexia in both the pembrolizumab monotherapy group (17.2 vs. 35.8 months, p < 0.001) and the ICI/chemotherapy group (27.0 months vs. not reached, p = 0.044). However, after stratifying by cancer cachexia status, no significant difference in OS was observed between the pembrolizumab monotherapy and chemoimmunotherapy groups, regardless of cachexia. In conclusion, ICI/chemotherapy offers limited benefits for NSCLC patients with high PD-L1 expression and concurrent cancer cachexia. Considering the frailty associated with cachexia, ICI monotherapy may be preferred to ICI/chemotherapy for these patients. New interventions that can better address the negative prognostic impact of cachexia in patients treated using ICIs with or without chemotherapy remain warranted.
KEYWORDS: Cancer cachexia, combination therapy, immune checkpoint inhibitor, non-small cell lung cancer, treatment outcome
Introduction
Lung cancer is a major cause of cancer-related death worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases, and in the majority of cases, NSCLC is diagnosed at advanced, unresectable, or metastatic disease stages.1 Immune checkpoint inhibitors (ICIs) and antibodies targeting programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) have demonstrated outstanding efficacy against advanced NSCLC.2–4 In particular, ICIs provide a lasting treatment benefit for untreated patients having NSCLC with a PD-L1 tumor proportion score (TPS) of ≥50%.2–4 For advanced NSCLC, irrespective of the PD-L1 TPS, combination therapy with ICI plus chemotherapy (ICI/chemotherapy) has efficacy superior to chemotherapy.5–9 Therefore, both ICI monotherapy and ICI/chemotherapy have been established as first-line standard treatments for patients having advanced NSCLC with high PD-L1 expression. The optimal treatment for NSCLC patients with a PD-L1 TPS of ≥50% between ICI with or without chemotherapy remains unclear.10,11 Therefore, another predictive factor that may provide clues for optimal treatment selection for this clinical population is warranted.
Cancer cachexia, defined as a multifactorial syndrome characterized by a persistent loss of skeletal muscle mass with or without fat loss that cannot be completely reversed by conventional nutritional therapy and progresses to functional impairment, is observed in approximately 20% of patients with lung cancer.12,13 It is associated with worsening of prognosis and quality of life. Cancer cachexia is associated with poor progression-free survival (PFS) and overall survival (OS) in patients receiving ICI monotherapy or ICI/chemotherapy.14–16 We have previously reported an association between cancer cachexia and poorer outcomes in an overall NSCLC population treated with ICI/chemotherapy; however, treatment outcomes did not significantly differ between those with and without cancer cachexia in the subgroup of patients with a PD-L1 TPS of ≥50%.15 This finding suggests that ICI/chemotherapy mitigates the negative impact of cancer cachexia in this PD-L1 high TPS population. Based on these findings, we hypothesized that ICI/chemotherapy might provide greater efficacy than ICI monotherapy in patients with PD-L1 ≥ 50%, including those with cancer cachexia, and ICI/chemotherapy is a more reasonable treatment option than ICI monotherapy as first-line therapy in patients having NSCLC with cancer cachexia and a PD-L1 TPS of ≥50%. The aim of this study is to determine whether cancer cachexia is a clinical biomarker for optimal treatment selection in this patient population.
Materials and methods
Study design and patients
This retrospective multicenter cohort study was conducted at 13 institutions in Japan and included consecutive patients with advanced NSCLC (stage IV, including postoperative recurrence according to the American Joint Committee on Cancer Staging Manual, version 8) of a PD-L1 tumor proportion score of ≥50% who had received pembrolizumab monotherapy or combination therapy with ICIs plus chemotherapy as the initial treatment between March 2017 and December 2020 were included.17 Patients with recurrence were eligible if the recurrence occurred more than 24 weeks after the last administration of perioperative chemotherapy, and those who received a combination of uracil and tegafur as perioperative chemotherapy were eligible regardless of the duration of recurrence.
Clinical data at the time of first-line treatment initiation were collected from electronic medical records. PD-L1 TPS in tumor cells was analyzed using the PD-L1 immunohistochemistry 22C3 pharmDx antibody (clone 22C3; Dako North America, Inc. Carpinteria, CA). Based on previous reports, cancer cachexia was defined as a weight loss of >5% of the body weight within the 6 months before chemoimmunotherapy initiation or weight loss of >2% of the body weight when the body mass index (BMI) was <20 kg/m2, along with laboratory values above the expected reference values (C-reactive protein [CRP] level: > 0.5 mg/dL, serum albumin [Alb] level: <3.2 g/dL, and hemoglobin [Hb] level: <12 g/dL).18,19 The body weight of the patients during the 6 months preceding chemoimmunotherapy was determined by interviewing the patients or their family members or by weight measurement in the hospitals.15 Patients who received systemic steroids at the initiation of ICI/chemotherapy were excluded. The study was approved by the ethics review board of Kyoto Prefectural University of Medicine and was conducted with the consent of the ethics review board of each hospital (approval no. ERB-C-2113). Informed consent was not required because of the retrospective nature of the study.
Efficacy assessments
The aim of the present study is to clarify the clinical impact of cancer cachexia on patients with advanced NSCLC receiving pembrolizumab monotherapy or ICI/chemotherapy as a first-line treatment. As such, the association between cancer cachexia and treatment outcomes such as PFS and OS in patients receiving pembrolizumab monotherapy or ICI/chemotherapy was evaluated. Thereafter, the treatment outcomes of pembrolizumab monotherapy and ICI/chemotherapy in patients with or without cancer cachexia were investigated, and treatment responses were evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1.20 PFS was measured from the start of first-line treatment until the first instance of lung cancer progression or death from any cause. OS was measured from the start of first-line treatment until death from any cause. The data cutoff date was February 28, 2023. When comparing the treatment outcomes of pembrolizumab monotherapy and ICI/chemotherapy, rigorous adjustments were performed for significant differences in the baseline characteristics of patients using propensity score matching (PSM) and the following variables were included: age (<75 years or ≥75 years), sex (male or female), smoking status (never-smoker or smoker), Eastern Cooperative Oncology Group performance status (ECOG PS) (0–1 or 2–4), histology (squamous cell carcinoma or non-squamous cell carcinoma), PD-L1 status (50–89% or 90–100%), stage (non-recurrence or recurrence), liver metastasis (present or absent), and brain metastasis (present or absent). Nearest-neighbor matching was performed at a ratio of 1:1 without replacement. Caliper was set at 0.2.
Statistical analysis
Continuous variables were compared using the Wilcoxon rank-sum test. Dichotomous variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Logistic regression analysis was performed to evaluate the risk of cancer cachexia associated with other patient characteristics. Survival outcomes were estimated using the Kaplan – Meier method and compared using the log-rank test. Cox proportional hazard models were used to determine the association between patient characteristics and survival outcomes. The results are expressed as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs) as appropriate. All analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA). Statistical significance was defined as a two-tailed P-value of <0.05.
Results
Patient characteristics
Overall, 446 consecutive NSCLC patients with a PD-L1 TPS ≥50% were enrolled in this study (Supplementary Figure S1), of which 35 were excluded due to receiving systemic steroids. Finally, 411 patients were included in the analysis. Of these, 255 and 156 patients were treated with pembrolizumab monotherapy and ICI/chemotherapy, respectively, as the first-line treatment. The baseline patient characteristics are summarized in Table 1. Compared with the ICI/chemotherapy group, the pembrolizumab group had a significantly higher proportion of older patients (72 [range: 43–90] years vs. 68 [range: 36–86 years], p <0.001), patients aged ≥75 years (100/255 vs. 19/156, p < 0.001), and patients with poor ECOG PS (45/255 vs. 12/156, p = 0.003). Additionally, there were significant differences in tumor stage (p = 0.001) between the two groups. Of the 411 patients, 85 (21%) were diagnosed with cancer cachexia, and there were no significant intergroup differences in the proportions of patients with cancer cachexia (53/255 [pembrolizumab group] vs. 32/156 [ICI/chemotherapy group], p = 0.947). To elucidate the characteristics of patients with cancer cachexia, we analyzed patient factors associated with cancer cachexia using logistic regression analysis. Multivariable logistic regression analyses revealed that the presence of driver mutations (p = 0.040), poor ECOG PS (p = 0.021), and underweight (p < 0.001) were associated with cancer cachexia, independent of other patient characteristics (Table 2).
Table 1.
Patient characteristics in all patients (N = 411).
| Patient characteristics | Pembrolizumab group (N = 255) |
ICI plus chemotherapy group (N = 156) |
p value |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 72 (43–90) | 68 (36–86) | <.001 |
| <75 years | 155 (61) | 137 (88) | <.001 |
| ≧75 years | 100 (39) | 19 (12) | |
| Sex | |||
| Male | 201 (79) | 119 (76) | .548 |
| Female | 54 (21) | 37 (24) | |
| Smoking status | |||
| Never-smoker | 31 (12) | 30 (19) | .053 |
| Current or former smoker | 224 (88) | 126 (89) | |
| EGFR mutation | |||
| Yes | 7 (3) | 7 (4) | .352 |
| ALK fusion | |||
| Yes | 2 (1) | 4 (3) | .152 |
| ROS1 rearrangement | |||
| Yes | 0 (0) | 1 (1) | .164 |
| ECOG PS | |||
| 0–1 | 210(82) | 144 (92) | .003 |
| 2–4 | 45 (18) | 12 (8) | |
| Histology | |||
| Squamous cell carcinoma | 76 (30) | 39 (25) | .290 |
| Adenocarcinoma | 144 (56) | 95 (61) | |
| Other | 35 (14) | 22 (14) | |
| PD-L1 status | |||
| 50–89% | 154 (60) | 105 (67) | .157 |
| 90–100% | 101 (40) | 51 (33) | |
| Stage | |||
| IVA | 88 (35) | 49 (31) | .001 |
| IVB | ea108 (42) | 90 (58) | |
| Recurrence | 59 (23) | 17 (11) | |
| BMI | |||
| <20 | 71 (28) | 51 (33) | .298 |
| ≧20 | 184 (72) | 105 (67) | |
| Liver metastasis | 31 (12) | 25 (16) | .271 |
| Brain metastasis | 39 (15) | 28 (18) | .482 |
| Cancer cachexia | 53 (21) | 32 (20) | .947 |
| Treatment regimen | |||
| Pembrolizumab | 255 (100) | ||
| CBDCA/PTX/Pembrolizumab | 1 (1) | ||
| CBDCA/nab-PTX/Pembrolizumab | 51 (33) | ||
| CBDCA/PEM/Pembrolizumab | 41 (26) | ||
| CDDP/PEM/Pembrolizumab | 28 (18) | ||
| CBDCA/PEM/Atezolizumab | 1 (1) | ||
| CBDCA/PTX/Atezolizumab | 1 (2) | ||
| CBDCA/PTX/BEV/Atezolizumab | 23 (15) | ||
| CBDCA/nab-PTX/Atezolizumab | 10 (6) |
ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS proto-oncogene 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed death ligand 1; BMI, body mass index; CBDCA, carboplatin; CDDP, cisplatin; PEM, pemetrexed; nab-PTX, nanoparticle albumin-bound paclitaxel; PTX, paclitaxel; BEV, bevacizumab.
Table 2.
Logistic regression analysis for factors associated with cancer cachexia (N = 411).
| Characteristics | Multivariate |
|
|---|---|---|
| OR (95% CI) | P-value | |
| Age | ||
| ≥75 years (vs. <75 years) | 1.21 (0.66–2.21) | .531 |
| Sex | ||
| female (vs. male) | 0.52 (0.24–1.14) | .102 |
| Smoking status | ||
| current or former smoker (vs. never) | 0.55 (0.22–1.35) | .192 |
| ECOG PS | ||
| 2–4 (vs. 0–1) | 2.29 (0.87–2.79) | .138 |
| Histology | ||
| squamous (vs. non-squamous) | 1.22 (0.74–2.00) | .429 |
| Driver mutation | ||
| positive (vs. negative or not investigated) | 3.19 (1.06–9.60) | .040 |
| ECOG PS | ||
| 2–4 (vs. 0–1) | 2.29 (1.13–4.64) | .021 |
| PD-L1 status | ||
| 50–89% (vs.≧90%) | 0.98 (0.55–1.74) | .933 |
| Stage | ||
| IVA or IVB (vs. Recurrence) | 1.60 (0.72–3.55) | .247 |
| BMI | ||
| <20 (vs.≧20) | 7.59 (4.36–13.2) | <.001 |
| Liver metastasis | ||
| yes (vs. no) | 1.26 (0.60–2.63) | .548 |
| Brain metastasis | ||
| yes (vs. no) | 0.93 (0.43–2.02) | .858 |
ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed death ligand 1; BMI, body mass index; OR, odds ratio; CI, confidence interval.
Comparison according to cancer cachexia in each treatment group
The baseline characteristics of the pembrolizumab group stratified by the presence or absence of cancer cachexia are summarized in Table 3. Compared with the group without cancer cachexia, that with cancer cachexia had significantly higher proportions of patients with poor ECOG PS (16/53 vs. 29/202, p = 0.011) and higher proportions of underweight patients (31/53 vs. 40/202, p < 0.001). Additionally, there were significant differences in cancer stage (p = 0.043) between the groups with and without cancer cachexia. In the pembrolizumab group, the median PFS and OS in patients with cancer cachexia were significantly shorter than in those without cancer cachexia (5.7 vs. 11.1 months, p = 0.007; 17.2 vs. 35.8 months, p < 0.001, respectively; Figure 1a,b).
Table 3.
Patient characteristics in pembrolizumab group (N = 255).
| Patient characteristics | With cancer cachexia (N = 53) |
Without cancer cachexia (N = 202) |
P value |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 74 (48–90) | 72 (43–88) | .087 |
| <75 years | 24 (45) | 126 (62) | .312 |
| ≧75 years | 29 (55) | 76 (38) | |
| Sex | |||
| Male | 42 (79) | 159 (79) | .933 |
| Female | 11 (21) | 43 (21) | |
| Smoking status | |||
| Never-smoker | 5 (9) | 26 (13) | .484 |
| Current or former smoker | 48 (91) | 176 (87) | |
| EGFR mutation | |||
| Yes | 3 (6) | 4 (2) | .181 |
| ALK fusion | |||
| Yes | 0 (0) | 2 (1) | .333 |
| ROS1 rearrangement | |||
| Yes | 0 (0) | 0 (0) | NA |
| ECOG PS | |||
| 0–1 | 37 (70) | 173 (86) | .011 |
| 2–4 | 16 (30) | 29 (14) | |
| Histology | |||
| Squamous cell carcinoma | 16 (30) | 60 (30) | .945 |
| Adenocarcinoma | 27 (51) | 117 (58) | |
| Other | 10 (19) | 25 (12) | |
| PD-L1 status | |||
| 50–89% | 31 (58) | 123 (61) | .751 |
| 90–100% | 22 (42) | 79 (39) | |
| Stage | |||
| IVA | 13 (25) | 75 (37) | .043 |
| IVB | 33 (62) | 75 (37) | |
| Recurrence | 7 (13) | 52 (26) | |
| BMI | |||
| <20 | 31 (58) | 40 (20) | <.001 |
| ≧20 | 22 (42) | 162 (80) | |
| Liver metastasis | 9 (17) | 22 (11) | .244 |
| Brain metastasis | 6 (11) | 33 (16) | .352 |
| Treatment regimen | |||
| Pembrolizumab | 53 (100) | 202 (100) |
EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS proto-oncogene 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed death ligand 1; BMI, body mass index.
Figure 1.
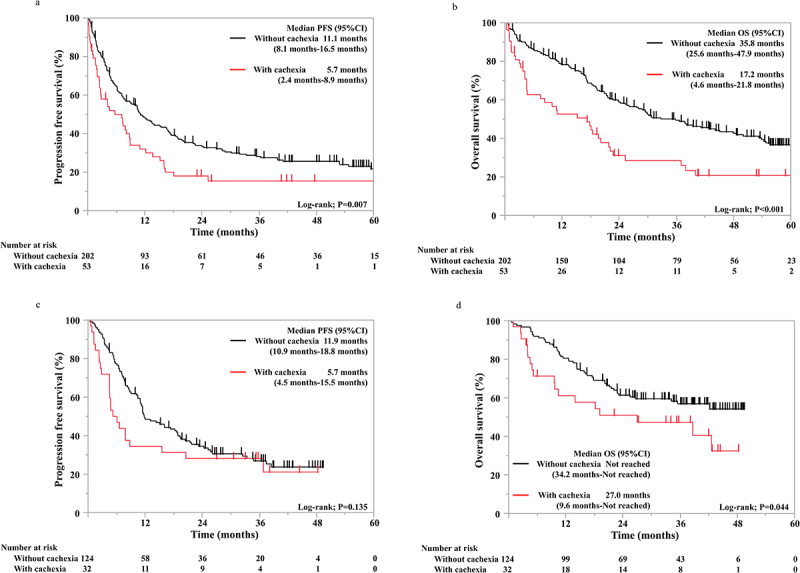
Kaplan – Meier survival curves showing the progression-free survival (a) and overall survival (b) in pembrolizumab monotherapy group (N = 255) and the progression-free survival (c) and overall survival (d) in the ICI plus chemotherapy group (N = 156).
The baseline characteristics of the ICI/chemotherapy group are summarized in Table 4. Compared with the group without cancer cachexia, the group with cancer cachexia had significantly higher proportions of patients with EGFR mutations (5/32 vs. 2/124, p < 0.001), ROS1 rearrangement (1/32 vs. 0/124, p = 0.048), and underweight (24/32 vs. 27/124, p <0.001). In the ICI/chemotherapy group, there was no significant difference in PFS between patients with and without cancer cachexia. (5.7 vs. 11.9 months, p = 0.135; Figure 1c). In contrast, the median OS of patients with cancer cachexia was significantly shorter than that of patients without cancer cachexia (27.0 months vs. not reached; p = 0.044; Figure 1d).
Table 4.
Patient characteristics in ICI/Chemo group (N = 156).
| Patient characteristics | With cancer cachexia (N = 32) |
Without cancer cachexia (N = 124) |
P value |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 67.5 (40–79) | 69 (36–86) | .310 |
| <75 years | 29 (91) | 108 (87) | .586 |
| ≧75 years | 3 (9) | 16 (13) | |
| Sex | |||
| Male | 24 (75) | 95 (77) | .848 |
| Female | 8 (25) | 29 (23) | |
| Smoking status | |||
| Never-smoker | 10 (31) | 20 (16) | .053 |
| Current or former smoker | 22 (69) | 104 (84) | |
| EGFR mutation | |||
| Yes | 5 (16) | 2 (2) | <.001 |
| ALK fusion | |||
| Yes | 0 (0) | 4 (3) | .303 |
| ROS1 rearrangement | |||
| Yes | 1 (3) | 0 (0) | .048 |
| ECOG PS | |||
| 0–1 | 27 (84) | 117 (94) | .058 |
| 2–4 | 5 (16) | 7 (6) | |
| Histology | |||
| Squamous cell carcinoma | 7 (22) | 32 (26) | .828 |
| Adenocarcinoma | 21 (66) | 74 (60) | |
| Other | 4 (13) | 18 (15) | |
| PD-L1 status | |||
| 50–89% | 20 (63) | 85 (69) | .516 |
| 90–100% | 12 (38) | 39 (31) | |
| Stage | |||
| IVA | 7 (22) | 42 (34) | .349 |
| IVB | 22 (69) | 68 (55) | |
| Recurrence | 3 (9) | 14 (11) | |
| BMI | |||
| <20 | 24 (75) | 27 (22) | <.001 |
| ≧20 | 8 (25) | 97 (78) | |
| Liver metastasis | 6 (19) | 19 (15) | .638 |
| Brain metastasis | 8 (25) | 20 (16) | .244 |
| Treatment regimen | |||
| CBDCA/PTX/Pembrolizumab | 0 (0) | 1 (1) | |
| CBDCA/nab-PTX/Pembrolizumab | 11 (34) | 40 (32) | |
| CBDCA/PEM/Pembrolizumab | 8 (25) | 33 (27) | |
| CDDP/PEM/Pembrolizumab | 6 (19) | 22 (18) | |
| CBDCA/PEM/Atezolizumab | 0 (0) | 1 (1) | |
| CBDCA/PTX/Atezolizumab | 0 (0) | 1 (1) | |
| CBDCA/PTX/BEV/Atezolizumab | 0 (0) | 9 (7) | |
| CBDCA/nab-PTX/Atezolizumab | 7 (22) | 17 (14) |
ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, ROS proto-oncogene 1; ECOG PS, Eastern Cooperative Oncology Group Performance Status; PD-L1, programmed death ligand 1; BMI, body mass index; CBDCA, carboplatin; CDDP, cisplatin; PEM, pemetrexed; nab-PTX, nanoparticle albumin-bound paclitaxel; PTX, paclitaxel; BEV, bevacizumab.
Treatment outcomes in patients with and without cancer cachexia
We compared the treatment outcomes in patients with and without cancer cachexia between the pembrolizumab monotherapy and ICI/chemotherapy groups. After weighting by PSM, 21 patients with cancer cachexia and 102 without cancer cachexia were included in each group. There were no significant differences in baseline characteristics between the two groups in patients with or without cancer cachexia (Supplementary Table S1 and Table 2). In patients with cancer cachexia, both the median PFS (7.2 vs. 7.9 months, p = 0.515; Figure 2a) and the median OS (20.2 vs. 38.7 months, p = 0.501; Figure 2b) were not significantly different between the pembrolizumab and ICI/chemotherapy groups. Similarly, in patients without cancer cachexia, both the median PFS (11.7 vs. 11.9 months, p = 0.693; Figure 2c) and the median OS (41.1 months vs. not reached, p = 0.681; Figure 2d) were not significantly different between the pembrolizumab and ICI/chemotherapy groups.
Figure 2.
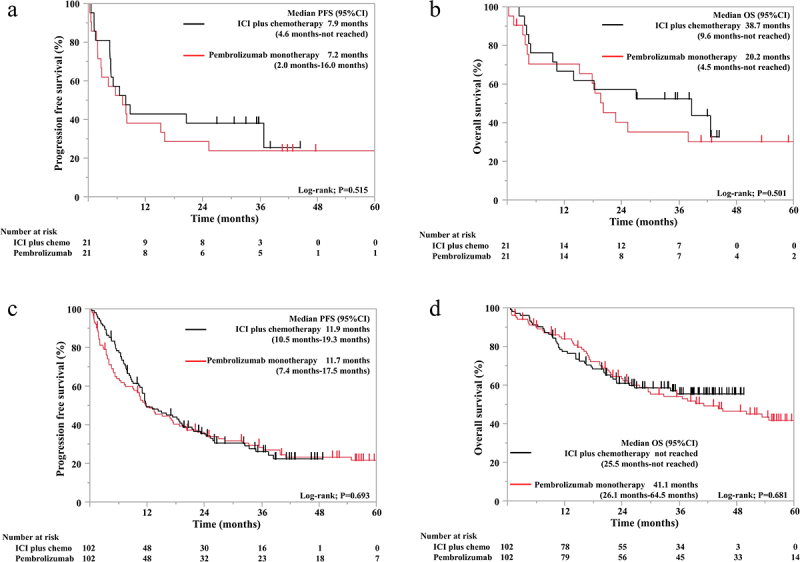
Kaplan – Meier survival curves showing the progression-free survival (a) and overall survival (b) in the cancer cachexia group (N = 42) and the progression-free survival (c) and overall survival (d) in the non-cancer cachexia group (N = 204) after propensity score matching.
Discussion
Cancer cachexia is a complex condition of tissue wasting that develops as a secondary disorder in patients with cancer and leads to progressive functional impairment. It is characterized by systemic inflammation, negative protein and energy balance, and involuntary loss of lean body mass with or without adipose tissue wasting, resulting in poor prognoses. Currently, the clinical management of cancer cachexia is both limited and complex; therefore, further investigations are warranted to improve the quality of life and prognosis of patients with cancer cachexia. We observed no significant differences in treatment outcomes between ICI monotherapy and chemoimmunotherapy in patients with advanced NSCLC with a high PD-L1 TPS, regardless of cancer cachexia. These findings suggest that cancer cachexia does not act as a predictive biomarker for guiding treatment choice between these modalities. To the best of our knowledge, this is the first study to show the clinical impact of cancer cachexia on treatment outcomes of patients having NSCLC with a PD-L1 TPS of ≥ 50% receiving ICI with or without chemotherapy, which may provide an optimal treatment strategy for this clinical population.
The present study showed that the complications of cancer cachexia were correlated with poor treatment outcomes in patients with NSCLC who were administered ICI and ICI/chemotherapy. Regardless of cancer cachexia, the additional benefit of chemotherapy over monotherapy with ICIs was limited in patients having advanced NSCLC with a high PD-L1 TPS. These results suggest that the additional benefit of combining ICIs with chemotherapy is limited in improving therapeutic responses for patients with cancer cachexia. In our study, poor ECOG PS was independently associated with cancer cachexia. We have previously reported that in a frail population, ICI/chemotherapy did not confer PFS or OS benefits compared with pembrolizumab monotherapy.15 Additionally, in a real-world study of patients having non-squamous NSCLC treated with ICI/chemotherapy containing pembrolizumab and pemetrexed, the incidence of severe adverse events was higher in patients with a poor PS than in those with a good PS.21 Several previous studies have shown the efficacy and safety of pembrolizumab monotherapy in frail populations.22,23 Thus, considering treatment effectiveness and safety and frailty induced by cancer cachexia, ICI monotherapy may be a more reasonable treatment option for NSCLC patients with a high PD-L1 TPS and cancer cachexia.
Our study suggests that novel treatment interventions may be needed to overcome the negative impact of cancer cachexia on the prognosis of patients with advanced NSCLC treated using ICIs with or without chemotherapy. Anamorelin is a ghrelin agonist and has been shown to substantially increase lean body mass and alleviate anorexia.24,25 It was the first drug with anticancer activity against cachexia approved in Japan. However, anamorelin did not improve motor function, and its impact on the efficacy and safety of ICIs with or without chemotherapy remains unclear.24 Our research group is currently conducting a prospective observational study to investigate the association between anamorelin and the therapeutic outcome of ICI/chemotherapy in patients having advanced NSCLC with cancer cachexia (SPIRAL-ANA, jRCT1071210053), which warrants further investigation. Additionally, neutralizing antibodies against growth differentiation factor-15 (GDF-15), which has been reported to induce anorexia by acting on the brain’s feeding center and is involved in the reduction of lean body mass in patients with cancer, may also be of interest as promising anti-cachexia drugs.26–28 In phase 1 trials, GDF-15-neutralizing antibodies were well tolerated, and some phase 2 trials are currently ongoing.27–29 Additionally, it was previously reported that serum GDF-15 levels were strongly correlated with the failure of PD-1-based ICI therapy in patients with melanoma, and neutralization of GDF-15 improved both T cell trafficking and therapy efficiency in murine tumor models.30 From this translational perspective, GDF-15-neutralizing cancer treatments can potentially ameliorate cancer cachexia and improve response to cancer immunotherapy.
The present study has some limitations. First, this was a multicenter, retrospective study. Therefore, the possibility of selection bias cannot be ruled out. However, the patients were consecutively enrolled, and PSM was conducted to reduce selection bias. Nevertheless, since the treatment decision was based on the clinical fitness of each patient, the possibility of selection bias cannot be completely ruled out. Second, since PSM was used to reduce patient background bias, a smaller sample size than that of the overall population was inevitable. Third, skeletal muscle mass, which is used to define cachexia, was not evaluated. However, approximately 90% of the patients with cachexia can be diagnosed using weight loss of >5% or BMI of <20 kg/m2 and weight loss of >2% alone. Fourth, there may have been bias in obtaining information on body weight within the 6 months preceding chemoimmunotherapy initiation. Fifth, this study included only Japanese patients. Sixth, this study was a retrospective observational study conducted in real-world clinical settings, where the timing of treatment response evaluation and imaging for PFS assessment was determined by the discretion of the treating physicians or participating centers. As standardized protocols for imaging timing remain lacking, this variability might have influenced PFS evaluation. Seventh, adverse events and complications remain a concern in patients with cancer cachexia; however, this study did discuss them in detail. Eighth, this study has a relatively short follow-up duration, with a median follow-up time of 22.9 months as of the data cutoff date. As a result, the OS data remain immature, particularly for patients enrolled in the later years of the study. Furthermore, our study has limitations related to statistical power, particularly in exploring potential interactions between cancer cachexia and treatment choice. This study included subgroup analyses to evaluate the impact of cancer cachexia on treatment outcomes, including PFS and OS, in patients receiving ICI/chemotherapy. However, the sample size within certain subgroups was limited. The lack of statistical significance in the observed differences and the interaction between cachexia status and treatment choice with respect to outcome may reflect insufficient statistical power. These limitations highlight the need for larger, adequately powered studies to validate our findings and provide deeper insights into the relationship between cancer cachexia and treatment efficacy. Despite these limitations, this study offers valuable insights into the potential impact of cancer cachexia on treatment outcomes in real-world clinical practice. Although the results of our study are clinically important, they may not be conclusive or generalizable. Therefore, confirmation using a larger global cohort is required.
In conclusion, the complications of cancer cachexia were associated with poor treatment outcomes in patients having NSCLC who were treated with ICIs and those who were treated with ICI/chemotherapy. Regardless of the presence of cancer cachexia, the benefits of adding chemotherapy to ICIs were limited in patients having NSCLC with a high PD-L1 TPS. In terms of frailty in cancer cachexia, ICI monotherapy may be a more suitable treatment option than ICI/chemotherapy for NSCLC patients with a high PD-L1 TPS and cancer cachexia. Novel treatment interventions remain warranted to overcome the negative impact of cancer cachexia on the prognosis of patients with advanced NSCLC receiving ICIs with or without chemotherapy.
Supplementary Material
Acknowledgments
We are grateful to all the patients and investigators involved in this study.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
Hayato Kawachi received personal fees from Bristol-Myers Squibb, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK outside the purview of the submitted work. Tadaaki Yamada received research grants from Ono Pharmaceutical, Janssen, AstraZeneca, and Takeda Pharmaceutical and speaking honoraria from Eli Lilly outside the purview of the submitted work. Motohiro Tamiya received research grants from Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Daiichi-Sankyo, Eisai, Chugai Pharmaceutical Co. Ltd., and Janssen and personal fees from Chugai Pharmaceutical Co. Ltd., Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical, Eli Lilly, Novartis, Pfizer, Asahi Kasei Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Bayer, Amgen, Kyowa-Kirin, and Nippon Kayaku outside the purview of the submitted work. Asuka Okada received personal fees from Chugai-Roshe, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Japan, Nippon Kayaku, and Bristol-Myers Squibb outside the purview of the submitted work. Takashi Kijima received personal fees from Chugai Pharmaceutical Co., Ltd. and MSD KK outside the purview of the submitted work. Koichi Takayama received research grants from Chugai Pharmaceutical Co. Ltd. and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical Co. Ltd., MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi-Sankyo outside the purview of the submitted work.
Author contributions
Conceptualization: Kawachi, Yamada.Data curation: All authors.Formal analysis: Kawachi, Yamada, Shimose.Investigation: All authors.Methodology: Kawachi, Yamada.Project administration: Kawachi, Yamada.Resources: All authors.Software: NoneSupervision: Takayama.Validation: None.Visualization: Kawachi, Yamada.Writing – original draft: Kawachi, Yamada.Writing – review & editing: All authors.
Data availability statement
The datasets generated in this study are available from the corresponding author upon request.
Ethics approval
All procedures involving human participants performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and the 1964 helsinki Declaration and its later amendments or comparable ethical standards. The review board of each institution approved the study protocol.
Informed consent
The requirement for informed consent from patients was waived owing to the retrospective nature of the study, and an opt-out method was included so that patients and families could opt out of participating in the study.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2442116
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–10. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G, Srimuninnimit V, Laktionov KK, Bondarenko I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-Cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, et al. Atezolizumab for first-line treatment of PD-L1–selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, Çay Şenler F, Csőszi T, Fülöp A, et al. Pembrolizumab plus chemotherapy for squamous non–small-Cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus chemotherapy in metastatic non–small-Cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 7.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J, Novello S, Orlandi F. et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 8.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp H-G, Daniel D, McCune S, Mekhail T, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 9.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 10.Pérol M, Felip E, Dafni U, Polito L, Pal N, Tsourti Z, Ton TGN, Merritt D, Morris S, Stahel R, et al. Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol. 2022;33(5):511–521. doi: 10.1016/j.annonc.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Akinboro O, Vallejo JJ, Nakajima EC, Ren Y, Mishra-Kalyani PS, Larkins EA, Vellanki PJ, Drezner NL, Mathieu LN, Donoghue MB, et al. Outcomes of anti–PD-(L)1 therapy with or without chemotherapy (chemo) for first-line (1L) treatment of advanced non–small cell lung cancer (NSCLC) with PD-L1 score ≥50%: FDA pooled analysis. J Clin Oncol. 2022;40(16_suppl):9000–9000. doi: 10.1200/JCO.2022.40.16_suppl.9000. [DOI] [Google Scholar]
- 12.Shukuya T, Takahashi K, Shintani Y, Miura K, Sekine I, Takayama K, Inoue A, Okamoto I, Kiura K, Kawaguchi T, et al. Epidemiology, risk factors and impact of cachexia on patient outcome: results from the Japanese lung cancer registry study. J Cachexia Sarcopenia Muscle. 2023;14(3):1274–1285. doi: 10.1002/jcsm.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita-Tanaka S, Yamada T, Takayama K. The landscape of cancer cachexia in advanced non-small cell lung cancer: a narrative review. Transl Lung Cancer Res. 2023;12(1):168–180. doi: 10.21037/tlcr-22-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyawaki T, Naito T, Kodama A, Nishioka N, Miyawaki E, Mamesaya N, Kawamura T, Kobayashi H, Omori S, Wakuda K, et al. Desensitizing effect of cancer cachexia on immune checkpoint inhibitors in patients with advanced NSCLC. JTO Clin Res Rep. 2020;1(2):100020. doi: 10.1016/j.jtocrr.2020.100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimoto K, Uchino J, Yokoi T, Kijima T, Goto Y, Nakao A, Hibino M, Takeda T, Yamaguchi H, Takumi C, et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non-small cell lung cancer: a retrospective study. Oncoimmunology. 2021;10(1):1950411. doi: 10.1080/2162402X.2021.1950411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakozaki T, Nolin-Lapalme A, Kogawa M, Okuma Y, Nakamura S, Moreau-Amaru D, Tamura T, Hosomi Y, Takeyama H, Richard C, et al. Cancer cachexia among patients with advanced non-small-cell lung cancer on immunotherapy: an observational study with exploratory gut microbiota analysis. Cancers (Basel). 2022;14(21):5405. doi: 10.3390/cancers14215405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 18.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Fujimoto D, Miura S, Yoshimura K, Wakuda K, Oya Y, Haratani K, Itoh S, Uemura T, Morinaga R, Takahama T, et al. A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin Res Rep. 2022;3(2):100265. doi: 10.1016/j.jtocrr.2021.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiotsu S, Yoshimura A, Yamada T, Morimoto K, Tsuchiya M, Yoshioka H, Hiranuma O, Chihara Y, Yamada T, Hasegawa I, et al. Pembrolizumab monotherapy for untreated PD-L1-Positive non-small cell lung cancer in the elderly or those with poor performance status: a prospective observational study. Front Oncol. 2022;12:904644. doi: 10.3389/fonc.2022.904644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SM, Schulz C, Prabhash K, Kowalski D, Szczesna A, Han B, Rittmeyer A, Talbot T, Vicente D, Califano R, et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): a phase 3, global, multicentre, open-label, randomised controlled study. Lancet. 2023;402(10400):451–463. doi: 10.1016/S0140-6736(23)00774-2. [DOI] [PubMed] [Google Scholar]
- 24.Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, Kitajima H, Yoshimori K, Sato K, Saito H, et al. Anamorelin (ONO-7643) for the treatment of patients with non–small cell lung cancer and cachexia: results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 2018;124(3):606–616. doi: 10.1002/cncr.31128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniguchi J, Mikura S, da Silva Lopes K. The efficacy and safety of anamorelin for patients with cancer-related anorexia/cachexia syndrome: a systematic review and meta-analysis. Sci Rep. 2023;13(1):15257. doi: 10.1038/s41598-023-42446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saarma M, Goldman A. Obesity: receptors identified for a weight regulator. Nature. 2017;550(7675):195–197. doi: 10.1038/nature24143. [DOI] [PubMed] [Google Scholar]
- 27.Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15(6):656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 28.Hong DS, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM, Stecher M, Mohanty P, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33(3):621–631. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melero I, De Miguel MJ, Alonso Casal G, Goebeler M-E, Ramelyte E, Calvo E, Garralda E, Dummer R, Rodríguez-Ruiz ME, Sayehli CM, et al. 729MO final results of the first-in-human clinical trial of the GDF-15 neutralizing antibody CTL-002 in combination with nivolumab in subjects with solid tumors relapsed/refractory to prior anti-PD1/PD-L1 treatment. Ann Of Oncol. 2022;33:S876. doi: 10.1016/j.annonc.2022.07.855. [DOI] [Google Scholar]
- 30.Haake M, Haack B, Schäfer T, Harter PN, Mattavelli G, Eiring P, Vashist N, Wedekink F, Genssler S, Fischer B, et al. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat Commun. 2023;14(1):4253. doi: 10.1038/s41467-023-39817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated in this study are available from the corresponding author upon request.


