ABSTRACT
This post-marketing surveillance study evaluated the safety of the adjuvanted recombinant zoster vaccine (RZV) in Chinese adults, given the limited country-specific safety data accumulated since the 2019 licensure of RZV in China for adults ≥ 50 years of age (YOA). This descriptive, prospective cohort study enrolled adults ≥ 50 YOA who voluntarily received RZV per routine clinical practice in six centers in China. The primary outcomes were occurrence, intensity, and causal relationship to vaccination of medically attended adverse events (MAEs) within 30 days post-any dose. The occurrence and causal relationship to RZV of serious AEs (SAEs) within 30 days post-any dose, and of SAEs and potential immune-mediated diseases (pIMDs) from dose 1 until 12 months post-last dose were secondary outcomes. The exposed set included 3,300 adults (mean age [standard deviation]: 61.2 [7.4] years; 67.1% female), of whom 3,175 completed the study. Fifty-six MAEs were recorded in 42 (1.3%, 95% confidence interval [CI]: 0.9–1.7%) participants; ≥1 grade 3 MAE was reported in six (0.2%, 0.1–0.4%) participants; 15 MAEs (in nine [0.3%, 0.1–0.5%] participants) were considered RZV-related. Within 30 days post-any dose, 12 SAEs were reported in 10 (0.3%, 0.1–0.6%) participants, while 29 SAEs in 22 (0.7%, 0.4–1.0%) participants were reported from post-dose 1 until 12 months post-last dose. The three reported fatal SAEs were not considered RZV-related. Three of the total seven pIMDs were considered RZV-related. The observed descriptive patterns of MAEs, SAEs, and pIMDs did not indicate safety concerns following RZV administration among Chinese adults ≥ 50 YOA.
KEYWORDS: Herpes zoster, adjuvanted recombinant zoster vaccine (RZV), post-marketing, safety, adverse events, potential immune-mediated diseases, Chinese adults
Plain Language Summary
What is the context?
Herpes zoster, or shingles, is a painful rash caused by the reactivation of the latent chickenpox virus.The adjuvanted recombinant zoster vaccine, or RZV, was shown to be well tolerated and to effectively prevent shingles in healthy adults aged 50 years or older.Limited data on real-world safety of RZV, in terms of health problems occurring after vaccination in routine medical practice, are available in China, where RZV was licensed for use in 2019.
What is new?
We analyzed data from 3,300 Chinese adults aged 50 years or older who voluntarily received at least one dose of RZV as part of routine clinical practice.No safety concerns following receipt of RZV were identified in this population.
What is the impact?
The results add important knowledge to the body of evidence regarding the safety of RZV in Chinese populations.
Introduction
Herpes zoster (HZ; also known as shingles), caused by the reactivation of latent varicella zoster virus, is a disease characterized by a painful unilateral rash.1,2 The HZ rash typically resolves within a few weeks but can evolve into post-herpetic neuralgia, a long-lasting neuralgic pain that is challenging to manage.1,2 The risk of HZ increases with age due to declining cell-mediated immunity.2,3 Without effective countermeasures, the global burden of HZ is expected to increase due to aging of the world population.3
Several studies evaluating the HZ burden in China have found an HZ incidence of ≥ 6.6 per 1,000 person-years in adults ≥50 years of age (YOA), with older age and immunocompromised status as the prominent risk factors for HZ onset.4–7 This is consistent with the HZ incidence reported globally and regionally, ranging from 2.8 to 22.4 per 1,000 person-years in adults ≥ 50 YOA, depending on the country and specific age group evaluated.8,9
The 2019 approval of the adjuvanted recombinant zoster vaccine (RZV; Shingrix, manufactured by GSK) marked the first vaccine licensed in China for the prevention of HZ in adults ≥ 50 YOA.7 RZV is administered as a two-dose series with the second dose administered 2–6 months after the first dose.10 The estimated RZV efficacy against HZ in Asian populations, including mainland Chinese, was reported to range from 95.6% to 100%.11–13 A real-world effectiveness study in ethnic Chinese ≥ 50 YOA in the United States estimated an RZV effectiveness of 87.6% against HZ.14
Although RZV efficacy/effectiveness data in Chinese individuals are available and the multi-country safety data are abundant,15–21 information regarding RZV safety in individuals of Chinese ethnicity was reported in only two studies.12,13 In the post-hoc analysis of phase III trials, the reactogenicity and safety profiles of RZV in Asian subpopulations were found to be comparable to the entire population.12 Similarly, a recent phase IV trial in adults from mainland China found that RZV had an acceptable safety profile.11,13 Solicited adverse events (AEs) were more frequent in the RZV than the placebo group, but in line with previous reports.15,17,18 The frequencies of serious AEs (SAEs), potential immune-mediated diseases (pIMDs), and deaths were similar between RZV and placebo groups, and none of these events were considered causally related to RZV.13
As illustrated by the above-described studies, the available real-world RZV safety data specific for Chinese populations are limited, particularly in terms of the occurrence of medically attended AEs (MAEs). The aim of this post-authorization, prospective, observational safety surveillance study committed to the Chinese regulatory authorities following the approval and launch of RZV in China was to address this knowledge gap and describe the real-world safety of RZV among Chinese adults ≥ 50 YOA who received this vaccine on a voluntary basis and as per local routine practice.
Methods
Study ethics
The study was designed and conducted in accordance with the International Society for Pharmacoepidemiology guidelines for Good Pharmacoepidemiology Practices,22 the Human Genetics Resources Administration of China23 regulations, the guiding principles of the Declaration of Helsinki,24 local ethical committee requirements, and other applicable guidelines and participant privacy requirements. The study sponsor obtained required approvals prior to study initiation, including approval from the Center for Drug Evaluation in China (approval number: 2019S00364) and the Ethics Committee of Beijing Center for Diseases Prevention and Control (approval number: 2020 No. [2]).
All participants or their legally acceptable representatives voluntarily provided a written or witnessed/thumb printed informed consent, prior to participation in the study. Participants voluntarily received RZV according to the prescribing information10,25 and as per local routine practice. This study complied with STROBE guidelines for reporting results of observational studies and the corresponding checklist has been included in the Supplemental material.
Study outcomes
The primary outcome was the occurrence, intensity, and causal relationship to vaccination of MAEs occurring within 30 days post-each dose. Secondary outcomes were the occurrence and causal relationship to vaccination of SAEs within 30 days post-each dose, and occurrence and causal relationship to vaccination of SAEs and pIMDs from dose 1 until 12 months post-last dose.
MAEs were defined as AEs leading to an otherwise unscheduled visit to or from medical personnel for any reason, including emergency room visits. Medical Dictionary for Regulatory Activities System Organ Class (MedDRA SOC)26 was used to define MAE classes. SAEs were defined as any untoward medical occurrences that resulted in death, were life threatening, required hospitalization or prolongation of existing hospitalization, resulted in disability or incapacity, or were a congenital anomaly or birth defect in the offspring of a study participant. If an MAE led to hospitalization (or met any other SAE criteria), it was also reported as an SAE; pIMDs included autoimmune diseases and other inflammatory and/or neurological disorders of interest that may or may not have had an autoimmune etiology.
Evaluation of outcomes of interest
The intensity level (grades 1–3) and causal relationship to vaccination of the outcomes were assessed based on the investigators’ clinical judgment, as outlined in the protocol (available, together with the Statistical Analysis Plan, on the GSK study register, study ID212290).27 The investigators assessed the maximum intensity that occurred over the duration of the event for all MAEs reported during the study. Grade 1 (mild) was assigned to AEs that were easily tolerated, caused minimal discomfort, and did not interfere with everyday activities. Grade 2 (moderate) was assigned to outcomes that were sufficiently discomforting to interfere with normal everyday activities, while grade 3 (severe) was used for outcomes found to prevent the normal daily activities of study participants. The investigators evaluated the causal relationship of vaccination to each outcome by considering if there was a reasonable possibility that the MAE, SAE, or pIMD may have been caused by the vaccine, also considering the local product information and investigating alternative causes such as natural history of any underlying diseases, other concomitant therapy, and risk factors. As per protocol,27 the investigator assessed causality using the following question: Is there a reasonable possibility that the MAE, SAE, or pIMD may have been caused by the vaccine? The investigator responded “yes” if there was a reasonable possibility that the vaccine contributed to the MAE, SAE, or pIMD, and responded “no” if there was no reasonable possibility that the vaccine contributed to the MAE, SAE, or pIMD, if there were other, more likely causes and administration of the vaccine was not suspected to have contributed to the MAE, SAE, or pIMD.
Study population
Potential participants were screened to determine if they meet eligibility criteria, and eligible participants were enrolled.
Study participants were male and female Chinese adults, who voluntarily received dose 1 of RZV at ≥ 50 YOA between 27 September 2020 and 8 March 2022, planned to receive dose 2 on the same voluntary basis and for whom the investigators believed they would comply with the protocol requirements (e.g., return for follow-up visits, telephone contacts). The participants’ age was computed using June 30th as day and month of birth.
Participants were excluded if they were unable to comply with study requirements specified in the protocol, were participating in another study within three months prior to enrollment in the present study, or, if per investigators, they failed to meet vaccination requirements or had any contraindications.
The study aimed to enroll 3,300 participants. As the study was descriptive, the sample size was estimated considering the precision of the exact 95% confidence intervals (CIs), based on the range of reported proportions of MAEs and SAEs in the Asian population from previously published phase III trials.12,15,17 A sample size of 3,300 was also required by the Center for Drug Evaluation in China. The study population comprised of the exposed set (ES), which included all enrolled participants who received at least one vaccine dose, and the per-protocol set, which included participants in the ES who received two doses of vaccination administered within the recommended 2–6-month interval. We here disclose the results of the ES, as these safety data are the most clinically relevant for this population.
Study design
Study procedures and exposure
This post-marketing surveillance study implemented a prospective, observational, multi-center cohort design. Cohort entry began after dose 1 was administered (on a voluntary basis per local prescribing practices) and consent to participate in the study was obtained. Participants were followed from enrollment until 12 months after the last dose (Figure 1). Vaccinations were not study interventions but needed to be given according to the schedule in the local prescribing information.25 Data (including those for MAEs, SAEs, and pIMDs) were collected using active and enhanced passive surveillance methods and were recorded in electronic case report forms. Participants were required to complete two in-person visits: visit 1 (dose 1) and visit 2 at 2–6 months post-dose 1 (dose 2). Participants also underwent three follow-up telephone calls 30 days post-dose 1 (call 1), 30 days post-dose 2 (call 2), and 12 months post-last dose (call 3) (Figure 1). During these visits and telephone calls, the investigator enquired about the MAEs, SAEs, or pIMDs that may have occurred since the last contact with the participant. Outside of these active follow-up points, the participants could also directly report the outcomes of interest (MAEs, SAEs, pIMDs) to the study investigators by telephone or e-mail. Additionally, healthcare professionals not involved in the study could contact the study investigators or their delegate to report any suspected AEs. Data were collected from 27 September 2020 until 14 September 2023.
Figure 1.
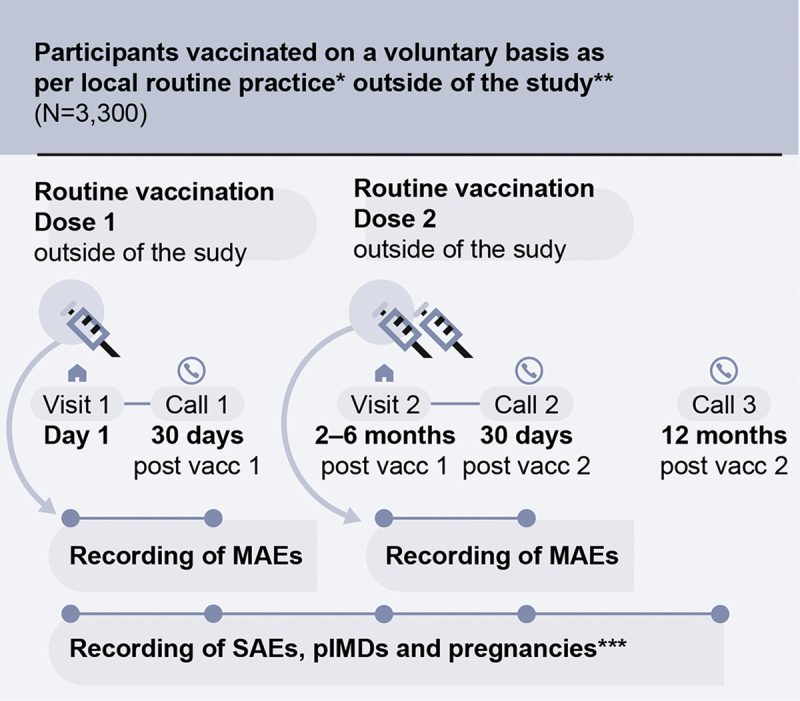
Study design.
* Local routine practice: two doses of the adjuvanted recombinant zoster vaccine (RZV) administered intramuscularly according to the local prescribing information (i.e., dose 2 administered 2–6 months post-dose 1); ** Outside of the study means that vaccinations were not study procedures (i.e., this was a non-interventional study). The participants’ decision to receive RZV was “outside of the study,” i.e., independent of their decision to participate in this study; *** Any exposure to RZV during pregnancy (any stage of gestation) needed to be reported to the study sponsor; MAEs, medically attended adverse events; N, number of participants; SAEs, serious adverse events; pIMDs, potential immune-mediated disorders; vacc, vaccine dose.
Baseline data collection
Baseline information collected from participants at study enrollment included date of enrollment, date of RZV dose 1, vaccination center, demographic data (age [birth year], sex, ethnicity), medical history of any preexisting conditions or signs/symptoms present prior to the first vaccination, medications taken by the participant at the time of enrollment, or any vaccine administered within 12 months preceding visit 1.
Follow-up data collection
Follow-up information was collected at study visits, at telephone follow-up contacts and through spontaneous reporting by the participant or a physician. In addition to information regarding MAEs, SAEs, and pIMDs (see “Study outcomes”), follow-up data included: date of dose 2, concomitant vaccinations or administration of any other vaccine during the study period, use of concomitant medications that may help explain the occurrence of an MAE/SAE/pIMD (i.e., medications that may have caused or were used to treat any outcome of interest) during the study period, use of any other concomitant medications during the 30 days post-each dose, and RZV administration during any stage of pregnancy.
Data analysis
Age of participants at dose 1 administration was summarized as mean (with standard deviation [SD]) and categorized in age groups 50–69 YOA and ≥ 70 YOA. Other demographic characteristics were described using number and percentage of participants.
The number and percentage (with exact 95% CI) of participants who reported at least one MAE or SAE within 30 days post-each dose were tabulated after each dose, overall per dose, and overall per participant, as well as per intensity level (for MAEs only). The number and percentage (with exact 95% CI) of participants who reported an SAE or a pIMD from dose 1 until 12 months post-last dose were also tabulated. All outcomes, i.e., MAEs, SAEs, and pIMDs, were summarized by age group and overall.
Missing data were handled in several ways depending on the variable of missingness.27 Missing severity, relationship with study vaccine, and outcomes of MAEs, SAEs, and pIMDs were not imputed. When partially completed dates (i.e., with missing day or month) were used in calculations, standard derivation rules were applied where a missing day was replaced by the 15th of the month and a missing day and month was replaced by June 30th (with some exceptions as detailed in the Supplemental material).
Results
Participant characteristics
A total of 3,300 participants were enrolled across six centers in China (three in Beijing and three in Shanghai) and received at least one RZV dose. The proportion of participants enrolled per center ranged from 11.8% to 28.3% (Table 1). Of the 3,300 participants, 3,160 received two RZV doses and 3,175 completed the study (Figure 2). The main reasons for withdrawal from the study (125 [3.8%] participants) were withdrawal by the participant and loss to follow-up, while only three participants withdrew due to an AE requiring expedited reporting.
Table 1.
Baseline characteristics of study participants.
| Characteristic | Total N = 3,300 |
|
|---|---|---|
| Value or n | % | |
| Mean (SD) agea in years | 61.2 (7.4) | |
| Agea group | ||
| 50–69 years | 2,867 | 86.9 |
| ≥70 years | 433 | 13.1 |
| Female sex | 2,213 | 67.1 |
| Race/ethnicity | ||
| Asian – Han Chinese | 3,197 | 96.9 |
| Other Asian – Chinese | 102 | 3.1 |
| Missing (non-Chinese ethnicity) | 1 | 0.03 |
| Study center | ||
| Center A | 443 | 13.4 |
| Center B | 636 | 19.3 |
| Center C | 490 | 14.8 |
| Center D | 390 | 11.8 |
| Center E | 407 | 12.3 |
| Center F | 934 | 28.3 |
aThe age (at first vaccination) was computed using June 30th as month and day of birth; N, total number of participants; n (%), number and percentage of participants in each category; SD, standard deviation.
Figure 2.
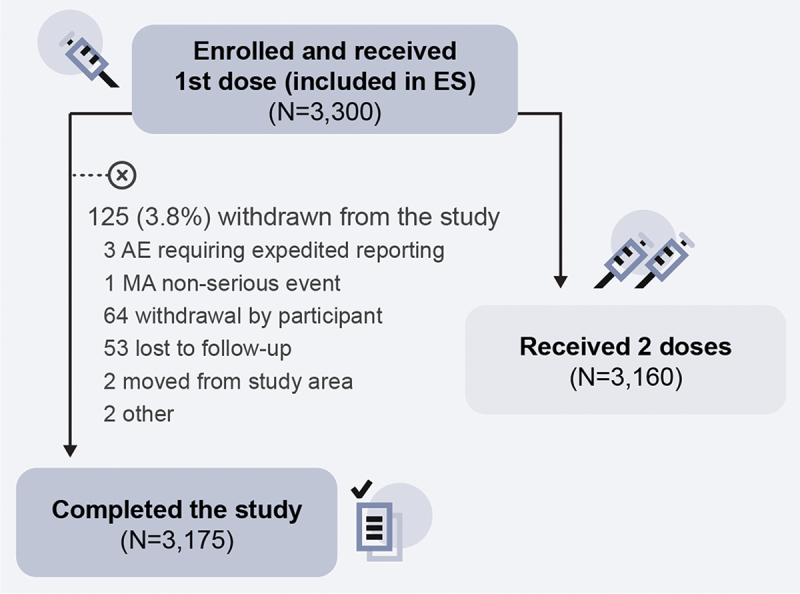
Participant flow chart.
AE, adverse event; ES, exposed set; MA, medically attended; N, number of participants. Note: RZV is a two-dose vaccine. The reason for representing the values for exposed (3,175) and two-dose recipients (3,160) separately is that the eligible participants were those who received at least one dose (but not necessarily both doses) of RZV. The population that completed the study was comprised of both individuals who received only one dose of RZV and of individuals who received two doses. The circled x symbol in the figure designates withdrawal from the study.
The mean (SD) age of enrolled participants at dose 1 administration was 61.2 (7.4) years. Out of the 3,300 participants, most were aged 50–69 years (86.9%), female (67.1%), and Asian – Han Chinese (96.9%) (Table 1). The interval between dose 1 and dose 2 varied from one to nine months, with a mean (SD) duration of 2.4 (1.0) months. Information on the participants’ medical history and concomitant medication use is provided in Supplementary tables S3 and S4.
Occurrence of MAEs within 30 days post-each dose
A total of 56 MAEs were reported in 42 (1.3%, 95% CI: 0.9–1.7%) participants; 15 MAEs in nine (0.3%, 95% CI: 0.1–0.5%) participants were assessed by the investigator as causally related to RZV (Figure 3a). As per the MedDRA SOC definition, the most frequently reported MAEs (of the total 56) were infections and infestations (in 0.5%, 95% CI: 0.3–0.7% of all participants), musculoskeletal and connective tissue disorders, and skin and subcutaneous tissue disorders (each in 0.2%, 95% CI: 0.0–0.4% of all participants).
Figure 3.
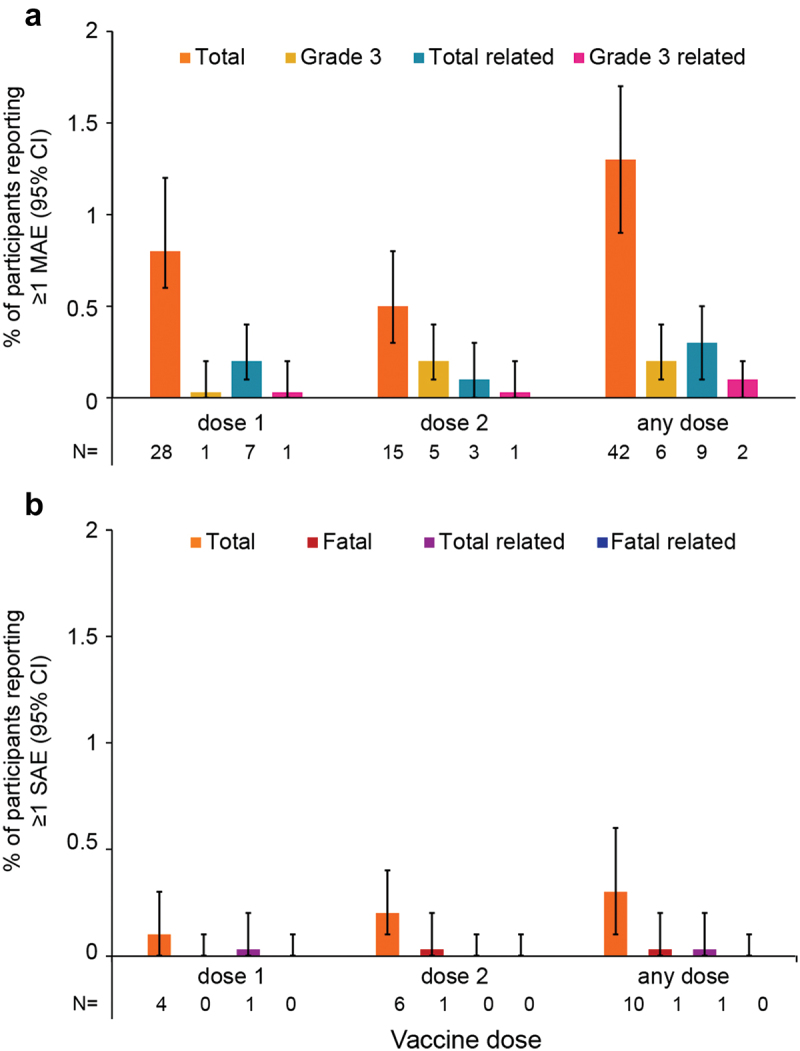
Number* and proportion of participants reporting at least one MAE (a) or SAE (b) within 30 days after vaccination, stratified by dose and overall.
* The numbers below each graph (N) correspond to numbers of participants reporting a given adverse event; CI, confidence interval; MAE, medically attended adverse event; SAE, serious adverse event.
The term “Total” refers to all reported MAEs or SAEs, “Grade 3” refers to MAEs with a grade 3 intensity, “related” refers to MAEs or SAEs assessed by the investigator to be related to vaccination. Error bars represent 95% CIs.
At least one grade 3 MAE was reported in six (0.2%, 95% CI: 0.1–0.4%) participants with onset within 30 days post-any dose. Two of these grade 3 MAEs were assessed by the investigator as related to vaccination. Supplementary figure S1A reports on MAEs stratified by age group.
Occurrence of SAEs within 30 days post-each dose
A total of 10 (0.3%, 95% CI: 0.1–0.6%) participants reported 12 SAEs occurring within 30 days post-any dose (Figure 3b). The most frequently reported SAEs per the MedDRA SOC were neoplasms (benign, malignant, and unspecified [e.g., cysts and polyps]), infections and infestations, and nervous system disorders (each in 0.1%, 95% CI: 0.0–0.3% of participants). Of the 12 SAEs, one SAE in one participant was assessed by the investigator as causally related to vaccination. Additionally, one fatal SAE was reported within 30 days post-dose 2; this SAE was assessed by the investigator as not causally related to RZV. Supplementary figure S1B reports on SAEs stratified by age group.
Occurrence of SAEs and pIMDs between dose 1 and 12 months post-last dose
From dose 1 until 12 months post-last dose, 29 SAEs were reported in 22 (0.7%, 95% CI: 0.4–1.0%) participants (Supplementary figure S2). Of these events, two SAEs in two (0.1%, 95% CI: 0.0–0.2%) participants were assessed as causally related to vaccination. Three fatal SAEs were reported in two (0.1%, 95% CI: 0.0–0.2%) participants, but these events were not considered causally related to RZV per the investigator’s assessment.
From dose 1 until 12 months post-last dose, seven pIMDs were reported in seven (0.2%, 95% CI: 0.1–0.4%) participants (Supplementary figure S2). Of these, three pIMDs in three (0.1%, 95% CI: 0.0–0.3%) participants were assessed by the investigator as RZV-related. The most commonly reported pIMDs were musculoskeletal and connective tissue disorders, and nervous system disorders (each in 0.1%, 95% CI: 0.0–0.2% of participants) per the MedDRA SOC.
Discussion
This was the first post-marketing surveillance study of the RZV safety profile in Chinese adults ≥ 50 YOA, who received the vaccine according to local prescribing information. This study observed no patterns that would indicate a safety concern for the reported MAEs, SAEs, and pIMDs following immunization with RZV. Most reported MAEs were considered unrelated to RZV administration, and only 0.2% of participants reported a grade 3 MAE. From dose 1 until 12 months post-last dose, SAEs were reported in up to 1.2% of all study participants (overall and per age group), with the majority being unrelated, and with 0.1% of all participants having a fatal (but not vaccine-related) SAE. Within the same time frame, up to 0.5% of all study participants (overall and per age group) reported a pIMD, with only 0.1% reporting a pIMD that was considered related. The present real-world safety surveillance results in this Chinese population are thus in line with results in adults ≥ 50 YOA from the multi-country phase III12,15,17,18 and post-licensure safety surveillance studies,16,19,21 as well as from the recent randomized phase IV clinical trial conducted in China.13
This study was distinct from clinical trials in similar age groups of adults ≥ 50 YOA, in that it did not include a placebo group and evaluated occurrence of MAEs, rather than solicited/unsolicited AEs. Although MAEs were not similarly defined in previously published studies, the proportion of participants reporting MAEs does not indicate different conclusions related to the safety profile of RZV compared to other studies.12,13,15,17,18 Furthermore, the proportions of RZV recipients reporting at least one SAE or pIMD in our study were within the published values.12,13,15,17,18 Analyses of pooled multi-country and Asian populations from phase III trials reported on comparable occurrences of SAEs (RZV: 10.1%–14.2%; placebo: 10.4%–13.2%), fatal AEs (RZV: 1.1%–5.2%; placebo: 1.1%–5.7%) and pIMDs (RZV: 0.6%–1.0%; placebo: 0.7%–0.8%) during one year after the last vaccination in RZV and placebo recipients.12,18 By comparison, within 30 days after each vaccination, 0.3% of participants in the current study reported at least one SAE, with 0.7% of participants reporting an SAE and 0.1% an SAE considered causally related to vaccination up to 12 months post-last dose. Consistent with previous studies in Asian populations,12,13 no fatal SAEs in this study were considered as RZV-related. With respect to pIMDs, a similar proportion of patients experienced a pIMD in the current study (seven cases in seven [0.2%] participants) as previously reported in Asian populations.12,13
Limitations of this study include its sample size, descriptive nature, lack of a control group, inclusion of only one country, and the possibility of selection or reporting bias. The study included 3,300 participants, which represent a small proportion of the Chinese population who have received the vaccine. The descriptive nature and lack of an unvaccinated control group did not allow for a quantification or estimation of the magnitude of an association (e.g., in terms of risk or odds) between a given AE and vaccination. The lack of an unvaccinated control group limited the interpretation of the study results as we were unable to assess whether there was a disproportion in the reporting of AEs between vaccinated and unvaccinated individuals. Without a control group the results cannot be interpreted comparatively or inferentially. As the study was conducted in only one country, generalizability is limited to the Chinese population, and, on its own, generalizability of this study’s specific findings to global public health, policy, and practice is limited. This study collected data as reported by participants, therefore, misreporting is possible. Additionally, participants who decided to enroll in the study may be different from the general population of individuals who have been vaccinated, potentially introducing selection bias. Lastly, as per protocol, causality assessments were based on clinical judgment of the medically trained investigators. We did not evaluate inter-rater reliability of the intensity or causality assessment between investigators. However, we expect limited variability of the causality assessment between investigators given clear criteria in the protocol and the investigators’ medical training, as is common practice in surveillance research. The main strengths of the study are that it enrolled Chinese adults who received vaccination voluntarily and that the eligibility criteria for participation were broad. Therefore, these safety results are likely generalizable to the population of Chinese adults who seek voluntary vaccinations.
This study was a non-interventional, post-authorization safety surveillance study committed to regulatory authorities in China following the approval of RZV in that country. This study found no descriptive patterns of MAEs, SAEs, or pIMDs to indicate a safety concern following RZV vaccination among Chinese adults ≥ 50 YOA. Together with all other published and known safety data related to the vaccine, this study further adds to the information demonstrating the safety of RZV in another population.
Trademark statement
Shingrix is a trademark owned by or licensed to GSK.
Supplementary Material
Acknowledgments
The authors would like to thank the study team members Michal Tokarczyk, Pedro De Almeida, Sridevi Pallem, Chiyu Ye, and Xiaoqing Sun for their contributions during study conduct, as well as Huizi Zhang for performing statistical analyses and Hao Wang for having contributed to two interim analyses. The authors thank Akkodis Belgium for editorial assistance, manuscript coordination, and design support on behalf of GSK; Irena Zurnic Bönisch provided medical writing support.
Biography
Dr. O’Mareen Spence is a Director, Epidemiology at GSK, specializing in vaccine pharmacoepidemiology.
Funding Statement
This study and related publication were sponsored by GSK.
Disclosure statement
OS, NP, JW, AC, SPS, and HY are employees of GSK. MY was an employee of GSK at the time the study was conducted and is currently an employee at another pharmaceutical company. OS, NP, AC, and HY hold financial equities in GSK. XP, TZ, and XG have nothing to disclose. The authors declare no other financial or non-financial relationships or activities.
Author contributions
XP and NP were involved in study concept or design. XP, TZ, and XG were involved in data acquisition. JW was involved in data analysis. All authors (XP, OS, NP, JW, TZ, XG, AC, SPS, MY, and HY) were involved in data interpretation.
Data availability statement
For requests to access anonymized subject-level data, please contact the corresponding author.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2024.2439031
References
- 1.Bricout H, Haugh M, Olatunde O, Prieto RG.. Herpes zoster-associated mortality in Europe: a systematic review. BMC Public Health. 2015;15(1):466. doi: 10.1186/s12889-015-1753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller NH, Gilden DH, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus infection: clinical features, molecular pathogenesis of disease, and latency. Neurol Clin. 2008;26(3):675–8. doi: 10.1016/j.ncl.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Oorschot D, Vroling H, Bunge E, Diaz-Decaro J, Curran D, Yawn B. A systematic literature review of herpes zoster incidence worldwide. Hum Vacc Immunother. 2021;17(6):1714–1732. doi: 10.1080/21645515.2020.1847582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang W, Li, G, Xu, Y, Pei, S, Yan, Y, Tong, H, Wang, W, Xu, C, Liu, Y and Yin, D. Epidemiological characteristics of herpes zoster in urban areas of Yichang city during 2016-2017 based on the Yichang big data platform for health management. Chin J Vaccines Immun. 2019;(4):432–435. [Google Scholar]
- 5.Sun X, Wei Z, Lin H, Jit M, Li Z, Fu C. Incidence and disease burden of herpes zoster in the population aged ≥50 years in China: data from an integrated health care network. J Infect. 2021;82(2):253–260. doi: 10.1016/j.jinf.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Chen T, Lin H, Zhan S, Mao S, Fu C. Incidence and economic burden of herpes zoster and its complications in immunocompromised adults: a retrospective cohort study in an eastern county, China. J Infect. 2022;85(5):e155–e157. doi: 10.1016/j.jinf.2022.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Liu X, Suo L, Zhao D, Pan J, Lu L. The incidence of herpes zoster in China: a meta-analysis and evidence quality assessment. Hum Vaccin Immunother. 2023;19(2):2228169. doi: 10.1080/21645515.2023.2228169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L-K, Arai H, Chen L-Y, Chou M-Y, Djauzi S, Dong B, Kojima T, Kwon KT, Leong HN, Leung EMF, et al. Looking back to move forward: a twenty-year audit of herpes zoster in Asia-Pacific. BMC Infect Dis. 2017;17(1):213. doi: 10.1186/s12879-017-2198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535. doi: 10.1177/25151355221084535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration . Package insert_shingrix. 2023. [Accessed 2024 Mar 4]. https://www.fda.gov/media/108597/download?attachment.
- 11.GSK . New shingrix data demonstrate 100% vaccine efficacy in the prevention of shingles in adults aged 50 and over in China. 2023. [Accessed 2024 Feb 6]. https://www.gsk.com/en-gb/media/press-releases/new-shingrix-data-demonstrate-100-vaccine-efficacy-in-prevention-shingles/.
- 12.Kim JH, Diaz-Decaro J, Jiang N, Hwang S-J, Choo EJ, Co M, Hastie A, Hui DSC, Irimajiri J, Lee J, et al. The adjuvanted recombinant zoster vaccine is efficacious and safe in Asian adults ≥50 years of age: a sub-cohort analysis of the ZOE-50 and ZOE-70 randomized trials. Hum Vaccin Immunother. 2021;17(7):2050–2057. doi: 10.1080/21645515.2020.1859321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandra Echeverria Proano D, Zhu F, Sun X, Zoco J, Soni J, Parmar N, Ali SO, Zhuoying H, Xiang G, Zhi L, et al. Efficacy, reactogenicity, and safety of the adjuvanted recombinant zoster vaccine for the prevention of herpes zoster in Chinese adults ≥ 50 years: A randomized, placebo-controlled trial. Hum Vaccin Immunother. 2024;20(1):2351584. doi: 10.1080/21645515.2024.2351584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Florea A, Sy LS, Qian L, Ackerson B, Luo Y, Wu J, Cheng Y, Ku JH, Vega Daily LI, Takhar H, et al. 1156. Real-world effectiveness of recombinant zoster vaccine in Chinese adults aged ≥50 years in the US. Open Forum Infect Dis. 2023;10(Supplement_2):ofad500.996. doi: 10.1093/ofid/ofad500.996. [DOI] [Google Scholar]
- 15.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang S-J, Díez-Domingo J, Godeaux O, Levin MJ, McElhaney JE, Puig-Barberà J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 16.Hesse EM, Shimabukuro TT, Su JR, Hibbs BF, Dooling KL, Goud R, Lewis P, Ng CS, Cano MV. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix) — United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(4):91–94. doi: 10.15585/mmwr.mm6804a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin MJ, McElhaney JE, Poder A, Puig-Barberà J, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 18.López-Fauqued M, Campora L, Delannois F, El Idrissi M, Oostvogels L, De Looze FJ, Diez-Domingo J, Heineman TC, Lal H, McElhaney JE, et al. Safety profile of the adjuvanted recombinant zoster vaccine: Pooled analysis of two large randomised phase 3 trials. Vaccine. 2019;37(18):2482–2493. doi: 10.1016/j.vaccine.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Ulloa-Pérez E, Yu O, Cook AJ, Jackson ML, Belongia EA, Daley MF, Harpaz R, Kharbanda EO, Klein NP, et al. Active postlicensure safety surveillance for recombinant zoster vaccine using electronic health record data. Am J Epidemiol. 2023;192(2):205–216. doi: 10.1093/aje/kwac170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimabukuro T. Update on post-licensure safety monitoring of recombinant zoster vaccine (RZV, Shingrix) - June 2019 advisory committee on immunization practices (ACIP) meeting. 2019. [Accessed: 2024 Mar 18]. https://stacks.cdc.gov/view/cdc/85339.
- 21.Tavares-Da-Silva F, Co MM, Dessart C, Hervé C, López-Fauqued M, Mahaux O, Van Holle L, Stegmann J-U. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020;38(18):3489–3500. doi: 10.1016/j.vaccine.2019.11.058. [DOI] [PubMed] [Google Scholar]
- 22.International Society for Pharmacoepidemiology . Guidelines for good pharmacoepidemiology practices (GPP), revision 3. 2015. [Accessed 2024 Aug 1]. https://www.pharmacoepi.org/resources/policies/guidelines-08027/.
- 23.Human Genetics Resources Administration of China . 登录系统 - 人类遗传资源管理平台. 2024. [Accessed 2024 Nov 13]. https://apply.hgrg.net/login.
- 24.World Medical Association . Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 25.China GSK. Recombinant herpes zoster vaccine - instructions. 2024. [Accessed: 2024 Aug 8]. https://www.gsk-china.com/media/6581/shingrix%E8%AF%B4%E6%98%8E%E4%B9%A6.pdf.
- 26.Medical Dictionary for Regulatory Activities . MedDRA Homepage. 2024. [Accessed 2024 Aug 1]. https://www.meddra.org/how-to-use/support-documentation/english/welcome.
- 27.GSK Study Register . A POst marketing surveillance (pms) study to assess thE safety of Shingrix given according to prescribing information in China. 2024. [Accessed: 2024 Aug 1]. https://www.gsk-studyregister.com/en/trial-details/?id=212290#documents-section.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For requests to access anonymized subject-level data, please contact the corresponding author.


