Abstract
BACKGROUND:
No disease-specific therapy currently exists for arrhythmogenic right ventricular cardiomyopathy (ARVC), a progressive cardiogenetic condition conferring elevated risk for ventricular arrhythmias, heart failure, and sudden cardiac death. Emerging gene therapies have the potential to fill this gap. However, little is known about how adults with ARVC, or any other inherited cardiomyopathy or arrhythmia syndrome, appraise the risks and benefits of gene therapy research and which considerations may influence their decisions about clinical trial participation.
METHODS:
Twenty adults with clinically diagnosed and gene-positive ARVC participated in semi-structured interviews that explored perceptions of gene therapy and hypothetical decision-making for gene therapy clinical trial participation. Interview transcripts were qualitatively coded and analyzed.
RESULTS:
Participants expressed enthusiasm for gene therapy with varied levels of personal interest in trial participation. Although clinical severity appeared to be associated with an elevated interest in early trial participation, participants anticipated weighing both personal and trial-specific factors including life stage, trial stage, risks, benefits, participation burden, study leadership, and anticipated cost of future gene therapy. Adaptation to living with ARVC and involvement in the ARVC patient community were also relevant to decision-making about trial participation. Potential ethical concerns included unquestioning trust in clinical teams collaborating on industry-led trials and vulnerability of those recently diagnosed or with high perceived severity of ARVC symptoms.
CONCLUSIONS:
Several characteristics of the individual and trial warrant consideration during the informed consent process. Insights from this study may affect trial planning and communication with participants who have inherited cardiac conditions.
Keywords: cardiomyopathies, clinical trials, decision making, genetic therapy, patients
Gene therapies for a range of inherited cardiomyopathy and arrhythmia syndromes are on the horizon. Clinical trials have been announced for hypertrophic cardiomyopathy,1,2 Danon disease,3 and arrhythmogenic right ventricular cardiomyopathy (ARVC),4–6 whereas preclinical research continues for other conditions such as long QT syndrome7,8 and catecholaminergic polymorphic ventricular tachycardia.9 Results from translational studies have been promising, leading to hope among patients and providers.10–13
ARVC is an autosomal dominant cardiogenetic condition associated with elevated risk for sudden cardiac death caused by progressive fibro-fatty cardiomyocyte replacement associated with ventricular arrhythmias and dysfunction.14 Pathogenic or likely pathogenic variants in desmosome-related genes can be identified in up to 60% of patients, with loss of function variants in PKP2 the most common.15 Early ARVC gene therapy strategies use adeno-associated viral vectors to systemically deliver PKP2 gene replacement targeting myocardial tissue.16–18
Gene therapy trials involve significant risks. Because of the concern for severe immune responses, immunosuppression is generally recommended for a limited period immediately before, during, and after treatment induction.19,20 As the administration of adeno-associated viral vector–based gene therapy leads to antibody formation against adeno-associated viral vector, those who have participated in prior gene therapy clinical trials may be ineligible for other future adeno-associated viral vector–based approaches.20 Off-target effects, such as cancer or other conditions caused by insertional mutagenesis, are also theoretically possible.21 Inflammatory responses to viral vectors may be of particular concern for people with ARVC because of the acute proarrhythmic effects of cardiac inflammation.10,22,23 Because of these risks, the participation burden will be highly intensive and likely involve hospitalization during therapy induction, as well as frequent in-person monitoring visits over several years.21 The likelihood of individual benefit from early clinical trial participation is unknown.
It is unclear how people with ARVC, or other cardiogenetic conditions, will weigh these potential risks and benefits and make decisions about joining gene therapy clinical trials. Studies among people with other genetic diseases suggest high levels of enthusiasm for gene therapy but nuanced disease-specific decision-making about clinical trial participation.24–34 Inherited cardiomyopathy and arrhythmia syndromes differ from previously studied conditions in that stabilizing treatment and interventions are available for most, despite being uncertain and imperfect. For instance, exercise restriction is a standard of care for PKP2-associated ARVC,35 and antiarrhythmic drugs and epicardial ablation have proven successful in reducing arrhythmia burden. Implantable cardioverter defibrillators (ICDs) have greatly reduced the risk of sudden death and heart transplantation has been successfully used to improve prognosis in patients with ARVC. In addition, gene therapy–related myocardial inflammation may be particularly worrisome for patients with ARVC as a potential arrhythmia trigger, given their high rates of ventricular arrhythmias, ICD discharges, and associated anxiety.36,37
As gene therapies for cardiogenetic conditions progress, it is critical to understand how patients with these conditions will perceive gene therapy, appraise risks and benefits, and approach decision-making. Understanding these perspectives is needed to inform patient education, informed consent processes, and clinical trial design. Therefore, we conducted a cross-sectional, qualitative semistructured interview study with adults with ARVC to gain insight into their perspectives on gene therapy and decision-making regarding clinical trial research participation.
Methods
The data that support the findings of this study may be available from the corresponding author on reasonable request. This study was approved by the Johns Hopkins Medicine institutional review board and participants provided oral consent. Methods are available as Supplemental Methods.
Results
Population
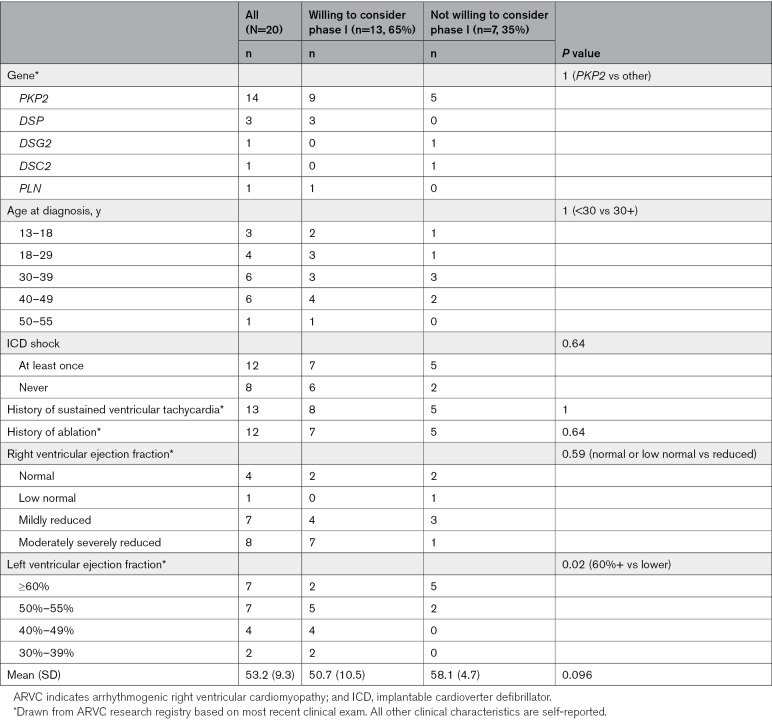
Twenty adults, mean age 43.3 years (SD=11.6), with ARVC participated in the interviews. Table 1 describes their demographics and Table 2 describes their clinical characteristics. Participants had been living with ARVC for a median 8.5 years (interquartile range, 6–13.5).
Table 1.
Participant Demographics (Self-Reported, N=20)
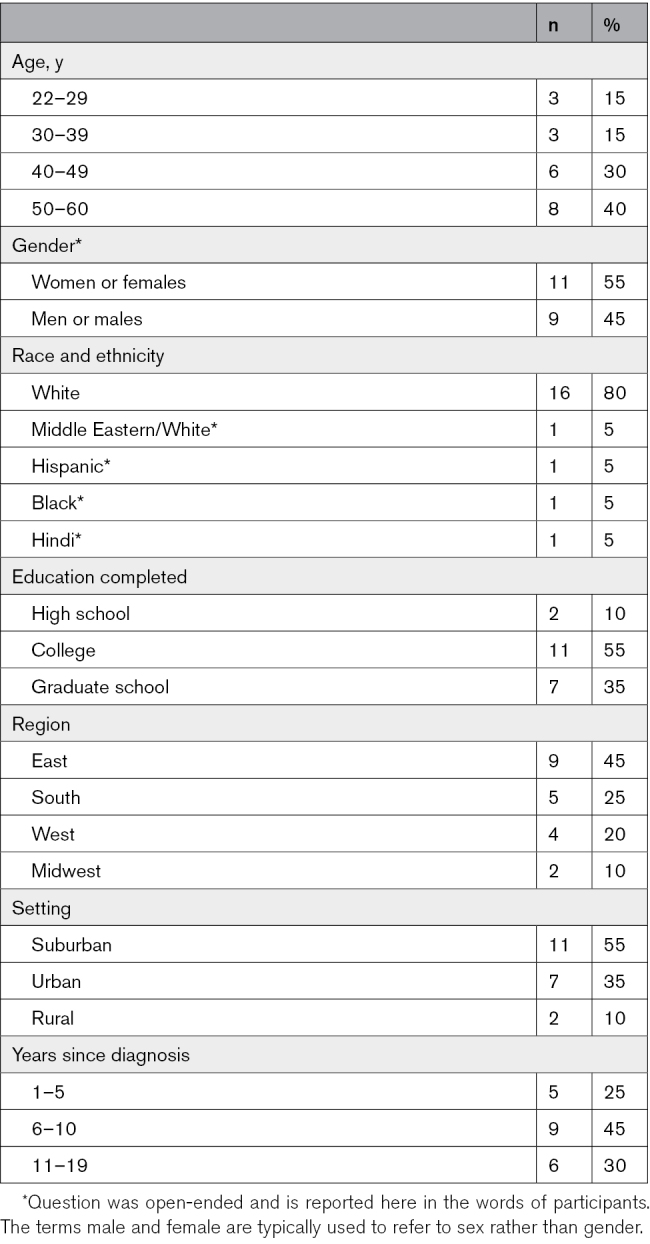
Table 2.
Clinical Characteristics of Participants
Interview Findings
Our interview findings fell into 3 broad categories. We begin by reporting gene therapy attitudes, which were predominantly positive, and interest in clinical trial participation, which ranged broadly. Next, we describe the personal characteristics of interviewees which influenced their hypothetical participation decision-making. Finally, we identify participants’ perceptions about features of a clinical trial that they believed would be important to these decisions. Additional participant quotations are available for each results section in Tables S1 through S3.
Participant Awareness of and Attitudes Toward Gene Therapy
Participants were typically already aware of gene therapy generally and specifically in the context of ARVC. Most participants were enthusiastic about the idea of gene therapy. Much of their hope centered on the view that gene therapy corrected the "root cause" of ARVC rather than just mitigating symptoms, and many described gene therapy as a cure despite this word not being used in the interview guide. A few participants expressed skepticism or concerns, largely about the use of a viral vector and perceived invasiveness, although many said their concerns may be ameliorated by further education about gene therapy mechanisms. Exemplar quotes addressing participants’ awareness and attitudes toward gene therapy are available in Table S1.
Most Participants Would Consider Joining Gene Therapy Clinical Trials
Interest in participating in gene therapy research was high, but comfort with early- versus late-stage trials varied considerably. No participant ruled out the possibility of participating in gene therapy clinical trials in the future. Interest in participation fell along a spectrum from certainly willing to participate immediately to presently unwilling to participate. Most (65%) would at least consider participating in phase I clinical trials if given the opportunity. No clear associations emerged between participation intentions and age, gender, education, race/ethnicity, home setting (urban/suburban/rural), age at diagnosis, years living with disease, or parental status. Table 2 summarizes clinical characteristics by willingness to consider participation in phase I clinical trials if given the opportunity. Table S1 contains quotations from participants representing a range of interest in participating in gene therapy clinical trials.
Characteristics of the Patient and Hypothetical Decision-Making
Several patient-specific factors were relevant to decision-making. These factors all ultimately relate to the relevance of ARVC to a person’s life.
ARVC permeates participants’ lives in multifaceted ways. Participants described an interconnected web of hyperawareness of physical symptoms, stress and anxiety about experiencing an ICD shock or sudden cardiac death, and drastic lifestyle modifications impacting not only exercise and athletics, but also social, career, financial, recreational, and reproductive decisions, as well as management of other health conditions. Consequently, many aspects of the reality of life with ARVC were highly salient to participation decisions. These findings are detailed below, and additional quotations from participants for each section are available in Table S2.
Perceived Disease Severity
The potential to benefit personally was a major motivator for participation, and a relative lack of concern about current disease status appeared to be a demotivator. Based on a preliminary analysis of clinical markers and participation intentions, those with lower left ventricular ejection fraction seem more likely to consider participation in phase I clinical trials (Table 2). There were no clear relationships between interest in participation and other objective clinical markers, such as experience with ICD shock, ablation, history of ventricular tachycardia, or right ventricular function. In describing disease severity, most participants focused on present stability more than prior clinical experiences or clinical measures. Many participants cited the stable status of their disease as a demotivator, even mentioning fears about the potential to jeopardize current stability. Correspondingly, many mentioned that if their disease increased in severity or interfered more with their life, participation would be highly desirable or even necessary. The participant who perceived his disease status as most severe and unstable was the most eager to participate in gene therapy clinical trials at any stage, stating the following: “A hundred percent I would participate, no questions asked … I’d say yes … Right away”—Participant 114.
Adaptation to Disease
Many participants described a years-long process of adaptation to living with ARVC, which was often complicated by experiencing periods of more severe symptoms or other reminders of their condition. Adaptation to disease was an important consideration in research participation.
Participants described how the impact of their condition had changed since their diagnosis. In discussing the transition to living with ARVC after diagnosis, many described drastic shifts in exercise level, social activities, and physical and mental health that affected their lives nearly every day. Over time, participants described their adaptation to living with their condition. For example, a participant diagnosed seven years ago states, “You know, I haven’t been living with this disease for a very long time, but it’s been long enough where I basically have had to accept that my life, the way I’m living my life is different from the way I want to be living my life”—Participant 111. Many participants who had lived with the condition for years speculated that this adaptation to disease resulted in a different view on gene therapy clinical trials than they may have had if they were more recently diagnosed: “Cause I think it was just such a radical upset in my life that I would have done anything to get rid of it. And now I’m like okay, well it’s been 10 years. I’m fine for the most part, like I’ve adjusted”—Participant 115.
The most recently diagnosed participant had been living with the condition for ≈1 year. She described the extreme impact that her diagnosis has had on her life and appeared to be very early in the adaptation process. When asked about her worries related to ARVC, she became tearful: “Umm [crying] that I’m going to die soon and I won’t be able to see my [kids] grow up … I think about it every day. Every day. [crying] I’m going to die soon and I’m going to die alone. And nobody’s going to find me”—Participant 119. On being asked about her interest in gene therapy clinical trial participation, she responded similarly to how many participants who had lived with ARVC for an extended period said they may have responded when first diagnosed: “I haven’t really been living with it that long, but I just want to go back to when I didn’t have any issues with my heart. So, if there’s a possibility of having that happen, then I’ll go for it … we know nothing’s guaranteed, but at least trying”—Participant 119.
However, some participants mentioned aspects of adaptation that made them more interested in participation. For example, 1 participant discussed changes in her identity and social sphere because of the inability to participate in sports which have become increasingly apparent over the years and mentioned that dissatisfaction with these changes is a motivator of participation for her.
Involvement With ARVC Community
Many of those who were interested in participation cited altruistic desires as major motivators. Participants described wanting to participate in research to help others with ARVC, including the current ARVC community and future generations of one’s own family. Some participants felt a sense of duty to help their community if personal risks of participation were within reason. Altruistic desires were expressed by many individuals but seemed to be more common among those who were more involved in the ARVC community and had friends or family members who were also affected with the condition. For example, a participant who described herself as highly involved in the ARVC disease advocacy community remarked: “I think it’s going to take very special people to be in phase one and phase two, brand new clinical trials. And I was thinking, would I do that? And it’s just a really hard concept because if, you know, ‘if not me, who?’ you have that kind of a response”—Participant 118.
Coping Styles
Conversely, some participants reported strategic distancing from the ARVC community as beneficial to mental health and described a degree of forgetfulness about their disease as therapeutic. Some participants who had previously participated in clinical trial research also described the increased time they spent thinking about their condition due to the demands of participating in research. Among participants who reported strategic distancing, concerns about potential risks and participation burden appeared to conflict with the desire for personal and community benefit. For example, in response to a question about immune suppression, 1 participant reacts: “I just had this sensation where I was just telling you that I don’t go to all these meetings and stuff because I want to distance myself from it. And I was just saying how I would like to help. And then there was a part of me that just said, ‘Well, just run away from all of this and just do your thing until you run out and, and I mean, why do this if you’re—? If there’s a risk and you don’t know about the outcome.’ But, I mean, I guess, I mean, if I’m being totally honest, I guess I feel both ways right now … Like I have this altruistic side that really wants to help … and then there’s a side that scares the crap out of me and, and, and look, I mean, you’re going to die from this thing anyway, so just don’t get involved in all of this and just let it run its course”—Participant 110.
Life Stage
Life stages, including family life and children, played a multifaceted role in anticipated decisions around research participation. Many participants talked about hoping for gene therapy to mitigate the risks of ARVC to ensure they could take care of and experience the lives of their children for years to come. Similarly, some participants hoped that gene therapy could restore physical activity limitations to enable them to physically care for, lift, and play with their children without anxiety. Desiring a cure for currently affected children was also a major motivator for participation. However, these hopes were tempered by concerns about potential harm to their children as a result of their research participation, such as taking time away from children to participate or becoming more ill because of research participation. Several parents mentioned that risks felt weightier after having children. Therefore, the potential implications that gene therapy research participation could have on one’s children’s lives were not seen as unilateral. Participation could risk the possibility of harm to a parent who is needed to provide care for them, but could also result in treatment advances that could save or improve the lives of both the affected parent and at-risk children.
Perceived Characteristics of the Trial Important to Decision-Making
Several trial-level factors were relevant to decision-making. Additional quotations from participants for each topic are available in Table S3.
Perceived Trial Benefits
Participants primarily hoped for personal therapeutic benefits from clinical trials, as well as possible benefits to one’s family members and community members. Hopes about the outcomes of gene therapy, and the definition of the word cure, appeared to vary significantly among participants. Specific hopes for personal benefit included being cured, no longer needing to think about ARVC, repairing prior cardiac damage, stopping other treatments or removing their ICD, resuming prior exercise levels or activities, and preventing future disease progression.
Perceived Trial Risks
Participant responses to the potential risks of trials varied widely. Some were accepting of the risks, many were highly daunted by the risks, and all desired further information. When asked which risk would be most important to clinical trial participation decisions, every risk presented was selected at least once. Long-term unknown off-target effects of gene therapy and the potential for ineligibility for future gene therapy treatments were the most frequently selected, and when others were chosen it was most often described as because of negative personal experience. Reasoning for selecting risks often centered on uncertainty, irreversibility, and feeling afraid. Certain risks felt less intimidating when viewed as comparable to risks already experienced living with ARVC.
Trial Stage and Data Availability
The stage of the trial and availability of clinical data regarding safety and effectiveness were very important to the majority of participants. Many individuals mentioned they would consider Phase I but would be more highly interested in phase III trials when safety had been more established. Many expressed a desire to wait for further data from early trials regarding safety and effectiveness before deciding about participation in later phases. They expressed the need for more data to inform personal risk-benefit analyses. For example, Participant 105 mentioned that he was less interested in stage I trials because he viewed his current condition as “not life-threatening,” but his desire to stop progression as early as possible informed his interest in taking on the lower risks of stage III trials. Participants who were most ready to participate in phase I trials either viewed their disease stage as severe enough to justify the risks or viewed the risks as minimal and worth the potential for community and personal benefit. Participants who would consider participation in stage I trials (and other stages) also expressed a desire for more information, including data from laboratory studies and animal models as well as from trials of other gene therapies to inform their decision.
Perceived Burden of Trial Participation
Appraisal of the potential time and energy burdens required to participate in gene therapy research varied widely between participants. Some participants described the participation burdens as the central factor in their decision, often mentioning an inability to travel due to obligations to career or family, such as raising young children. Others, including some with young children, described the time and energy burdens as playing little to no role in their participation decisions in comparison to weighing the medical risks and benefits of participation.
Trust in Clinicians and Researchers
Many participants noted that the opinion of trusted members of their healthcare team would be pivotal to their decision to participate. All participants were familiar with the ARVC team at Johns Hopkins Medicine, and some had very longstanding relationships with their providers, regardless of institution. In addition to consulting them for personalized clinical expertise, they relied on the ARVC team to conduct research advancing treatments and keep them abreast of developments at yearly patient-focused conferences. As a result, trust in clinicians and trust in researchers were heavily entangled. Participants mentioned strong beliefs in the benevolent intentions of their clinician-research team, for example, “I totally trust the doctors … I guess there’s a blind faith that if they ask me to participate, that they’re going to use me in some way to advance their knowledge”—Participant 110. Many participants also had positive experiences participating in prior research conducted with their clinical team, and this appeared to heighten their willingness to participate. For example, when asked about interest in participating in gene therapy trials, 1 participant stated the following: “Yeah. I’ll do it. I guess, like I said, I’ve kind of done everything that you guys presented, because I feel like it’s been good. It’s for my own betterment and ultimately a lot of other people’s betterment, and, yeah, as long as I feel that you guys pick a good study … I guess I trust myself to make a good decision for myself because I have a lot of trust in the information you guys provide”—Participant 107.
Conversely, some participants expressed ambivalence about the importance of personal trust in study leadership, focusing more on the expertise of the team. Finally, participants emphasized their desire to be fully informed about the research itself and expressed that secrecy may lead to loss of trust.
Anticipated Cost of Gene Therapy
Disparities in potential future access may impact research participation decisions. Some participants had preexisting concerns about future gene therapy costs, whereas others became aware of the high cost associated with prior approved gene therapies near the end of the interview. A few participants, particularly those with primarily altruistic motivations to participate, responded by expressing unwillingness to participate in research if the gene therapies were ultimately only likely to be financially accessible to a minority of affected individuals. Many desired estimated cost and insurance coverage information before participation decisions. Others mentioned that concern about being unable to access the treatment once approved was a motivating factor to participate in research and increased their willingness to accept risks. Other participants viewed the likely high costs as expected and outside of their control, and expressed this would not affect their willingness to participate.
Gene Specificity
Individuals were mostly accurately aware of whether their genetic variant was likely to be eligible for early trials. Although non-PKP2 participants expressed some impatience for gene therapies to address their disease, they overall felt that progress for any gene was likely to benefit all in the long run.
Discussion
This study explored how patients with ARVC appraise the risks and benefits of gene therapy research and identified which clinical, personal, and trial-specific factors may influence their decisions about clinical trial participation. We found that although attitudes toward gene therapy were positive, interest in participating in gene therapy clinical trials varied. Various characteristics of the potential participant, their experiences with ARVC, and perceived features of the trial and its outcomes were relevant to decision-making. As described below, our findings extend the results of research on decision-making for participation in gene therapy research to patients with cardiogenetic disease for the first time. Our results can also guide both healthcare providers facilitating participation decision-making and informed consent, as well as investigators designing trials that will maximize acceptability to participants.
Considerations for Recruitment Education and Informed Consent
As was seen in most other research among people with genetic conditions,24,27,34,38–41 gene therapy was highly acceptable to participants, with a minority having any concerns. However, participant perceptions of gene therapy revealed important considerations for the consent process.
Hope around gene therapy and the use of the word cure highlight the need for careful alignment of expectations with expected trial outcomes. Current evidence from animal models suggests that gene therapy may slow or prevent the progression of the disease,42 but the degree to which this may improve the lives of patients for whom the disease has already significantly progressed is not clear. Currently, the removal of an existing ICD after gene therapy would not be recommended, and receiving gene therapy would not change the recommendations for exercise restriction. In this sample, hopes for personal benefit far outstripped current evidence. In addition, the word cure was frequently applied by participants to gene therapy but was not clearly defined. Careful and explicit discussion of likely outcomes of a trial during informed consent is essential, with particular consideration to the emotional valence and hopes that gene therapy clinical trials evoke for patients. In similar research on other conditions, the belief and desire for an absolute cure has also driven participation decisions.26,28,32 Clarifying patients’ differing expectations of meaningful trial outcomes was noted as of major importance.25 Further exploration of willingness to take on risks given the likelihood of particular outcomes in ARVC is warranted, especially in larger samples of patients and using quantitative measures.
Participants responded to risks in varied ways. Many felt most concerned about risks that are intrinsically related to the uncertainties of research. In-depth presentation of the limited available data about trial risks is both necessary to facilitate informed consent and highly desired by participants, as seen in similar studies conducted in other disease groups.24 Many participants in this study also compared the risks of gene therapy trials to those they already experienced related to ARVC or in life, and some were most worried about risks that reminded them of a personal experience. Careful consideration of whether and how to differentiate between the risks of early-stage gene therapy trials to those of standard of care and other potential comparators is warranted.
Future studies may further explore demographic or disease-related predictors of participation intentions, including further investigation of the potential relationship between the extent of cardiac dysfunction and participation interest. This study was poorly suited to quantify these relationships, and potential associations have been observed in other studies.24
Participants’ perceptions of their disease were also important considerations for consent. Perceiving one’s disease status as unstable and life-threatening may strongly motivate participation, especially for those who feel they lack other options. These participants may also be more vulnerable to underestimating the risks of gene therapy and overestimating its benefits.43 This is of particular relevance given the possibility that those with highly severe clinical disease may be ineligible for early trials given increased risks of side effects, while individuals who perceive their disease as more severe than clinically suggested are more likely to be eligible to enroll. Although remaining aware that individuals will have divergent appraisals of risk, ensuring potential participants are well-informed of their risk stratification and evaluation by their clinical team is essential, particularly given some participants reported their severity as unknown to them. Furthermore, the ARVC community may expect justification for trial exclusion based on severe clinical markers given the extremely high desire for participation among those individuals and the widespread perception that more severely affected individuals are the most appropriate potential participants.
We also found that participants who were early in the process of adapting to life with ARVC viewed gene therapy differently compared with those who had more opportunity to adapt to living with ARVC. Although the adaptation process can be nonlinear, one’s perceived ability to live with the risks of ARVC will likely change over time, and researchers should engage with how to ensure individuals early in the disease adaptation process are making truly informed decisions. This finding corroborates the observation that individuals with inherited eye conditions who have lower self-acceptance regarding vision loss were more motivated to participate in gene therapy trials.25
Considerations for Trial Design
Providing information is important for any clinical trial. Presenting prospective participants with specific data from preclinical activities and other gene therapy trials was noted many times by our study participants as especially important.
With regard to the participation burden, the projected psychological toll of constant reminders about ARVC was noted as significant. Many participants described coping with their condition by maintaining a level of distance from their disease community and other reminders of their condition. Given the many follow-up visits likely to be necessary for safety and data collection, clinical trial planners may consider how best to support mental health during the research process. Trial designers should be mindful to consider the time and energy burden of participation in a clinical trial as well, such as the need for childcare for parents during both study visits and periods of immune suppression.
The extremely high levels of trust and reliance on recommendations by the participants’ medical team were notable, apparently beyond what has been observed in other gene therapy studies.25,27 This may be related to the specific partnerships of ARVC providers with the community, given how few ARVC programs exist and how rare the condition is, as well as the fact that participants were recruited from a research registry. Regardless, this finding highlights that clinical teams with longstanding patient relationships should be very clear about their level of involvement with the trials when discussing them with patients. Those who are directly involved in trials should communicate their roles and what they can or cannot control about the trial. Imprecise communication about this may jeopardize their deeply trusting relationships with these patients and unduly influence participant decisions.
Finally, concern about the future cost of gene therapy was substantial and has rarely been noted in prior studies.26 This may reflect increasing awareness of the potential future costs of gene therapy and/or the high levels of gene therapy awareness among participants. Trial organizers should be aware that participants may have questions and expectations of answers with regard to future cost and accessibility of gene therapy, and for some, this may be a demotivator for participation.
Limitations
Qualitative methodology is not intended to prove causal relationships and is considered transferable rather than generalizable; instead, this methodology enables us to explore or interpret concepts that are not easily captured in quantitative analyses and to highlight areas for future exploration.44
Despite purposive sampling, the demographic diversity was limited most notably with regard to race (people identifying as White were overrepresented) and education (people with at least a college education were overrepresented). This is of particular note given that most gene therapy perceptions research has been conducted largely among highly educated White individuals, and some studies including higher numbers of participants of other backgrounds have found markedly more negative perceptions of gene therapy.30,31 In addition, participants in this study likely have more positive orientations toward research than the general population of individuals with ARVC, given that they agreed to participate in this study and had previously agreed to be contacted for research when enrolling in the Johns Hopkins ARVC Registry.
Conclusions
Several considerations for gene therapy participation from other conditions can be applied to those with cardiogenetic conditions, such as the need to clarify perceived expectations and risks. Additional factors are also at play, such as variable adaptation to disease and perceived severity, as well as aspects of the trials themselves. Overall, these findings support a holistic approach to informed consent and participation procedures, including not only transparency with detailed data provision but individualized, grounded decision support and ongoing support for participants throughout the trial.
ARTICLE INFORMATION
Acknowledgments
The authors are deeply grateful to the patients with arrhythmogenic right ventricular cardiomyopathy and families who have made this work possible. This work was completed in partial fulfillment of E.M. Schopp’s graduate degree in genetic counseling.
Sources of Funding
This research was funded by the National Human Genome Research Institute in collaboration with the National Cancer Institute through the intramural program of the National Institutes of Health (HG200353-17).
Disclosures
Dr James performs modest consulting for Lexeo Therapeutics and Pfizer. Dr Calkins is a consultant for Medtronic, Inc, Biosense Webster, Pfizer, StrideBio, Rocket, and Abbott. B. Murray is a consultant for MyGeneCounsel. C. Tichnell is a consultant for StrideBio Inc. Dr Barth is a consultant for Solid Biosciences. Dr James receives research funding from Lexeo Therapeutics; Tenaya Therapeutics, Arvada Therapeutics, StrideBio Inc, EicOsis. C. Tichnell receives salary support on these grants. L. Okwara receives salary support on a grant from Lexeo Therapeutics. Dr Calkins receives research support from Boston Scientific Corp. C. Tichnell and Dr James receive salary support from this grant. Dr Calkins receives research support from Tenaya Inc. C. Tichnell, L. Okwara, and C. James receive salary support on this grant. Dr Calkins receives research support from Medtronic, Biosense Webster, Farapulse, and Adagio. E.M. Schopp, Dr Jamal, and A. Turiff are funded by the Intramural Research Program of the National Institutes of Health. The opinions and assertions expressed herein are those of the author(s) and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences or the Department of Defense.
Supplemental Material
Supplemental Methods
Interview Guide
Educational Material
Tables S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ARVC
- arrhythmogenic right ventricular cardiomyopathy
- ICD
- implantable cardioverter defibrillator
L. Jamal and C.A. James contributed equally as co-senior authors.
For Sources of Funding and Disclosures, see page 546.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.124.004759.
Contributor Information
Leonore Okwara, Email: lokwara1@jhmi.edu.
Crystal Tichnell, Email: ctichne1@jhmi.edu.
Amy Turriff, Email: amy.turriff@nih.gov.
Brittney Murray, Email: bdye1@jhmi.edu.
Andreas S. Barth, Email: abarth3@jhmi.edu.
Hugh Calkins, Email: hcalkin1@jhmi.edu.
Leila Jamal, Email: leila.jamal@nih.gov.
Cynthia A. James, Email: cjames7@jhmi.edu.
References
- 1.Tenaya Therapeutics. Tenaya Therapeutics doses first patient in the MyPeak-1™ Phase 1b clinical trial of TN-201 for the treatment of MYBPC3-associated hypertrophic cardiomyopathy. Globe Newswire. October 5, 2023. [Google Scholar]
- 2.Tenaya Therapeutics. Study of safety and tolerability of TN-201 in adults with symptomatic MYBPC3 mutation-associated HCM (MyPEAK-1). ClinicalTrials.gov Identifier: NCT05836259. Accessed April 21, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05836259 [Google Scholar]
- 3.Rocket Pharmaceuticals Inc. A multi-center, open label gene therapy study of RP-A501 in male patients with Danon disease. Clinicaltrials.gov identifier: NCT06092034. Accessed April 21, 2024. https://clinicaltrials.gov/study/NCT06092034 [Google Scholar]
- 4.Rocket Pharmaceuticals Inc. A phase 1, dose escalation trial of RP-A601 in subjects with PKP2 variant-mediated arrhythmogenic cardiomyopathy (PKP2-ACM). ClinicalTrials.gov identifier: NCT05885412. Accessed April 21, 2024. https://clinicaltrials.gov/study/NCT05885412 [Google Scholar]
- 5.Lexeo Therapeutics. Gene therapy for ACM due to a PKP2 pathogenic variant. ClinicalTrials.gov identifier: NCT06109181. Accessed April 21, 2024. https://clinicaltrials.gov/study/NCT06109181 [Google Scholar]
- 6.Tenaya Therapeutics. Open-label, dose escalation study of safety and preliminary efficacy of TN-401 in adults with PKP2 mutation-associated ARVC (RIDGE-1). ClinicalTrials.gov identifier: NCT06228924. Accessed April 21, 2024. https://clinicaltrials.gov/study/NCT06228924 [Google Scholar]
- 7.Dotzler SM, Kim CJ, Gendron WA, Zhou W, Ye D, Bos JM, Tester DJ, Barry MA, Ackerman MJ. Suppression-replacement KCNQ1 gene therapy for type 1 long QT syndrome. Circulation. 2021;143:1411–1425. doi: 10.1161/circulationaha.120.051836 [DOI] [PubMed] [Google Scholar]
- 8.Bains S, Zhou W, Dotzler SM, Martinez K, Kim CJ, Tester DJ, Ye D, Ackerman MJ. Suppression and replacement gene therapy for KCNH2-mediated arrhythmias. Circ Genom Precis Med. 2022;15:e003719. doi: 10.1161/CIRCGEN.122.003719 [DOI] [PubMed] [Google Scholar]
- 9.Bezzerides VJ, Caballero A, Wang S, Ai Y, Hylind RJ, Lu F, Heims-Waldron DA, Chambers KD, Zhang D, Abrams DJ, et al. Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca2+/calmodulin-dependent kinase II. Circulation. 2019;140:405–419. doi: 10.1161/CIRCULATIONAHA.118.038514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezzerides VJ, Prondzynski M, Carrier L, Pu WT. Gene therapy for inherited arrhythmias. Cardiovasc Res. 2020;116:1635–1650. doi: 10.1093/cvr/cvaa107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Boer RA, Heymans S, Backs J, Carrier L, Coats AJS, Dimmeler S, Eschenhagen T, Filippatos G, Gepstein L, Hulot JS, et al. Targeted therapies in genetic dilated and hypertrophic cardiomyopathies: from molecular mechanisms to therapeutic targets. A position paper from the Heart Failure Association (HFA) and the Working Group on Myocardial Function of the European Society of Cardiology (ESC). Eur J Heart Fail. 2022;24:406–420. doi: 10.1002/ejhf.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan T, Roberts JD. Emerging targeted therapies for inherited cardiomyopathies and arrhythmias. Card Electrophysiol Clin. 2023;15:261–271. doi: 10.1016/j.ccep.2023.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Paratz ED, Mundisugih J, Rowe SJ, Kizana E, Semsarian C. Gene therapy in cardiology: is a cure for hypertrophic cardiomyopathy on the horizon? Can J Cardiol. 2023;40:777–788. doi: 10.1016/j.cjca.2023.11.024 [DOI] [PubMed] [Google Scholar]
- 14.James CA, Jongbloed JDH, Hershberger RE, Morales A, Judge DP, Syrris P, Pilichou K, Domingo AM, Murray B, Cadrin-Tourigny J, et al. International evidence based reappraisal of genes associated with arrhythmogenic right ventricular cardiomyopathy using the clinical genome resource framework. Circ Genom Precis Med. 2021;14:e003273. doi: 10.1161/CIRCGEN.120.003273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dries AM, Kirillova A, Reuter CM, Garcia J, Zouk H, Hawley M, Murray B, Tichnell C, Pilichou K, Protonotarios A, et al. ; Regeneron Genetics Center. The genetic architecture of plakophilin 2 cardiomyopathy. Genet Med. 2021;23:1961–1968. doi: 10.1038/s41436-021-01233-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford W, Liang Y, Mataraarachchi N, Do A, Gu Y, Peterson K, Sheikh F. Plakophilin-2 gene therapy prevents arrhythmogenic right ventricular cardiomyopathy development in a novel mouse model harboring patient genetics. FASEB J. 2021;35. doi: 10.1096/fasebj.2021.35.S1.03193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyriakopoulou E, Versteeg D, de Ruiter H, Perini I, Seibertz F, Döring Y, Zentilin L, Tsui H, van Kampen SJ, Tiburcy M, et al. Therapeutic efficacy of AAV-mediated restoration of PKP2 in arrhythmogenic cardiomyopathy. Nat Cardiovasc Res. 2023;2:1262–1276. doi: 10.1038/s44161-023-00378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Opbergen CJ, Narayanan B, Sacramento CB, Stiles KM, Mishra V, Frenk E, Ricks D, Chen G, Zhang M, Yarabe P, et al. AAV-mediated delivery of plakophilin-2a arrests progression of arrhythmogenic right ventricular cardiomyopathy in murine hearts: preclinical evidence supporting gene therapy in humans. Circ Genom Precis Med. 2024;17:e004305. doi: 10.1161/CIRCGEN.123.004305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingozzi F, High KA. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K, Liao MZ. Clinical pharmacology considerations on recombinant adeno-associated virus-based gene therapy. J Clin Pharmacol. 2022;62(Suppl 2):S79–S94. doi: 10.1002/jcph.2141 [DOI] [PubMed] [Google Scholar]
- 21.Gene therapy needs a long-term approach. Nat Med. 2021;27:563–563. doi: 10.1038/s41591-021-01333-6 [DOI] [PubMed] [Google Scholar]
- 22.van der Voorn SM, Te Riele ASJM, Basso C, Calkins H, Remme CA, van Veen TAB. Arrhythmogenic cardiomyopathy: pathogenesis, pro-arrhythmic remodelling, and novel approaches for risk stratification and therapy. Cardiovasc Res. 2020;116:1571–1584. doi: 10.1093/cvr/cvaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasperetti A, James CA, Cerrone M, Delmar M, Calkins H, Duru F. Arrhythmogenic right ventricular cardiomyopathy and sports activity: from molecular pathways in diseased hearts to new insights into the athletic heart mimicry. Eur Heart J. 2021;42:1231–1243. doi: 10.1093/eurheartj/ehaa821 [DOI] [PubMed] [Google Scholar]
- 24.Aiyegbusi OL, Macpherson K, Elston L, Myles S, Washington J, Sungum N, Briggs M, Newsome PN, Calvert MJ. Patient and public perspectives on cell and gene therapies: a systematic review. Nat Commun. 2020;11:6265. doi: 10.1038/s41467-020-20096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napier MP, Selvan K, Hayeems RZ, Shuman C, Chitayat D, Sutherland JE, Day MA, Heon E. Gene therapy: perspectives from young adults with Leber’s congenital amaurosis. Eye (Lond). 2022;36:2088–2093. doi: 10.1038/s41433-021-01763-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quarmyne MO, Ross D, Sinha C, Bakshi N, Boudreaux J, Krishnamurti L. Decision-making about gene therapy in transfusion dependent thalassemia. BMC Pediatr. 2022;22:536. doi: 10.1186/s12887-022-03598-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turriff A, Blain D, Similuk M, Biesecker B, Wiley H, Cukras C, Sieving PA. Motivations and decision making processes of men with X-linked retinoschisis considering participation in an ocular gene therapy trial. Am J Ophthalmol. 2019;204:90–96. doi: 10.1016/j.ajo.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho HL, Kim SYH, Fitzhugh C, Hsieh M, Tisdale J, Grady C. Motivations and decision-making of adult sickle cell patients in high-risk clinical research. Biol Blood Marrow Transplant. 2020;26:1225–1232. doi: 10.1016/j.bbmt.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peay HL, Scharff H, Tibben A, Wilfond B, Bowie J, Johnson J, Nagaraju K, Escolar D, Piacentino J, Biesecker BB. “Watching time tick by…”: decision making for Duchenne muscular dystrophy trials. Contemp Clin Trials. 2016;46:1–6. doi: 10.1016/j.cct.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubé K, Simoni J, Louella M, Sylla L, Mohamed ZH, Patel H, Luter S, Collier AC. Acceptability of cell and gene therapy for curing HIV infection among people living with HIV in the northwestern United States: a qualitative study. AIDS Res Hum Retroviruses. 2019;35:649–659. doi: 10.1089/AID.2019.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King WD, Wyatt GE, Liu H, Williams JK, DiNardo AD, Mitsuyasu RT. Pilot assessment of HIV gene therapy-hematopoietic stem cell clinical trial acceptability among minority patients and their advisors. J Natl Med Assoc. 2010;102:1123–1128. doi: 10.1016/s0027-9684(15)30766-5 [DOI] [PubMed] [Google Scholar]
- 32.Regier DS, Bąk A, Bausell H, O’Reilly E, Cowsert LM. Starting the conversation on gene therapy for phenylketonuria: current perspectives of patients, caregivers, and advocates. Mol Genet Metab Rep. 2022;31:100855. doi: 10.1016/j.ymgmr.2022.100855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desine S, Hollister BM, Abdallah KE, Persaud A, Hull SC, Bonham VL. The meaning of informed consent: genome editing clinical trials for sickle cell disease. AJOB Empir Bioeth. 2020;11:195–207. doi: 10.1080/23294515.2020.1818876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Overbeeke E, Hauber B, Michelsen S, Peerlinck K, Lambert C, Hermans C, Lê PQ, Goldman M, Simoens S, Huys I. Patient preferences for gene therapy in haemophilia: results from the PAVING threshold technique survey. Haemophilia. 2021;27:957–966. doi: 10.1111/hae.14401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corrado D, Wichter T, Link MS, Hauer RNW, Marchlinski FE, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015;132:441–453. doi: 10.1161/CIRCULATIONAHA.115.017944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James CA, Tichnell C, Murray B, Daly A, Sears SF, Calkins H. General and disease-specific psychosocial adjustment in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy with implantable cardioverter defibrillators: a large cohort study. Circ Cardiovasc Genet. 2012;5:18–24. doi: 10.1161/CIRCGENETICS.111.960898 [DOI] [PubMed] [Google Scholar]
- 37.Asatryan B, Asimaki A, Landstrom AP, Khanji MY, Odening KE, Cooper LT, Marchlinski FE, Gelzer AR, Semsarian C, Reichlin T, et al. Inflammation and immune response in arrhythmogenic cardiomyopathy: state-of-the-art review. Circulation. 2021;144:1646–1655. doi: 10.1161/CIRCULATIONAHA.121.055890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelles M, Stieger K, Preising MN, Kruse J, Lorenz B. Shared decision-making, control preferences and psychological well-being in patients with RPE65 deficiency awaiting experimental gene therapy. Ophthalmic Res. 2015;54:96–102. doi: 10.1159/000435887 [DOI] [PubMed] [Google Scholar]
- 39.Woollacott I, Morgan G, Chowdary P, O’Hara J, Franks B, van Overbeeke E, Dunn N, Michelsen S, Huys I, Martin A, et al. Examining patient and professional perspectives in the UK for gene therapy in haemophilia. Haemophilia. 2022;28:588–609. doi: 10.1111/hae.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack HG, Britten-Jones AC, McGuinness MB, Chen FK, Grigg JR, Jamieson RV, Edwards TL, De Roach J, O’Hare F, Martin KR, et al. Survey of perspectives of people with inherited retinal diseases on ocular gene therapy in Australia. Gene Ther. 2022;30:336–346. doi: 10.1038/s41434-022-00364-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landrum Peay H, Fischer R, Tzeng JP, Hesterlee SE, Morris C, Strong Martin A, Rensch C, Smith E, Ricotti V, Beaverson K, et al. Gene therapy as a potential therapeutic option for Duchenne muscular dystrophy: a qualitative preference study of patients and parents. PLoS One. 2019;14:e0213649. doi: 10.1371/journal.pone.0213649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheikh F, Zhang J, Wang J, Bradford WH, Nair A, Fargnoli A, Selvan N, Gutierrez S, Law K, Fenn T, et al. LX2020, an adeno associated viral-based plakophilin 2 gene therapy stabilizes cardiac disease phenotype in a severe mouse model of arrhythmogenic right ventricular cardiomyopathy. Circulation. 2022;146:A13599–A13599. [Google Scholar]
- 43.Jansen LA. Two concepts of therapeutic optimism. J Med Ethics. 2011;37:563–566. doi: 10.1136/jme.2010.038943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green J, Britten N. Qualitative research and evidence based medicine. BMJ. 1998;316:1230–1232. doi: 10.1136/bmj.316.7139.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmermans S, Tavory I. Data Analysis in Qualitative Research: Theorizing With Abductive Analysis. University of Chicago Press; 2022. [Google Scholar]
- 46.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daimee UA, Assis FR, Murray B, Tichnell C, James CA, Calkins H, Tandri H. Clinical outcomes of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy: insights from the Johns Hopkins ARVC Program. Heart Rhythm. 2021;18:1369–1376. doi: 10.1016/j.hrthm.2021.04.028 [DOI] [PubMed] [Google Scholar]
- 48.Campbell S, Greenwood M, Prior S, Shearer T, Walkem K, Young S, Bywaters D, Walker K. Purposive sampling: complex or simple? Research case examples. J Res Nurs. 2020;25:652–661. doi: 10.1177/1744987120927206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ASGCT Patient Education. American society of gene & cell therapy. Accessed February 2023. https://patienteducation.asgct.org/. 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.