Abstract
BACKGROUND
Standard therapy for advanced endometrial cancer after failure of platinum-based chemotherapy remains unclear.
METHODS
In this phase 3 trial, we randomly assigned, in a 1:1 ratio, patients with advanced endometrial cancer who had previously received at least one platinum-based chemotherapy regimen to receive either lenvatinib (20 mg, administered orally once daily) plus pembrolizumab (200 mg, administered intravenously every 3 weeks) or chemotherapy of the treating physician’s choice (doxorubicin at 60 mg per square meter of body-surface area, administered intravenously every 3 weeks, or paclitaxel at 80 mg per square meter, administered intravenously weekly [with a cycle of 3 weeks on and 1 week off]). The two primary end points were progression-free survival as assessed on blinded independent central review according to the Response Evaluation Criteria in Solid Tumors, version 1.1, and overall survival. The end points were evaluated in patients with mismatch repair–proficient (pMMR) disease and in all patients. Safety was also assessed.
RESULTS
A total of 827 patients (697 with pMMR disease and 130 with mismatch repair– deficient disease) were randomly assigned to receive lenvatinib plus pembrolizumab (411 patients) or chemotherapy (416 patients). The median progression-free survival was longer with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 6.6 vs. 3.8 months; hazard ratio for progression or death, 0.60; 95% confidence interval [CI], 0.50 to 0.72; P<0.001; overall: 7.2 vs. 3.8 months; hazard ratio, 0.56; 95% CI, 0.47 to 0.66; P<0.001). The median overall survival was longer with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 17.4 vs. 12.0 months; hazard ratio for death, 0.68; 95% CI, 0.56 to 0.84; P<0.001; overall: 18.3 vs. 11.4 months; hazard ratio, 0.62; 95% CI, 0.51 to 0.75; P<0.001). Adverse events of grade 3 or higher occurred in 88.9% of the patients who received lenvatinib plus pembrolizumab and in 72.7% of those who received chemotherapy.
CONCLUSIONS
Lenvatinib plus pembrolizumab led to significantly longer progression-free survival and overall survival than chemotherapy among patients with advanced endometrial cancer. (Funded by Eisai and Merck Sharp and Dohme [a subsidiary of Merck]; Study 309–KEYNOTE-775 ClinicalTrials.gov number, NCT03517449.)
The incidence of endometrial cancer is increasing worldwide.1–4 Approximately 10 to 15% of patients with endometrial cancer present with advanced-stage disease,5 and 5-year survival among patients with distant metastases has been reported to be 17%.6 No treatments have been globally accepted as the standard of care for advanced or recurrent endometrial cancer after the failure of platinum-based chemotherapy.7,8 Targeted therapies and chemotherapy have had limited efficacy, substantial toxic effects, or both.9–16 Lenvatinib, a multitargeted tyrosine kinase inhibitor of vascular endothelial growth factor receptors 1 through 3, fibroblast growth factor receptors 1 through 4, platelet-derived growth factor receptor α, RET, and KIT,17 has limited efficacy as second-line treatment for recurrent endometrial carcinoma (objective response in 14.3% [95% confidence interval {CI}, 8.8 to 21.4] of patients, as assessed on independent review according to Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1).18 In a nonrandomized study, checkpoint inhibitors, including pembrolizumab (a programmed cell death 1 [PD-1] inhibitor), had compelling antitumor activity, as assessed on the basis of objective response and duration of response, in patients with microsatellite instability–high (MSI-H) or mismatch repair–deficient (dMMR) advanced endometrial carcinoma.19 However, only 16 to 31% of endometrial cancers are MSI-H or dMMR.20–23 Furthermore, pembrolizumab monotherapy has shown less activity in patients with microsatellite-stable or mismatch repair–proficient (pMMR) disease than in those with MSI-H or dMMR disease.19,24
In the Study 111–KEYNOTE-146 trial,25 treatment with lenvatinib in combination with pembrolizumab had compelling efficacy in patients with previously treated advanced endometrial carcinoma; high-grade adverse events were managed with supportive therapy and dose modifications, with a relatively low incidence of discontinuation due to adverse events. We conducted the Study 309–KEYNOTE-775 trial to confirm the results of the earlier trial by comparing the efficacy and safety of lenvatinib plus pembrolizumab with the physician’s choice of doxorubicin or paclitaxel chemotherapy in patients with advanced endometrial cancer who had disease progression after the receipt of at least one platinum-based therapy.
METHODS
Patients
We enrolled women 18 years of age or older with confirmed advanced, recurrent, or metastatic endometrial cancer of any histologic subtype, except carcinosarcoma and sarcoma. Eligible women had disease progression after the receipt of one previous platinum-based chemotherapy regimen, with no history of exposure to vascular endothelial growth factor– or PD-1–targeting regimens. Patients may have received two lines of platinum-based chemotherapy if one was given as neoadjuvant or adjuvant therapy. There was no restriction regarding the previous receipt of hormonal therapy. Other inclusion criteria were the following: at least one measurable lesion according to RECIST, version 1.1; available biopsy specimens for the determination of MMR status; and an Eastern Cooperative Oncology Group (ECOG) performance-status score of 0 or 1 (on a 5-point scale, with higher scores indicating greater disability). The full lists of the inclusion and exclusion criteria are provided in the protocol, available with the full text of this article at NEJM.org.
Trial Design and Treatments
This multicenter, open-label, phase 3 trial had a screening phase of up to 28 days, after which eligible patients were randomly assigned (in a 1:1 ratio) to receive either lenvatinib plus pembrolizumab or chemotherapy of the treating physician’s choice (with doxorubicin or paclitaxel chosen before randomization; chemotherapy group). In the lenvatinib–pembrolizumab group, patients received lenvatinib at a dose of 20 mg, administered orally once daily, plus pembrolizumab at a dose of 200 mg, administered intravenously as a 30-minute infusion every 3 weeks, on the basis of previous dose-finding studies.26 Patients could have received up to 35 doses of pembrolizumab in the trial. In the chemotherapy group, patients received doxorubicin at a dose of 60 mg per square meter of body-surface area, administered intravenously as a 1-hour infusion or according to institutional guidelines, every 3 weeks, or paclitaxel at a dose of 80 mg per square meter, administered intravenously as a 1-hour infusion or according to institutional guidelines, weekly (with a cycle of 3 weeks on and 1 week off). Randomization to treatment group was initially stratified according to MMR status (deficient [dMMR] or proficient [pMMR]). Furthermore, within the pMMR population, patients were stratified according to ECOG performance-status score (0 or 1), geographic region (region 1 [Australia, Canada, Europe, Israel, New Zealand, and the United States] or region 2 [rest of the world]), and history of pelvic irradiation (yes or no). Details regarding treatment duration, discontinuation, and dose modifications are provided in the Supplementary Appendix, available at NEJM.org.
Trial Oversight
The trial was conducted in accordance with the Good Clinical Practice guidelines of the International Council for Harmonisation and ethical principles originating from the Declaration of Helsinki. All the patients provided written informed consent. Institutional review boards or independent ethics committees approved the trial protocol at each site. Data from the interim analysis were collected by the investigator, monitored by an external data and safety monitoring committee, and analyzed by independent central reviewers. The trial was designed by academic authors and employees of the sponsors (Eisai and Merck Sharp and Dohme [a subsidiary of Merck]). All the authors had full access to the data and attest to their participation in the preparation and review of the manuscript. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. A medical writer, funded by the sponsors, assisted with the preparation of an earlier version of the manuscript.
End Points
The two primary end points were progression-free survival as assessed on blinded independent central review according to RECIST, version 1.1, and overall survival. Secondary end points included objective response as assessed on blinded independent central review according to RECIST, version 1.1; safety and side-effect profile; and health-related quality of life (assessed with the use of the global health status and quality-of-life score of the European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire–Core 30 [QLQ-C30]); and the exposure–safety relationship of lenvatinib therapy. A full list of the secondary end points is available in the protocol. Duration of response was an exploratory end point.
All the end points were assessed in the pMMR population and among all patients. Subgroup analyses were prespecified. Details regarding tumor assessments are provided in the Supplementary Methods section. Adverse events occurring from the time of treatment assignment until 30 days after treatment discontinuation were recorded and were graded according to the Common Terminology Criteria for Adverse Events, version 4.03, of the National Cancer Institute.
Statistical Analysis
The planned sample was approximately 780 patients (with 660 patients in the pMMR population and 120 in the dMMR population). The first interim analysis was the final efficacy analysis for progression-free survival and an interim efficacy analysis for overall survival. This analysis was to be conducted at least 6 months after the last patient had undergone randomization and when approximately 368 deaths had occurred in the pMMR population. Sample-size and power calculations for the analysis of overall survival were based on the pMMR population. We calculated that with 660 patients in the pMMR population, a total of 526 deaths would provide the trial with 90% power to detect a significant difference at the 0.049 level in the final analysis of overall survival, under the assumptions that the hazard ratio for death would be 0.75, that the first and second interim analyses would be performed when approximately 368 and 463 deaths, respectively, had occurred; and that a Lan–DeMets spending function with the O’Brien– Fleming boundary would be used. For the analysis of progression-free survival, we estimated that 564 events of progression or death would provide the trial with more than 99% power to detect a significant difference at the 0.001 level, under the assumption that the hazard ratio for progression or death would be 0.55. Primary efficacy analyses were conducted in the intention-to-treat population (defined as all the patients who underwent randomization).
Treatment differences in the analyses of progression-free survival and overall survival were assessed by means of the stratified log-rank test (with two-sided P values); the nonparametric Kaplan–Meier method was used to estimate curves. A stratified Cox proportional-hazards model with Efron’s method of tie handling was used to assess hazard ratios. The stratified Miettinen and Nurminen’s method was used to determine between-group differences in objective response. The difference in the percentages of patients with an objective response and the 95% confidence intervals with strata weighting according to sample size were reported, and a separate analysis in which missing data were accounted for through multiple imputation was also conducted (see the Supplementary Methods section). Confidence intervals were not adjusted for multiplicity, so definitive treatment effects cannot be inferred.
The stratification factors that were used for randomization were applied to the analyses. The total familywise error rate (type I error) among the two primary efficacy end points and the secondary end point of objective response were strongly controlled at a two-sided alpha of 0.05. A prespecified graphical approach for multiplicity to control for type 1 error was used to test progression-free survival first in the pMMR population, then among all patients, followed by overall survival (first in the pMMR population and then among all patients) and then objective response (first in the pMMR population and then among all patients) (Fig. S1 in the Supplementary Appendix). For health-related quality-of-life analyses, global health status scores were summarized according to treatment group over time. The safety analysis population included all the patients who underwent randomization and received at least one dose of trial treatment. Further details are included in the Supplementary Methods section and the statistical analysis plan (see the protocol).
RESULTS
Patients and Treatments
Across 167 sites in 21 countries, 827 patients (697 in the pMMR population and 130 in the dMMR population) were randomly assigned to a treatment group between June 11, 2018, and February 3, 2020 (Fig. S2). Data cutoff occurred on October 26, 2020, for the final analysis of progression-free survival and the first interim analysis of overall survival. The median follow-up was 12.2 months in the lenvatinib–pembrolizumab group and 10.7 months in the chemotherapy group. At the data-cutoff date, treatment was ongoing in 27.8% of the patients in the pMMR population and in 30.5% of all the patients who started treatment in the lenvatinib–pembrolizumab group and in 2.8% and 2.6%, respectively, of those who started chemotherapy. The primary reason for treatment discontinuation among all the patients in all the groups was disease progression.
The demographic and disease characteristics of the patients at baseline were balanced between the treatment groups, both overall and in the pMMR population (Tables 1, S1, and S2). These populations were also determined to be equivalent to real-word populations (Table S3). Among all the patients, 84.2% of the patients in the lenvatinib–pembrolizumab group and 84.4% of those in the chemotherapy group had confirmed pMMR status, and 35.0% and 38.2% of the patients, respectively, had previously received systemic treatment only as neoadjuvant or adjuvant therapy. Treatment with one previous platinum-based therapy was reported for 79.3% of the patients in the lenvatinib–pembrolizumab group and for 75.7% of those in the chemotherapy group; 20.2% and 24.3% of the patients, respectively, had received two platinum-based therapies previously; 8.8% and 10.6% had received palliative hormonal therapy previously; and 46.0% and 47.8% had received external-beam radiotherapy previously.
Table 1.
Demographic and Disease Characteristics of All the Trial Patients at Baseline.*
| Characteristic | Lenvatinib plus Pembrolizumab (N = 411) | Chemotherapy (N = 416) |
|---|---|---|
| Age | ||
| Median (range) — yr | 64 (30–82) | 65 (35–86) |
| <65 yr — no. (%) | 206 (50.1) | 204 (49.0) |
| Race — no. (%)† | ||
| White | 261 (63.5) | 246 (59.1) |
| Black | 17 (4.1) | 14 (3.4) |
| Asian | 85 (20.7) | 92 (22.1) |
| Geographic region — no. (%)‡ | ||
| Region 1 | 234 (56.9) | 240 (57.7) |
| Region 2 | 177 (43.1) | 176 (42.3) |
| MMR status — no. (%) | ||
| pMMR | 346 (84.2) | 351 (84.4) |
| dMMR | 65 (15.8) | 65 (15.6) |
| ECOG performance-status score — no. (%)§ | ||
| 0 | 246 (59.9) | 241 (57.9) |
| 1 | 164 (39.9) | 175 (42.1) |
| History of pelvic irradiation — no. (%) | 174 (42.3) | 186 (44.7) |
| Histologic features at initial diagnosis — no. (%)¶ | ||
| Endometrioid carcinoma | 243 (59.1) | 254 (61.1) |
| High grade | 94 (22.9) | 90 (21.6) |
| Low grade | 59 (14.4) | 54 (13.0) |
| Not specified‖ | 90 (21.9) | 110 (26.4) |
| Serous carcinoma | 103 (25.1) | 115 (27.6) |
| Clear-cell carcinoma | 30 (7.3) | 17 (4.1) |
| Mixed features | 22 (5.4) | 16 (3.8) |
Percentages may not total 100 because of rounding. The term dMMR denotes mismatch repair–deficient, MMR mismatch repair, and pMMR mismatch repair–proficient.
Race was reported by the patient. Data on race were missing for 36 patients (8.8%) in the lenvatinib-pembrolizumab group and for 44 (10.6%) in the chemotherapy group. Other races or ethnic groups (reported by 12 patients [2.9%] in the lenvatinib-pembrolizumab group and by 20 [4.8%] in the chemotherapy group) included American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and multiple.
Region 1 was defined as Australia, Canada, Europe, Israel, New Zealand, and the United States, and region 2 as the rest of the world.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability. One patient in the lenvatinib–pembrolizumab group had an ECOG performance-status score of 3 (was enrolled in error).
Information regarding histologic features at diagnosis for categories that included less than 5% of the patients is provided in Table S2.
The “not specified” category included endometrioid carcinoma (grade not specified) nd endometrioid carcinoma with squamous differentiation.
Efficacy
In the pMMR population, progression-free survival as assessed on blinded independent central review according to RECIST, version 1.1, was significantly longer with lenvatinib plus pembrolizumab (median, 6.6 months; 95% CI, 5.6 to 7.4) than with chemotherapy (median, 3.8 months; 95% CI, 3.6 to 5.0) (hazard ratio for progression or death, 0.60; 95% CI, 0.50 to 0.72; P<0.001) (Fig. 1A). Similar results were seen in the overall trial population; the median progression-free survival was 7.2 months (95% CI, 5.7 to 7.6) with lenvatinib plus pembrolizumab, as compared with 3.8 months (95% CI, 3.6 to 4.2) with chemotherapy (hazard ratio, 0.56; 95% CI, 0.47 to 0.66; P<0.001) (Fig. 1B).
Figure 1: Progression-free Survival.
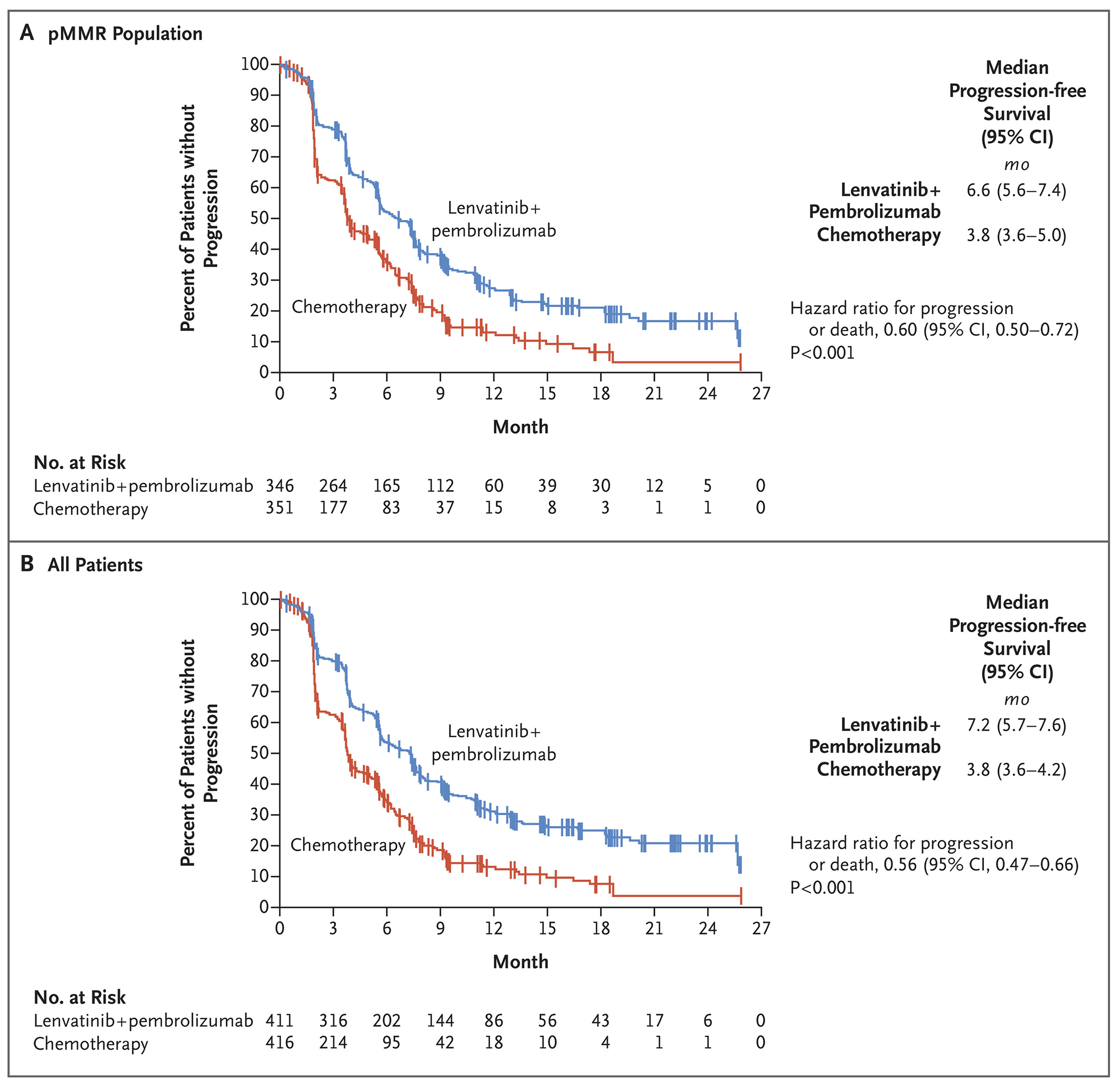
Panel A shows progression-free survival in the population of patients with advanced endometrial cancer with mismatch repair–proficient (pMMR) disease, and Panel B shows progression-free survival among all the patients. Tick marks indicate censored data.
Overall survival in the pMMR population was significantly longer with lenvatinib plus pembrolizumab (median, 17.4 months; 95% CI, 14.2 to 19.9) than with chemotherapy (median, 12.0 months; 95% CI, 10.8 to 13.3) (hazard ratio for death, 0.68; 95% CI, 0.56 to 0.84; P<0.001) (Fig. 2A). Similar results were seen in the overall trial population; the median overall survival was 18.3 months (95% CI, 15.2 to 20.5) with lenvatinib plus pembrolizumab, as compared with 11.4 months (95% CI, 10.5 to 12.9) with chemotherapy (hazard ratio, 0.62; 95% CI, 0.51 to 0.75; P<0.001) (Fig. 2B). The proportional-hazards assumptions of progression-free survival and overall survival among all the patients were met, thus the hazards were deemed to be proportional (Fig. S3).
Figure 2: Overall Survival.
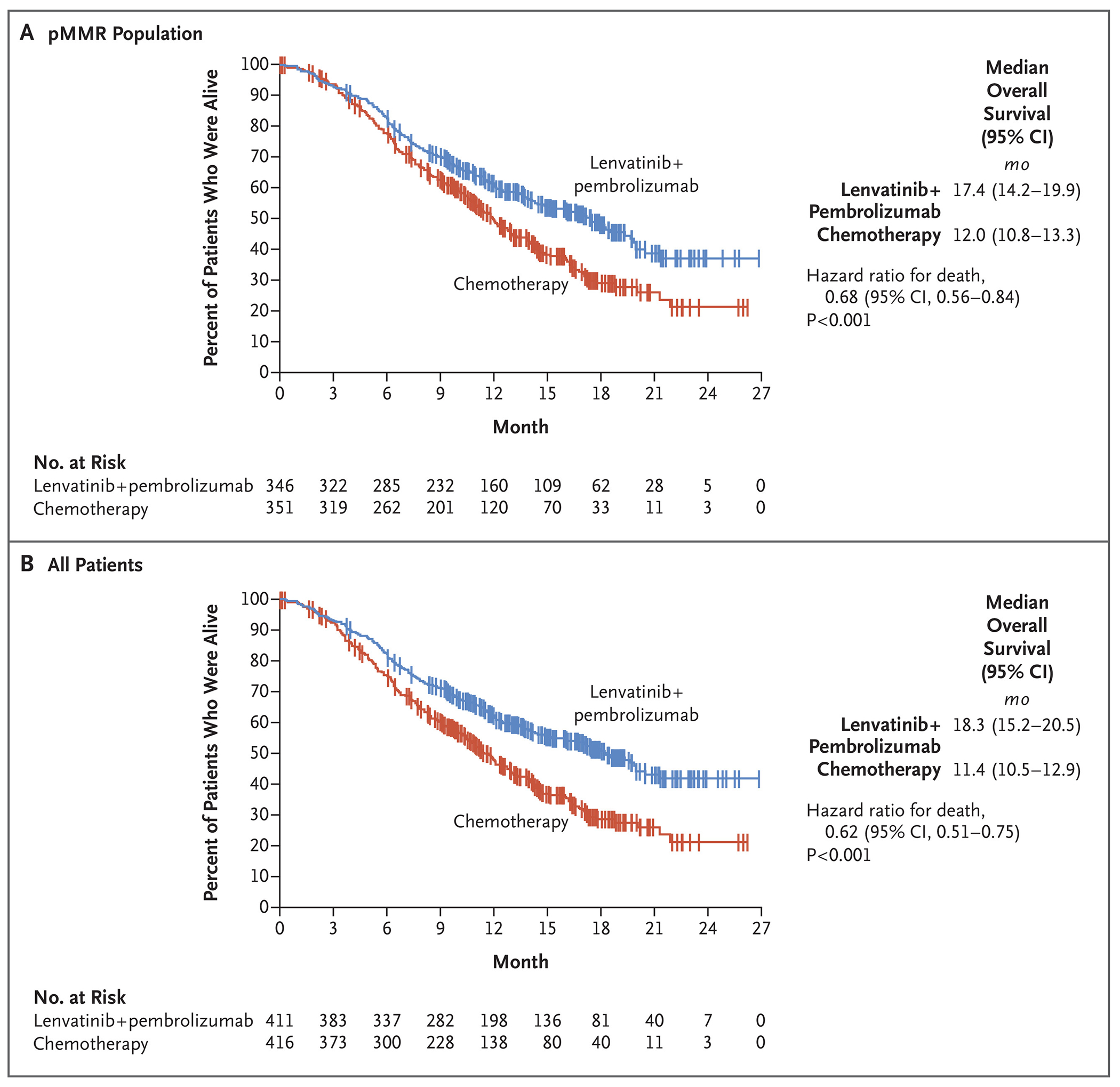
Tick marks indicate censored data.
The results of the analyses of progression-free survival and overall survival favored the lenvatinib plus pembrolizumab group over the chemotherapy group across all the evaluated subgroups, including subgroups defined according to age, histologic features, and previous lines of therapy. Results were similar both in the pMMR population and among all the patients (Figs. S4 and S5).
In the pMMR population, the percentage of patients with a confirmed objective response as assessed on blinded independent central review according to RECIST, version 1.1, was higher with lenvatinib plus pembrolizumab (30.3%) than with chemotherapy (15.1%); in the overall trial population, the percentages were 31.9% and 14.7%, respectively (Table 2). The results of the prespecified and multiple imputation analyses of objective response are reported in Table S4. In the pMMR population, 5.2% of the patients in the lenvatinib–pembrolizumab group and 2.6% of those in the chemotherapy group had a complete response; the corresponding percentages in the overall trial population were 6.6% and 2.6%. More patients (approximately twice as many) in the chemotherapy group than in the lenvatinib–pembrolizumab group had a best response of progressive disease. Among patients with a response, the median duration of response in the pMMR population was 9.2 months (range, 1.6 to 23.7) with lenvatinib plus pembrolizumab and 5.7 months (range, 0.0 to 24.2) with chemotherapy; among patients with a response, the median duration of response in the overall trial population was 14.4 months (range, 1.6 to 23.7) and 5.7 months (95% CI, 0.0 to 24.2), respectively (Table 2 and Fig. S6).
Table 2.
Confirmed Tumor Responses.*
| End Point | pMMR Population | All Patients | dMMR Population | |||
|---|---|---|---|---|---|---|
| Lenvatinib plus Pembrolizumab (N = 346) | Chemotherapy (N = 351) | Lenvatinib plus Pembrolizumab (N = 411) | Chemotherapy (N = 416) | Lerivatiriib plus Pembrolizumab (N =65) | Chemotherapy (N =65) | |
| Objective response | ||||||
| No. of patients | 105 | 53 | 131 | 61 | 26 | 8 |
| Percent (95% CI) | 30.3 (25.5 to 35.5) | 15.1 (11.5 to 19.3) | 31.9 (27.4 to 36.6) | 14.7 (11.4 to 18.4) | 40 (28 to 53) | 12 (5 to 23) |
|
| ||||||
| Best overall response | ||||||
|
| ||||||
| Complete response | ||||||
| No. of patients | 18 | 9 | 27 | 11 | 9 | 2 |
| Percent (95% CI) | 5.2 (3.1 to 8.1) | 2.6 (1.2 to 4.8) | 6.6 (4.4 to 9.4) | 2.6 (1.3 to 4.7) | 14 (7 to 25) | 3 (<1 to 11) |
|
| ||||||
| Partial response | ||||||
| No. of patients | 87 | 44 | 104 | 50 | 17 | 6 |
| Percent (95% CI) | 25.1 (20.7 to 30.1) | 12.5 (9.3 to 16.5) | 25.3 (21.2 to 29.8) | 12.0 (9.1 to 15.5) | 26 (16 to 39) | 9 (3 to 19) |
|
| ||||||
| Stable disease | ||||||
| No. of patients | 168 | 139 | 193 | 167 | 25 | 28 |
| Percent (95% CI) | 48.6 (43.2 to 54.0) | 39.6 (34.4 to 44.9) | 47.0 (42.0 to 51.9) | 40.1 (35.4 to 45.0) | 38 (27 to 51) | 43 (31 to 56) |
|
| ||||||
| Progressive disease | ||||||
| No. of patients | 54 | 108 | 61 | 123 | 7 | 15 |
| Percent (95% CI) | 15.6 (11.9 to 19.9) | 30.8 (26.0 to 35.9) | 14.8 (11.5 to 18.7) | 29.6 (25.2 to 34.2) | 11 (4 to 21) | 23 (14 to 35) |
|
| ||||||
| Could not be evaluated† | ||||||
| No. of patients | 2 | 7 | 5 | 8 | 3 | 1 |
| Percent (95% CI) | 0.6 (0.1 to 2.1) | 2.0 (0.8 to 4.1) | 1.2 (0.4 to 2.8) | 1.9 (0.8 to 3.8) | 5 (1 to 13) | 2 (0 to 8) |
|
| ||||||
| Not assessed‡ | ||||||
| No. of patients | 17 | 44 | 21 | 57 | 4 | 13 |
| Percent (95% CI) | 4.9 (2.9 to 7.8) | 12.5 (9.3 to 16.5) | 5.1 (3.2 to 7.7) | 13.7 (10.5 to 17.4) | 6 (2 to 15) | 20 (11 to 32) |
|
| ||||||
| Median duration of response (range) — mo§ | 9.2 (1.6 to 23.7) | 5.7 (0.0 to 24.2) | 14.4 (1.6 to 23.7) | 5.7 (0.0 to 24.2) | NR (2.1 to 20.4) | 4.1 (1.9 to 15.6) |
|
| ||||||
| Median time to response (range) — mo | 2.1 (1.5 to 9.4) | 3.5 (1.0 to 7.4) | 2.1 (1.5 to 16.3) | 2.1 (1.0 to 7.4) | 2.9 (1.7 to 16.3) | 1.9 (1.8 to 3.7) |
|
| ||||||
| Disease control¶ | ||||||
| No. of patients | 248 | 163 | 296 | 194 | 48 | 31 |
| Percent (95% CI) | 71.7 (66.6 to 76.4) | 46.4 (41.1 to 51.8) | 72 (67.4 to 76.3) | 46.6 (41.8 to 51.6) | 74 (61 to 84) | 48 (35 to 60) |
NR denotes not reached.
A postbaseline assessment was available for these patients, but they could not be evaluated for response by the independent imaging vendor (e.g., owing to poor radiographic technique, poorly defined tumor margins, lesions that had been identified at screening not being imaged at a subsequent time point, or the lesion being obstructed by another body part and thus being unable to be measured or evaluated).
No postbaseline assessment was available for response evaluation.
Duration of response was assessed only in patients with a complete or partial response.
Disease control was defined as a best overall response of complete response, partial response, or stable disease at 7 weeks or more after randomization.
Overall, more patients in the lenvatinib–pembrolizumab group than in the chemotherapy group had tumor shrinkage (Fig. S7). Although the trial was not designed or powered to compare lenvatinib plus pembrolizumab with chemotherapy in the dMMR population, clinically meaningful improvement was observed across efficacy end points (Table 2 and Figs. S4, S5, S6, S8, and S9).
Exposure and Safety
In the safety analysis population, the median duration of treatment was 231 days (range, 1 to 817) with lenvatinib plus pembrolizumab and 104.5 days (range, 1 to 785) with chemotherapy (Table S5). Among patients receiving lenvatinib plus pembrolizumab, the median dose intensity of lenvatinib was 13.8 mg per day, and the median number of cycles of pembrolizumab was 10. Among patients receiving chemotherapy, the median number of cycles was 5 for doxorubicin and 6 for paclitaxel. More patients in the lenvatinib–pembrolizumab group than in the chemotherapy group had durations of exposure of at least 6 months, at least 12 months, and at least 18 months (Table S6). The median time to the first dose reduction of lenvatinib was 1.9 months (range, 0.1 to 22.8); 45.6% of the patients in the lenvatinib–pembrolizumab group had two or more dose reductions of lenvatinib (Table S7).
Almost all the patients in the two treatment groups (>99%) had adverse events during treatment, with the most common being hypertension (in 64.0% of the patients) with lenvatinib plus pembrolizumab and anemia (in 48.7%) with chemotherapy (Table 3). Grade 3 or higher adverse events occurred in 88.9% of the patients receiving lenvatinib plus pembrolizumab and in 72.7% of those receiving chemotherapy. The most common serious adverse events were hypertension (in 4.2% of the patients) with lenvatinib plus pembrolizumab and febrile neutropenia (in 4.1%) with chemotherapy. Grade 5 adverse events (regardless of the investigator’s assessment of relation to treatment) occurred in 5.7% of the patients receiving lenvatinib plus pembrolizumab and in 4.9% of those receiving chemotherapy.
Table 3.
Adverse Events of Any Cause with an Incidence of 25% or More among All the Patients in Either Treatment Group, According to Preferred Term.
| Event | Lenvatinib plus Pembrolizumab (N = 406) | Chemotherapy (N = 388) | ||
|---|---|---|---|---|
| Any Grade | Grade ≥3* | Any Grade | Grade ≥3* | |
| Any adverse event | 405 (99.8) | 361 (88.9) | 386 (99.5) | 282 (72.7) |
| Hypertension† | 260 (64.0) | 154 (37.9) | 20 (5.2) | 9 (2.3) |
| Hypothyroidism†‡ | 233 (57.4) | 5 (1.2) | 3 (0.8) | 0 |
| Diarrhea | 220 (54.2) | 31 (7.6) | 78 (20.1) | 8 (2.1) |
| Nausea | 201 (49.5) | 14 (3.4) | 179 (46.1) | 5 (1.3) |
| Decreased appetite | 182 (44.8) | 32 (7.9) | 82 (21.1) | 2 (0.5) |
| Vomiting | 149 (36.7) | 11 (2.7) | 81 (20.9) | 9 (2.3) |
| Weight decrease | 138 (34.0) | 42 (10.3) | 22 (5.7) | 1 (0.3) |
| Fatigue | 134 (33.0) | 21 (5.2) | 107 (27.6) | 12 (3.1) |
| Arthralgia | 124 (30.5) | 7 (1.7) | 31 (8.0) | 0 |
| Proteinuria† | 117 (28.8) | 22 (5.4) | 11 (2.8) | 1 (0.3) |
| Anemia | 106 (26.1) | 25 (6.2) | 189 (48.7) | 57 (14.7) |
| Constipation | 105 (25.9) | 3 (0.7) | 96 (24.7) | 2 (0.5) |
| Urinary tract infection | 104 (25.6) | 16 (3.9) | 39 (10.1) | 4 (1.0) |
| Neutropenia | 30 (7.4) | 7 (1.7) | 131 (33.8) | 100 (25.8) |
| Alopecia | 22 (5.4) | 0 | 120 (30.9) | 2 (0.5) |
Among the patients who received lenvatinib plus pembrolizumab, 5.7% died owing to grade 5 adverse events (gastrointestinal disorder in 1.2% of the patients, cardiac disorder in 0.5%, general disorder in 1.5%, infection in 0.7%, decreased appetite in 0.2%, and neoplasms, nervous system disorder, psychiatric disorder, renal disorder, reproductive disorder, or respiratory disorder in 0.2% each). Among the patients who received chemotherapy, 4.9% died owing to grade 5 adverse events (cardiac disorder in 1.0%, general disorder in 1.3%, infection in 1.5%, subdural hematoma in 0.3%, and respiratory disorder in 0.8%).
This event was a clinically significant adverse event with lenvatinib therapy (Table S14).
This event was an adverse event of interest with pembrolizumab therapy (Table S13).
The most frequent adverse events leading to dose reduction, treatment interruption, and trial-drug discontinuation are listed in Tables S8, S9, and S10, respectively. Among patients receiving lenvatinib plus pembrolizumab, adverse events of any grade led to dose reduction of lenvatinib in 66.5%, to interruption (of lenvatinib, pembrolizumab, or both) in 69.2%, and to trial-drug discontinuation in 33.0% (discontinuation of lenvatinib in 30.8%, of pembrolizumab in 18.7%, and of both in 14.0%). Among patients receiving chemotherapy, adverse events of any grade led to dose reduction in 12.9%, to interruption in 27.1%, and to trial-drug discontinuation in 8.0%. Details are provided in Table S11.
Adverse events related to trial therapy are elucidated in Table S12. Adverse events of interest with regard to pembrolizumab occurred in 67.2% of the patients; hypothyroidism was the most common, with an incidence of 57.6% (grade 1 in 17.2% and grade 2 in 38.9%) among patients who received lenvatinib plus pembrolizumab (Table S13). Clinically significant adverse events with lenvatinib therapy are listed in Table S14, and serious adverse events that occurred in at least 1% of all the treated patients are listed in Table S15.
Health-Related Quality of Life
The QLQ-C30 was completed for more than 95% of the patients in the two treatment groups at baseline; scores at 12 weeks after randomization were available for 80% of the patients in the lenvatinib–pembrolizumab group and for 62% of those in the chemotherapy group. No substantial between-group differences were observed in the QLQ-C30 global health status quality-of-life scores over time (Fig. S10).
Subsequent Therapy
In the intention-to-treat population, 28.0% of the patients in the lenvatinib–pembrolizumab group and 48.1% of those in the chemotherapy group received subsequent systemic anticancer medications. In the chemotherapy group, 9.1% of the patients in the pMMR population received lenvatinib plus pembrolizumab as subsequent therapy, and 16.9% of the patients in the dMMR population received PD-1 pathway–targeting monotherapy or combination regimens as subsequent therapies.
DISCUSSION
In this phase 3 trial, we compared lenvatinib plus pembrolizumab with physician’s choice of chemotherapy in patients with advanced endometrial cancer whose disease had progressed or recurred after the receipt of at least one previous platinum-based chemotherapy regimen. With respect to the two primary efficacy end points, both progression-free survival and overall survival were significantly longer with lenvatinib plus pembrolizumab than with chemotherapy, both in the pMMR population and among all the patients; these results address a need for effective therapy in these patient populations. The efficacy curves separated early during the course of trial therapy and remained consistently separated throughout the evaluation period. These benefits in progression-free survival and overall survival were seen across all evaluated subgroups, including subgroups defined according to less-common yet aggressive histologic features, history of pelvic irradiation, and previous lines of therapy. Patients in the lenvatinib–pembrolizumab group had improved outcomes, including prolonged overall survival, as compared with those in the chemotherapy group, despite 9.1% of the patients in the pMMR population who had been assigned to the chemotherapy group receiving lenvatinib plus pembrolizumab as subsequent anticancer treatment and 16.9% of those in the dMMR population who had been assigned to the chemotherapy group receiving PD-1 pathway– targeting checkpoint monotherapy or combination therapies as subsequent treatments. The percentages of patients with an objective response associated with lenvatinib plus pembrolizumab treatment were consistent with previous findings from the phase 2 Study 111–KEYNOTE-146 trial25 and were higher than the percentages observed in the chemotherapy group. Efficacy results with chemotherapy were consistent with findings from phase 3 trials in the context of second-line or later treatment.9,10
The most frequent adverse events (incidence of ≥30% in the respective treatment groups) were hypertension, hypothyroidism, diarrhea, nausea, decreased appetite, vomiting, decreased body weight, fatigue, and arthralgia among patients receiving lenvatinib plus pembrolizumab and anemia, nausea, neutropenia, and alopecia among those receiving chemotherapy. The safety data for the combination therapy in our trial were generally consistent with the results observed in the Study 111–KEYNOTE-146 trial and the known adverse-event profiles of each agent.19,24,25,27–31 The incidence of hypothyroidism (an adverse event that has been associated with both lenvatinib and pembrolizumab treatment),17,32 although higher than with chemotherapy in this trial or individual monotherapies in earlier trials,29,30 was detected by means of surveillance and corrected easily with oral medication, and most events were of grade 1 or 2 severity. The strategy of administering lenvatinib therapy by starting at the established dose and reducing as necessary has previously been successful.25 Although a small-scale retrospective study33 has suggested that a starting dose of 14 mg per day does not compromise efficacy, that study involved a small number of patients (70 enrolled, of which only 16 were treated at a dose of 20 mg per day). Prospective studies (with larger populations) have involved patients with other tumor types and have indicated that lower starting doses were not noninferior to the approved starting dose of lenvatinib for the treatment of renal-cell carcinoma and differentiated thyroid cancer.34,35 Moreover, safety was not noticeably improved at lower starting doses.34,35 In our trial, adverse events of any cause led to dose reductions in 66.5% of the patients who received lenvatinib plus pembrolizumab.
A limitation of this trial is the relatively short duration of follow-up, which may mean that responses are evolving. Although the protocol-specified criteria were met for the efficacy analyses, safety and efficacy monitoring is ongoing.
This trial showed that treatment with lenvatinib plus pembrolizumab led to significantly longer progression-free survival and overall survival than chemotherapy of the treating physician’s choice, both in the pMMR population and in the overall trial population of patients with advanced endometrial cancer who had disease progression after the receipt of previous systemic platinum-based therapy.
Supplementary Material
Acknowledgements:
We thank all the patients and the trial teams who participated in the trial; all the personnel at Eisai and Merck who assisted with the trial, particularly Corina Dutcus, Jodi McKenzie, Jie Huang, Gursel Aktan, Greg Lubiniecki, and Elizabeth Rafeiro, given the challenges presented during the coronavirus disease 2019 pandemic; and Swati Khare, of Oxford PharmaGenesis, for medical writing assistance with an earlier version of the manuscript
Funding:
Supported by Eisai and Merck Sharp and Dohme, a subsidiary of Merck.
Footnotes
A list of the Study 309–KEYNOTE-775 investigators is provided in the Supplementary Appendix, available at NEJM.org.
Presented in part at the Annual Meeting on Women’s Cancer held by the Society of Gynecologic Oncology, March 19–25, 2021; the Annual Meeting of the American Society of Clinical Oncology, June 4–8, 2021; the Annual Meeting of the Japan Society of Gynecologic Oncology, July 16–18, 2021; the European Society for Medical Oncology Congress, September 16–21, 2021; the Annual Global Meeting of the International Gynecologic Cancer Society, August 30–September 2, 2021; and the European Society of Gynaecological Oncology Congress, October 23–25, 2021.
Disclosures: Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Data Sharing Statement:
The data will not be available for sharing at this time because the data are considered commercially confidential. However, Eisai will consider written requests to share the anonymized data on a case-by-case basis.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Gong TT, Liu FH, et al. Global, regional, and national burden of endometrial cancer, 1990-2017: results from the Global Burden of Disease study, 2017. Front Oncol 2019;9:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst 2018;110:354–61. [DOI] [PubMed] [Google Scholar]
- 4.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 5.Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin 2019;69:258–79. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review 1975-2017. National Cancer Institute. April 15, 2020. (https://seer.cancer.gov/csr/1975_2017/). [Google Scholar]
- 7.National Comprehensive Cancer Network. Clinical practice guidelines in oncology: uterine neoplasms. Version 1. 2021. (https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf). [Google Scholar]
- 8.Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021;31: 12–39. [DOI] [PubMed] [Google Scholar]
- 9.McMeekin S, Dizon D, Barter J, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol 2015;138:18–23. [DOI] [PubMed] [Google Scholar]
- 10.Miller DS, Scambia G, Bondarenko I, et al. ZoptEC: phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer. J Clin Oncol 2018; 36(Suppl 15):5503. abstract. [Google Scholar]
- 11.Heudel PE, Fabbro M, Roemer-Becuwe C, et al. Phase II study of the PI3K inhibitor BKM120 in patients with advanced or recurrent endometrial carcinoma: a stratified type I-type II study from the GINECO group. Br J Cancer 2017;116:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pautier P, Vergote I, Joly F, et al. A phase 2, randomized, open-label study of irosustat versus megestrol acetate in advanced endometrial cancer. Int J Gynecol Cancer 2017;27:258–66. [DOI] [PubMed] [Google Scholar]
- 13.Emons G, Kurzeder C, Schmalfeldt B, et al. Temsirolimus in women with platinum-refractory/resistant ovarian cancer or advanced/recurrent endometrial carcinoma. A phase II study of the AGO-study group (AGO-GYN8). Gynecol Oncol 2016; 140:450–6. [DOI] [PubMed] [Google Scholar]
- 14.Oza AM, Pignata S, Poveda A, et al. Randomized phase II trial of ridaforolimus in advanced endometrial carcinoma. J Clin Oncol 2015;33:3576–82. [DOI] [PubMed] [Google Scholar]
- 15.Konecny GE, Finkler N, Garcia AA, et al. Second-line dovitinib (TKI258) in patients with FGFR2-mutated or FGFR2- non-mutated advanced or metastatic endometrial cancer: a non-randomised, open-label, two-group, two-stage, phase 2 study. Lancet Oncol 2015;16:686–94. [DOI] [PubMed] [Google Scholar]
- 16.Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev 2012;(8): CD003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenvima (lenvatinib) prescribing information. Woodcliff Lake, NJ: Eisai, 2020. [Google Scholar]
- 18.Vergote I, Powell MA, Teneriello MG, et al. Second-line lenvatinib in patients with recurrent endometrial cancer. Gynecol Oncol 2020;156:575–82. [DOI] [PubMed] [Google Scholar]
- 19.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol 2017;2017:17.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prendergast EN, Holman LL, Liu AY, et al. Comprehensive genomic profiling of recurrent endometrial cancer: implications for selection of systemic therapy. Gynecol Oncol 2019;154:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soumerai TE, Donoghue MTA, Bandlamudi C, et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res 2018;24:5939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenzi M, Amonkar M, Zhang J, Mehta S, Liaw K-L. Epidemiology of microsatellite instability high (MSI-H) and deficient mismatch repair (dMMR) in solid tumors: a structured literature review. J Oncol 2020;2020:1807929. [Google Scholar]
- 24.Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol 2017;35:2535–41. [DOI] [PubMed] [Google Scholar]
- 25.Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020;38:2981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor M, Dutcus CE, Schmidt E, et al. A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients with selected solid tumors. Ann Oncol 2016; 27(Suppl 6):vi267. abstract. [Google Scholar]
- 27.O’Malley D, Marabelle A, De Jesus-Acosta A, et al. Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann Oncol 2019;30:Suppl 5:v425–v426. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergote I, Teneriello M, Powell MA, et al. A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: Angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol 2013;31:(Suppl 15):5520. abstract. [Google Scholar]
- 29.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol 2015;16:1473–82. [DOI] [PubMed] [Google Scholar]
- 30.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521–32. [DOI] [PubMed] [Google Scholar]
- 31.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- 32.Keytruda (pembrolizumab) package insert. Whitehouse Station, NJ: Merck Sharp & Dohme, 2021. [Google Scholar]
- 33.How JA, Patel S, Fellman B, et al. Toxicity and efficacy of the combination of pembrolizumab with recommended or reduced starting doses of lenvatinib for treatment of recurrent endometrial cancer. Gynecol Oncol 2021;162:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal SK, Puente J, Heng DYC, et al. Phase 2 trial of lenvatinib at 2 starting doses + everolimus in renal cell carcinoma (RCC). Kidney Cancer J 2020;18(Suppl 4): 34–5. abstract. [Google Scholar]
- 35.Brose MS, Panaseykin Y, Konda B, et al. A multicenter, randomized, double-blind, phase II study of lenvatinib (LEN) in patients (pts) with radioiodine-refractory differentiated thyroid cancer (RR-DTC) to evaluate the safety and efficacy of a daily oral starting dose of 18 mg vs 24 mg. Ann Oncol 2020;31:(Suppl 6):S1409. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will not be available for sharing at this time because the data are considered commercially confidential. However, Eisai will consider written requests to share the anonymized data on a case-by-case basis.


