Abstract
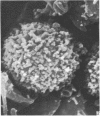
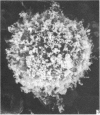
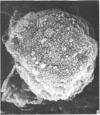
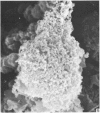
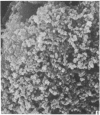
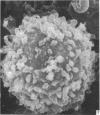
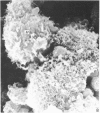
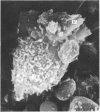
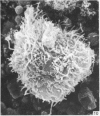
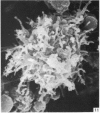
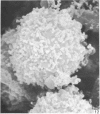
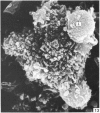
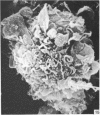
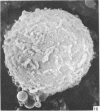
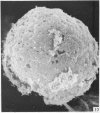
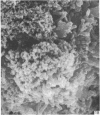
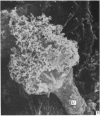
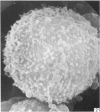
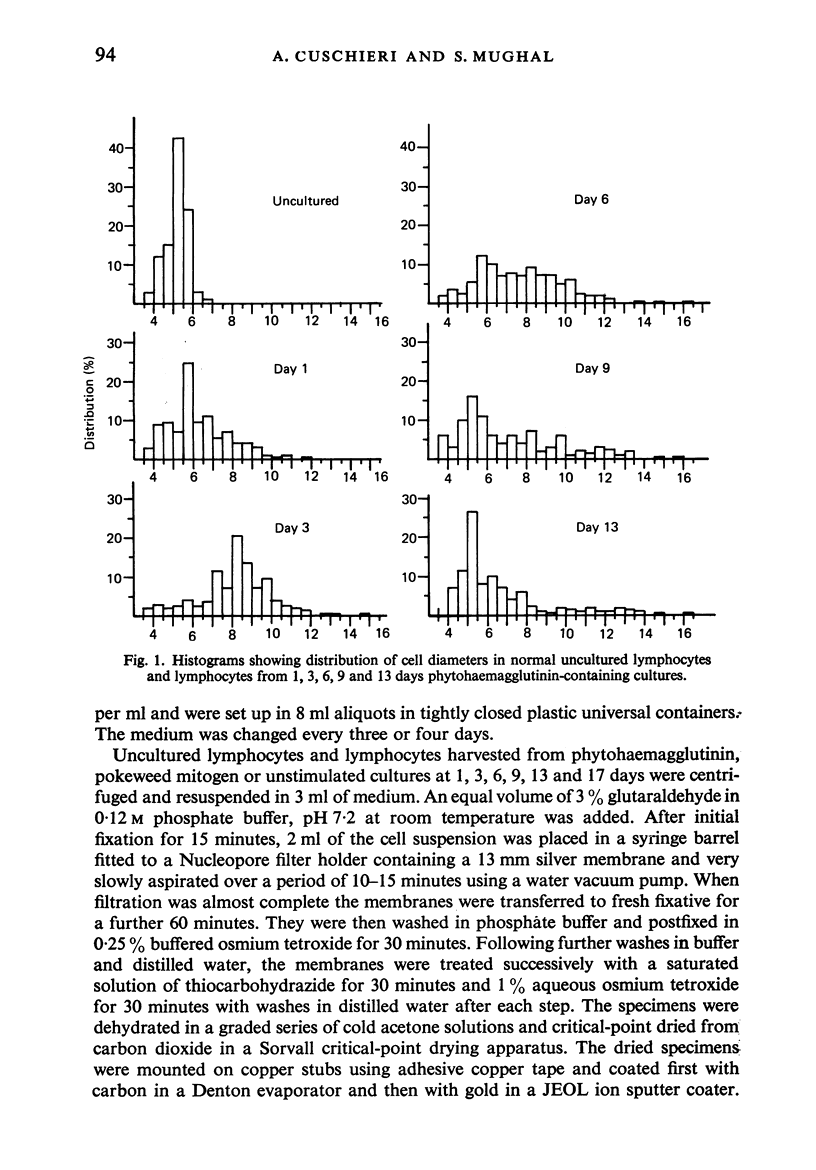
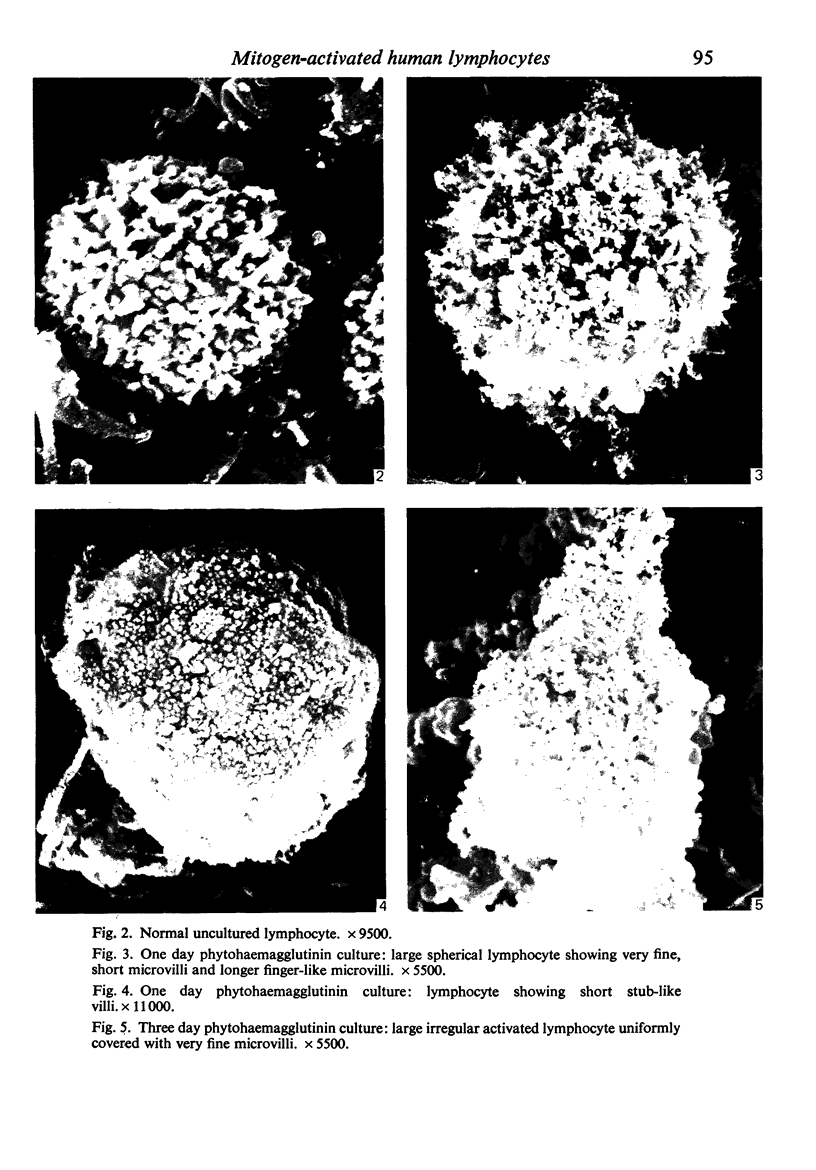
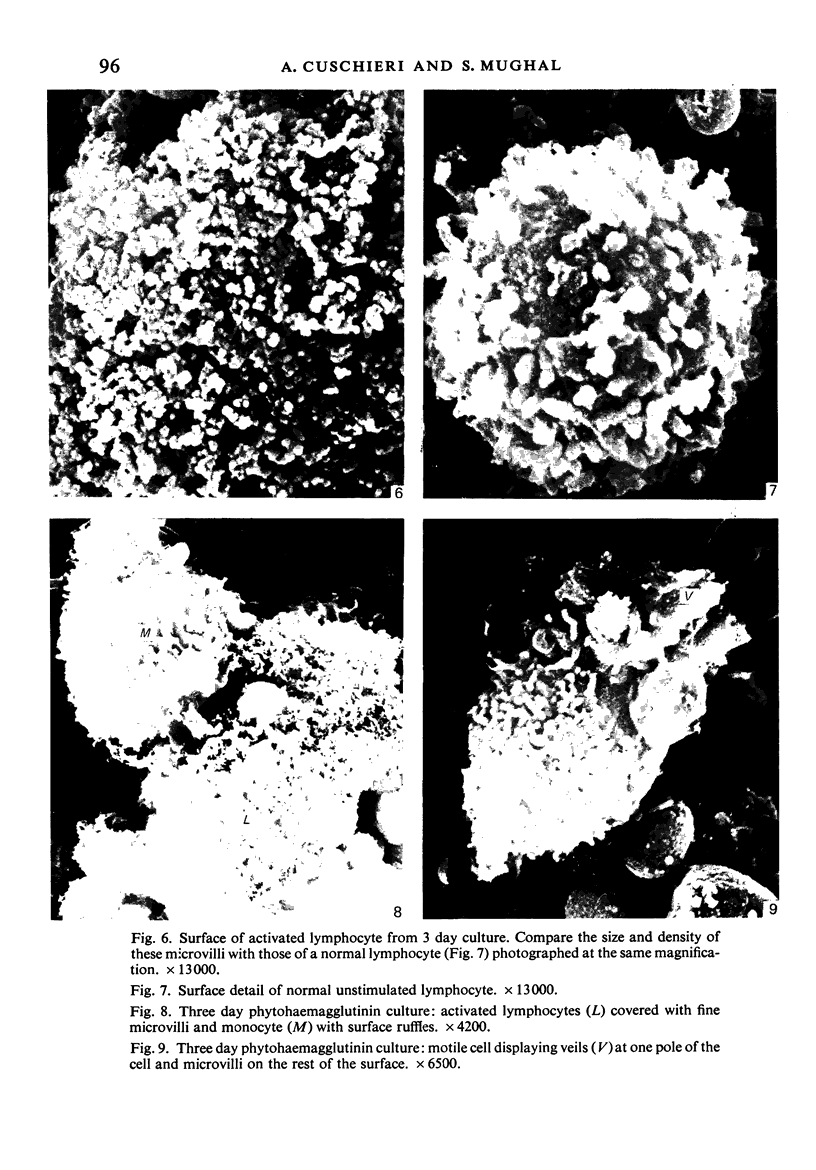
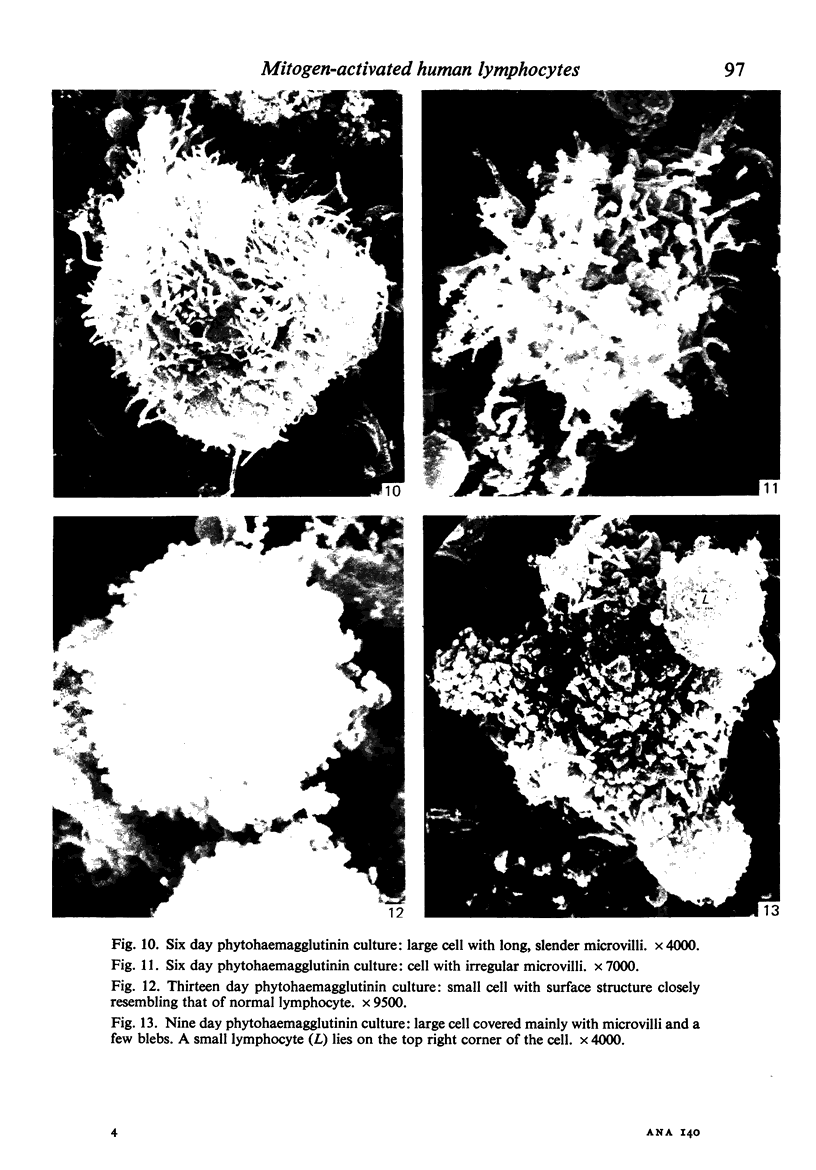
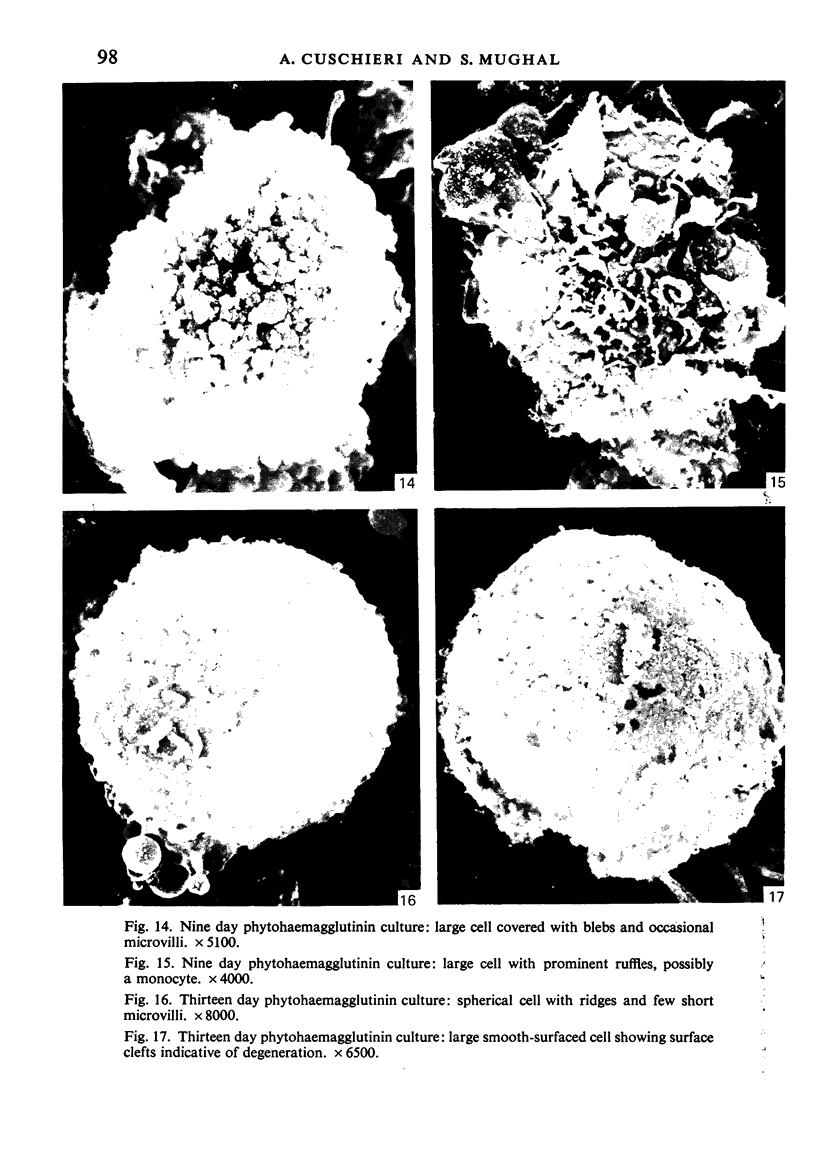
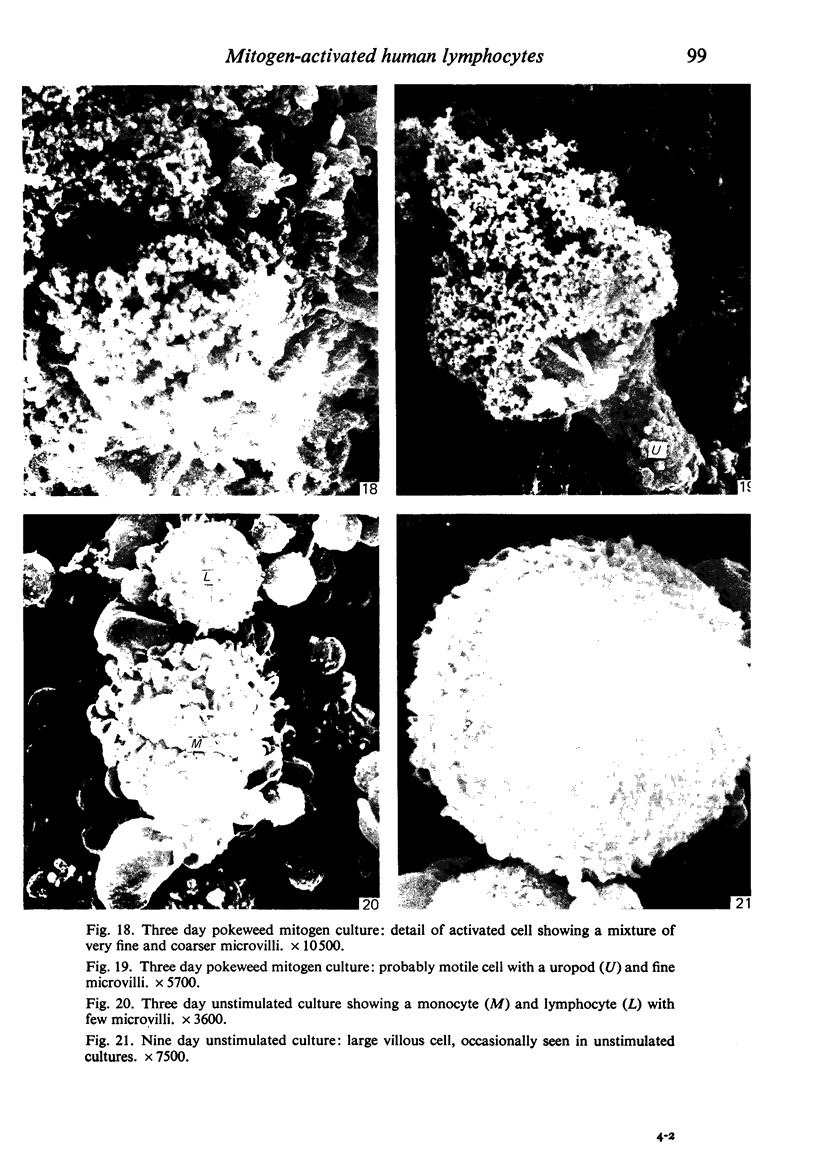
Human peripheral blood lymphocytes were cultured with phytohaemagglutinin or pokeweed mitogen for various intervals up to 17 days and studied by scanning electron microscopy. Activated lymphocytes in 3 day cultures were large irregular cells characterised mainly by an abundance of very fine microvilli, which were much thinner, shorter and more densely packed than the microvilli on uncultured lymphocytes. Cells intermediate in size and surface morphology between these and unstimulated lymphocytes were numerous in 1 day cultures. Some motile cells and large cells with finger-like or conical microvilli were also present. Cell counts showed that after 6 days in phytohaemagglutinin culture small villous cells resembling normal healthy lymphocytes were progressively more numerous, suggesting that most of the activated cells reverted to small lymphocytes. Very large cells were also present and displayed a heterogeneous surface morphology of villi, ridges, blebs and ruffles. A few of these cells had typical monocytoid features but others were predominantly villous. In pokeweed mitogen cultures there was extensive cellular degeneration affecting all cell types after 6 days. In unstimulated cultures the lymphocytes had fewer microvilli or were smooth-surfaced. There was extensive lymphocyte degeneration after 3 days and eventually typical monocytes were the predominant cells. Large, villous cells, which could be activated lymphocytes, were occasionally encountered in unstimulated cultures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Wetzel B. Human lymphocytes: similarity of B and T cell surface morphology. Science. 1975 May 16;188(4189):732–734. doi: 10.1126/science.1079095. [DOI] [PubMed] [Google Scholar]
- Biberfeld P. Uropod formation in phytohaemagglutinin (PHA) stimulated lymphocytes. Exp Cell Res. 1971 Jun;66(2):433–445. doi: 10.1016/0014-4827(71)90698-7. [DOI] [PubMed] [Google Scholar]
- Boyde A., Weiss R. A., Veselý P. Scanning electron microscopy of cells in culture. Exp Cell Res. 1972;71(2):313–324. doi: 10.1016/0014-4827(72)90299-6. [DOI] [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J., Willoughby D. A. Some scanning electron-microscope observations on stimulated lymphocytes. J Pathol. 1971 Jun;104(2):115–118. doi: 10.1002/path.1711040205. [DOI] [PubMed] [Google Scholar]
- Criswell B. S., Rich R. R., Dardano J., Kimzey S. L. Scanning electron microscopy of normal and mitogen-stimulated mouse lymphoid cells. Cell Immunol. 1975 Oct;19(2):336–348. doi: 10.1016/0008-8749(75)90215-4. [DOI] [PubMed] [Google Scholar]
- Cuschieri A., Mughal S., Kharbat B. A. The fate of phytohaemagglutinin-activated human lymphocytes following their peak proliferative activity. J Anat. 1985 Jan;140(Pt 1):79–92. [PMC free article] [PubMed] [Google Scholar]
- Gormley I. P., Ross A. The morphology of phytohaemagglutinin (PHA)-stimulated lymphocytes and permanent lymphoid cell lines seen by the scanning electron microscope. Eur J Cancer. 1972 Oct;8(5):491–494. doi: 10.1016/0014-2964(72)90099-0. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Hoffmann C. C., Moore K. C., Shih C. Y., Blakley R. L. Scanning electron microscopy of human lymphocytes during transformation and subsequent treatment with methotrexate. J Cell Sci. 1977 Dec;28:151–165. doi: 10.1242/jcs.28.1.151. [DOI] [PubMed] [Google Scholar]
- Holt P. J., Pal S. G., Catovsky D., Lewis S. M. Surface structure of normal and leukaemic lymphocytes. I. Effect of mitogens. Clin Exp Immunol. 1972 Apr;10(4):555–570. [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Belohradsky B., Yee K., Vogel J., Butcher D., Wybran J., Fudenberg H. H. Cellular interactions: scanning electron microscopy of human thymus-derived rosette-forming lymphocytes. Clin Immunol Immunopathol. 1974 Apr;2(3):301–309. doi: 10.1016/0090-1229(74)90048-8. [DOI] [PubMed] [Google Scholar]
- Kelly G. E., Nockolds C. E. Morphological differences between sub-populations of human lymphocytes revealed by scanning electron microscopy. Scand J Haematol. 1977 Aug;19(2):172–184. doi: 10.1111/j.1600-0609.1977.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Lin P. S., Cooper A. G., Wortis H. H. Scanning electron microscopy of human T-cell and B-cell rosettes. N Engl J Med. 1973 Sep 13;289(11):548–551. doi: 10.1056/NEJM197309132891102. [DOI] [PubMed] [Google Scholar]
- Lin P. S., Wallach D. F., Tsai S. Temperature-induced variations in the surface topology of cultured lymphocytes are revealed by scanning electron microscopy. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2492–2496. doi: 10.1073/pnas.70.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G., Roath S. The surface morphology of mitogen-stimulated human peripheral blood lymphocytes. Br J Haematol. 1978 Aug;39(4):615–622. doi: 10.1111/j.1365-2141.1978.tb03632.x. [DOI] [PubMed] [Google Scholar]
- Parakkal P., Pinto J., Hanifin J. M. Surface morphology of human mononuclear phagocytes during maturation and phagocytosis. J Ultrastruct Res. 1974 Aug;48(2):216–226. doi: 10.1016/s0022-5320(74)80078-x. [DOI] [PubMed] [Google Scholar]
- Polgar P. R., Kibrick S., Foster J. M. Reversal of PHA-induced blastogenesis in human lymphocyte cultures. Nature. 1968 May 11;218(5141):596–597. doi: 10.1038/218596a0. [DOI] [PubMed] [Google Scholar]
- Polliack A., Touraine J. L., de Harven E., Lampen N., Hadden J. W. Scanning electron microscopy of mitogen-transformed human lymphocytes. Isr J Med Sci. 1975 Dec;11(12):1285–1298. [PubMed] [Google Scholar]
- Porter K., Prescott D., Frye J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J Cell Biol. 1973 Jun;57(3):815–836. doi: 10.1083/jcb.57.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. W., Everhart L. P. The effect of cell-to-cell contact on the surface morphology of Chinese hamster ovary cells. J Cell Biol. 1973 Jun;57(3):837–844. doi: 10.1083/jcb.57.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]