ABSTRACT
Transcatheter pulmonary valve implantation (TPVI) is the standard of care in patients with repaired tetralogy of Fallot (rTOF) presenting with right ventricular outflow tract (RVOT) dysfunction. However, the feasibility of TPVI is limited by the high cost and nonavailability of larger-sized valves for dilated native RVOT of rTOF patients. We report the first successful TPVI with a custom-made 35 mm balloon-expandable valve (Myval™) in a 30-year-old rTOF patient with severe pulmonary regurgitation and RV dysfunction.
Keywords: Balloon-expandable valve, dysfunctional right ventricular outflow tract, large percutaneous valves
INTRODUCTION
Transcatheter pulmonary valve implantation (TPVI) has become the standard of care for right ventricular outflow tract (RVOT) dysfunction in patients with repaired tetralogy of Fallot (rTOF). The major limiting factors for TPVI are the higher cost and limited size options. Besides the effects of long-standing pulmonary regurgitation (PR), late presentation in countries like India also makes RVOT of these patients further dilated, thus posing challenges in TPVI using routinely available valve sizes. We hereby share our experience of the first-ever use of a custom-made 35 mm transcatheter balloon-expandable heart valve (Myval™) for TPVI in a rTOF patient having severe PR, a dilated RVOT, and RV dysfunction.
CASE REPORT
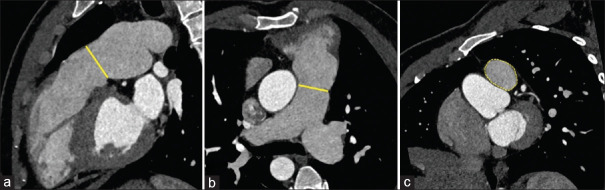
A 30-year-old male had undergone intracardiac repair for TOF in 1996. Postoperatively, he had severe PR because of the transannular patch and was on regular follow-up. He was asymptomatic and could do his daily activities and work without limitations. On follow-up echocardiography, he was found to have severe PR with RV dilatation and RV dysfunction. There was no residual ventricular septal defect, tricuspid regurgitation, or RVOT obstruction (RVOTO). His electrocardiogram showed a right bundle branch block with a QRS duration of 170 ms. A cardiac magnetic resonance imaging (MRI) showed indexed RV end-diastolic (RVEDVi) and end-systolic (RVESVi) volumes of 201 mL/m2 and 115 mL/m2, respectively, suggesting significant RV dilatation. The RV ejection fraction (RVEF) was 42%, and the PR fraction was 60%. The left ventricular (LV) volume and function were within the normal limits. The patient was thus planned for pulmonary valve replacement. A computed tomography angiogram was done to assess the RVOT anatomy for TPVI, which showed a cylindrical-shaped RVOT with a diameter of 33 mm × 28 mm [Figure 1]. The dilated RVOT was an essential concern while planning for TPVI, as the available valves were unsuitable for RVOT dimensions over 32 mm. However, self-expanding valves of 36 mm were available, and a need for considerable oversizing in self-expanding valves prevented using those in this patient. Thus, a custom-made 35 mm balloon-expandable valve (Meril’s Life Sciences Pvt Ltd, Vapi, India) was planned for this patient. After informed consent from the patient, he was taken up for TPVI.
Figure 1.
Computed tomography angiogram in sagittal (a) and axial (b) plane showing right ventricular dilatation and cylindrical-shaped dilated right ventricular outflow tract with a diameter of 33 mm × 28 mm (yellow lines) and area of 725 mm2 (c) at the annulus (yellow dotted area)
The procedure was done under general anesthesia. The baseline RV pressures were 35/10 mmHg, and the pulmonary artery (PA) pressures were 34/10 mmHg with a mean of 20 mmHg. Through 8F right femoral venous access, PA was entered with a 5F pigtail catheter and J-tipped 0.035 hydrophilic wire, and a PA angiogram was done in the right anterior oblique 30° and lateral views. With a 5F Judkins right (JR) catheter and 0.035” J-tipped hydrophilic wire; it was parked deep in the lower lobe branch of the left PA (LPA). This was exchanged with 0.035” Amplatz extra stiff wire and a 7F JR guiding catheter. A Lunderquist guide wire (Cook Medical, Bloomington, IN, USA) was introduced through the guiding catheter, and the extra stiff wire was removed. The 8F femoral vein short sheath was upgraded to a 14F Python sheath (Meril’s Life Sciences Pvt. Ltd., Vapi, India). Through 6F left femoral artery access, the left coronary artery was engaged with a 5F Judkins left catheter, and through 6F right internal jugular venous access, a 6F pigtail was placed in the RVOT. A 5F balloon-tipped temporary pacemaker lead was placed in the RV through a 7F Mullins sheath from a left femoral vein (Cook Medical, Bloomington, IN, USA). Through the 14F Python sheath, a 30 mm × 50 mm Z-med balloon (Numed Inc., TX, USA) was introduced over the wire and placed across the RVOT. The balloon was inflated with RV pacing at 200 bpm, and a simultaneous angiogram was done from RVOT and the left coronary artery. There was no coronary compression, the balloon completely occluded the RVOT, and the measurement at the waist was 29 mm × 30 mm [Figure 2]. The area at the narrowest part of RVOT calculated from this was 684 mm2, and with 25% oversizing, the expected area for the valve was 854 mm2. This will be slightly more than the valve area that a 32 mm valve can achieve, so we planned to implant a 35 mm valve. With a 7F JR guiding catheter, a second Lunderquist wire was placed in the LPA to act as a buddy wire. A Myval™ 35 mm XXL transcatheter heart valve (Meril’s Life Sciences Pvt. Ltd., Vapi, India) was crimped over a 35 mm Navigator™ Transcatheter heart valve delivery system (Meril’s Life Sciences Pvt. Ltd., Vapi, India) with a Val-de-crimp™ crimping tool. The crimped valve profile was 8 mm in diameter and a length of 31 mm. The nominal volume of the navigator balloon for 35 mm was 50 mL. The valve mounted on the delivery system balloon was introduced over one of the Lunderquist wires and was positioned across the RVOT with angiographic guidance. Then, the buddy wire was removed. After confirmation of the position, with RV pacing at 200 bpm and simultaneous RV angiogram, the valve was deployed at the annulus by balloon inflation with 50 mL [Figure 3]. A stable valve position was achieved, and the balloon was deflated and withdrawn. The final valve diameter was 35 mm, and the height was 24 mm. A coronary angiogram was done, which showed unobstructed coronaries, and a PA angiogram was done after the valve implantation, which showed no PR [Figure 4]. Postprocedure RV systolic pressure was 33 mmHg with an end-diastolic pressure of 10 mmHg, and the PA pressure was 32/17 mmHg with a mean PA pressure of 23 mmHg. The postprocedure echocardiogram showed no RVOTO, PR, or mild tricuspid regurgitation with normal RV function. At 11-month follow-up, the patient remains symptom-free, and the echocardiogram shows no PR and RVOTO. A cardiac MRI done on follow-up showed indexed RVEDVi and RVESVi volumes of 133 mL/m2 and 64 mL/m2. The RVEF was 51.6%, and the PR fraction was 1.3%.
Figure 2.
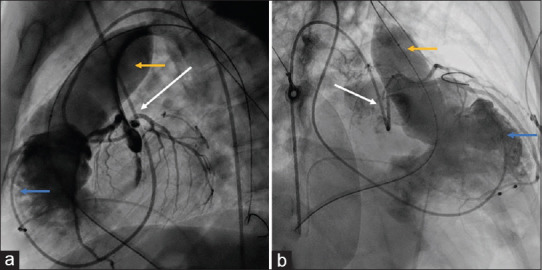
Lateral (a) and right anterior oblique 30 (b) projections showing balloon interrogation with 30 mm × 50 mm Z-Med balloon (yellow arrow) over Lunderquist wire, simultaneous right ventricular angiogram with pigtail catheter (blue arrow), and left coronary injection with Judkins left catheter (white arrow). There was no coronary compression, and the right ventricular outflow tract size was measured
Figure 3.
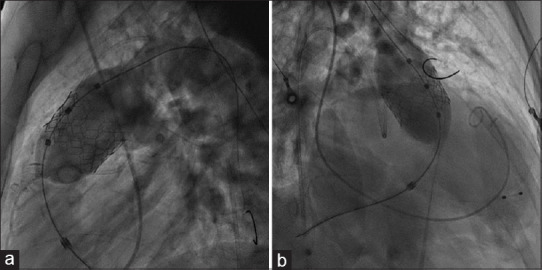
Lateral (a) and right anterior oblique 30 (b) projections showing 35 mm Myval THV being deployed using a navigator balloon with a nominal volume of 50 mL
Figure 4.
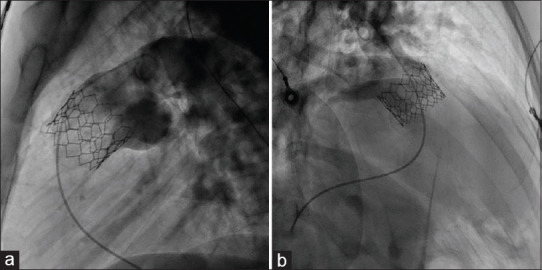
This pulmonary artery angiogram, taken after valve deployment, showing normal leaflet movement and no pulmonary regurgitation in lateral (a) and right anterior oblique 30 (b) projection
DISCUSSION
TPVI was first done by Bonhoeffer et al. in 2000 using a stented valve made from a bovine jugular vein in a boy with RV to PA conduit dysfunction.[1] This was an essential milestone in the care of patients with dysfunctional RVOT, especially those postintracardiac rTOF. These patients used to undergo multiple surgeries in their lives, starting with shunt surgeries in early infancy, intracardiac repair, and later pulmonary valve replacement for RVOT dysfunction. The introduction of TPVI reduced the need for repeated open-heart surgeries for pulmonary valve replacement. Over the past 24 years, TPVI has become the standard of care for RVOT dysfunction at most centers. Initial transcatheter heart valves introduced were the Melody™ valve from Medtronic and the SAPIEN™ heart valve from Edwards Lifesciences. These valves had limited size options, and their utility was mainly restricted to those patients with RV to PA conduits and small or normal-sized RVOT.[2,3] TPVI in developing countries like India was limited by the cost of the available valves and by the larger RVOT dimensions due to delayed presentation.[4] Table 1 summarizes all the available platforms used in TPVI. Until now, only Venus Medtech, a self-expanding porcine valve with a nitinol frame, is available in sizes up to 36 mm. This valve has been successfully used in patients with large RVOT.[5]
Table 1.
Pulmonary valves available for transcatheter implantation
| Name | BEV/SEV | Material | Valve diameter | Anatomy |
|---|---|---|---|---|
| Melody™ (Medtronic Inc., MN, US) | Balloon expandable | Bovine jugular vein valve in platinum–iridium frame | 16 and 18 mm | Conduit ViV |
| SAPIEN (Edwards Life Sciences, CA, US) | Balloon expandable | Bovine pericardium in cobalt–chromium frame | 20, 23, 26, 29 | Conduit ViV Native RVOT |
| Myval (Meril’s Life Sciences Pvt. Ltd., Gujarat, India) | Balloon expandable | Bovine pericardium in nickel–cobalt frame | 20, 21.5, 23, 24.5, 26, 27.5, 29, 30.5, 32 mm | Conduit ViV Native RVOT |
| Harmony (Medtronic Inc., MN, US) | Self-expanding | Porcine pericardium in nitinol frame | Waist 22, 25 | Native RVOT |
| Med-Zenith PT (Beijing MedZenith, Beijing, China) | Self-expanding | Porcine pericardium in nitinol frame | Waist 20, 23, 28 | Native RVOT |
| Pulsta (TaeWoong Medical Co, Gyeonggido, South Korea) | Self-expanding | Porcine pericardium in nitinol frame | Waist 18, 20, 22, 24, 26, 28, 30, and 32 | Native RVOT |
| Venus P Valve (Venus MedTech, Shanghai, China) | Self-expanding | Porcine pericardium in nitinol frame | Waist 18, 20, 22, 24, 26, 28, 30, 32, 34, and 36 | Native RVOT |
BEV: Balloon-expandable valve, SEV: Self-expanding valve, ViV: Valve-in-valve, RVOT: Right ventricular outflow tract
Myval™ is a balloon expandable trileaflet valve made of the bovine pericardium on a nickel-cobalt alloy frame for high radial strength and radiopacity with a hybrid honeycomb cell design.[6] Myval™ valve was initially designed for use in transcatheter aortic valve implantation and is available in a wide range of sizes from 20 to 32 mm with increments of 1.5 mm. Early experience from India with Myval™ in TPVI showed good short-term outcomes in seven patients with dysfunctional RVOT.[7]
Our patient had a significant native RVOT dilatation. The preprocedural evaluation showed that the available valves were unsuitable for implantation in this patient. Hence, a custom-made valve of size 35 mm Myval™ was manufactured by Meril’s Life Sciences, India. The patient underwent successful TPVI with a 35 mm balloon-expandable valve. This is the first report of a 35 mm balloon-expandable valve implanted in native RVOT, probably TPVI, with the largest RVOT dimension ever done with a balloon-expandable valve. This case is a starting point for the inclusion of patients with dilated native RVOT for TPVI who are otherwise offered surgical pulmonary valve implantation. This is particularly important in patients from low-middle income countries where the patients present late with dilated and dysfunctional RVOT and are forced to be offered surgical PVI, increasing the burden on already over-burdened and depleted surgical facilities. Careful preprocedural evaluation, planning, and collaboration with valve manufacturers can improve the care of these patients and help us offer what is best and most comfortable for them.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–5. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 2.Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the U. S. Clinical trial. J Am Coll Cardiol. 2009;54:1722–9. doi: 10.1016/j.jacc.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Martin MH, Meadows J, McElhinney DB, Goldstein BH, Bergersen L, Qureshi AM, et al. Safety and feasibility of melody transcatheter pulmonary valve replacement in the native right ventricular outflow tract: A multicenter pediatric heart network scholar study. JACC Cardiovasc Interv. 2018;11:1642–50. doi: 10.1016/j.jcin.2018.05.051. [DOI] [PubMed] [Google Scholar]
- 4.Patel ND, Levi DS, Cheatham JP, Qureshi SA, Shahanavaz S, Zahn EM. Transcatheter pulmonary valve replacement: A review of current valve technologies. J Soc Cardiovasc Angiogr Interv. 2022;1:100452. doi: 10.1016/j.jscai.2022.100452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivakumar K, Sagar P, Qureshi S, Promphan W, Sasidharan B, Awasthy N, et al. Outcomes of Venus p-valve for dysfunctional right ventricular outflow tracts from Indian Venus P-valve database. Ann Pediatr Cardiol. 2021;14:281–92. doi: 10.4103/apc.APC_175_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. [[Last accessed on 2024 Sep 02]]. Available from: https://www.merillife.com/medical-devices/vascular-intervention/heart-valves/tavr/myval .
- 7.Sivaprakasam MC, Reddy JR, Gunasekaran S, Sivakumar K, Pavithran S, Rohitraj GR, et al. Early multicenter experience of a new balloon expandable MyVal transcatheter heart valve in dysfunctional stenosed right ventricular outflow tract conduits. Ann Pediatr Cardiol. 2021;14:293–301. doi: 10.4103/apc.apc_242_20. [DOI] [PMC free article] [PubMed] [Google Scholar]