ABSTRACT
Cardiac strangulation is a rare phenomenon in children following epicardial pacemaker implantation, caused by compression of the heart and great vessels by the epicardial pacemaker wires. We report a rare case of cardiac strangulation presenting after 8 years of epicardial pacemaker implantation. On routine follow-up, computed tomography angiography showed significant extrinsic compression of the mid-left anterior descending (LAD) artery by the epicardial pacing wire. She was referred to our department for myocardial perfusion imaging (MPI), which showed significant inducible ischemia in the LAD territory. Following this, she underwent a successful pacemaker reimplantation. MPI can, thus, act as a good tool to assess the functional significance of the compression caused by strangulation of the heart by pacemaker leads in asymptomatic patients.
Keywords: Congenital heart block, myocardial ischemia, pacing in children
INTRODUCTION
Cardiac strangulation is a rare life-threatening complication following epicardial pacemaker implantation in growing children due to mechanical compression of the heart and the great vessels. It can be subclinical or may present with symptoms such as syncope, chest pain, new-onset murmur, and impaired exercise tolerance. The incidence of such cases has been reported to be 0.016%. Here, we report a similar case with findings of subclinical inducible ischemia on the myocardial perfusion imaging (MPI) scan. To the best of our knowledge, only two such cases with nuclear imaging have been reported so far.
CASE REPORT
An 8-year-old child with a history of permanent epicardial pacemaker implantation in 2015 at 1 month of age for congenital complete heart block presented with heart failure in 2018, which did not improve significantly with medical management. As a result, it was converted to a cardiac resynchronization therapy defibrillator.
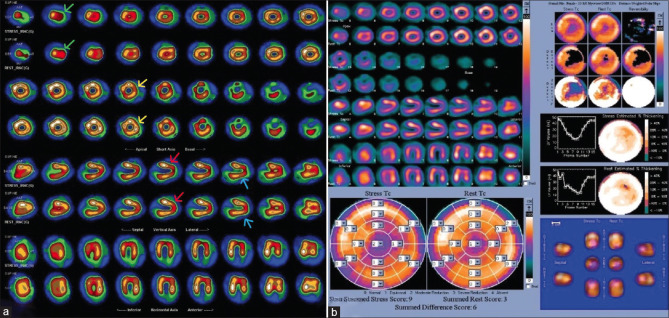
Routine follow-up electrocardiogram in 2023 showed paced rhythm, right bundle branch block with left anterior fascicular block, and relatively narrow QRS. The pacemaker interrogation suggested issues with right atrial lead (high resistance with capture failure). Two-dimensional echocardiography showed right ventricular outflow tract (RVOT) outpouching with turbulence across it, mild RVOT obstruction, and a peak systolic gradient of 19 mmHg. Computed tomography (CT) angiography [Figure 1] showed epicardial pacemaker leads tightened and getting embedded in the myocardium around the right and left ventricle (LV), causing (a) saccular aneurysmal dilation of the right ventricle with a narrow neck and aneurysmal sac, (b) small LV apical aneurysm neck and aneurysmal sac, (c) dilated LV lateral wall aneurysmal sac, (d) mild anteroposterior compression of the RVOT, and (e) significant extrinsic compression of the mid-left anterior descending (LAD) by the epicardial pacing wire with no forward flow noted in mid/distal LAD, distal to the compression. She was referred for an MPI scan. Resting images were acquired first. After this, the patient underwent pharmacological stress with adenosine, which was infused over 4 min at 140 mg/kg/min. At 2 min, 99mTc-tetrofosmin was injected IV. After 60 min, poststress images were acquired. Poststress images showed mild to moderate hypoperfusion in the apex, apical anterior, apical inferior, and anterolateral walls. Resting images showed partial improvement in these defects. Gated images showed resting left ventricular ejection fraction (LVEF) = 44% and poststress LVEF = 45%. Overall scan findings showed large areas of inducible ischemia (~8.8% of the total myocardium) in the apex, apical anterior, apical inferior, and anterolateral wall (LAD territory), with surrounding infarct in the same region [Figure 2].
Figure 1.
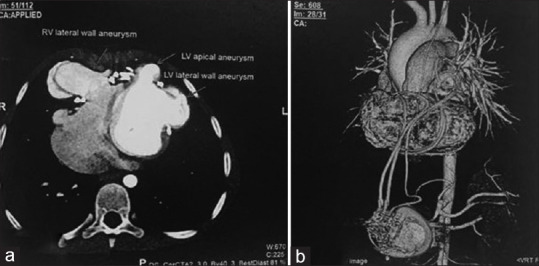
Computed tomography angiography: (a) Three-dimensional reconstructed image showing status postcardiac pacemaker implantation with epicardial pacemaker wires encircling the heart and causing significant extrinsic compression of the mid left anterior descending; and (b) axial section showing right ventricular lateral wall and left ventricular apical and lateral wall aneurysms
Figure 2.
(a) STEP10 images of myocardial perfusion imaging – short, vertical, and long axis showing mild-to-moderate hypoperfusion in apex (green arrows), apical anterior (red arrows), apical inferior (blue errors), and anterolateral wall (yellow areas) on stress images, which show partial improvement on resting images, (b) polar map showing summed difference score (summed stress score – summed rest score) of 6
Following all the investigations, she was posted for a pacemaker change. A median sternotomy was done. Adhesions were noted all over the pericardium, and vascular adhesions were also seen. Both electrodes were isolated and changed. She was shifted to the intensive care unit for close monitoring [Figure 3]. The inotropic support was gradually tapered, and she was discharged in hemodynamically stable condition. Echocardiography at discharge showed normal left ventricular function (LVEF: 55%).
Figure 3.
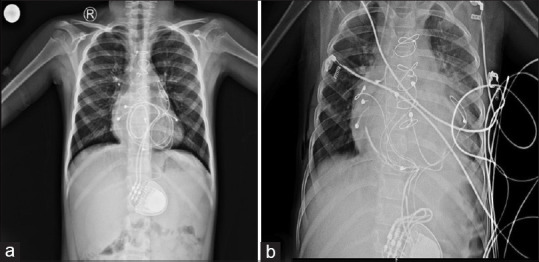
(a) Preoperative chest X-ray (CXR) shows looping of pacemaker wires around the heart and (b) postoperative CXR shows successful pacemaker change
DISCUSSION
Cardiac strangulation is a rare, life-threatening complication that occurs due to the growing heart in children getting mechanically strangulated by the epicardial pacemaker leads. It can lead to coronary artery stenosis, impaired filling of the heart, valvular insufficiency, arrhythmia, ventricular dysfunction, and sudden cardiac death. The patients can either be asymptomatic or present with dizziness, chest pain, new-onset murmur, impaired exercise tolerance, myocardial infarction, heart failure, etc. Presentation depends on the area of maximum compression. The overall incidence of such reported cases worldwide is 0.016%. However, according to Carreras et al., incidences were higher with 2.3% incidence and 1.2% mortality.[1] Ideal methods of implantation include minimal length of redundant lead and avoiding lead loops to be placed on the anterior surface of the heart.[2] A study by Carreras et al. recommended screening posteroanterior (PA) and lateral chest radiographs every 3 years during the child’s growth period, usually until 20 years of age.[1] However, given its low sensitivity, patients with suspicious symptoms but equivocal X-rays should undergo Cine CT or coronary angiography.[3] CT has disadvantages, like streak artifacts from epicardial leads, which make assessing the coronary arteries difficult. The only treatment option is to remove the leads by surgery and replace them with new leads. However, lead removal becomes difficult with increasing duration of implantation as the leads adhere to the heart with encapsulating fibrous tissue.
MPI is a nuclear medicine functional imaging modality to assess coronary blood flow. Resting and poststress (exercise or pharmacological stress) images are acquired and compared to evaluate inducible ischemia. Indications include detecting coronary artery disease, assessing the significance of coronary artery stenosis, viability, response to treatment, and risk stratification. It is a well-established imaging modality in adults. However, its use in the pediatric age group has been very limited because of fewer referrals from cardiologists, radiation exposure, and the inability to stress kids adequately.[4] The main indications in the pediatric population include Kawasaki disease, arterial switch operation posttransposition of great arteries, and anomalous origin of the left coronary artery from the pulmonary artery, among many others.[5] In our case, we used MPI to study the significance of the extrinsic compression of mid-LAD by the epicardial wire and found a large area of inducible ischemia.
Catheter angiography is usually recommended in equivocal and even in positive cardiac CT cases to confirm “strangulation” even in the presence of coronary artery compression and to assess the severity of the lesions.[3] However, it is invasive and involves considerable radiation exposure (around 10 mSv), which is more than an MPI study (2–8 mSv). Hence, MPI can be a good tool to evaluate asymptomatic patients with high clinical suspicion, study the functional significance of coronary compression, analyze reversibility of strangulation postleads replacement, and also potentially avoid the need for invasive catheter angiograms. Further, long-term studies are required to establish the importance and incremental benefits of MPI over other modalities in cardiac strangulation. We found only two previous case reports with MPI findings demonstrated in a case of cardiac strangulation.[6,7]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Carreras EM, Duncan WJ, Djurdjev O, Campbell AI. Cardiac strangulation following epicardial pacemaker implantation: A rare pediatric complication. J Thorac Cardiovasc Surg. 2015;149:522–7. doi: 10.1016/j.jtcvs.2014.10.094. [DOI] [PubMed] [Google Scholar]
- 2.Morrison ML, Karayiannis S, Speggiorin S, Sands AJ, Rosenthal E. Cardiac strangulation after epicardial pacing: The importance of non-specific symptoms and a low index of suspicion. Cardiol Young. 2021;31:159–62. doi: 10.1017/S104795112000342X. [DOI] [PubMed] [Google Scholar]
- 3.Mah DY, Prakash A, Porras D, Fynn-Thompson F, DeWitt ES, Banka P. Coronary artery compression from epicardial leads: More common than we think. Heart Rhythm. 2018;15:1439–47. doi: 10.1016/j.hrthm.2018.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Sundaram PS, Padma S. Role of myocardial perfusion single photon emission computed tomography in pediatric cardiology practice. Ann Pediatr Cardiol. 2009;2:127–39. doi: 10.4103/0974-2069.58314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moscatelli S, Bianco F, Cimini A, Panebianco M, Leo I, Bucciarelli-Ducci C, et al. The use of stress cardiovascular imaging in pediatric population. Children (Basel) 2023;10:218. doi: 10.3390/children10020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muneuchi J, Doi H, Watanabe M, Ochiai Y. Sub-clinical myocardial ischaemia due to cardiac strangulation by an epicardial pacemaker lead. Cardiol Young. 2020;30:1335–6. doi: 10.1017/S1047951120002395. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe H, Hayashi J, Sugawara M, Hashimoto T, Sato S, Takeuchi K. Cardiac strangulation in a neonatal case: A rare complication of permanent epicardial pacemaker leads. Thorac Cardiovasc Surg. 2000;48:103–5. doi: 10.1055/s-2000-9935. [DOI] [PubMed] [Google Scholar]