Abstract
Background:
Our previous studies suggested that bone mineral density (BMD) correlated with the severity of chronic heart failure (HF) as classified by the New York Heart Association (NYHA) and that blood routine test (BRT)-based biomarkers, including hemoglobin, red blood cells (RBCs), lymphocytes, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and systemic immune-inflammation index, were significantly related with BMD in general population.
Objective:
This work aimed to evaluate the relationship between BRT-based biomarkers and BMD in elderly patients with chronic HF.
Methods:
It was a retrospective study. BRT and BMD were measured on the same day. Chi-square analysis and 1-way analysis of variance or the Wilcoxon rank-sum test were used to compare categorical variables and continuous variables, respectively. Correlation analysis was performed by the Spearman correlation test.
Results:
A total of 1049 participants were enrolled. Hemoglobin, RBCs, white blood cells, neutrophils, monocytes, eosinophils, lymphocyte-to-monocyte ratio, and systemic immune-inflammation index were significantly different among different NYHA groups. The Spearman correlation test showed that lumbar vertebrae 2-4 (L2–L4) BMD was closely related to hemoglobin and RBC, and that femoral neck BMD was also significantly correlated with hemoglobin and RBC, white blood cells, neutrophils, monocytes, and platelets. Furthermore, stratified analysis in different NYHA classes demonstrated, only in NYHA class I and II groups, hemoglobin was significantly related to L2–L4 and femoral neck BMD.
Conclusion:
BRT-based biomarkers were significantly different among different NYHA groups, which deserves further investigation and application in the future.
Keywords: bone mineral density, hemoglobin, heart failure, neutrophil-to-lymphocyte ratio, red blood cells, systemic immune-inflammation index
1. Introduction
Blood routine test (BRT), one of the most common test items in clinical practice, contained a large amount of useful information. In addition to the conventional information about hemoglobin, red blood cells (RBC), white blood cells (WBC), and platelets, it could also obtain a lot of immune and systemic inflammation biomarkers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR) and systemic immune-inflammation index (SII).[1] Increasing evidence suggested a close relationship between BRT-based biomarkers and inflammatory diseases, such as chronic obstructive pulmonary disease, pulmonary hypertension, ulcerative colitis, antineutrophil cytoplasmic antibody-associated vasculitis, malignancy, kidney stone.[2–8]
In addition, our previous work and other studies also demonstrated these BRT-based biomarkers were associated with chronic heart failure (HF) and osteoporosis (OP), both being part of the multiple comorbidities of the elderly.[9–18] For example, Durmus E et al found that NLR and PLR of HF patients were significantly higher compared to those of the controls (P < .01).[9] Similarly, Bai B et al showed that high NLR, coupled with transcriptional activation of neutrophils, correlated with systemic inflammation and functional impairment in HF patients.[10] Moreover, NLR was related with higher mortality risk in HF patients.[11] On the other hand, a lot of studies demonstrated that NLR was inversely related with bone mineral density (BMD).[12–14] Eroglu S et al found PLR was an important marker in the diagnosis of OP in postmenopausal women.[15] In another study from Du YN et al, a significant inverse association was observed between SII and BMD in postmenopausal women.[16] Additionally, Gao K et al reported MLR and PLR levels were remarkably higher in OP patients than in osteopenia patients and that MLR had a higher diagnostic value for OP.[17] More recently, our retrospective study, which included 1366 participants attending the health promotion center of Zhejiang hospital for evaluation of bone health, showed that gender, body mass index (BMI), hemoglobin, RBC, lymphocyte, NLR, PLR, LMR, and SII were significantly related with BMD, and that, after adjustment for gender, age and BMI, hemoglobin, RBC and lymphocyte were independent risk factors for OP.[18] Interestingly, a few researches had indicated bone mass status was related to New York Heart Association (NYHA) classification, the modality most frequently used in clinical practice to express the severity of HF.[19–23] Thus, it is reasonable to speculate that BRT-based parameters was related with BMD in HF patients. However, there had been no relevant researches so far.
Here, using the data from 1049 subjects,[19] we evaluated the relationship between BRT-based biomarkers and BMD in elderly patients with chronic HF.
2. Methods
2.1. Research subjects and materials
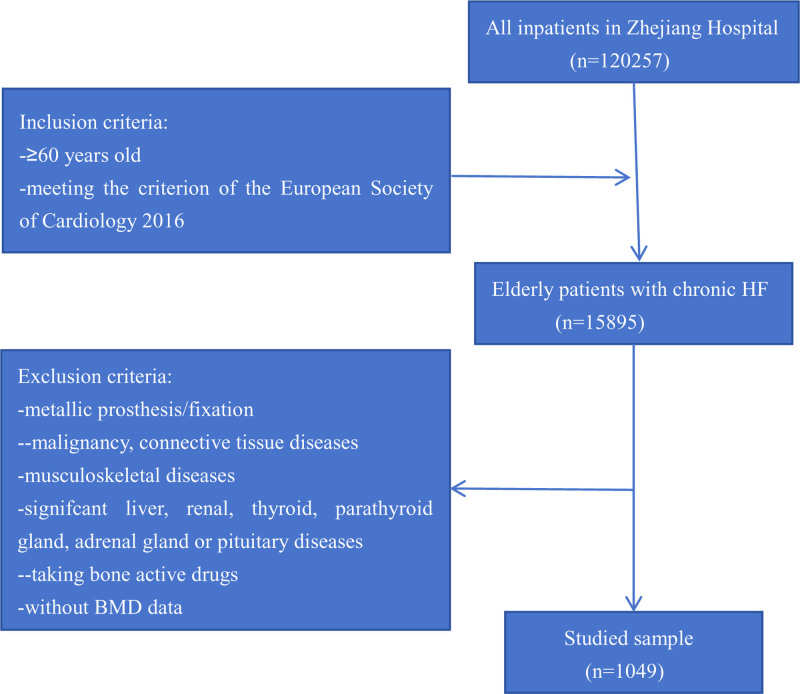
Participants inclusion and exclusion criteria, evaluation of HF severity and NYHA class and BMD measurement were presented in previous study.[19] A detailed overview of the studied sample was provided in Figure 1. It was approved by the Medical Ethics Committee of Zhejiang Hospital (2023-45K).
Figure 1.
Flow chart for the studied sample selection.
2.2. Measurement of BRT-based biomarkers
At the same day of BMD analysis, BRT was conducted. In addition to conventional parameters, including hemoglobin, RBC count, platelet count, WBC count, and leukocyte subpopulation, NLR, PLR, LMR, and SII were calculated. Briefly, NLR was the division of the neutrophile count and the lymphocyte count; PLR was the division of the platelet count and the lymphocyte count; MLR was the division of the monocyte count and the lymphocyte count. In addition, SII was defined as platelet counts × neutrophil counts/lymphocyte counts.
2.3. Statistical analysis
First, a normality check was performed on the continuous variables using the Kolmogorov–Smirnov test. Then, their difference among different groups were analyzed using the one-way ANOVA for variables with normal distribution and the Wilcoxon rank-sum test for the noncategorical variables with non-normal distribution, respectively. In addition, chi-square analysis was used to compare categorical variables. Finally, correlation analysis was performed using Spearman correlation test. All statistical analyses were completed using the SPSS 21.0 software package. P < .05 was considered statistically significant.
3. Results
3.1. BRT-based biomarkers in HF patients with different NYHA functional classes
From October 2008 to September 2016, a total of 1049 chronic HF patients aged over 60 years attending in Zhejiang Hospital for evaluation of heart failure were recruited. Hemoglobin, RBC, WBC, neutrophil, monocyte, eosinophil, LMR, and SII were significantly different among different NYHA groups. However, lymphocyte, basophil, platelets, NLR, and PLR showed no obvious difference. Results were presented in Table 1. And, other clinical parameters, BMD and lab data in different NYHA functional classes were reported before.[19]
Table 1.
Comparison of BRT-based biomarkers in different NYHA group.
| NYHA I (n = 109) | NYHA II (n = 662) | NYHA III (n = 243) | NYHA IV (n = 35) | F/Z value | |
|---|---|---|---|---|---|
| Hemoglobin (g/L) | 132.96 ± 16.29 | 126.32 ± 16.31 | 121.45 ± 17.26 | 120.57 ± 17.05 | 13.755* |
| RBC (1012/L) | 4.41 ± 0.72 | 4.08 ± 0.62 | 3.98 ± 0.66 | 3.95 ± 0.5 | 11.819* |
| WBC (109/L) | 4.99 ± 1.82 | 5.69 ± 1.48 | 7.06 ± 1.84 | 8.15 ± 1.74 | 77.44* |
| Neutrophil (109/L) | 2.86 ± 1.64 | 3.50 ± 1.15 | 4.81 ± 1.58 | 6.21 ± 1.83 | 112.545* |
| Monocyte (109/L) | 0.41 ± 0.16 | 0.47 ± 0.19 | 0.55 ± 0.21 | 0.57 ± 0.22 | 16.896* |
| Lymphocyte (109/L) | 1.54 ± 0.66 | 1.50 ± 0.61 | 1.51 ± 0.59 | 1.26 ± 0.48 | 2.041 |
| Eosinophil (109/L) | 0.13 ± 0.10 | 0.18 ± 0.21 | 0.16 ± 0.14 | 0.09 ± 0.09 | 5.105** |
| Basophil (109/L) | 0.00 ± 0.01 | 0.00 ± 0.04 | 0.01 ± 003 | 0.01 ± 0.03 | 2.110 |
| Platelets (109/L) | 164.50 ± 56.02 | 170.34 ± 85.03 | 176.76 ± 56.36 | 178.14 ± 52.82 | 0.845 |
| NLR | 0.02 ± 0.01 | 0.03 ± 0.08 | 0.04 ± 0.20 | 0.04 ± 0.02 | 1.567 |
| PLR | 121.94 ± 70.91 | 131.99 ± 105.00 | 131.94 ± 59.34 | 160.08 ± 69.96 | 1.521 |
| LMR | 4.16 ± 1.92 | 3.55 ± 2.01 | 3.14 ± 1.78 | 2.44 ± 1.39 | 10.555* |
| SII | 394.78 ± 613.07 | 483.44 ± 642.61 | 651.08 ± 399.47 | 1045.25 ± 638.68 | 15.492* |
BRT = blood routine test, F = the one-way ANOVA, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, NYHA = New York Heart Association, PLR = platelet-to-lymphocyte ratio, RBC = red blood cells, SII = systemic immune-inflammation index, WBC = white blood cells, Z = Wilcoxon rank-sum test.
P < .01.
P < .05.
3.2. Correlation analysis between BRT-based biomarkers and BMD in HF patients
First, Spearman correlation test was used to determine the correlation between BRT-based biomarkers and BMD in HF patients. In the present study, BMD at the level of lumbar vertebrae 2–4 (L2–L4) and femoral neck (FN) were measured using dual-energy X-ray absorptiometry for all participants. As shown in Table 2, L2–L4 BMD was closely related to hemoglobin and RBC. In contrast, besides hemoglobin and RBC, FN BMD was also significantly correlated with WBC, neutrophil, monocyte, and platelets.
Table 2.
Correlation analysis between BRT-based biomarkers and BMD in chronic HF patients.
| BMD L2–L4 | BMD FN | |||
|---|---|---|---|---|
| r | P value | r | P value | |
| Hemoglobin (g/L) | 0.143 | <.0001 | 0.174 | <.0001 |
| RBC (1012/L) | 0.122 | <.0001 | 0.105 | .001 |
| WBC (109/L) | ‐0.022 | .476 | ‐0.072 | .020 |
| Neutrophil (109/L) | ‐0.040 | .201 | ‐0.077 | .013 |
| Monocyte (109/L) | 0.044 | .157 | ‐0.063 | .041 |
| Lymphocyte (109/L) | ‐0.031 | .320 | 0.005 | .879 |
| Eosinophil (109/L) | 0.059 | .055 | 0.041 | .180 |
| Basophil (109/L) | ‐0.014 | .641 | 0.015 | .632 |
| Platelets (109/L) | ‐0.057 | .067 | ‐0.069 | .025 |
| NLR | 0.036 | .244 | 0.057 | .063 |
| PLR | ‐0.031 | .309 | ‐0.056 | .071 |
| LMR | ‐0.056 | .069 | 0.022 | .477 |
| SII | ‐0.013 | .679 | ‐0.050 | .108 |
BMD = bone mineral density, BRT = blood routine test, FN = femoral neck, HF = heart failure, LMR = lymphocyte-to-monocyte ratio, L2–L4 = lumbar vertebrae 2 to 4, NLR = neutrophil-to-lymphocyte ratio, PLR = platelet-to-lymphocyte ratio, RBC = red blood cells, SII = systemic immune-inflammation index, WBC = white blood cells.
3.3. Correlative analysis between BRT-based biomarkers and BMD in different HF groups
Then, to further explored the association of BRT-based biomarkers with BMD, we conducted a stratified analysis in different NYHA classes. In NYHA class I group, only hemoglobin was significantly related with L2–L4 and FN BMD. As for NYHA class II, the parameters related to L2–L4 and FN BMD included hemoglobin, RBC and neutrophil. However, no BRT- based biomarkers showed relationship with BMD, except for that WBC and neutrophil with L2–L4 BMD and that neutrophil with FN BMD. The results of stratified analysis were summarized in Table 3.
Table 3.
Correlation analysis between biomarkers originated from BRT and BMD in different NYHA class.
| BMD L2–L4 | BMD FN | ||||
|---|---|---|---|---|---|
| r | P value | r | P value | ||
| NYHA I | Hemoglobin (g/L) | 0.239 | .012 | 0.247 | .01 |
| RBC (1012/L) | 0.155 | .106 | 0.153 | .113 | |
| WBC (109/L) | 0.012 | .902 | 0.014 | .881 | |
| Neutrophil (109/L) | 0.074 | .445 | 0.089 | .355 | |
| Monocyte (109/L) | ‐0.042 | .664 | 0.085 | .380 | |
| Lymphocyte (109/L) | ‐0.188 | .050 | ‐0.137 | .156 | |
| Eosinophil (109/L) | ‐0.039 | .685 | ‐0.166 | .084 | |
| Basophil (109/L) | 0.297 | .002 | ‐0.034 | .725 | |
| Platelets (109/L) | ‐0.043 | .659 | ‐0.013 | .897 | |
| NLR | 0.121 | .209 | 0.115 | .235 | |
| PLR | 0.088 | .364 | 0.078 | .419 | |
| LMR | ‐0.147 | .127 | ‐0.187 | .051 | |
| SII | 0.084 | .386 | 0.036 | .707 | |
| NYHA II | Hemoglobin (g/L) | 0.108 | .005 | 0.160 | <.0001 |
| RBC (1012/L) | 0.146 | <.0001 | 0.113 | .004 | |
| WBC (109/L) | ‐0.064 | .099 | ‐0.066 | .092 | |
| Neutrophil (109/L) | ‐0.109 | .005 | ‐0.082 | .034 | |
| Monocyte (109/L) | 0.077 | .049 | ‐0.001 | .975 | |
| Lymphocyte (109/L) | ‐0.028 | .475 | ‐0.014 | .711 | |
| Eosinophil (109/L) | 0.031 | .423 | 0.077 | .049 | |
| Basophil (109/L) | ‐0.041 | .288 | ‐0.003 | .938 | |
| Platelets (109/L) | ‐0.071 | .069 | ‐0.055 | .161 | |
| NLR | 0.046 | .235 | 0.083 | .033 | |
| PLR | ‐0.041 | .295 | ‐0.067 | .087 | |
| LMR | ‐0.058 | .133 | ‐0.021 | .596 | |
| SII | ‐0.020 | .614 | ‐0.028 | .466 | |
| NYHA III | Hemoglobin (g/L) | 0.138 | .032 | 0.092 | .152 |
| RBC (1012/L) | 0.029 | .657 | 0.004 | .946 | |
| WBC (109/L) | 0.203 | .001 | 0.095 | .139 | |
| Neutrophil (109/L) | 0.216 | .001 | 0.104 | .107 | |
| Monocyte (109/L) | 0.088 | .170 | ‐0.067 | .296 | |
| Lymphocyte (109/L) | ‐0.012 | .856 | 0.053 | .407 | |
| Eosinophil (109/L) | 0.141 | .028 | ‐0.003 | .964 | |
| Basophil (109/L) | 0.019 | .767 | 0.083 | .199 | |
| Platelets (109/L) | 0.006 | .927 | ‐0.108 | .093 | |
| NLR | 0.044 | .495 | 0.068 | .294 | |
| PLR | ‐0.017 | .789 | ‐0.053 | .409 | |
| LMR | ‐0.104 | .107 | 0.041 | .528 | |
| SII | 0.094 | .143 | ‐0.009 | .890 | |
| NYHA IV | Hemoglobin (g/L) | 0.378 | .025 | 0.198 | .253 |
| RBC (1012/L) | 0.274 | .111 | 0.094 | .593 | |
| WBC (109/L) | 0.138 | .429 | 0.483 | .003 | |
| Neutrophil (109/L) | 0.193 | .267 | 0.537 | .001 | |
| Monocyte (109/L) | ‐0.202 | .244 | ‐0.166 | .340 | |
| Lymphocyte (109/L) | ‐0.090 | .607 | ‐0.140 | .422 | |
| Eosinophil (109/L) | ‐0.126 | .472 | ‐0.326 | .056 | |
| Basophil (109/L) | 0.020 | .910 | 0.263 | .127 | |
| Platelets (109/L) | ‐0.088 | .616 | ‐0.060 | .732 | |
| NLR | 0.143 | .413 | 0.292 | .088 | |
| PLR | 0.080 | .649 | 0.066 | .705 | |
| LMR | 0.094 | .597 | 0.171 | .334 | |
| SII | 0.162 | .354 | 0.266 | .123 | |
BMD = bone mineral density, BRT = blood routine test, FN = femoral neck, LMR = lymphocyte-to-monocyte ratio, L2–L4 = lumbar vertebrae 2 to 4, NLR = neutrophil-to-lymphocyte ratio, NYHA = New York Heart Association, PLR = platelet-to-lymphocyte ratio, RBC = red blood cells, SII = systemic immune-inflammation index, WBC = white blood cells.
4. Discussion
Our retrospective research showed many BRT-based biomarkers, such as hemoglobin, RBC, WBC, neutrophil, monocyte, eosinophil, LMR, and SII, were significantly related to the HF severity expressed by NYHA classes. In agreement with our results, through a 6-month follow-up on 390 patients with HF, Silva N et al found that patients who died of HF had significantly lower values of LMR and that WBC and monocyte counts revealed a multivariate-adjusted risk for both endpoints of HF and all-cause mortality.[24] However, contrary to the findings from Durmus E and Bai B et al which displayed a negative correlation between NLR, PLR and HF,[9,10] our study failed to confirm the relationship between NLR, PLR and HF. The reasons for these inconsistencies might be the differences in sample size, research subjects, and methods. However, the exact reason was not very clear and required further research in the future.
This study contained a relatively large sample size, allowing us to conduct stratified analysis of the chronic HF patients in different NYHA classes. The results of the subgroup analysis indicated that, in NYHA class I and II, hemoglobin was closed related to BMD both at L2–L4 and FN. Interestingly, our previous work demonstrated that, after adjustment for gender, age and BMI, hemoglobin was an independent risk factor for OP in general population.[18] Consistently, using a conditional logistic regression model among 69,760 OP patients, Kim et al estimated the association of hemoglobin level with OP and found that the hemoglobin level was associated with 0.98-fold lower odds for OP (95% CI 0.97–0.99, P < .001).[25] Correspondingly, hemoglobin level was related with a 0.97-fold lower risk of OP in patients with type 2 diabetes mellitus.[26] Taken together, the above results suggested that there was a significant correlation between hemoglobin and BMD not only in ordinary population but also in patients with HF or diabetes.
The possible mechanism of how hemoglobin influenced BMD might be hypoxemia, which was reported to mediate the risk of OP. It is well known that adaptations to hypoxia were mediated by hypoxia-inducible factor (HIF) transcription factors, of which there were 2 transcriptionally active isoforms (HIF-1α and HIF-2α). Via osteocyte-specific loss-of-function and gain-of-function HIF-1α and HIF-2α mutations in C57BL/6 female mice, Mendoza SV et al found that degradation-resistant HIF-2α, but not HIF-1α, generated dramatic increases in bone mass, enhanced osteoclast activity.[27]
Additionally, both inflammation and cytokines might also underlying the link between hemoglobin and BMD.[28,29] For example, Zha L et al found that postmenopausal women with OP had increased levels of tumor necrosis factor-α, compared with those without OP, and that tumor necrosis factor-α synergistically promoted receptor activator of nuclear factor-kB ligand-induced osteoclast formation by activation of nuclear factor kappa-B and phosphatidylinositol 3-kinase/protein kinase B signaling.[28] Finally, erythropoietin might contribute to bone formation both directly by communication pathways and indirectly by increasing vascular endothelial growth factor expression.[30] However, the exact mechanism underlying the link between hemoglobin and BMD remained unclear. In addition, the reason why hemoglobin only rose in the early stages of HF was not yet clear.
As mentioned above, the present study had relatively large sample size. Moreover, it had BMD data at different sites. However, there were some limitations. First, it was a retrospective, observational association study, which might leave uncertainty. Second, the current data lacked clinical outcome, such as survival time and fracture. Third, although the overall sample size was relatively large, the sample size of certain subgroups was relatively small. For example, there were only 35 patients in NYHA class IV. Finally, the participants were heterogeneous, with diversities of medication and different morbidity, which might confound the results.
5. Conclusion
Our study showed that there was a significant correlation between BRT-based biomarkers and HF severity and that hemoglobin was closely related to L2–L4 and FN BMD in NYHA class I and II HF patients, implying that we should pay more attention to the hemoglobin value in patients with HF, in clinical practice. Furthermore, considering that BRT was a convenient and inexpensive detection method in clinic, application of biomarkers originated from BRT in HF were worth further research.
Author contributions
Conceptualization: Lan Chen.
Data curation: Lan Chen.
Investigation: Fan Xu, Qian Tong.
Methodology: Guofu Wang, Qian Tong.
Software: Fan Xu.
Validation: Qian Tong.
Writing – review & editing: Guofu Wang.
Writing – original draft: Fan Xu.
Abbreviations:
- BMD
- bone mineral density
- BMI
- body mass index
- BRT
- blood routine test
- FN
- femoral neck
- HF
- heart failure
- HIF
- hypoxia-inducible factor
- L2–L4
- lumbar vertebrae 2–4
- LMR
- lymphocyte-to-monocyte ratio
- NLR
- neutrophil-to-lymphocyte ratio
- NYHA
- New York Heart Association
- OP
- osteoporosis
- PLR
- platelet-to-lymphocyte ratio
- RBC
- red blood cells
- SII
- systemic immune-inflammation index
- WBC
- white blood cells
The Medical Ethics Committee of Zhejiang Hospital approved this study (2023-45K). This was a retrospective study which used patients’ medical records from October 2008 to September 2016 rather than directly contacting patients. The risk of this research was no more than the minimum risk. More importantly, the researchers have promised to protect the privacy of participants. Hence, according to the 39th provisions of Chinese law, the Ethical Review of Human Biomedical Research (2016), the Medical Ethics Committee of Zhejiang Hospital agreed to exempt the patients consent.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Chen L, Xu F, Tong Q, Wang G. Blood routine test-based biomarkers is related to bone mineral density in elderly patients with chronic heart failure: A retrospective study. Medicine 2024;103:50(e40946).
Contributor Information
Lan Chen, Email: chenl70817@163.com.
Fan Xu, Email: 165553273@qq.com.
Qian Tong, Email: 1169198943@qq.com.
References
- [1].Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune-inflammation index, based on platelet counts and neutrophil–lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297–304. [DOI] [PubMed] [Google Scholar]
- [2].Lan CC, Su WL, Yang MC, Chen SY, Wu YK. Predictive role of neutrophil-percentage-to-albumin, neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratios for mortality in patients with COPD: evidence from NHANES 2011-2018. Respirology. 2023;28:1136–46. [DOI] [PubMed] [Google Scholar]
- [3].Yao CY, Liu XL, Tang Z. Prognostic role of neutrophil–lymphocyte ratio and platelet-lymphocyte ratio for hospital mortality in patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2017;12:2285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zuo H, Xie X, Peng J, Wang L, Zhu R. Predictive value of novel inflammation-based biomarkers for pulmonary hypertension in the acute exacerbation of chronic obstructive pulmonary disease. Anal Cell Pathol (Amst). 2019;2019:5189165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9:3295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Akpinar MY, Ozin YO, Kaplan M, et al. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict mucosal disease severity in ulcerative colitis. J Med Biochem. 2018;37:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim Y, Choi H, Jung SM, Song JJ, Park YB, Lee SW. Systemic immune-inflammation index could estimate the cross-sectional high activity and the poor outcomes in immunosuppressive drug-naïve patients with antineutrophil cytoplasmic antibody-associated vasculitis. Nephrology (Carlton). 2018;24:711–7. [DOI] [PubMed] [Google Scholar]
- [8].Mao W, Wu J, Zhang Z, Xu Z, Xu B, Chen M. Neutrophil-lymphocyte ratio acts as a novel diagnostic biomarker for kidney stone prevalence and number of stones passed. Transl Androl Urol. 2021;10:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Durmus E, Kivrak T, Gerin F, Sunbul M, Sari I, Erdogan O. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol. 2015;105:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bai B, Cheng M, Jiang L, Xu J, Chen H, Xu Y. High neutrophil to lymphocyte ratio and its gene signatures correlate with diastolic dysfunction in heart failure with preserved ejection fraction. Front Cardiovasc Med. 2021;8:614757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vakhshoori M, Nemati S, Sabouhi S, et al. Neutrophil to lymphocyte ratio (NLR) prognostic effects on heart failure; a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23:555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang C, Li S. Association of blood neutrophil lymphocyte ratio in the patients with postmenopausal osteoporosis. Pak J Med Sci. 2016;32:762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Salimi M, Khanzadeh M, Nabipoorashrafi SA, et al. Association of neutrophil to lymphocyte ratio with bone mineral density in post-menopausal women: a systematic review and meta-analysis. BMC Women Health. 2024;24:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yu XY, Li XS, Li Y, Liu T, Wang R. Neutrophil–lymphocyte ratio is associated with arterial stiffness in postmenopausal women with osteoporosis. Arch Gerontol Geriatr. 2015;61:76–80. [DOI] [PubMed] [Google Scholar]
- [15].Eroglu S, Karatas G. Platelet/lymphocyte ratio is an independent predictor for osteoporosis. Saudi Med J. 2019;40:360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Du YN, Chen YJ, Zhang HY, Wang X, Zhang ZF. Inverse association between systemic immune-inflammation index and bone mineral density in postmenopausal women. Gynecol Endocrinol. 2021;37:650–4. [DOI] [PubMed] [Google Scholar]
- [17].Gao K, Zhu W, Liu W, et al. The predictive role of monocyte-to-lymphocyte ratio in osteoporosis patient. Medicine (Baltimore). 2019;98:e16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen L, Wu Q, Lv X, Tong Q, Xu F, Wang G. The predictive role of blood routine test-based biomarkers in patients with osteoporosis. Ann Clin Case Rep. 2023;8:2445. [Google Scholar]
- [19].Fang Y, Wang L, Xing W, et al. Bone mineral density in older patients with chronic heart failure is related to NYHA classification: a retrospective study. Eur Geriatr Med. 2018;9:183–9. [DOI] [PubMed] [Google Scholar]
- [20].Loncar G, Cvetinovic N, Lainscak M, Isaković A, von Haehling S. Bone in heart failure. J Cachexia Sarcopenia Muscle. 2020;11:381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nedeljkovic BB, Loncar G, Vizin T, Radojicic Z, Brkic VP, Kos J. Relationship of high circulating cystatin C to biochemical markers of bone turnover and bone mineral density in elderly males with a chronic heart failure. J Med Biochem. 2019;38:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xing W, Lv X, Gao W, et al. Bone mineral density in patients with chronic heart failure: a meta-analysis. Clin Interv Aging. 2018;13:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- [24].Silva N, Bettencourt P, Guimarães JT. The lymphocyte-to-monocyte ratio: an added value for death prediction in heart failure. Nutr Metab Cardiovasc Dis. 2015;25:1033–40. [DOI] [PubMed] [Google Scholar]
- [25].Kim SY, Yoo DM, Min C, Choi HG. Association between osteoporosis and low hemoglobin levels: a nested case-control study using a national health screening cohort. Int J Environ Res Public Health. 2021;18:8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xiu S, Mu Z, Sun L, Zhao L, Fu J. Hemoglobin level and osteoporosis in Chinese elders with type 2 diabetes mellitus. Nutr Diabetes. 2022;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mendoza SV, Murugesh DK, Christiansen BA, et al. Degradation-resistant hypoxia inducible factor-2alpha in murine osteocytes promotes a high bone mass phenotype. JBMR Plus. 2023;7:e10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zha L, He L, Liang Y, et al. TNF-α contributes to postmenopausal osteoporosis by synergistically promoting RANKL-induced osteoclast formation. Biomed Pharmacother. 2018;102:369–74. [DOI] [PubMed] [Google Scholar]
- [29].Shahen VA, Gerbaix M, Koeppenkastrop S, et al. Multifactorial effects of hyperglycaemia, hyperinsulinemia and inflammation on bone remodelling in type 2 diabetes mellitus. Cytokine Growth Factor Rev. 2020;55:109–18. [DOI] [PubMed] [Google Scholar]
- [30].Shiozawa Y, Taichman RS. Bone: elucidating which cell erythropoietin targets in bone. Nat Rev Endocrinol. 2015;11:263–4. [DOI] [PubMed] [Google Scholar]